Abstract
There is an increasing demand for silver nanoparticles due to its wide applicability in various area of biological science such as in field of antimicrobial and therapeutics, biosensing, drug delivery etc. To use in bioprocess the silver nanoparticles should be biocompatible and free from toxic chemicals. In the present study we report a cost effective and environment friendly route for green synthesis of silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract as reducing as well as capping agent. This plant has been opted for the present study for its known medicinal properties and it is easily available. The biosynthesized silver nanoparticles are characterized by UV–Vis spectroscopy and TEM analysis. It is observed the nanoparticles are well shaped and the average particle size is 20 nm in the range of 5–50 nm. The antibacterial activity of these nanoparticles against Pseudomonas aeruginosa MTCC 741 has been measured by disc diffusion method, agar cup assay and serial dilution turbidity measurement assay. The results show green synthesized silver nanoparticles, using Vasaka leaf extract, have a potential to inhibit the growth of bacteria.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-015-0512-1) contains supplementary material, which is available to authorized users.
Keywords: Antibacterial activity, Vasaka (Justicia adhatoda L.) leaf extract, Pseudomonas aeruginosa MTCC 741, Silver nanoparticles, Green synthesis
Introduction
In terms of resistance, the battle between bacteria and antibacterial forced the researchers to have a re-look on the ways to attack bacteria. It is revealed that bacteria within biofilm are responsible for more than 80 % infectious diseases as well as more resistance to antibiotics [1]. In recent years, due to unique physical and chemical properties of nanoparticles, its uses to disrupt biofilm of bacteria are revolutionary steps in antimicrobial research [2–13]. The uses of silver and silver compounds are very well known from ancient time in case of dental work, catheters and burn wounds etc. Thus, among the various inorganic metal nanoparticles, silver (Ag) nanoparticles have received substantial attention in the field of biological system, living organisms and medicine [3–5, 7–13]. To use in biological field the metal nanoparticles should be biocompatible and free from toxic chemicals which are used to synthesis nanoparticles in traditional chemical process. The need of biocompatible silver nanoparticles opens the various biosynthesis routes of nanoparticles. Among those, the silver nanoparticles synthesis using plant parts extract become an emerging field because these methods are very efficient in the production of silver nanoparticles due to of their simplicity, accuracy and cost effectiveness, and also from the green chemistry perspective and their biomedical application [3–5, 9, 13–20]. Environment friendly reducing and capping agents such as carbohydrates, polysaccharides, alkaloids, flavonoids present in the plant extract plays an important role in case of green synthesis of silver nanoparticles [21]. The synthesis of silver nanoparticles using various plant leaf extract are widely reported but antibacterial activities of few of those biosynthesized silver nanoparticles are reported [3–5, 7, 9, 11, 12, 15, 18].
In this present study an attempt has been done to synthesis of silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract from the green chemistry perspective and to investigate the antibacterial activities of this biosynthesized silver nanoparticles on Pseudomonas aeruginosa MTCC 741 strain as a model gram negative bacteria.
Materials and Method
Synthesis of Ag Nanoparticles Using Plant Extract
About 5 g of fresh matured leaves of Vasaka was crashed and mixed with 60 ml of double distilled water. Adding 0.2 ml of vasaka leaf extract in 20 ml of 1 mM of AgNO3 (Meark) solution, the mixture was stirred for 30 min at 60 °C. The colour of the solution was gradually changed from light yellow to reddish brown colour (Fig. 1).
Fig. 1.

Ag nanoparticle solutions, synthesized by reducing AgNO3 using Vasaka leaf extract
Characterization of Ag-Nanoparticles
The formation of Ag nanoparticles was confirmed by recording the UV–visible spectra (SYSTRONICS, India). The morphology and size are analyzed by TEM (CM 12 PHILIPS) using standard method.
Microbiological Assays
The model gram negative organism P. aeruginosa MTCC 741 strain was maintained in both Nutrient agar (Hi-media, India) and Nutrient Broth medium (Hi-media, India) at 37 °C. For the disc diffusion method, 100 µl of 24 h old bacterial culture was uniformly spread over the nutrient agar surface. Freshly prepared Ag nanoparticles solution soaked sterile paper discs of 5 mm diameter were placed on that agar surface. The zones of inhibition were measured after 24 h incubation (at 37°). In case of agar cup assay, 100 µl of 24 h old bacterial culture was uniformly spread over the nutrient agar surface. Then, 50 µl of Ag nanoparticles solutions synthesized from three different concentrations of AgNO3 solution were added into the wells (1 cm diameter) on that nutrient agar surface. The zones of inhibition (ZoI) were measured after 24 h incubation (at 37 °C). For serial dilution turbidity measurement assay, nutrients broths were prepared and distributed in several culture tubes. Each tube contains 10 ml of nutrient broth. The stock solution of the Ag nanoparticles solution was serially diluted at 10−1, 10−2, 10−3 level. The tubes were inoculated with 50 µl of 24 h old bacterial suspension. Each tube was mixed with 1 ml of Ag nanoparticles solution of different concentration. The tubes were incubated for 24 h at 37 °C and OD were taken at 540 nm to measure the turbidity against a sterile blank. A negative control without bacterium and a positive control without Ag nanoparticles were incubated for comparative purpose. All the experiments were done in triplicate.
Results and Discussion
The vasaka is easily available and well known medicinal plant. Leaf extract of it is used to treat cough, chronic bronchitis, asthma, phythisis etc. [22]. It contains a large number of secondary metabolites which includes vasicine, vasicinine, vasicinol, vasicinone, essential oil, maiontone, deoxyvasicinone, vasicol, glucoside sitosterol, kaempferol. It is reported that the alkaloids [23] present in leaf extract are responsible for reducing Ag+ ions and capping for Ag nanoparticles. In this study some of the alkaloids present in working leaf extract may act as reducing and/or capping agent to synthesis Ag nanoparticles.
UV–visible Spectroscopy
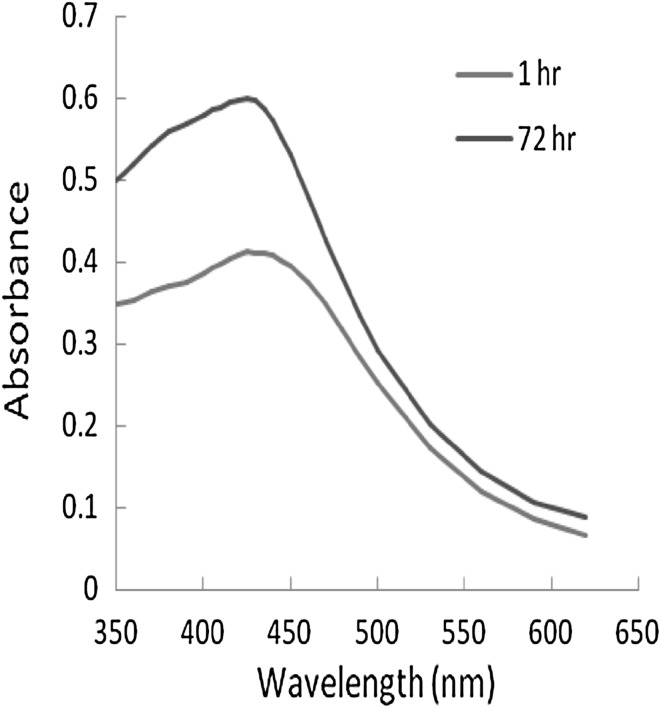
Reduction of Ag+ ion into Ag nanoparticles using leaf extract of vasaka was evidenced by visual change of colour of reaction mixture from colourless to yellow then to yellowish brown (Fig. 1). Figure 2 shows the UV–Vis spectra which confirmed the formation of Ag nanoparticles. The surface plasmon resonances (SPR) of silver nanoparticle were observed at 430 nm. The intensity of the SRP peaks increase as reaction time increase, which indicates the increase concentration of the silver nanoparticles. However, the overall result reflects that the Ag nanoparticles prepared by leaf extract are very stable without aggregation.
Fig. 2.
UV–Vis spectra of Ag nanoparticle, synthesized by reducing AgNO3 using Vasaka leaf extract
TEM
From Fig. 3 it is observed that most of the Ag nanoparticles spherical in shape, a few agglomerated Ag nanoparticles were also observed in some place. Figures also show that there is variation in particle size, the estimated average particle size is 20 nm in the range is from 5 to 50 nm. The size of these biosynthesized Ag nanoparticles is conformity with the size of earlier reported biosynthesized Ag nanoparticles using the leaf extract of Mangifera indica (50–65 nm), Eucalyptus tereticornis (60–150 nm), Carica papaya (25–40 nm), Musa paradisiaca (10–50 nm) [5].
Fig. 3.
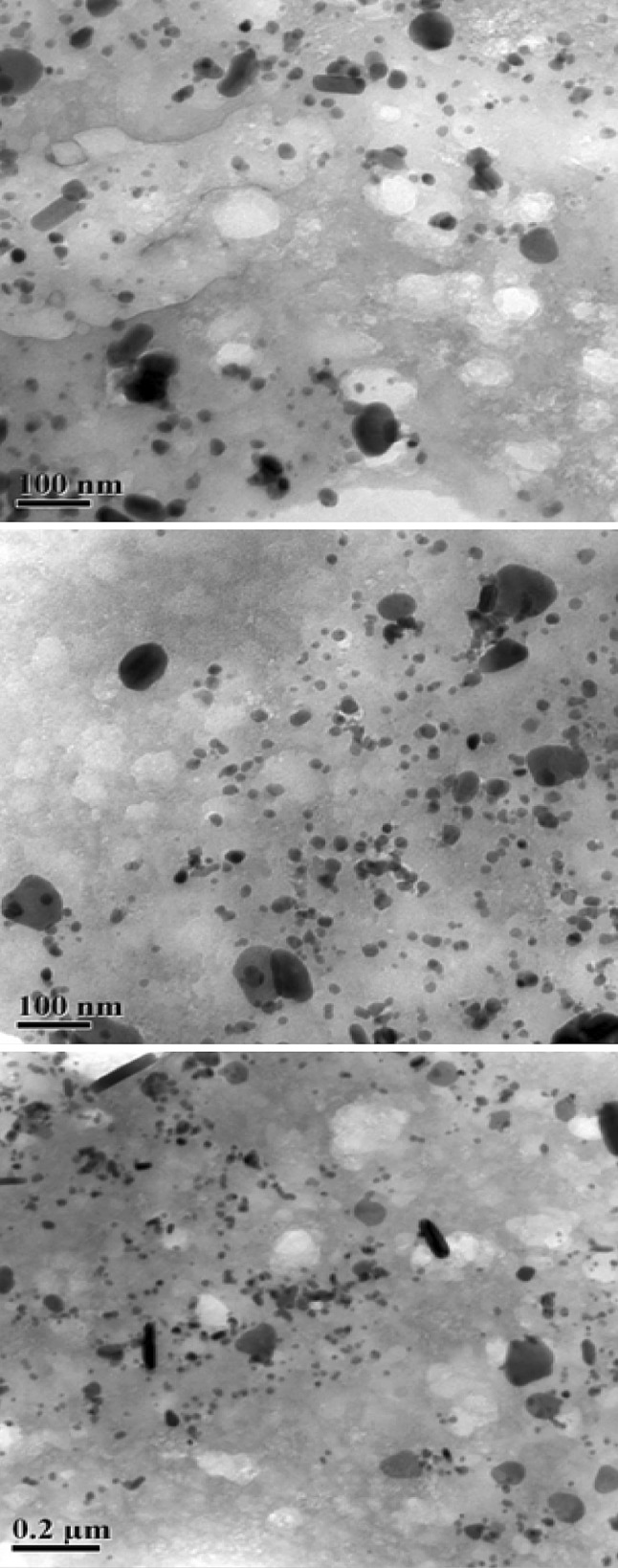
TEM images Ag nanoparticles synthesized from AgNO3 using Vasaka leaf extract
Microbiological Assays
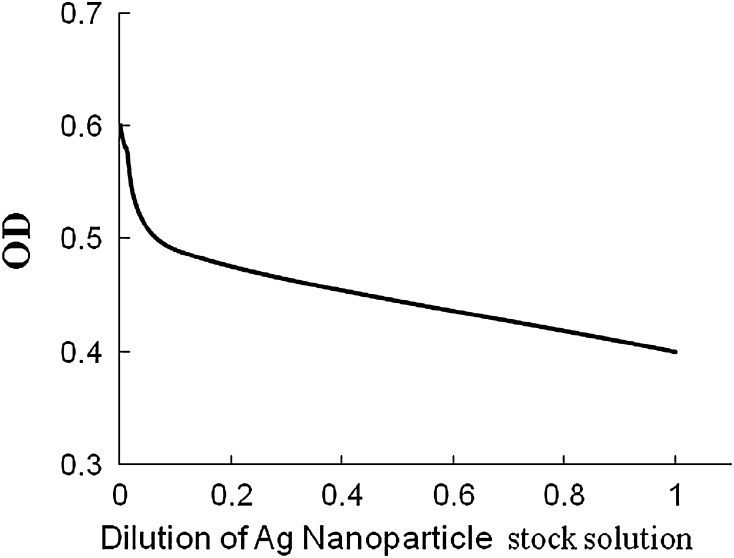
In the present study, the potentiality of silver nanoparticles as a common antimicrobial substance has been evaluated against P. aeruginosa MTCC 741 by standard methods. P. aeruginosa is an opportunistic human pathogen and the genus itself is a potent plant pathogen also. It produces soft rot disease in Arabidopsis thaliana [24] and Lactuca sativa [25]. The results of disc diffusion assays indicate that the antimicrobial activities of silver nanoparticles (ZoI = 7–9 mm) are as effective as silver ions (ZoI = 8–10 mm) whereas the raw leaf extract has no visible inhibitory effect on P. aeruginosa MTCC 741 strain. However, the result of disc diffusion assays is too preliminary to conclude about the antibacterial properties of silver nanoparticles. The Agar-cup assay method and serial dilution turbidity measurement assay methods are more sensitive than disc diffusion assay method. The results of agar-cup assay reveal that biosynthesized silver nanoparticles using vasaka leaf extract have strong efficiency to inhibit (average ZoI = 1.36–1.4 cm) the bacterial growth. The serial dilution method (Fig. 4) has selective advantage over other method of assay. The results of serial dilution method are illustrated in Fig. 5. The nanoparticles when added to the liquid medium start their antibacterial activities in terms of inhibition of the growth of bacteria. This inhibition is measured in terms of optical density values (OD). The MIC value indicates that the green synthesized silver nanoparticles using the leaf extract of vasaka can inhibit the growth of P. aeruginosa MTCC 741 strain at the concentration of 10−5 (M). This study is tuned with earlier reported studies: anti bacterial activity of Ag nanoparticles on fish pathogen and other microbes [3–5].
Fig. 4.

Serial dilution method of anti-microbial activity assay of Ag-nanoparticles synthesized from Vasaka leaf extract
Fig. 5.
Calculation of MIC of Ag nanoparticles (synthesized by reducing AgNO3 using Vasaka Leaf extract)
Several mechanisms regarding antimicrobial activity of Ag nanoparticles have been proposed. It seems that sulphur and phosphorus containing proteins or enzymes or phosphorous moiety of DNA of bacterial system may be affected by the Ag nanoparticles which lead to the inhibition of enzyme system of the organism [9]. Le et al. [26] clearly demonstrated the Ag nanoparticles get attached to the cell surface of E. coli and V. Cholera and then penetrated into the cell, destroyed the cell cytoplasm and killed the organism. Le et al. [26] also found that Ag nanoparticles significantly increase the cell permeability and affect the proper transport through plasma membrane. Thus the regulation of transport through the membrane become disrupted which leads the cell death.
In conclusion, this study revealed an economical, efficient and environment friendly route for green synthesis of Ag nanoparticles and evaluated the efficacy of the inhibitory effect of this biosynthesized Ag nanoparticles against P. aeruginosa. If further inhibitory studies of this biosynthesized Ag nanoparticles against other plant pathogenic pseudomonades like P. syringae, P. tolaasii and P. agarici agree with our present finding, then the green synthesized Ag nanoparticles could be more economical and promising alternate antibacterial agent in the field of agriculture.
Electronic supplementary material
Acknowledgments
Authors acknowledge the UGC (ERO) Kolkata for providing financial help in terms of minor research project. Authors also acknowledge the help and support from Prof. N. C. Mondal, Visva-Bharati, Santiniketan, WB for providing bacterial strain.
Contributor Information
Debadin Bose, Email: debadin@hotmail.com.
Someswar Chatterjee, Email: someswar_76@yahoo.com.
References
- 1.Kalia VC. Microbes, antimicrobials and resistance: the battle goes on. Indian J Microbiol. 2014;54:1–2. doi: 10.1007/s12088-013-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SK, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK. Immobilization of Laccase on SiO2 nanocarriers improves the its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Barua S, Hazarika SN, Manhar AK, Nath D, Karak N, Namsa ND, Mukhopadhyay R, Kalia VC, Mandal M. Green silver nanoparticles: enhanced antimicrobial and antibiofilm activity with effects on DNA replication and cell cytotoxicity. RSC Adv. 2014;4:52845–52855. doi: 10.1039/C4RA08791G. [DOI] [Google Scholar]
- 4.Velmurugan P, Iydroose M, Lee SM, Cho M, Park JH, Balachandar V, Oh BT. Synthesis os silver and gold nanoparticles using cashew nut shell liquid and its antimicrobial activity against fish pathogens. Indian J Microbiol. 2014;54:196–202. doi: 10.1007/s12088-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahanty A, Mishra S, Basu R, Maurya UK, Netam SR, Sarkar B. Phytoextract-synthesized silver nanoparticles inhibit bacterial fish pathogen Aeromonas hydrophila. Indian J Microbiol. 2013;53:438–446. doi: 10.1007/s12088-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwala M, Choudhury B, Yadav RNS. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogen. Indian J Microbiol. 2014;54:365–368. doi: 10.1007/s12088-014-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shameli K, Ahmad BM, Jazayeri SD, Shabanzadah P, Sangpour P, Jahangirin H. Investigation of antibacterial properties silver nano particles prepared via green method. Chem Cent J. 2012;6:73. doi: 10.1186/1752-153X-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Sulaiman GM, Mohammed WH, Marzoog TR, Al-Amiery AAA, Kadhum AAH, Mohamad AB. Green synthesis, antimicrobial and cytotoxic effect of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac J Trop Biomed. 2013;3:58–63. doi: 10.1016/S2221-1691(13)60024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya D, Gupta RK. Nanotechnology and potential of microorganisms. Crit Rev Biotechnol. 2005;25:199–204. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 11.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 13.Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–588. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 15.Singhal GR, Bhavesh K, Kasariya AR, Sharma R, Singh P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanoparticle Res. 2011;13:2981–2988. doi: 10.1007/s11051-010-0193-y. [DOI] [Google Scholar]
- 16.Amkamwar B, Damle C, Ahamad A, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica officinails fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 2005;5:1665–1671. doi: 10.1166/jnn.2005.184. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C. Biosynthesis of silver and gold nanoparticles by novel sun dried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104–105115. doi: 10.1088/0957-4484/18/10/105104. [DOI] [Google Scholar]
- 18.Prasad TNVKV, Elumalai EK. Biofabrication of Ag nanoparticles using Moriga olefera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed. 2011;1:439–443. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha AK, Prasad K. Green fruit of chilli (Capsicum annum L.) synthesizes nano silver. Dig J Nanomat Bios. 2011;6:1717–1723. [Google Scholar]
- 20.Harris AT, Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res. 2008;10:691–695. doi: 10.1007/s11051-007-9288-5. [DOI] [Google Scholar]
- 21.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 22.Amin AH, Mehta DR. A bronchodilator alkaloid (Vasicinone) from Adhatoda vasica Nees. Nature. 1959;184:1317. doi: 10.1038/1841317a0. [DOI] [PubMed] [Google Scholar]
- 23.Mehta DR, Naravane JS, Desai RM. Vasicinone: a bronchodilator principle from Adhatoda vasica Nees (N. O. Acanthaceae) J Org Chem. 1963;28:445–448. doi: 10.1021/jo01037a041. [DOI] [Google Scholar]
- 24.Walker TS, Bais HP, Déziel E, Schweizer HP, Rahme LG, Fall R, Vivanco JM. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol. 2004;134:320–331. doi: 10.1104/pp.103.027888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahme LG, Tan MW, Le L, Wong SM, Tompkins RG, Calderwood SB, Ausubel FM. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci USA. 1997;94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le AT, Le TT, Nguyen VQ, Tran HH, Dang DA, Tran QH, Vu DL. Powerful Silver nanoparticles for the prevention of Gastrointestinal bacterial infection. Adv Nat Sci Nanosci Nanotechnol. 2012;3:045007. doi: 10.1088/2043-6262/3/4/045007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.