Infliximab is approved for the treatment of patients with moderate to severe active ulcerative colitis (UC) who have had an inadequate response to conventional therapy. While the Active Ulcerative Colitis Trials (ACT) 1 and 2 were conducted in outpatients1–3, infliximab rescue of patients hospitalized with severe UC has become common practice4 since the publication of successful rescue therapy in severe UC with a single 5 mg/kg dose of infliximab5. The optimal induction regimen for patients hospitalized with severe UC is not known, but Gibson, et al., tested accelerated infliximab induction of remission in this issue of Clinical Gastroenterology and Hepatology.
This paper retrospectively compares 15 patients receiving all 3 infliximab induction doses at 5 mg/kg over 2 weeks followed by q8 weekly maintenance to 35 patients receiving standard 6 week infliximab induction and q8 weekly maintenance. At 3 months after the initiation of infliximab therapy, significantly fewer patients needed colectomy in the accelerated compared to the conventional dosing group (1/15; 6.7%) vs. 14/35; 40%), but no differences in colectomy rate were observed at 6 and 12 months of therapy. Higher albumin levels and the use of accelerated induction were associated with avoidance of colectomy in a multivariate analysis. Patients who achieved a CRP nadir of <=5 mg/L had a rate of colectomy of 3.2%, vs. a rate of 68.4% in those who did not achieve this level of control of inflammation.
This accelerated dosing of infliximab has solid theoretical underpinnings, provided by a number of findings which suggest that severe UC may require more infliximab during induction than the usual 5 mg/kg outpatient dosing at weeks 0, 2, and 6. Several pieces of evidence strongly support the importance of adequate serum trough concentrations of infliximab in UC. Higher infliximab serum levels have been shown to positively correlate with treatment success in the ACT 1 and 2 studies as well as in a retrospective analysis of a single center cohort.6–8 Known factors influencing infliximab drug levels include high baseline inflammatory load with elevated levels of TNF and CRP, body size, gender, antibodies to infliximab, and concomitant use of immunosuppressants.9–12 Pharmacokinetic analyses of sera of the ACT 1 and 2 studies revealed that higher serum albumin concentrations were associated with lower infliximab clearance and subsequently longer infliximab half-life.13 Inpatients with severe UC have high inflammatory loads, low albumin levels, and a severely damaged mucosal barrier in the colon.
Another pharmacokinetic concern is the reported loss of infliximab into the intestinal lumen.11 This loss appears to accelerate depletion of circulating infliximab and may be important for the success or failure of infliximab therapy. Taken together the limited pharmacokinetic data presently available suggest that in patients with severe steroid refractory UC the initial aim should be to establish adequate infliximab drug levels either by an increased infliximab dose and /or shorter dosing intervals. It is not clear whether rapid measurement of infliximab levels is required or cost effective. The finding of Gibson, et al., that achieving a CRP nadir of ≤5mg/L was strongly predictive of colectomy outcomes, suggests that close monitoring of serum CRP may be an important component of the care of severe inpatient UC.
Once the patient is in remission, standard infliximab dosing and interval may be adequate, but the diminishing returns reported by Gibson, et al. suggest that there may be value in monitoring of CRP or infliximab levels to guide dosing and interval adjustment, as in the TAXIT trial in Crohn’s disease.14 At this point, we also have limited information about the safety of a more aggressive anti-TNF approach with accelerated dosing. As has been shown in patients with rheumatoid arthritis, increased dose infliximab therapy might lead to higher frequency of infectious complications during induction.15 Furthermore, those patients requiring colectomy after failing high dose infliximab therapy might also face a higher risk for peri-operative infectious complications, which is not the case with the current standard induction.16
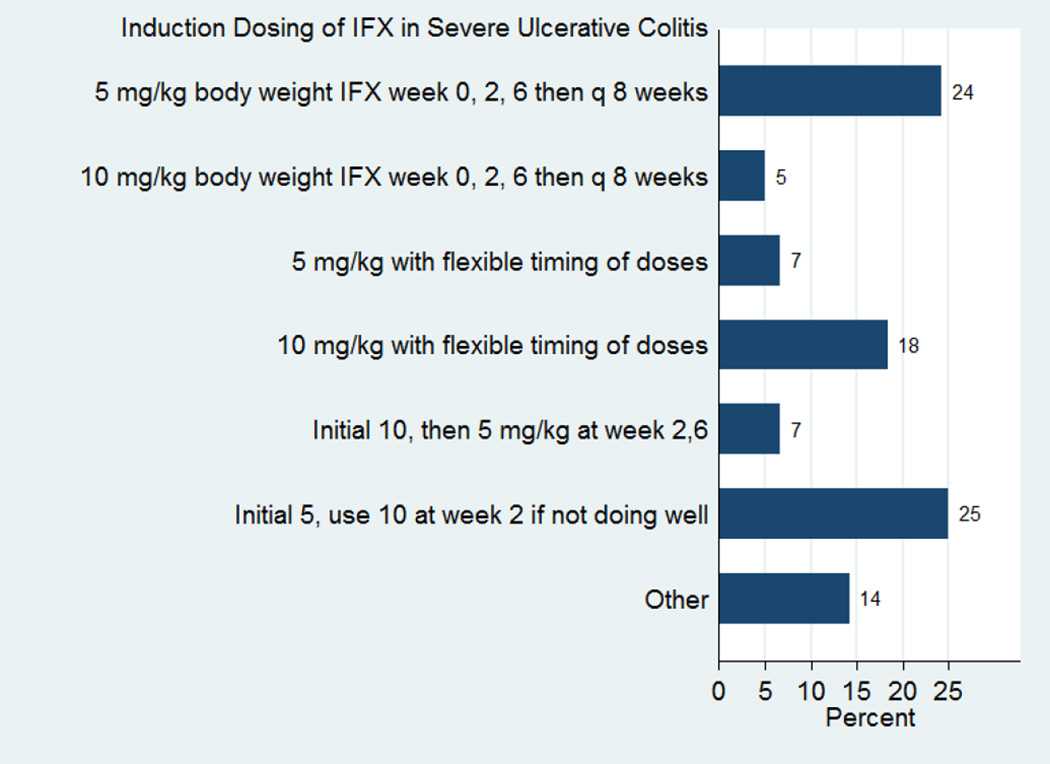
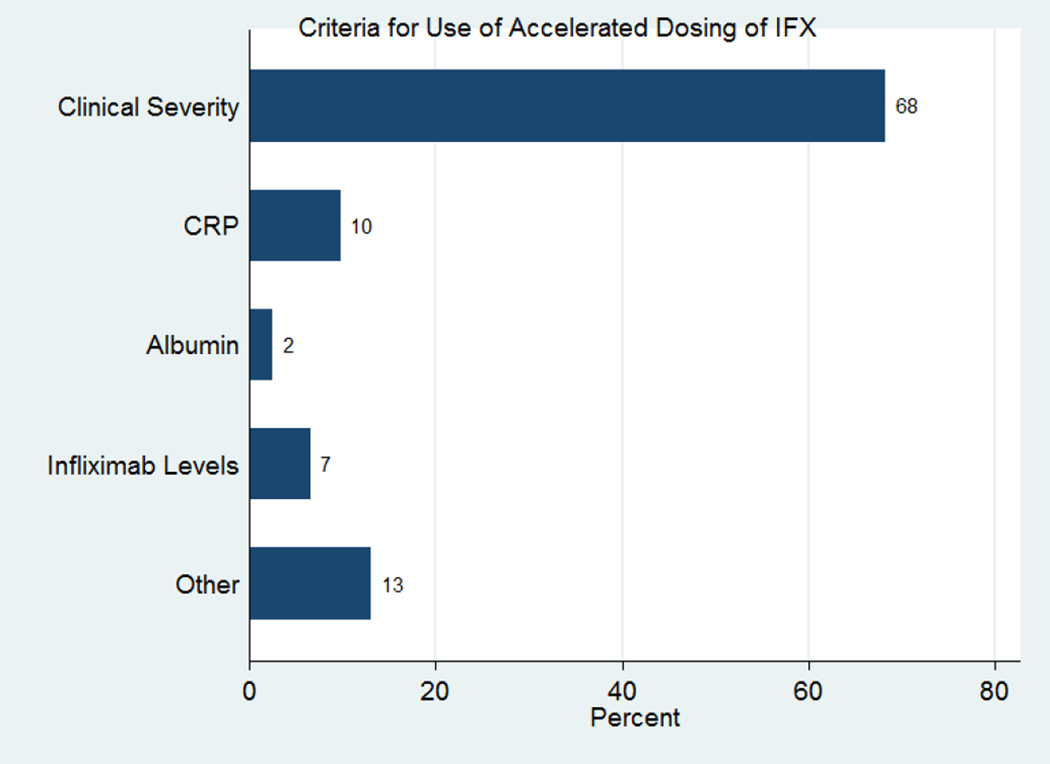
Given the growing evidence that infliximab may be rapidly cleared in severe UC patients, we recently assessed the use of accelerated infliximab dosing in practice. We performed an internet-based survey among all members of the Crohn’s and Colitis Foundation of American Clinical Research Alliance (CCFA-CRA) and active members of the International Organization for Inflammatory Bowel Disease (IOIBD). In this survey 69% of participants practiced in the US (n=85) and 31% outside of the US (n=38). In hospitalized patients with severe UC, only 24% of respondents used the standard dosing of infliximab (5 mg/kg at 0, 2, and 6 weeks) for induction of remission (see Figure 1). Participants were asked what criteria they used to determine which patients should receive accelerated dosing of infliximab in severe inpatient UC. The most common response was clinical symptoms, followed by C-reactive protein (CRP) and infliximab levels, as seen in Figure 2. Participants were asked what criteria they used to determine when patients should receive their next dose of infliximab, if earlier than the standard 2 and 6 weeks. Most chose “Clinical Severity”, but 22% chose “Other” (usually a combination of factors including “Clinical Severity”, CRP, Albumin, and “Endoscopic Severity”), and 11% chose both CRP and infliximab level.
Figure 1. Induction Dosing of Infliximab in Severe Ulcerative Colitis.
Most respondents do not use standard dosing in severe ulcerative colitis. The most common approach was to begin with 5 mg/kg, and increase to 10 mg/kg at week 2 if the patient did not respond well, but there was extensive practice variation.
Figure 2. Criteria for Use of Accelerated Dosing of Infliximab.
More than two thirds of respondents used solely clinical criteria to decide which patients should receive accelerated dosing of infliximab, though many included biomarkers including CRP, ESR, fecal calprotectin, albumin, and infliximab levels when available.
The report of Gibson et al and our survey results are somewhat surprising since current recommendations and guidelines do not mention the 10 mg/kg infliximab dosage in the treatment algorithm for patients with steroid refractory UC.17, 18 No difference was seen in a small pilot trial with 8 patients using a different infliximab dosing schedule (n=3; 5 mg/kg, n=3; 10 mg/kg, n=2; 20 mg/kg) in steroid refractory patients with regard to treatment failures (33.3%, 67.3% and 50%, respectively).19 Two prospective trials comparing the efficacy of infliximab with placebo and of infliximab with cyclosporine in hospitalized patients with steroid refractory UC only used 5 mg/kg infliximab.5, 20 Also, there was no significant difference in treatment outcome comparing infliximab at a dosage of 5 mg/kg versus 10 mg/ kg in the ACT 1 and ACT 2 trials, which evaluated the efficacy of infliximab in the outpatient setting.1 Interestingly, in ACT 2, a trend towards higher remission rates (35.8% with 10 mg/kg vs. 25.6% with 5 mg/kg) and mucosal healing (56.7% for 10 mg/kg vs. 46.3% for 5 mg/kg) was observed in week 30 in the 10 mg/kg infliximab group. However, this trend was not present at week 8. Additionally, an evaluation of the endpoint of colectomy, demonstrated the need for significantly fewer colectomies in the 10 mg/kg infliximab group (8%, p<0.007) compared to placebo therapy (17%).21 This difference in colectomy rates was not significant for the 5 mg/kg infliximab group (12% p<.16).
In conclusion, our cross sectional survey of IBD specialists clearly indicates enormous variation in practice, and frequent non-evidence based accelerated dosing of infliximab in steroid-refractory hospitalized patients. There appears to be a sub rosa movement in the IBD community to try accelerated infliximab in severe UC, and the Gibson study provides the first data to support this approach. This retrospective study generates important hypotheses and questions about the treatment of steroid-refractory severe UC: (1) is accelerated dosing of infliximab superior to standard dosing? (2) is a CRP of ≤5 mg/L the optimal short-term goal of infliximab induction? (3) is monitoring with infliximab levels more cost-effective than monitoring with CRP? (4) After accelerated induction, what is the best strategy to maintain patients in remission on infliximab?
At this point, it is critical to the field to conduct randomized multi-center clinical trials to identify the optimal dosing regimen for anti-TNF therapy in severe inpatient UC, and to identify the best monitoring strategies, evaluating the value of serum CRP, serum albumin levels, and serum and fecal infliximab levels. Additional studies of long-term maintenance strategies are also needed, as the promising results seen at 3 months in this retrospective study were not durable with standard q8 weekly dosing.
Acknowledgments
HH and PDRH are supported by the Crohn’s and Colitis Foundation of America (CCFA).
HH is supported by a National Institutes of Health grant 5U01DK092239.
PDRH is supported by a National Institutes of Health grant R01 GM097117.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to declare in relation to this manuscript.
References
- 1.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 2.Probert CS, Hearing SD, Schreiber S, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52:998–1002. doi: 10.1136/gut.52.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2014;39:660–671. doi: 10.1111/apt.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reich KM, Chang HJ, Rezaie A, et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: a time-trend study. Aliment Pharmacol Ther. 2014;40:629–638. doi: 10.1111/apt.12873. [DOI] [PubMed] [Google Scholar]
- 5.Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebocontrolled study. Gastroenterology. 2005;128:1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 7.Reinisch W, Feagan BG, Rutgeerts PJ, et al. Infliximab Concentration and Clinical Outcome in Patients With Ulcerative Colitis. Gastroenterology. 2012;142:S–114. [Google Scholar]
- 8.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association Between Serum Concentration of Infliximab and Efficacy in Adult Patients with Ulcerative Colitis. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Khanna R, Sattin BD, Afif W, et al. Review article: a clinician's guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:447–459. doi: 10.1111/apt.12407. [DOI] [PubMed] [Google Scholar]
- 10.Ordas I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635–646. doi: 10.1038/clpt.2011.328. [DOI] [PubMed] [Google Scholar]
- 11.Brandse JF, Wildenberg M, de Bruyn JR, et al. Fecal Loss of Infliximab As a Cause of Lack of Response in Severe Inflammatory Bowel Disease. Gastroenterology. 2013;144:S–36. [Google Scholar]
- 12.Panaccione R, Ghosh S, Middleton S, et al. Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis. Gastroenterology. 2014;146:392–400. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65:1211–1228. doi: 10.1007/s00228-009-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vande Casteele N, Gils A, Compernolle G, et al. Drug Level Versus Clinically Based Dosing of Infliximab Maintenance Therapy In IBD: Final Results of the Randomized Controlled Taxit Trial. Inflammatory Bowel Diseases. 2013;19:S2–S3. [Google Scholar]
- 15.Westhovens R, Yocum D, Han J, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54:1075–1086. doi: 10.1002/art.21734. [DOI] [PubMed] [Google Scholar]
- 16.Narula N, Charleton D, Marshall JK. Meta-analysis: peri-operative anti- TNFalpha treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:1057–1064. doi: 10.1111/apt.12313. [DOI] [PubMed] [Google Scholar]
- 17.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 19.Sands BE, Tremaine WJ, Sandborn WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–88. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–1260. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]