Abstract
Development of effective and safe therapeutic treatment of fungal infections remains one of the major challenge for modern medicine. The aim of presented investigation was to analyze the in vitro antifungal activity of selected essential oils, ethanolic extracts of propolis and silver nanoparticles dropped on TiO2 against azole-resistant C. albicans (n = 20), C. glabrata (n = 14) and C. krusei (n = 10) clinical isolates. Among tested essential oils, the highest activity has definitely been found in the case of the oil isolated from the bark of Cinnamomum cassia, with MIC and MFC values for all tested strains in the range of 0.0006–0.0097 % (v/v) and 0.0012–0.019 % (v/v), respectively. High activity was also observed for the Lemon, Basil, Thyme, Geranium and Clove (from buds) essential oils. Significant differences in fungicidal activity have been observed in the case of four tested propolis samples. Only one of them revealed high activity, with MFC values in the range from 0.156 to 1.25 % (v/v). Satisfactory fungicidal activity, against C. albicans and C. glabrata isolates, was also observed in the case of silver nanoparticles, however C. krusei isolates were mostly resistant. We also revealed that constituents of most of essential oils and propolis as well as silver nanoparticles are not substrates for drug transporters, which belong to the most important factors affecting resistance of Candida spp. clinical isolates to many of conventional antimycotics. To conclude, the results of our investigation revealed that essential oils, propolis and silver nanoparticles represent high potential for controlling and prevention candidiasis.
Keywords: Candida albicans, Candida glabrata, Candida krusei, Essential oils, Propolis, Silver nanoparticles
Introduction
The yeasts of the genus Candida are the most frequently recovered from human fungal infections. The Candida genus comprises over 150 heterogeneous species [1], but only a minority (about 20 species) are known to be etiological agents of human and animals’ infections [2, 3]. During the last three decades the number of fungal infections caused by yeasts of Candida spp. has increased dramatically, mainly due to the rise in the number of immunocompromised patients [4, 5]. Currently, candidiasis represents the fourth leading cause of nosocomial infections, at 8–10 %, and mortality due to systemic candidiasis remains high, ranging from 15 to 35 % depending on the infecting Candida species [6, 7].
The number of available and applied currently antifungal chemotherapeutics is very limited. Nearly all of them belong to four classes: azoles, polyenes, echinocandins and allylamines. In fact, none of currently used antifungal agents meets all expectations: polyenes such as amphotericin B are toxic and characterized by low solubility in water, echinocandins such as caspofungin can only be administered intravenously, allylamines such as terbinafine lack anticandidal activity, treating the infection with azoles (e.g. fluconazole or voriconazole) often results in acquisition of resistance among fungal etiological agents [8, 9]. Moreover, some species of the genus Candida e.g. C. glabrata and C. krusei are intrinsically resistant to fluconazole and other azoles, and frequency of isolations of these two species has significantly increased recently. Rapid acquisition of resistance is also observed in the case of a treatment regimen with the use of 5-fluorocytosine, which belongs to pyrimidine analogs [10]. Thus, there is an urgent need to look for new antifungal agents which could be used in treatment as well as prevention of infections caused by the yeasts of the genus Candida. The most promising alternatives for conventional treatment of microbial (including fungal) infections are plant-derived compounds as well as gold and silver nanoparticles [11, 12].
The aim of presented investigation was to analyze the in vitro antifungal activity of selected essential oils, ethanolic extracts of propolis as well as silver nanoparticles dropped on the TiO2 against azole resistant C. albicans, C. glabrata and C. krusei clinical isolates.
Materials and Methods
Strains
The group of 48 clinical isolates of Candida spp. were investigated. Twenty C. albicans isolates, 14 C. glabrata isolates and 10 C. krusei isolates were recovered from a variety of clinical specimens: urine, feces, blood, fluid from the peritoneal cavity, stoma, bronchopulmonary lavage, swabs of the mouth, throat and anus from patients of the Children’s Memorial Health Institute in Warsaw. All of them were fluconazole-resistant. Other four C. albicans isolates, assigned as B3, B4, Gu4 and Gu5 were kindly provided by Prof. Joachim Morschhäuser from University of Würzburg (Germany). Strains Gu4 and B3 are fluconazole-sensitive isolates obtained from early infection episodes, while Gu5 and B4 are the corresponding fluconazole-resistant isolates obtained from later episodes in the same patients treated with fluconazole. In the case of Gu5 the lack of susceptibility to fluconazole is a consequence of overexpression of CDR1/2 genes encoding ABC transporters whereas the resistance of B4 strain is caused by overexpression of MDR1 gene encoding a membrane transport protein of the major facilitator superfamily (MFS) [13]. Transporters of both groups, ABC as well as MFS have low substrate specificity, as a consequence they are able to remove from the cells broad spectrum of xenobiotics (toxic components). Microorganisms as well as cancer cells producing drug transporters usually characterize with MDR phenotype (Multi Drug Resistant) - are resistant to many chemotherapeutics belonging to different chemical groups.
Tested Agents
In the preliminary studies the antifungal activities of following essential oils were analyzed: Rosewood (Aniba rosaeodora), Lavender (Lavandula angustifolia), Mandarin (Citrus nobilis), Bergamot (Citrus aurantium bergamia), Rosemary (Rosmarinus officinalis), Petitgrain (Citrus aurantium amara), Juniper (Juniperus communis), Pine (Pinus silvestris), Eucalyptus (Eucalyptus globulus), Fir (Abies sibrica), Manuka (Leptospermum scoparium), Peppermint Essential (Mentha piperita), Cumin (Carum carvi), Hyssop (Hyssopus officinalis), Thyme (Thymus vulgaris), Ylang Ylang (Cananga odorata), Lemon (Citrus limonum), Ginger (Zingiber officinale), Fennel (Foeniculum vulgare), Roman Chamomile (Anthemis nobilis), Lime (Citrus aurantifolia), Orange (Citrus aurantium dulcis), Geranium (Pelargonium graveolens), Cedar (Juniperus virginiana), Sage (Salvia sclarea), Marjoran (Origanum majorana), Cardamon (Elettaria cardamomum), Cinnamon (from bark) (Cinnamomum aromaticum, called also Cinnamomum cassia), Tea Tree (Melaleuca alternifolia), Coriander (Coriandrum sativum), Sandalwood (Santalum album), Basil (Ocimum basilicum), Anise (Illicium verum), Grapefruit (Citrus grandis), Citronella (Cymbopogon nardus), Patchuoli (Pogostemon cablin), Clove (from buds) (Eugenia caryophyllus), Cypress (Cupressus sempervirens). All of the essential oils were purchased from Pollena Aroma (Poland). Eight the most effective oils were selected for more advanced analysis.
The analyzed propolis samples (n = 4) were provided by professional beekeepers from Silesia region (the south-western part of Poland). The samples of propolis were suspended in ethanol (96 %, POCH, Poland) at a weight ratio 1:2. The suspension was incubated for 48 h at room temperature and vigorously vortexed every six hours. To remove insoluble ingredients the suspensions were centrifuged (5,000 rpm, 10 min, 20 °C), the pellet was discharged and the supernatant (solution of propolis components in ethanol) was used for antifungal activity assay. The pure ethanol was used as a control in the propolis antifungal activity assay.
Silver-modified titanium dioxide nanocomposites (Ag-TiO2) were obtained in microemulsion system (water/AOT/cyclohexane) according to method described by Zielinska and coworkers [14]. The size of the nanoparticles was in the range of 5–10 nm (Fig. 1). The antifungal activity was tested for two different samples containing silver in the concentrations of: 2.5 and 6.5 %. The tested materials were used in the form of suspension in DMSO, at initial concentration of 6.4 mg/ml. Preparation of the suspension in DMSO was important for sterilization of the Ag-TiO2 nanocomposites without using high temperature or any other agent which could result in the destruction of their structure. Titanium dioxide suspension in DMSO (at the same initial concentration) not containing silver was used as a control for antifungal activity assay.
Fig. 1.
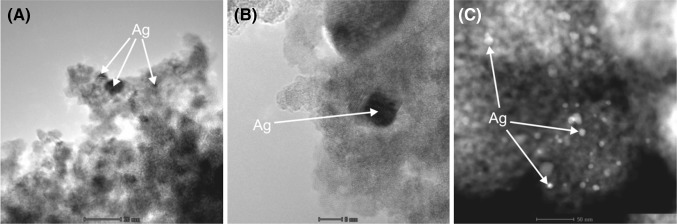
Pictures of silver nanoparticles immobilized on titanium dioxide (TiO2) in three different magnifications
Susceptibility Testing—MIC and MFC determination
The activity of essential oils was evaluated by determination of both minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) parameters. Due to the fact that the suspensions of propolis and Ag-TiO2 nanocomposites in the media used for the activity assay were not transparent, it was possible to determine only the MFC parameter. The assays were performed based on Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI document M27-A3) [15] with slight modifications as described below. Briefly, pure cultures of the isolates were grown on Sabouraud Dextrose Agar at 37 °C for 24 h. A small amount of biomass of each isolate was suspended in RPMI-1640 medium (Sigma, Germany) to get final cells concentration in the range of 1–2 × 105 CFU/ml.
100 µl of twofold dilutions of tested agents (essential oils, suspension of propolis samples in ethanol and suspension of Ag-TiO2 nanocomposites in DMSO) were prepared in the rows of 96-well plates using RPMI 1640 medium (Sigma, Germany) containing 2 % of glucose and buffered to a pH of 7.0 with 0.165 M MOPS buffer. Subsequently the plates were inoculated with equal volume (100 µl) of cells suspension prepared as described above. Finally the activity of essential oils was analyzed in the range of concentrations of 1.25–0.0006 % (v/v), propolis extracts in the range of concentrations of 5–0.0098 % (v/v) and 128–0.25 μg/ml in the case of Ag-TiO2 nanocomposites. Additionally, positive control of the growth of strains tested and control of the medium sterility were performed. The plates were incubated 24 h at 37 °C. After the incubation, the MIC values of the tested essential oils were determined by measuring the absorbance at 531 nm using a Victor3 microplate reader (Perkin Elmer, USA). The lowest concentration of the oil that caused inhibition of growth equal or higher than 90 % of growth control was taken as the MIC value.
To determine fungicidal activity (MFC) 10 µl of each well content in the case of propolis and Ag-TiO2 nanocomposites and from the wells containing essential oils in concentration of MIC, 2×MIC and 4×MIC were transferred on the Sabouraud Dextrose Agar without any antimicrobial agent. No visible colony growth after subsequent 24–48 h incubation at 37 °C was accepted as a MFC. All experiments in case of both MIC and MFC determination were conduced in triplicate.
Results
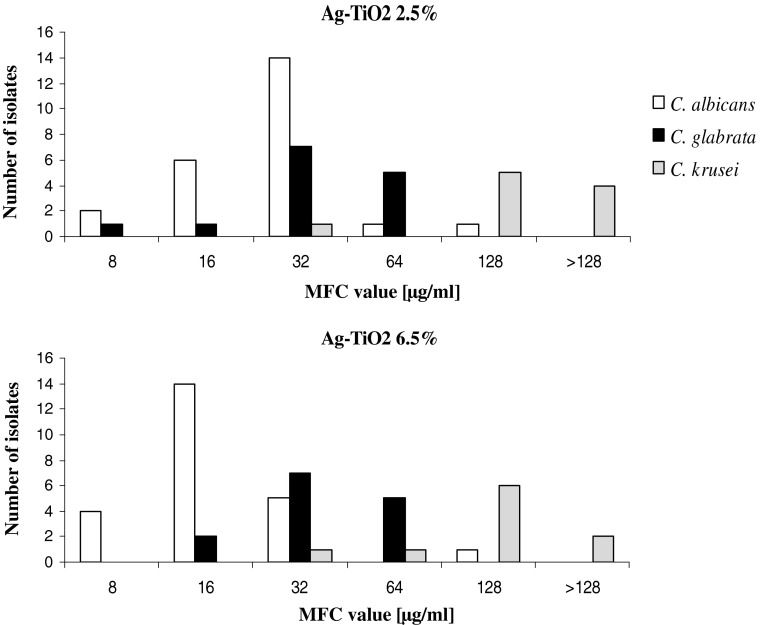
Results of preliminary tests, carried out using three randomly selected strains of each species, showed that 8 out of 37 investigated oils have promising antifungal activity. These oils are: Lavender, Lemon, Tea Tree, Basil, Thyme, Geranium, Cinnamon (from bark) and Clove (from buds). The results of detailed analysis of their activity, characterized with MIC and MFC values, against all strains tested are presented on Fig. 2. Definitely, the highest activity has been found in the case of Cinnamon oil. The MIC values for all strains tested, regardless of the yeast genus were in the range of 0.0006–0.0097 % (v/v). A small shift of MFC value in comparison to MIC has been observed in the case of many strains, however the values of MFC parameter were still on a very low level, in the range of concentrations from 0.0012 to 0.019 % (v/v), which confirms high fungicidal activity of components of the Cinnamon oil. Moreover, we did not observe any evident differences in its activity against B3, Gu4 and overproducing efflux pumps B4 and Gu5 strains (Table 1). The obtained results suggest that components of Cinnamon oil are not substrates for drug transporters, of both groups (neither ABC transporters produced in Gu5 strain, nor MFS transporters overproduced in B4 strain) and potentially could be used in treatment of infections caused by strains expressing these transporters, as a consequence resistant to many antifungal agents, especially azoles. High activity, particularly against C. glabrata and C. krusei isolates, has been also observed in the case of Lemon oil. The MIC and MFC values of this oil in the case of C. krusei were in the range of concentrations from 0.0024 to 0.0097 and from 0.0024 to 0.019 % (v/v) respectively, in the case of C. glabrata the values of both parameters were in the same range of concentrations from 0.0024 to 0.156 % (v/v). C. albicans isolates were generally less sensitive to the activity of Lemon oil, however its activity was also quite satisfactory [both MIC and MFC values in the range of 0.0097–0.312 % (v/v)]. A four-fold increase in resistance of Gu5 strain in comparison to Gu4 could suggest that some active components of Lemon oil are removed from the cells by the ABC drug transporters. High level of activity has been also found in the case of Basil oil. The highest sensitivity revealed C. krusei isolates [MIC value in the range 0.039–0.312 % (v/v); MFC values in the range 0.078–0.312 % (v/v)]. The fungicidal effect against C. glabrata and C. albicans was achieved in the range of concentrations from 0.078 to 0.625 % (v/v), and from 0.312 to 1.25 % (v/v), respectively. Although other tested essential oils exhibited lower fungistatic as well as fungicidal activities, some individual isolates revealed high sensitivity to Thyme and Geranium oils. In case of both these essential oils evident differences in susceptibility of B3 and B4 strains have been noticed, which suggest that some active ingredients are substrates for MFS transporters. No differences were observed in case of Gu4 and Gu5 strains, thus ABC efflux pumps are not able to remove the active constituents of these oils from the yeast cells.
Fig. 2.
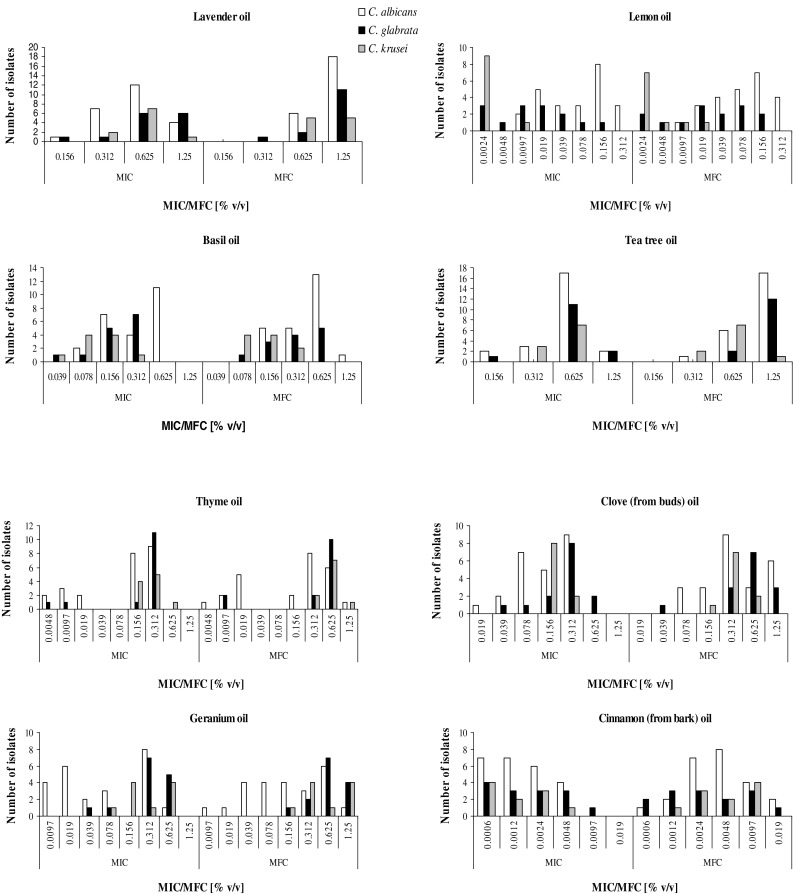
Fungistatic (characterized with MIC – minimal inhibitory concentration values) and fungicidal (characterized with MFC – minimal fungicidal concentration values) of selected eight essential oils
Table 1.
Comparison of activity of tested agents against overproducing (B4 and Gu5) and not-overproducing (B3 and Gu4) multi-drug efflux pumps C. albicans isolates, detailed explanation in the text
| Isolate | Silver 2.5 % MFC [µg/ml] | Silver 6.5 % MFC [µg/ml] | Propolis 1 MFC [% v/v] | Propolis 2 MFC [% v/v] | Propolis 3 MFC [% v/v] | Propolis 4 MFC [% v/v] | Lavender oil MIC/MFC [% v/v] | Lemon oil MIC/MFC [% v/v] | Basil oil MIC/MFC [% v/v] | Tea tree oil MIC/MFC [% v/v] | Thyme oil MIC/MFC [% v/v] | Clove oil MIC/MFC [% v/v] | Geranium oil MOC/MFC [% v/v] | Cinnamomum oil MIC/MFC [% v/v] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B3 | 16 | 16 | 2.5 | 0.312 | 2.5 | 5 | 1.25/1.25 | 0.312/0.312 | 0.312/0.625 | 1.25/1.25 | 0.0048/0.019 | 0.312/0.312 | 0.019/0.078 | 0.0006/0.0012 |
| B4 | 32 | 8 | 2.5 | 0.312 | 2.5 | 5 | 0.625/1.25 | 0.312/0.312 | 0.625/0.625 | 0.625/0.625 | 0.312/0.312 | 0.156/0.312 | 0.312/0.625 | 0.0006/0.0024 |
| Gu4 | 32 | 16 | 2.5 | 0.312 | 1.25 | 5 | 1.25/1.25 | 0.078/0.078 | 0.625/0.525 | 1.25/1.25 | 0.156/0.312 | 0.156/0.156 | 0.312/0.625 | 0.0012/0.0024 |
| Gu5 | 8 | 8 | 5 | 0.312 | 2.5 | 5 | 0.625/1.25 | 0.312/0.312 | 0.625/1.25 | 0.625/0.625 | 0.312/0.312 | 0.312/0.312 | 0.625/0.625 | 0.0048/0.0048 |
The evident differences in the susceptibility are marked with bold
Significant differences of fungicidal activity have been observed in the case of propolis samples Fig. 3. The highest activity was found in the case of sample no 2 with MFC values in the range from 0.156 to 1.25 % (v/v), and fungicidal effect for most of isolates (n = 25) was achieved at concentration of 0.312 % (v/v). Other samples, especially no 1 and no 4, were definitely less active. In general C. glabrata and especially C. krusei exhibited higher resistance to the activity of propolis constituents in comparison to C. albicans. Within the range of tested concentrations samples 1 and 4 did not exhibit any antifungal activity against 6 and 9 out of 10 C. krusei isolates, respectively. No differences were observed in the case of susceptibility of strains producing and not producing drug transporters to the propolis samples.
Fig. 3.
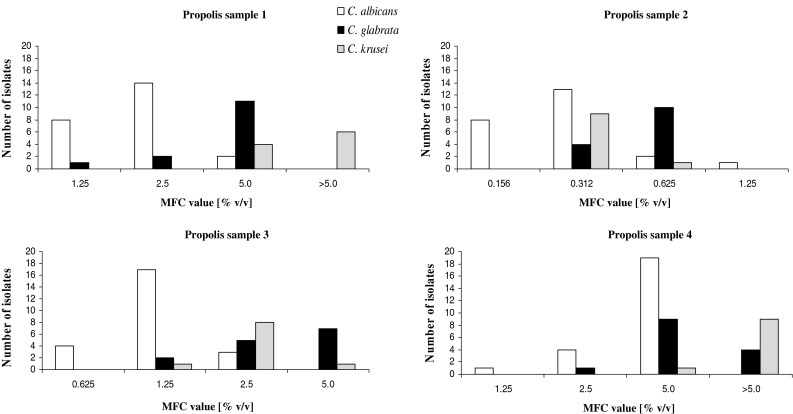
Fungicidal activity of ethanolic extracts of four propolis samples
Candida albicans and Candida glabrata isolates revealed comparable susceptibility to Ag-TiO2 nanocomposites, whilst C. krusei were definitely more resistant (Fig. 4). Similarly to the most of essential oils and propolis constituents the silver nanoparticles are not substrates of drug transporters—MFC values for strains producing these proteins (B4 and Gu5) are comparable (or even lower) to MFC values of B3 and Gu4, respectively (Table 1).
Fig. 4.
Fungicidal activity of silver nanoparticles dropped on TiO2
Discussion
Development of effective and safe therapeutic treatment of fungal infections remains one of the major challenge for modern medicine. Resistance, toxicity and problems with administration are the most important limitations of therapies carried out with conventional antimycotics [16, 17]. Thus, there is an urgent need to look for other active and safe chemotherapeutics which could be used in treatment of fungal infections. The results of our investigations clearly revealed that some of tested essential oils, e.g. isolated from C. limon, O. basilicums and especially from the bark of C. cassia, as well as propolis and silver nanoparticles could be considered as alternative antifungal agents. Besides of high antifungal activity the most important advantage of all tested agents, including silver, is their low toxicity [16, 18]. On the other hand, it is also important to remember about some limitations as well as drawbacks of using these agents in treatment of infections caused by fungi or bacteria. The chemical composition and consequently antimicrobial activity of natural products derived from plants, such as essential oils and propolis, depend on many factors e.g. growing conditions (climate, soil, fertilization), time of harvest as well as the way the essential oil or propolis extract is produced, transported and stored [19, 20]. Thus using of these products as therapeutic agents or even cosmetics would require confirmation of their antimicrobial activity (for each production batch). Interesting analysis of differences of chemical composition as well as antifungal activity of commercially available Lemon essential oils has been recently presented by Bialon and coworkers [21]. From 6 tested commercial oils only 4 showed antifungal potential. Additionally, the authors revealed that antifungal activity of the oils was related to content of monoterpenoids. The group of Pozzatti did not find any antifungal activity against Candida isolates in the case of Basi oil [22, 23]. In present studies, the oil obtained from O. basilicum was found as one of most active, with MIC values in the range of concentrations from 0.078 to 0.625 % (v/v). In comparison to results presented by Budzynska and coworkers [24] we noticed lower activity in the case of Lavender and Clove oils, activity of Geranium oil was comparable and activity of Lemon oil in our investigation especially in the case of C. glabrata and C. krusei was much higher. Interestingly in the case of both these studies, essential oils from the same source were tested (Pollena Aroma, Poland). The oils could be prepared from different batches of raw materials. Another source of observed differences in the activity of tested oils could be supplementation of the RPMI medium with 0.5 % of Tween20 by Budzynska and coworkers [24]. The highest activity among essential oils was found for the oil isolated from the bark of C. cassia, known traditional Chinese medicine. The antifungal activity of this oil was previously analyzed by the group of Ooi [25] and the observed MIC values were definitely higher (in the range from 100 to 450 µg/ml) in comparison to the results obtained in present investigation (0.0006–0.0097 % v/v). For easier comparison results of these two studies we recalculated activity of this oil taking into account its density (1.04 g/ml). Thus the range of MIC values in present studies was in the range of concentrations from 6.24 to 100.8 µg/ml. The mentioned authors revealed also that antimicrobial effectiveness of the oil was caused by its major constituent - cinnamaldehyde (MIC values for both oil and the aldehyde were almost equivalent). The observed much higher activity of the oil investigated in the presented studies is probably a consequence of presence of some ingredients acting synergistically with the cinnamaldehyde. Identification of these components as well as analysis of their synergistic effect is the subject of the research carried out currently in our group.
The health benefits as well as biological activities of propolis have been known and used in folk medicine for centuries. It contains resinous substances collected by honey bees, from various plants, thus forming a complex of biologically active substances [16]. Several research groups revealed high antifungal potential of propolis against Candida clinical isolates [26–29]. The results of our investigation confirmed high fungicidal activity of propolis against all strains tested, but only in the case of one of four analyzed samples. Significant variability of the chemical composition of propolis samples and hence their biological activity has been also found by other authors [30, 31]. Monzote and coworkers [32] stated lack of antifungal activity in the group of 20 Cuban propolis extracts. Thus using propolis as a therapeutic agent would require special attention. Only the samples with confirmed evident antimicrobial activity could be applied for production of medical or cosmetic products used for treatment or prevention of fungal infections.
Interesting results were also obtained in the case of investigation of silver nanoparticles, which are not natural, but promising, alternative antimicrobial agents. In the case of C. albicans but also C. glabrata its fungicidal activity was quite satisfactory and comparable to the results presented by other authors [18, 33–35]. Surprisingly C. krusei isolates were mostly resistant in the tested range of concentration. The silver nanoparticles used in presented studies were immobilized on the surface of titanium dioxide. After reaction with water the photo excided TiO2 can produce hydroxyl radicals with high redox oxidizing and antimicrobial potential [36]. Thus we expected the enhanced activity of silver nanoparticles. The carried out determination revealed however that the titanium dioxide without the silver did not reveal any activity against all strains tested, thus the observed fungicidal effect was only caused by the silver nanoparticles. As a consequence, the concentration of active compound—silver nanoparticles was in fact in the range of concentrations from 3.2 to 0.00625 µg/ml (in the case of sample containing 2.5 % of silver) and from 8.32 to 0.01625 µg/ml (in the case of sample containing 6.5 % of silver). Similar differences in sensitivity to silver and gold nanoparticles of different species within the genus Candida have been recently observed by Jebali and coworkers [34]. The authors found that C. tropicalis were more resistant than C. glabrata and C. albicans. Moreover, the authors observed an evident relationship between the shape and activity of silver nanoparticles. The highest activity was found in the case of nanocubes, nanowires and especially nanospheres were less active [34].
To conclude, the results of our investigation revealed that essential oils, propolis and silver nanoparticles represent high potential for controlling and prevention candidiasis, including infections caused by the strains resistant to azoles, the group of chemotherapeutics commonly used for treatment and prevention of these infections. However, a large variation in the composition (consequently antimicrobial activity) of natural products as well as the differences in the sensitivity of particular species of yeast of the genus Candida to silver nanoparticles should be taken into account.
Acknowledgments
This research was supported by the grant no N N405 151040 from the National Science Centre in Poland. The authors are also grateful to Teresa Tanczyk, from Pollena-Aroma and Marta Schielmann from Gdansk University of Technology, for their help in preparing the manuscript.
Conflict of interest
Authors do not declare any conflict of interest.
References
- 1.Calderone RA. Introduction and historical perspectives. Candida and Candidiasis. Washington, DC: ASM Press; 2002. pp. 15–25. [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 4.Low Ch-Y, Rotstein C. Emerging fungal infections in immunocompromised patients. Med Rep. 2011;3:14. doi: 10.3410/M3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9:e1003550. doi: 10.1371/journal.ppat.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlind TD, Katiyar SK. Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob Agents Chemother. 2010;54:4733–4738. doi: 10.1128/AAC.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanowska E (2008) Mechanizmy oporności grzybów na leki przeciwgrzybicze/Fungal resistance mechanisms on antifungal agents. In: Dzierżanowska D (ed) Zakażenia szpitalne. α – medica press, Bielsko-Biała, pp 497–507 (in Polish)
- 10.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 11.Kurek A, Grudniak AM, Kraczkiewicz-Dowjat A, Wolska KI. New antibacterial therapeutics and strategies. Pol J Microbiol. 2011;60:3–12. [PubMed] [Google Scholar]
- 12.Rajeshkumar R, Sundararman M. Emergence of Candida spp. and exploration of natural bioactive molecules for anticandidal therapy—status quo. Mycoses. 2012;55:e60–e73. doi: 10.1111/j.1439-0507.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- 13.Franz R, Ruhnke M, Morschhaüser J. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses. 1999;42:453–458. doi: 10.1046/j.1439-0507.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 14.Zielinska A, Kowalska E, Sobczak JW, Lacka I, Gazda M, Ohtani B, Hupka J, Zaleska A. Silver-doped TiO2 prepared by microemulsion method; surface properties, bio- and photoactivity. Sep Purif Tech. 2010;72:309–318. doi: 10.1016/j.seppur.2010.03.002. [DOI] [Google Scholar]
- 15.Clinical Laboratory Standards Institute (CLSI) Reference method for broth dilution antifungal susceptibility testing of yeasts: third edition (M27-A3) Wayne: CLSI; 2008. [Google Scholar]
- 16.de Castro PA, Bom VL, Brown NA, de Almeida RS, Ramalho LN, Savoldi M, Goldman MH, Berretta AA, Goldman GH. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet Biol. 2013;60:74–86. doi: 10.1016/j.fgb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Romanowska E (2010) Zakażenia grzybicze/Fungal infections. In: Piegdoń (ed) Pielęgniarstwo epidemiologiczne. Instytut Pomnik Centrum Zdrowia Dziecka, Warszawa, pp 58–65 (in Polish)
- 18.Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Krystof V, Hamal P, Zboril R, Kvitek L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333–6340. doi: 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 19.Kim KS, Kim YS, Han I, Kim M-H, Jung MH, Park H-K. Quantitative and qualitative analyses of the cell death process in Candida albicans treated by antifungal agents. PLoS One. 2011;6:e28176. doi: 10.1371/journal.pone.0028176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalhinho S, Costa AM, Coelho AC, Martins E, Sampaio A. Susceptibilities of Candida albicans mouth isolates to antifungal agents. Essential oils and mouth rinses. Mycophatologia. 2012;174:69–76. doi: 10.1007/s11046-012-9520-4. [DOI] [PubMed] [Google Scholar]
- 21.Bialon M, Krzysko-Lupicka T, Koszalkowska M, Wieczorek PP. The influence of chemical composition of commercial lemon essential oils on the growth of Candida strains. Mycopathologia. 2014;17:29–39. doi: 10.1007/s11046-013-9723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzatti P, Scheid LA, Spader TB, Atayde ML, Santurio JM, Alves SH. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can J Microbiol. 2008;54:950–956. doi: 10.1139/W08-097. [DOI] [PubMed] [Google Scholar]
- 23.Pozzatti P, Loreto ES, Lopes PG, Athayde ML, Santurio JM, Alves SH. Comparison of the susceptibilities of clinical isolates of Candida albicans and Candida dubliniensis to essential oils. Mycoses. 2010;53:12–15. doi: 10.1111/j.1439-0507.2008.01643.x. [DOI] [PubMed] [Google Scholar]
- 24.Budzynska A, Sadowska B, Lipowczan G, Maciag A, Kalemba D, Rozalska B. Activity of selected essential oils against Candida spp. trains. Evaluation of new aspects of their specific pharmacological properties, with special reference to Lemon balm. Adv Microbiol. 2013;3:317–325. doi: 10.4236/aim.2013.34045. [DOI] [Google Scholar]
- 25.Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chin Med. 2006;34:511–522. doi: 10.1142/S0192415X06004041. [DOI] [PubMed] [Google Scholar]
- 26.Dota KF, Consolaro ME, Svidzinski TI, Bruschi ML. Antifungal activity of Brazilian propolis microparticles against yeasts isolated from vulvovaginal candidiasis. Evid Based Complement Alternat Med. 2011;2011:201953. doi: 10.1093/ecam/neq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokri H, Khosravi AR, Yalfani R. Antifungal efficacy of propolis against fluconazole-resistant Candida glabrata isolates obtained from women with recurrent vulvovaginal candidiasis. Int J Gynaecol Obstet. 2011;114:158–159. doi: 10.1016/j.ijgo.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Astani A, Zimmermann S, Hassan E, Reichling J, Sensch KH, Schnitzler P. Antimicrobial activity of propolis special extract GH 2002 against multidrug-resistant clinical isolates. Pharmazie. 2013;68:695–701. [PubMed] [Google Scholar]
- 29.Capistrano HM, de Assis EM, Leal RM, Alvrez-Leite ME, Brener S, Bastos EM. Brazilian green propolis compared to miconazole gel in the treatment of Candida-associated denture stomatitis. Evid Based Complement Alternat Med. 2013;2013:947980. doi: 10.1155/2013/947980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isla MI, Dantur Y, Salas A, Danert C, Zampini C, Arias M, Ordonez R, Maldonado L, Bedascarrasbure E, Nieva Moreno MI. Effect of seasonality on chemical composition and antibacterial and anticandida activities of Argentine propolis. Design of a topical formulation. Nat Prod Commun. 2012;7:1315–1318. [PubMed] [Google Scholar]
- 31.Falcao SI, Vale N, Cos P, Gomes P, Freire C, Maes L, Vilas-Boas M. In vitro evaluation of portuguese propolis and floral sources for antiprotozoal, antibacterial and antifungal activity. Phytother Res. 2014;28:437–443. doi: 10.1002/ptr.5013. [DOI] [PubMed] [Google Scholar]
- 32.Monzote L, Cuesta-Rubio O, Campo Fernandez M, Marquez Hernandez I, Fraga J, Perez K, Kerstens M, Maes L, Cos P. In vitro antimicrobial assessment of Cuban propolis extracts. Mem Inst Oswaldo Cruz. 2012;107:978–984. doi: 10.1590/S0074-02762012000800003. [DOI] [PubMed] [Google Scholar]
- 33.Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 34.Jebali A, Hajjar FH, Pourdanesh F, Hekamtimoghaddam S, Kazemi B, Masoudi A, Daliri K, Sedighi N. Silver and gold nanostructures: antifungal property of different shapes of these nanostructures on Candida species. Med Mycol. 2014;52:65–72. doi: 10.3109/13693786.2013.822996. [DOI] [PubMed] [Google Scholar]
- 35.Gupta K, Barua S, Hazarika NS, Manhar AK, Nath D, Karak N, Namsa ND, Mukhopadhyay R, Kalia VC, Mandal M. Green silver nanoparticles: enhanced antimicrobial and antibiofilm activity with effects on DNA replication and cell cytotoxicity. RSC Adv. 2014;4:52845–52855. doi: 10.1039/C4RA08791G. [DOI] [Google Scholar]
- 36.Zaleska A. Doped TiO2; a review. Recent Patents Eng. 2008;2:157–164. doi: 10.2174/187221208786306289. [DOI] [Google Scholar]