Abstract
Diosgenin is an important precursor for synthesis of more than 200 steroidal hormone medicines. Rhizome of Dioscorea zingiberensis C. H. Wright (RDZ) contained the highest content of diosgenin in Dioscorea plant species. Diosgenin is traditionally extracted by acid hydrolysis from RDZ. However, the acid hydrolysis process produces massive wastewater which caused serious environment pollution. In this study, diosgenin extraction by direct biotransformation with Penicillium dioscin was investigated. The spawn cultivation conditions were optimized as: Czapeks liquid culture medium without sugar and agar (1,000 ml) + 6.0 g dioscin/6.0 g DL, 30 °C, 36 h; solid fermentation of RDZ: mycelia/RDZ of 0.05 g/kg, 30 °C, 50 h; the yield of diosgenin was over 90 %. Spawn cultivation was crucial for the direct biotransformation. In the spawn cultivation, amount and ratio of dioscin/DL were the key factors to promote biotransformation activity of P. dioscin. This biotransformation method was environment-friendly, simple and energy saving, and might be a potential substitute for acid hydrolysis in diosgenin extraction industry.
Keywords: Biotransformation, Diosgenin, Dioscin, Dioscorea zingiberensis, Penicillium dioscin, Rhizome
Introduction
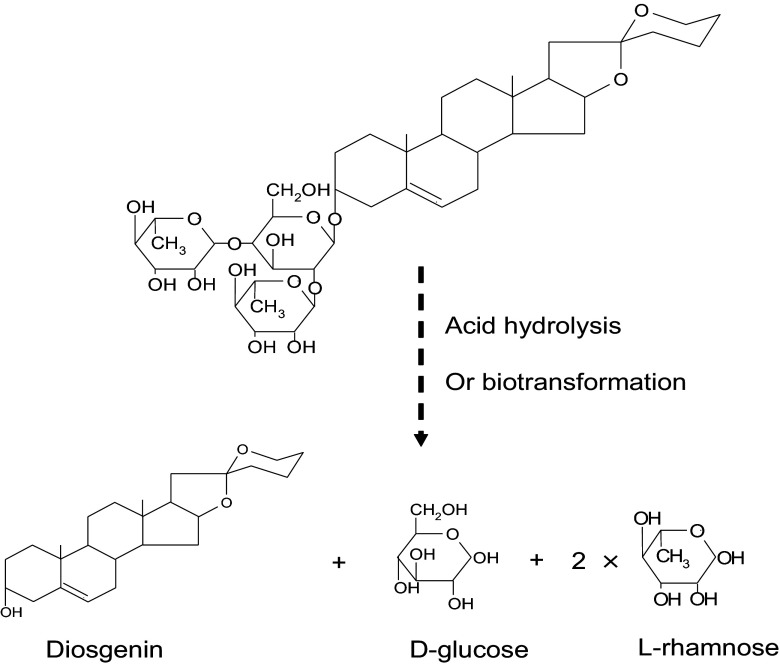
Dioscorea plants were used as Traditional Chinese Medicine (TCM) in China. In the Chinese medicine monographs shennongbencaojing and bencaogangmu, dioscorea plants were recorded as “reducing rheumatism, strengthening, nourishing muscles, enhancing ear and eyesight”. In recent years, there were many reports on the medicinal value of diosgenin, such as anticancer activity [1], antagonistic effect on rheumatoid arthritis, cardiovascular action, and antimalarial action [2–4]. To date, diosgenin present in dioscorea plants has been used as the precursor for synthesis of more than 200 kinds of steroids hormone medicines [5]. Dioscorea zingiberensis Wright, called Chinese yam, was determined of the highest content of diosgenin in Dioscorea species and was used as the main source of diosgenin. The average production of RDZ in China was more than 12 million tons per year. Diosgenin exists mostly in the form of dioscin in RDZ. In diosgenin extraction industry, preparation of diosgenin mainly uses sulphuric acid to hydrolyze dioscin from RDZ (Fig. 1), then followed by petroleum ether extraction. Unfortunately, the acid hydrolysis process produces massive acid wastewater [6, 7], which was the main bottleneck against diosgenin industry. Several processes trying to increase extraction rate of diosgenin and reduce acid wastewater were used in diosgenin extraction industry: (1) materials were fermented with complex enzymes to remove fiber, starch and sugars of the materials and then hydrolyzed with sulphuric acid; (2) materials were pre-fermented naturally and then acid hydrolyzed to enhance extraction rate by about 15 %; (3) dioscin was first extracted from RDZ and then hydrolyzed with sulphuric acid, which greatly decreased the amount of acid wastewater. All these methods greatly enhance extraction yield or reduce acid wastewater. However, sulphuric acid hydrolysis are still used, which is the main source of environment pollution and bottleneck of diosgenin extraction industry.
Fig. 1.
Chemical structure of dioscin and hydrolysis reaction
Biological hydrolysis of natural products has many advantages, such as high specificity, mild reaction conditions, and clean production process as well as low cost. Research on production of bioactive compounds through fungi biotransformation had been a hot point in biomedicine field [8–12]. However, to our knowledge, there were very few reports on screening of microorganism of highly efficient biotransformation of dioscin to diosgenin and no reports on direct biotransformation of RDZ, which was simple and more practical. Several literatures reported biotransformation of extracted steroidal saponins to diosgenin, but of low biotransformation rate [13–15]; furthermore, extraction of dioscin is relatively of high cost, which makes it less applicable in the biotransformation procedure. So related researches should be focused on screening of microorganism species with high activity and developing a highly efficient biotransformation system.
In this study, spawn cultivation of Penicillium dioscin and direct RDZ biotransformation by P. dioscin were investigated. This study provided a possibility for direct biotransformation of dioscin in RDZ to diosgenin, which was simple, and environment-friendly.
Materials and Methods
Materials, Chemicals and Determination Method
RDZ were collected from Shiyan cultivation base of D. zingiberensis, Shiyan, Hubei, China. Dioscin and DL used in spawn cultivation were prepared from RDZ. The fungal strain (P. dioscin) used for biotransformation was screened and identified by authors and preserved in China Center of Type Culture Collections (CCTCC), Wuhan University, China, CCTCC No: M206001.
Standard diosgenin and dioscin were purchased from Beijing Yingze Naxin Chemical Technology Institute (Beijing, China). Acetonitrile was of HPLC grade. Other chemical reagents were of analytical grade. HPLC system (Hangzhou Saizhi Sci & Tech Co., Ltd., China) consists of N2000 ChemStation, STI501 pump, STI 501 UV detector and Lichrospher C18 column (4.6 × 250 mm, Jiangsu Hanbon Sci & Tech Co., Ltd., Jiangsu, China). Contents of diosgenin and dioscin were simultaneously analyzed by the following HPLC method.
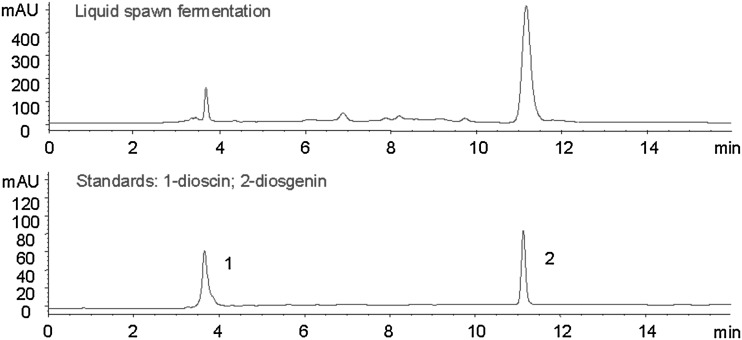
Dioscin and diosgenin standards were dissolved together with 95 % ethanol to different concentrations; acetonitrile was used as solvent A (0.5 % acetic acid as solvent B) 0–20 min, 50–100 %, 20–25 min, 100 %, 25–30 min, 100–50 %, column temperature of 25 °C, sample injection volume of 20.0 µl, flow rate of 1.0 ml/min, detection wavelength of 206 nm. With this HPLC method, dioscin and diosgenin standards were well separated (Fig. 2). The calibration curves were constructed by plotting the peak areas versus the mass concentration of each standard. The calibration curve of diosgenin were obtained as follows:
Fig. 2.
Diosgenin biotransformed by P. dioscin (1 dioscin; 2 diosgenin)
Analysis of diosgenin in the sample: Liquid culture medium or RDZ were vacuum dried and ground to powders. The powders were extracted with 95 % ethanol, and diosgenin of the extract was analyzed with HPLC method described above.
Determination of diosgenin in RDZ: 50 g ground RDZ was dried at 80 °C for 4 h, then weighted and hydrolyzed with 400 mL hydrochloric acid (1.0 M) at 95 °C water bath for 4 h, then regulated pH to 7.5 with ammonia, filtrate, washed with distilled water five times, dried at 80 °C for 4 h, then extracted with petroleum ether in Soxhlet extractor for 4 h. The extract was dried and redissolved with methanol for HPLC analysis.
Preparation of Dioscin and DL
Dioscin Preparation
500 g of dried RDZ powders were extracted with 1000 mL 90 % ethanol (v/v) at 80 °C for 12 h, three extraction cycles; the extracts were mixed and vacuum concentrated at 80 °C to 200 ml, and then extracted with 500 mL petroleum ether to remove oils; the deoiled extracts were finally extracted with 500 mL n-butyl alcohol; the n-butyl alcohol extracts were vacuum-dried at 80 °C and redissolved with 100 mL 95 % ethanol, and 500 mL acetone was added into deposit dioscin. The sediments were washed with acetone and filtered. The obtained sediments were vacuum dried at 80 °C, 9.35 g light yellow powders were obtained as dioscin sample. With this extraction process, more RDZ powders were extracted to obtain enough dioscin to be used in this study.
DL Preparation
The extracted dioscin was hydrolyzed with 1.0 M hydrochloric acid at 95 °C for 4 h, then neutralized with ammonia to pH 7.0. The neutralized solution was extracted with petroleum ether, and the left solution was vacuum dried at 60 °C. The dried residues were mostly DL (about 1/3 glucose and 2/3 rhamnose, Fig. 1) to be used in the liquid culture.
Spawn Cultivation
According to our previous study, dioscin and DL were the important factors for inducing and maintaining the biotransforming activity of P. dioscin (data not shown). So in the spawn cultivation of P. dioscin, amount and ratio of dioscin/DL was investigated. P. dioscin was cultured in the medium containing dioscin/DL of different ratios: NSM+dioscin/DL (0/8, 1/7, 2/6, 3/5, 4/4, 5/3, 6/2, 7/1, 8/0 g/L). Spawn cultivation was conducted in vibration with a speed of 200 r/min and at 25 °C. The growth of mycelia was determined according to absorbance of the cultured liquid at 600 nm (OD value). Diosgenin produced in the liquid media was extracted and determined with the method described above. Ratio of diosgenin/dioscin in the media was considered as DHR. Based on the analysis of OD and DHR, ratio of dioscin/DL was optimized.
Based on the optimized ratio of dioscin/DL, different amounts of dioscin were added into the liquid culture medium (g/L). OD and DHR were analyzed to select the right amount of dioscin that should be added in the liquid DSM (Czapeks culture medium with DL as the only carbon source, called DSM thereafter). Based on the optimized medium, the spawn cultivation parameters as temperature (15/20/25/30/35/40 °C) and time (12/24/36/48/60/72/84/96 h) were optimized.
RDZ Fermentation
100 g dried RDZ powders were directly incubated with liquid spawn. Mycelia contents of the incubated liquid spawn were analyzed: liquid spawn was centrifuged at 5,000 r/min to separate mycelia, the separated mycelia was weighted to calculate the fresh mycelia content (g/L). 1.0 g RDZ powders/0.8 mL liquid spawn, mixed well and fermented in darkness for 48 h at 28 °C. After fermentation, diosgenin of the RDZ was extracted and determined according to the methods described above. DD was calculated to select the right fermentation parameters as temperature, time and amount of incubated spawn.
Results
Spawn Cultivation: Optimal Ratio of Dioscin/DL, 1/1
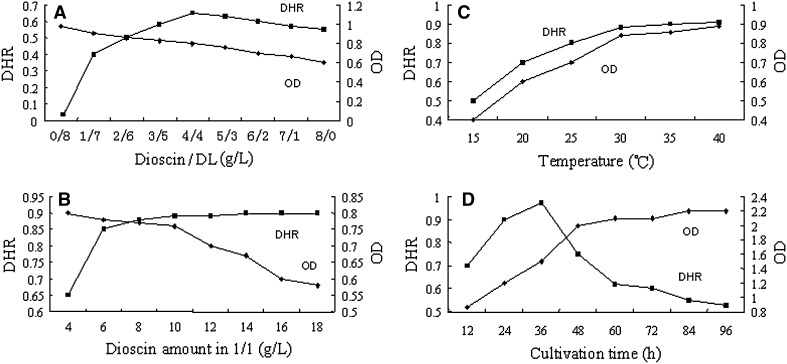
In the spawn cultivation, in order to promote biotransformation activity of P. dioscin, only dioscin or DL was used as carbon source for P. dioscin cultivation. Theoretically, in such cultivation, P. dioscin was induced to secrete dioscin hydrolysis enzymes to get carbon source (glucose and rhamnose) from dioscin. In Fig. 3a, mycelia growth (OD value) decreased with the ratio of dioscin/DL increasing, which indicated that it was more difficult for P. dioscin to utilize DL from dioscin by enzyme hydrolysis than to use DL directly added in the culture media. It might be concluded that dioscin had some inhibitory effect against mycelia growth of P. dioscin after dioscin/DL = 4/4, or dioscin over such concentration ratio acted as the environmental stress to activate the expression of dioscin hydrolysis enzymes. The DHR was increased positively proportional to the ratio of dioscin to DL and finally reached the highest at the amount of dioscin/DL = 4.0 g/4.0 g, and then decreased after 4.0 g/4.0 g. So in order to increase the mycelia growth and hydrolysis activity of P. dioscin, the ratio of dioscin/DL should be 1/1.
Fig. 3.
Liquid fermentation parameters (n = 3, RSD = 1.72–3.5 %; DHR diosgenin transformed from dioscin added in the media/the dioscin added in the media; OD absorbance of the liquid mycelia at 600 nm)
Spawn Cultivation: Optimal Amount of Dioscin/DL, 6.0 g/6.0 g, g/L
Based on the optimized ratio of dioscin/DL (1/1), different amounts of dioscin were added into the liquid culture medium. In Fig. 3b, DHR increased fast in positive proportion to the amount of dioscin before 6.0 g/L, but increased very slowly after 6 g/L, which indicated that at the ratio of dioscin/DL = 1/1, dioscin could enhance DHR. However, mycelia growth showed negative correlation with dioscin. So it could be concluded that dioscin enhanced expression of dioscin hydrolyzing enzyme and inhibited mycelia growth at the same time. The optimal amount of dioscin should be 6 g/L. Namely, dioscin/DL = 6.0 g/6.0 g g/L.
Spawn Cultivation: Optimal Temperature and Time: 30 °C, 36 h
In Fig. 3c, 30 °C proved to be the best temperature for DHR and mycelia growth. After the temperature of 30 °C, DHR and mycelia growth increased slowly. So 30 °C should be the optimal temperature for both mycelia growth and DHR. In Fig. 3d, mycelia growth increased with time within 12–96 h and increased fastest within 12–48 h. DHR showed time-dependent within 12–36 h, but decreased rapidly after 36 h, which may be attributed that some dioscin was absorbed and decomposed by mycelia or the secretion activity of the mycelia declined after 36 h although the mycelia amount increased within 12–96 h. So the cultivation time should be no more than 36 h.
Biotransformation in Solid Fermentation of RDZ: Liquid Spawn, 0.05 g Mycelia/kg RDZ, 30 °C, 50 h, over 90 % yield
In the biotransformation products by P. dioscin, the main component was diosgenin (Fig. 2), although RDZ contains several kinds of steroidal saponins [13]. In this study, P. dioscin showed the specific activity to produce diosgenin. So RDZ fermentation with P. dioscin is convenient to produce more purified diosgenin, while the traditional acid hydrolysis produces a complex mixture of many kinds of steroidal saponins that need further separation and purification [16].
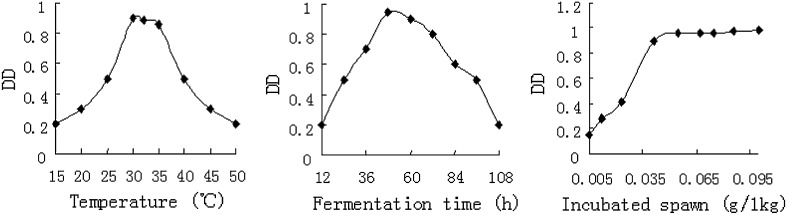
In Fig. 4, DD decreased significantly after fermentation of 50 h. The possible reason for the DD decreasing after 50 h might be the spawn activity decline or feedback inhibitory effects by dioscin against secretion of dioscin hydrolysis enzymes. The optimal parameters for the highest DHR were: temperature of 30 °C, fermentation time of 50 h, and incubated spawn amount of 0.05 g/kg. The diosgenin production (DD) was over 90 % of the total diosgenin of the RDZ by acid hydrolysis (Table 1). In the diosgenin extraction industry, the RDZ fermentation showed simple, economic and energy-saving (Table 1) and should be more applicable compared to the traditional methods and reported biotransformation methods [16].
Fig. 4.
Parameters of RDZ fermentations (each value is the mean of three replicates, RSD = 0.57–4.15 %; DD diosgenin by biotransformation/diosgenin by acid hydrolysis)
Table 1.
Comparison of diosgenin production by acid hydrolysis and RDZ fermentation (n = 3)
| Isolation methods | Isolation process | Diosgenin content (%) | DD | Heating treatment |
|---|---|---|---|---|
| Traditional method | Acid hydrolysis → neutralization → drying → diosgenin extraction | 2.357 ± 0.399 | 95 °C, 4 h, for acid hydrolysis; 80 °C, 4 h, before diosgenin extraction | |
| Direct biotransformation | Biotransformation → drying → diosgenin extraction | 2.143 ± 0.113 | 0.96 | 80 °C, 4 h before diosgenin extraction |
| p value | 0.167 | |||
DD diosgenin by biotransformation/diosgenin by acid hydrolysis
Discussion
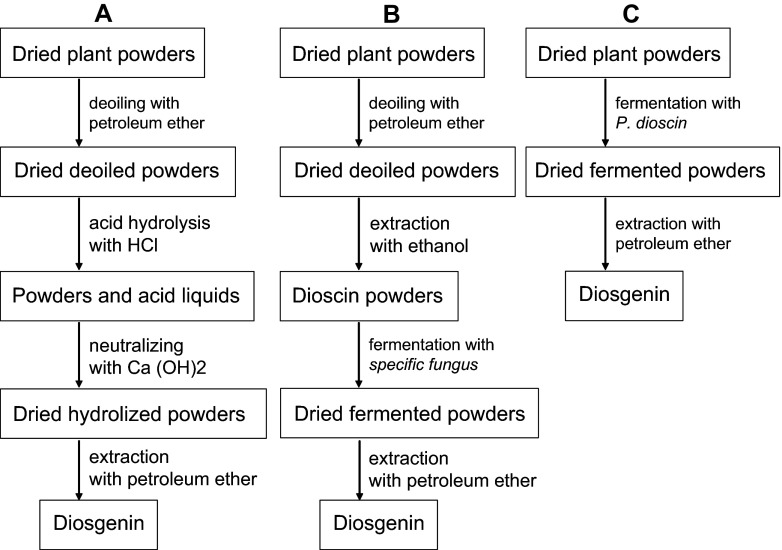
In the traditional extraction process, heating was used for acid hydrolysis (95 °C, 4 h) and it wasted much more energy than the direct biotransformation process (Table 1). So compared to the traditional acid hydrolysis process, the direct biotransformation was not only energy-saving but environment-friendly and simple (Table 1). Liu et al. [13] had reported another biotransformation with RDZ powders in water, in which dioscins were dissolved in water and then biotransformed into diosgenin by a fungus species of Trichoderma harzianum (Fig. 5b). However, in our study, RDZ could be directly fermented with the specific fungus species of P. dioscin (Fig. 5c) by solid fermentation without using large amounts of water, which could be industrially used. Although P. dioscin could transform dioscin into diosgenin by fermentation with extracted dioscin with a high yield of 90 % (data not shown), dissolving or extraction of dioscin from RDZ showed high cost in practical application. So this direct biotransformation of RDZ was more practical and economic than the traditional extraction (Fig. 5a) and reported biotransformation (Fig. 5b).
Fig. 5.
Comparison of diosgenin extraction methods from RDZ (a traditional extraction method; b reported extraction method by biotransformation; c extraction method by direct biotransformation of this study)
In this direct biotransformation method, mycelia amount proved to be a crucial factor of DHR in such solid fermentation. So liquid cultivation of P. dioscin spawn was the most important step for the direct biotransformation process. In the liquid cultivation, dioscin acted as an inducer of dioscin-hydrolysis activity and as an inhibitor against the mycelia growth at the same time, so dioscin/DL was the key factor for preparation of highly active spawns. The mechanism deserves further study.
Except for the traditional cultivation factors such as culture medium components and temperature, other new physical factors as selenium and lightening of special wavelengths [17] were reported to significantly enhance activity of fungus species as Cordyceps militaris. So new cultivation methods to promote hydrolysis activity of P. dioscin spawn deserves further research.
Conclusions
Conditions for spawn cultivation were primarily optimized: Czapeks culture medium (no sugar and agar) + dioscin/DL = 6 g/6 g, 30 °C, and 36 h fermentation. Optimal conditions for RDZ fermentation: liquid spawn incubated in the RDZ at the amount of 0.05 g mycelia/kg RDZ, fermentation temperature of 30 °C, fermentation time of 50 h. The production of diosgenin proved to be over 90 % of total diosgenin of the RDZ samples. Penicillium dioscin showed the specific activity to produce mostly diosgenin in the biotransformation products; in this biotransformation, no further purification was needed; amount and ratio of dioscin/DL were the key factors to promote biotransformation activity of P. dioscin. Completely no acid hydrolysis process used and no acid wastewater produced, this direct biotransformation method (RDZ fermented by P. dioscin) provided a basis for further development of environment-friendly bioprocess in diosgenin extraction industry.
Acknowledgments
This research was funded by Open Foundation of Key Laboratory of Biologic Resources Protection Utilization of Hubei Province (2011PKLHB1116) and by Scientific Foundation of Education Department of Hubei Province (D20141901). We are grateful to anonymous reviewers and scientific editor for critical review and valuable suggestions.
Abbreviations
- RDZ
Rhizome of Dioscorea zingiberensis, vacuum dried at 80 °C and ground into powders through 60 mesh screen
- DL
d-glucose and l-rhamnose prepared from dioscin
- NSM
Czapeks culture medium without sugar and agar
- DSM
Czapeks culture medium with sugars prepared from dioscin as the only carbon source
- DD
Ratio of diosgenin by biotransformation/diosgenin by acid hydrolysis
- DHR
Ratio of diosgenin by biotransformation/the dioscin added in the medium
- OD
Optical density
Contributor Information
Jingzhou Dong, Email: djz21cn@aliyun.com.
Ying Wang, Email: yingwang@wbgcas.cn.
References
- 1.Shu D, Qing Y, Tong Q, He Y, Xing Z, Zhao YL, Yi L, Wei YQ, Huang WWuXH. Deltonin isolated from Dioscorea zingiberensis inhibits cancer cell growth through inducing mitochondrial apoptosis and suppressing Akt and mitogen activated protein kinase signals. Biol Pharm Bull. 2011;34:1231–1239. doi: 10.1248/bpb.34.1231. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez G, Pabon A, Carmona J, Blair S. Evaluation of clastogenic potential of the antimalarial plant Solanum nudum. Phytother Res. 2004;18:845–848. doi: 10.1002/ptr.1534. [DOI] [PubMed] [Google Scholar]
- 3.Liagre B, Vergne-Salle P, Corbiere C, Charissoux JL, Beneytout JL. Diosgenin, a plant steroid, induces apoptosis in human rheumatoid arthritis synoviocytes with cyclooxygenase-2 over expression. Arthritis Res Ther. 2004;6:R373–R383. doi: 10.1186/ar1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trouillas P, Corbiere C, Liagre B, Duroux JL, Beneytout JL. Structure–function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: a molecular modelling approach of natural molecules structurally close to diosgenin. Bioorg Med Chem. 2005;13:1141–1149. doi: 10.1016/j.bmc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS. Microbial conversion of steroid compounds: recent developments. Enzym Microb Technol. 2003;32:688–705. doi: 10.1016/S0141-0229(03)00029-2. [DOI] [Google Scholar]
- 6.Li H, Fang Z. Resourceful treatment of Dioscorea zingiberensis wastewater using a double-chamber microbial fuel cell. Adv Mater Res. 2013;602:1081–1085. doi: 10.4028/www.scientific.net/AMR.634-638.1081. [DOI] [Google Scholar]
- 7.Li MM, Yan QQ, Sun XQ, Zhao YM, Zhou YF. A preliminary study on pollination biology of three species in Dioscorea (Dioscoreaceae) Life Sci J. 2014;11:436–444. [Google Scholar]
- 8.Wei M, Bai Y, Ao M, Jin W, Yu P, Zhu M, Yu L. Novel method utilizing microbial treatment for cleaner production of diosgenin from Dioscorea zingiberensis C.H. Wright (DZW) Bioresour Technol. 2003;146:549–555. doi: 10.1016/j.biortech.2013.07.090. [DOI] [PubMed] [Google Scholar]
- 9.Yan Q, Zhou W, Shi XL. Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces Bainier sp. 229. Process Biochem. 2010;45:1550–1556. doi: 10.1016/j.procbio.2010.06.007. [DOI] [Google Scholar]
- 10.Rodarte-Morales AI, Feijoo G, Moreira MT. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation. 2011;23:145–156. doi: 10.1007/s10532-011-9494-9. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho TC, Polizeli AM, Turatti ICC. Screening of filamentous fungi to identify biocatalysts for lupeol biotransformation. Molecules. 2010;15:6140–6151. doi: 10.3390/molecules15096140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabinya LV, Mafunga T, Brand JM. Bioconversion of ferulic acid and 4-vinylguaiacol by a white-rot fungus isolated from decaying wood. Afr J Biotechnol. 2010;9:1955–1958. [Google Scholar]
- 13.Liu L, Dong YS, Qi SS, Wang H, Xiu ZL. Biotransformation of steriodal saponins in Dioscorea zingiberensis C. H. Wright to diosgenin by Trichoderma harzianum. Appl Microbiol Biotechnol. 2010;85:933–940. doi: 10.1007/s00253-009-2098-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Li H, Lu Z, Shi J, Xu Z. Screening and condition optimization of a strain for efficiently biotransformation of saponins in Dioscorea zingiberensis into diosgenin. Chin J Biotechnol. 2013;29:848–852. [PubMed] [Google Scholar]
- 15.Yu LD, Zheng TX, Zhu YL. Microbial transformation of saponins in Dioscorea zingiberensis for diosgenin production with Trichoderma reesei. Adv Mater Res. 2013;709:805–809. doi: 10.4028/www.scientific.net/AMR.709.805. [DOI] [Google Scholar]
- 16.XU CJ (2000) The Chinese dioscorea plant resoures, SectionI, Sichuan Science Press (In Chinese) p 80
- 17.Dong J, Liu M, Lei C, Zheng X, Wang Y. Effects of selenium and light wavelengths on liquid culture of Cordyceps militaris link. Appl Biochem Biotechnol. 2012;166:2030–2036. doi: 10.1007/s12010-012-9628-5. [DOI] [PubMed] [Google Scholar]