Abstract
Background
Dabigatran and rivaroxaban are new oral anticoagulants that are eliminated through the kidneys. Their use in dialysis patients is discouraged because these drugs can bio-accumulate to precipitate inadvertent bleeding. We wanted to determine if prescription of dabigatran or rivaroxaban was occurring in the dialysis population and if these practices were safe.
Methods and Results
Prevalence plots were used to describe the point prevalence (monthly) of dabigatran and rivaroxaban use among 29,977 hemodialysis patients with atrial fibrillation (AF). Poisson regression compared the rate of bleeding, stroke, and arterial embolism in patients who started dabigatran, rivaroxaban, or warfarin. The first record of dabigatran prescription among hemodialysis patients occurred 45 days after the drug became available in the US. Since then, dabigatran and rivaroxaban use in the AF-ESRD population has steadily risen where 5.9% of anti-coagulated dialysis patients are started on dabigatrian or rivaroxaban. In covariate adjusted Poisson regression, dabigatran (RR=1.48; 95% CI 1.21-1.81, p=0.0001) and rivaroxaban (RR=1.38; 95% CI 1.03-1.83, p=0.04) associated with a higher risk of hospitalization or death from bleeding when compared to warfarin. The risk of hemorrhagic death was even larger with dabigatran (RR=1.78; 95% CI 1.18-2.68, p=0.006) and rivaroxaban (RR=1.71; 95% CI 0.94-3.12, p=0.07) relative to warfarin. There were too few events in the study to detect meaningful differences in stroke and arterial embolism between the drug groups.
Conclusions
More dialysis patients are being started on dabigatran and rivaroxaban, even when their use is contraindicated and there are no studies to support the benefits outweigh the risks of these drugs in ESRD.
Keywords: atrial fibrillation, warfarin, dabigatran, rivaroxaban, dialysis
INTRODUCTION
Dabigatran is a novel, orally available, direct thrombin inhibitor that is currently approved to prevent stroke in patients with atrial fibrillation. The anticoagulant is easier to use than warfarin because laboratory monitoring is not required and there are fewer drug-drug interactions.1
Accordingly, dabigatran use in the United States increased from 3.1% of all oral anticoagulation visits in 2010 to 18.9% in 2011.2 By virtue of dabigatran's growing availability, the drug is sometimes prescribed for other situations where the drug has not been rigorously studied. For example, in 2011, Kirkly et al. reported that 37% of treatment visits for dabigatran were for coronary heart disease, hypertensive heart disease, and venous thromboembolism.2 Similar trends will likely be seen with rivaroxaban, another novel anticoagulant that is directed against Factor Xa, which became available in 2011.3
Dabigatran and rivaroxaban have not been studied in end-stage renal disease patients who were specifically excluded from RE-LY and ROCKET AF, two large randomized trials that were conducted in patients without renal failure.4,5 Prescription of these novel oral anticoagulant drugs (NOAC) in dialysis patients is currently contraindicated because the drugs are cleared via the kidneys and drugs levels can bio-accumulate to precipitate bleeding.6,7 Dabigatran is cleared by dialysis, which leads to a precipitous drop in the drug level, while rivaroxaban is not cleared by dialysis because 95% of rivaroxaban is protein bound.
We surveyed a large ESRD population, to describe the prescribing patterns of dabigatran and rivaroxaban in chronic hemodialysis patients with atrial fibrillation. We also compared the rate of bleeding in dialysis patients taking warfarin, dabigatran, or rivaroxaban.
METHODS
Study population and data sources
We generated prevalence plots, from October 2010 to October 2014, to describe the prevalence of dabigatran and rivaroxaban within a population of chronic hemodialysis patients with atrial fibrillation. In a secondary analysis, we identified the time point where patients from the above hemodialysis population who were initiated (de novo) on dabigatran, rivaroxaban, warfarin, or aspirin only. These patients were then followed for up to two years for bleeding and stroke outcomes.
Data for the study were abstracted from the Fresenius Medical Care North America (FMCNA) ESRD database which prospectively captures 1,922 clinically pertinent and standardized data elements for the purpose of conducting large scale outcomes studies in the dialysis population. Over 1500 clinics in 48 States, the District of Columbia, and the Territory of Puerto Rico were represented in the Database which corresponds to approximately 30% of the US chronic dialysis population. The study protocol was approved and granted a waiver of informed consent by the New England Institutional Review Board.
All subjects registered in the Database are followed longitudinally, where data parameters are actively collected and entered at the point-of-care for each patient at every visit, which typically occurs three times per week. Demographic, comorbid, medication, vascular access, and dialysis treatment data are inputted by trained healthcare personnel into standardized electronic forms provided on touchscreen monitors located near each treatment station. The data collection tool includes predefined logic features and user alerts to check data as it is entered. Required fields are structured so that valid data must be entered before the system can authorize the hemodialysis treatment to begin. At the end of each dialysis shift, automatic edit checks are executed to identify inconsistent or out-of-range data which require immediate reconciliation by the facility charge nurse before the shift can be closed and submitted for billing. All laboratory processing was performed by a single accredited provider (Spectra Laboratories, Rockleigh NJ) and the results were directly downloaded into the Database.
Data auditing occurred on the second week of each month when facility data quality reports were generated for assessment by FMCNA Clinical Quality Managers. The reports were also disseminated back to facilities for auditing by the clinical manager and senior clinical staff.
Outcome measures
Among AF patients who started de novo oral anticoagulation after October 2010, we reported the percentage of patients who were initiated on dabigatran or rivaroxaban. Drug use was also reported by dose for patients on the full drug dose (dabigatran 150 mg BID and rivaroxaban 20 mg qD) and for patients on a reduced dose for moderate renal impairment (dabigatran 75 mg BID and rivaroxaban 15 mg qD).We used prevalence plots to graphically depict the longitudinal point prevalence of dabigatran and rivaroxaban in the full AF population on anticoagulation. Patients with a previous diagnosis of warfarin skin necrosis (n=4), protein S deficiency (n=0), protein C deficiency (n=0), or calciphylaxis (n=2) were excluded from the analysis. Caciphylaxis is a syndrome of vascular calcification, thrombosis, and skin necrosis that can be precipitated by warfarin use.
The endpoints of the secondary analysis were (a) bleeding (major and minor), and (b) embolic stroke or arterial embolism, within two years of medication initiation. Bleeding events were categorized into major or minor, and then further classified by the anatomical location of the bleed. Major bleeding was defined as a hemorrhagic event resulting in hospitalization or death. Cause of hospitalization or death was extracted from hospital discharge summaries or Form 2746-ESRD death notification, which is mandated by The Centers for Medicare & Medicaid Services for all dialysis deaths. The accuracy of the major bleeding endpoint was previously validated among 75 patients sampled from the Database (К=0.969).22 Minor bleeding was defined as a hemorrhagic event where hemostasis was achieved without the need for hospitalization and did not result in death. Minor bleeding events were identified by reviewing dialysis nursing notes. For this, KC reviewed more than 900,000 pages of nursing notes for all study patients to identify documentation of patient or nurse reported bleeding events where hemostasis was achieved without the need of medical attention. The adjudicator was blinded to patient, treatment group, and outcome. Access bleeding was defined as 1) spontaneous bleeding from the arteriovenous shunt or exit site between dialysis sessions; or 2) prolonged bleeding where more than 30 minutes of compression was required to achieve hemostasis after the needles were withdrawn from the vascular access at the end of the dialysis treatment. Pulmonary bleeding included hemoptysis and hemorrhagic effusion. Hematuria, vaginal, or penile bleeding was defined as urological. Embolism was defined as the acute occlusion of an arterial vessel, excluding the heart and brain.
Medication prescription and patient covariates
Patient prescriptions for oral and home medications were charted in the Research Database on standardized medication tables. Registered nurses at the dialysis facilities transcribed each drug name with its route, start date, and stop date as labeled on the medication bottle into the Database forms. Medication records were reconciled when patients initiated dialysis, routinely once per month, and in the immediate post-hospitalization period.
For this study, medication records for each patient were electronically parsed for occurrences of dabigatran, rivaroxaban, warfarin, aspirin, Aggrenox, and clopidogrel which were preceded by a diagnosis of atrial fibrillation in the patient record. The diagnostic accuracy of atrial fibrillation in the Database was previously found to be 75% when compared to electrocardiograms obtained from the same patient.8 Because the drug names in the medication records were entered as free text, KC reviewed each drug order for accuracy before being included in the study analysis. Patients were categorized into study groups based on their first ever prescribed anticoagulant drug (de novo). Patients were then enrolled in the analysis only if this first prescription date of dabigatran, rivaroxaban, warfarin, or aspirin occurred after October 10, 2010 which was the day dabigatran was approved for use in the US. Patient stroke risk was quantified by the CHADS2 Score, while the risk for major bleeding was estimated using the Outpatient Bleeding Risk Index.9,10
Statistical analysis
We calculated and graphed the point prevalence (monthly, October 2010 to October 2014) of dabigatran and rivaroxaban, among the AF hemodialysis population on anticoagulation. The point prevalence was defined as the number of hemodialysis patients on dabigatran or rivaroxaban divided by the number of patients on dabigatran, rivaroxaban, or warfarin on the first day of each month (per 100 patients). A second series of prevalence plots with 95% confidence bands were generated as Supplementary Figures 1, 2, and 3.
For the secondary analysis, subjects were classified and followed from the time they initiated dabigatran, rivaroxaban, warfarin, or aspirin; by which ever drug was started first. Patient time was counted up until death, withdrawal, transplantation, transfer to another dialysis unit, or discontinuation of dabigatran, rivaroxaban, warfarin, or aspirin for more than 14 days. Baseline characteristics of patients initiated on dabigatran, rivaroxaban, warfarin, and aspirin were tabulated and compared using ANOVA (continuous variable) or chi-square (categorical variable) testing.
Study endpoints were initially reported by event count and unadjusted Poisson event rate (per 100 patient years) for each of the dabigatran, rivaroxaban, warfarin, or aspirin groups. Formal comparisons between the drug groups were done by rate ratio (RR) with 95% confidence intervals using covariate adjusted Poisson regression. The final adjusted model included all parameters listed in Table 1 after backward variable selection with an exit criteria of p<0.05. A second analysis was performed to further evaluate the robustness of the main conclusion with covariate matching to decrease the potential bias from unmeasured confounding. For this, each dabigatran (1:2 matching ratio) and rivaroxaban (1:2) subject was matched to a warfarin subjects on the 20 baseline characteristics listed in Supplemental Table 1. Data matching was accomplished through a greedy algorithm supplied by Kosanke et al.11 In short, cases were matched to controls by the shortest matching distance between case and control variables. Matches were made based on the current available choices without consideration of future case-control selection, hence the term “greedy” algorithm. Groups were examined for covariate balance, before rate ratios for major and minor bleeding were calculated between the drug groups.
Table 1.
Baseline characteristics of patients initiated on warfarin, aspirin, dabigatran, or rivaroxaban
| Warfarin | Aspirin | p-value | Dabigatran | p-value | Rivaroxaban | p-value | |
|---|---|---|---|---|---|---|---|
| n | 8064 | 6018 | 281 | 244 | |||
| age-years | 70.6(11) | 71.7(11) | 0.006 | 68.4(12) | 0.002 | 66.9(12) | <0.0001 |
| male gender | 61.2%(4935) | 57.3%(3448) | 0.46 | 59.2%(166) | 0.29 | 60.5%(148) | 0.69 |
| Caucasian race | 75.9%(6120) | 73.3%(4411) | 0.27 | 73.3%(206) | 0.18 | 67.7%(165) | 0.0009 |
| diabetic | 67.9%(5475) | 66.8%(4020) | 0.16 | 70.4%(198) | 0.39 | 67.8%(165) | 0.96 |
| years on HD | 2.2(3.5) | 2.1(3.3) | 0.02 | 2.6(3.6) | 0.06 | 2.5(3.1) | 0.16 |
| catheter | 29.4%(2371) | 29.4%(1769) | 0.96 | 31.4%(88) | 0.47 | 19.3%(47) | 0.0008 |
| systolic BP-mmHg | 131(24) | 133(25) | <0.0001 | 128(25) | 0.07 | 136(26) | 0.003 |
| diastolic BP-mmHg | 68(14) | 68(15) | 0.006 | 67(14) | 0.07 | 70(14) | 0.30 |
| albumin-g/dL | 3.6(0.5) | 3.6(0.5) | <0.0001 | 3.6(0.5) | 0.08 | 3.6(0.5) | 0.67 |
| hemoglobin-g/dL | 10.6(1.3) | 10.5(1.3) | <0.0001 | 10.8(1.3) | 0.04 | 10.5(1.3) | 0.06 |
| thrombocytopenia | 0.2%(16) | 0.2%(12) | 0.98 | 0.3%(1) | 0.37 | 0.0%(0) | n/a |
| Epogen-units per HD | 4978(6059) | 5122(6248) | 0.17 | 6266(7051) | 0.0007 | 4947(5348) | 0.96 |
| heparin-units per HD | 2799((3135) | 2851(3186) | 0.33 | 3671(4126) | <0.0001 | 3342(3336) | 0.01 |
| anti-platelet (%) | 3.1%(250) | 100%(6018) | n/a | 5.6%(16) | 0.18 | 3.4%(8) | 0.76 |
| Charlson score | 5.5(1.9) | 5.5(2.0) | 0.10 | 5.4(1.7) | 0.32 | 5.5(2.0) | 0.72 |
| CHADS2 score | 2.4(1.0) | 2.4(1.1) | 0.003 | 2.3(1.0) | 0.07 | 2.2(1.0) | 0.01 |
| CHF | 20.8%(1677) | 21.3%(1282) | 0.55 | 14.6%(41) | 0.01 | 14.1%(34) | 0.01 |
| HTN | 88.5%(7137) | 88.9%(5350) | 0.44 | 86.9%(244) | 0.41 | 84.9%(207) | 0.09 |
| embolic CVA | 12.0%(968) | 12.8%(770) | 0.12 | 11.2%(31) | 0.94 | 14.6%(36) | 0.13 |
| bleeding index score | 1.9(0.6) | 1.9(0.6) | <0.0001 | 1.9(0.6) | 0.24 | 1.8(0.6) | 0.03 |
| GI bleed | 5.3%(427) | 7.5%(451) | <0.0001 | 7.5%(21) | 0.13 | 6.0%(15) | 0.66 |
| stroke | 12.7%(1024) | 14.3%(861) | 0.01 | 12.5%(35) | 0.93 | 16.0%(39) | 0.20 |
| minor bleed* | 2.0%(161) | 1.7%(102) | 0.13 | 2.8%(8) | 0.0004 | 4.3%(10) | 0.02 |
| major bleed* | 3.3%(266) | 0.7%(42) | <0.0001 | 4.1%(12) | 0.48 | 4.2%(10) | 0.41 |
A third sensitivity analysis was performed to determine the effect of warfarin management on the outcome of study; moreover, the increased bleeding in subjects on dabigatran and rivaroxaban may have been an artifact of suboptimal warfarin management where patients were being under dosed (ie INR<2). As such, we recalculated the rate ratios for major and minor bleeding including only warfarin patients with ≥60% of their INR readings between 2 to 3. Zoppo reported 60% of patients have an INR within the recommended range at any given time in usual clinical practice.12
We also explored the effect of drug dose on the risk of major bleeding associated with dabigatran and rivaroxaban. For this, we calculated the adjusted rate ratio for major bleeding in patients on full dose dabigatran (150 mg BID), reduced dose dabigatran (75 mg BID), full dose rivaroxaban (20 mg qD), and reduced dose rivaroxaban (15 mg qD) referent to patients on warfarin. Our final analysis explored the impact of dialysis efficiency on the risk of major bleeding associated with dabigatran and rivaroxaban. For this, we recalculated these rate ratios in dabigatran and rivaroxaban patients that were divided by urea clearance and dialysis time (top 50th percentile vs. bottom 50th percentile).
Statistical analyses were executed with SAS v9.1 (Cary, North Carolina).
RESULTS
Prevalence of anticoagulant drugs
Among 316,859 chronic hemodialysis patients from October 2010 to October 2014, we identified 29,977 (9.5%) patients with atrial fibrillation (AF). Within this AF cohort, the first record of dabigatran prescription occurred 45 days after the drug became available in the US. The first prescription of rivaroxaban occurred 161 days after it became FDA approved to prevent stroke from atrial fibrillation (Figure 1a). 15.3% of patients on dabigatran were prescribed the full dose (150 mg twice daily), while 84.7% of subjects were prescribed a reduced dose (75 mg twice daily) intended for patients with moderate chronic kidney impairment. Similarly for rivaroxaban, 32.1% of patients received the full dose (20 mg per day) while 67.8% received an adjusted dose (15 mg per day) for patients with a creatinine clearance between 15-50 cc/min (Figure 1b). Patients on dialysis typically have a creatinine clearance of less than 10 cc/minute. Among atrial fibrillation patients on dialysis who received anticoagulation over the four year study period, 3.1% were prescribed dabigatran, and 2.8% rivaroxaban. Overall, the utilization of dabigatran and rivaroxaban continues on an upwards trajectory where the prevalence of these drugs was 4.6 per 100 AF patients on anticoagulation in October 2014 (Figure 1a).
Figure 1.
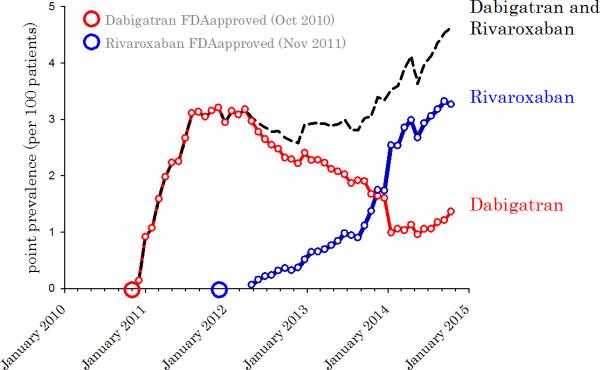
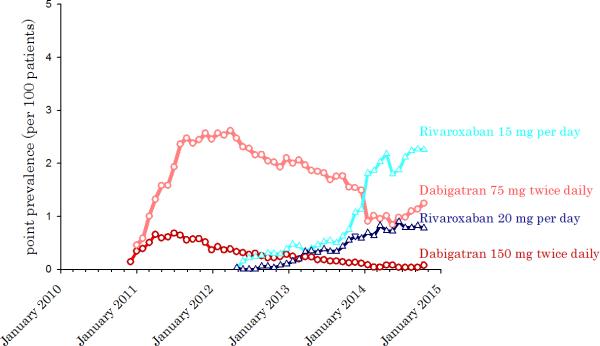
A. Point prevalence of dabigatran and rivaroxaban among anticoagulated chronic hemodialysis patients with atrial fibrillation. B. Point prevalence of dabigatran and rivaroxaban among anticoagulated chronic hemodialysis patients with atrial fibrillation, by drug dose.
Baseline patient characteristics
From the AF cohort, we identified 8,064 subjects who were started de novo on warfarin after October 10, 2010 for comparison to 6,018 subjects start on aspirin, 281 subjects started on dabigatran, and 244 subjects started on rivaroxaban. There were statistically significant differences in patient characteristics between the drug groups (Table 1). Dabigatran patients were more likely to have higher hemoglobin levels and heparin doses. Rivaroxaban patients were less likely to be Caucasian, less likely to dialyze with a catheter, and had higher systolic blood pressure readings relative to the warfarin group. All patients on NOAC's were also younger , on higher erythropoietin doses, and more likely to have a history of bleeding relative to the warfarin group. Aspirin patients had spent the least time on chronic dialysis. There were no clinically meaningful differences in CHADS2, bleeding index risk, and Charlson scores between the groups. The average time on drug was 168 days for dabigatran, 211 days for aspirin, and 175 days for warfarin subjects. The average time on drug for rivaroxaban was 106 days and expectedly lower because the drug did not become available until 2011. Among warfarin patients, the mean INR was 2.2 (standard deviation=0.9), but only 13.7% of patients had ≥60% of their INR readings within the target of 2-3.
Unadjusted event rates
The event rate of major bleeding was highest in the dabigatran (83.1 events per 100 patient-years) and rivaroxaban group (68.4 events per 100 patient-years), while lower in the warfarin group at 35.9 events per 100 patient-years (Table 2). The mortality rate from bleeding was higher in patients on NOAC's: dabigatran (19.2 deaths per 100 patient years), rivaroxaban (16.2), warfarin (10.2), and aspirin (7.7). The highest event rate for minor bleeding also occurred in dabigatran (120.6 events per 100 patient-years) and rivaroxaban patients (149.4 events per 100 patient-years) as shown in Table 3. The lowest major and minor bleeding rates were seen in patients on aspirin. The composite rate of stroke or arterial embolism was 6.2 events per 100 patient-years among warfarin users, yet the rate was significantly lower for patients aspirin (5.0 events per 100 patient-years). There were too few stroke and arterial embolism events in the study to detect any meaningful associations between warfarin, dabigatran, and rivaroxaban (Table 4).
Table 2.
Major bleeding in patients initiated on warfarin, aspirin, dabigatran, or rivaroxaban
| number of events | event rate (per 100 pt-years) | unadjusted rate ratios | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Warf | ASA | Dabi | Riva | Warf | ASA | Dabi | Riva | ASA vs Warf | Dabi vs Warf | Riva vs Warf | |
| Access | |||||||||||
| access bleed out | 123 | 57 | 9 | 3 | 3.2 | 1.7 | 7.3 | 4.2 | |||
| exit site bleeding | 7 | 1 | 0 | 0 | 0.03 | 0.22 | 0.0 | 0.0 | |||
| prolonged access bleeding | 405 | 268 | 36 | 15 | 10.3 | 8.2 | 26.9 | 20.9 | |||
| Total | 13.2 | 10.1 | 34.2 | 25.1 | 0.76 (0.66-0.87) | 2.59 (1.89-3.54) | 1.90 (1.19-3.04) | ||||
| Hemorrhagic stroke | 121 | 75 | 1 | 0 | 3.2 | 2.3 | 0.8 | 0.0 | 0.74 (0.55-0.98) | 0.26 (0.04-1.84) | N/A |
| Pulmonary | 27 | 22 | 2 | 0 | 0.7 | 0.7 | 1.6 | 0.0 | 0.97 (0.55-1.70) | 2.32 (0.55-9.74) | N/A |
| Gastrointestinal | |||||||||||
| intestine, stomach, abdomen | 742 | 498 | 26 | 13 | 19.3 | 15.4 | 21.2 | 18.2 | |||
| rectal | 84 | 74 | 11 | 2 | 2.2 | 2.3 | 9.0 | 2.8 | |||
| hematemesis | 26 | 21 | 4 | 0 | 0.7 | 0.7 | 3.3 | 0.0 | |||
| Total | 21.9 | 17.9 | 32.6 | 20.9 | 0.82 (0.73-0.91) | 1.49 (1.08-2.04) | 0.96 (0.57-1.59) | ||||
| Urological | 51 | 32 | 7 | 1 | 1.3 | 1.0 | 5.7 | 1.4 | 0.75 (0.48-1.16) | 4.30 (1.94-9.46) | 1.05 (0.14-7.60) |
| Epistaxis | 30 | 27 | 3 | 0 | 0.8 | 0.8 | 2.4 | 0 | 1.07 (0.64-1.80) | 2.13 (0.95-10.2) | N/A |
| Other | 242 | 100 | 7 | 12 | 6.2 | 3.0 | 16.8 | 5.7 | 0.49 (0.39-0.62) | 0.92 (0.43-1.95) | 2.70 (1.51-4.83) |
| Total Major Bleeds | 47.1 | 35.9 | 83.1 | 68.4 | 0.76 (0.71-0.82) | 1.76 (1.44-2.15) | 1.45 (1.09-1.93) | ||||
Warf warfarin; ASA aspirin; Dabi dabigatran; Riva rivaroxaban; HD hemodialysis
N/A not applicable because there we no events in one of the groups.
Total follow-up time: 3839 patient years for warfarin, 3226 patient years for aspirin, 123 patient years for dabigatran, 72 patient years for rivaroxaban.
Table 3.
Minor bleeding in patients initiated on warfarin, aspirin, dabigatran, or rivaroxaban
| number of events | event rate (per 100 pt years) | unadjusted rate ratios | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warf | ASA | Dabi | Riva | Warf | ASA | Dabi | Riva | ASA vs Warf | Dabi vs Warf | Riva vs Warf | ||
| Access | ||||||||||||
| access bleed out | 1006 | 554 | 48 | 39 | 26.0 | 16.9 | 37.5 | 50.3 | ||||
| exit site bleeding | 108 | 41 | 3 | 1 | 2.8 | 1.3 | 1.6 | 0.94 | ||||
| prolonged access bleeding | 1492 | 737 | 44 | 41 | 37.5 | 22.1 | 35.8 | 55.9 | 1.14 (0.93-1.42) | 1.63 (1.29-2.05) | ||
| Total | 65.2 | 39.9 | 74.9 | 106.1 | 0.61 | (0.57-0.65) | ||||||
| Ear-Nose-Throat | ||||||||||||
| epistaxis | 525 | 183 | 9 | 11 | 13.7 | 5.7 | 7.3 | 15.4 | ||||
| eye | 173 | 61 | 2 | 5 | 4.5 | 1.9 | 1.6 | 7.0 | ||||
| mouth-ear | 61 | 24 | 4 | 0 | 1.6 | 0.7 | 3.3 | 0.0 | 0.62 (0.37-1.03) | 1.13 (0.69-1.85) | ||
| Total | 19.7 | 8.3 | 12.2 | 22.3 | 0.42 | (0.37-0.48) | ||||||
| Pulmonary | 73 | 33 | 3 | 1 | 1.9 | 1.0 | 2.4 | 1.4 | 0.53 (0.36-0.80) | 1.29 (0.41-4.08) | 0.73 (0.10-5.28) | |
| Gastrointestinal | ||||||||||||
| intestine, stomach, abdomen | 132 | 52 | 3 | 2 | 3.4 | 1.6 | 2.4 | 2.8 | ||||
| rectal | 122 | 80 | 4 | 1 | 3.2 | 2.5 | 3.3 | 1.4 | ||||
| positive occult testing | 0 | 5 | 0 | 1 | 0.0 | 1.4 | 3.2 | 0.0 | ||||
| hematemesis | 10 | 7 | 0 | 0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.85 (0.40-1.79) | 0.82 (0.31-2.22) | ||
| Total | 6.7 | 4.4 | 5.7 | 5.6 | 0.66 | (0.53-0.80) | ||||||
| Other | ||||||||||||
| urological-gynecological | 158 | 78 | 6 | 5 | 4.1 | 2.4 | 4.9 | 7.0 | ||||
| wound-bruise | 297 | 4 | 13 | 2 | 7.7 | 0.1 | 10.6 | 2.8 | ||||
| other | 210 | 94 | 14 | 4 | 5.5 | 2.9 | 11.4 | 5.6 | 1.56 (1.11-2.21) | 0.89 (0.49-1.61) | ||
| Total | 17.2 | 5.5 | 26.9 | 15.4 | 0.32 | (0.27-0.37) | ||||||
| Total Minor Bleeds | 110.0 | 58.8 | 120.6 | 149.4 | 0.53 (0.51-0.56) | 1.10 (0.93-1.29) | 1.36 (1.12-1.64) | |||||
Warf warfarin; ASA aspirin; Dabi dabigatran; Riva rivaroxaban; HD hemodialysis
N/A not applicable because there we no events in one of the groups.
Total follow-up time: 3839 patient years for warfarin, 3226 patient years for aspirin, 123 patient years for dabigatran, 72 patient years for rivaroxaban.
Table 4.
Ischemic stroke and arterial embolism in patients initiated on warfarin, aspirin, dabigatran, or rivaroxaban
| number of events | event rate (per 100 pt years) | unadjusted rate ratios | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Warf | ASA | Dabi | Riva | Warf | ASA | Dabi | Riva | ASA vs Warf | Dabi vs Warf | Riva vs Warf | |
| embolic stroke | 221 | 157 | 11 | 8 | 5.8 | 4.9 | 9.0 | 11.2 | |||
| arterial embolism | 25 | 11 | 2 | 0 | 0.7 | 0.3 | 1.6 | 0.0 | |||
| Total embolic events | 244 | 168 | 13 | 8 | 6.2 | 5.0 | 10.6 | 11.2 | 0.81 (0.66-0.99) | 1.71 (0.97-2.99) | 1.80 (0.89-3.64) |
Warf warfarin; Dabi dabigatran; ASA aspirin; Riva rivaroxaban
Total follow-up time: 3839 patient years for warfarin, 3226 patient years for aspirin, 123 patient years for dabigatran, 72 patient years for rivaroxaban.
Covariate adjusted rate ratios
In covariate adjusted Poisson regression, dabigatran (RR=1.48; 95% CI 1.21-1.81, p=0.0001) and rivaroxaban (RR=1.38; 95% CI 1.03-1.83, p=0.04) associated with a higher risk of major bleeding when compared to warfarin. The risk of hemorrhagic death was even larger with dabigatran (RR=1.78; 95% CI 1.18-2.68, p=0.006) and rivaroxaban (RR=1.71 95% CI 0.93-3.12, p=0.07) referent to warfarin. Similar associations were seen with minor bleeding: dabigatran (RR=1.17; 95% CI 1.00-1.38, p=0.05) and rivaroxaban (RR=1.35; 95% CI 1.11-1.65, p=0.001) when compared to warfarin. Patients treated with aspirin had the lowest risk for major bleeding (RR=0.78; 95% CI 0.72-0.84, p<0.0001), minor bleeding (RR=0.39; 95% CI 0.34-0.46, p<0.0001), and hemorrhagic death (RR=0.42; 95% CI 0.28-0.64, p<0.0001) referent to warfarin.
Sensitivity analyses
Two additional analyses were conducted to evaluate the robustness of the primary analysis. To diminish the measured and unmeasured differences in patient characteristics between the study groups, we matched each dabigatran and rivaroxaban subject to two warfarin subjects on 20 data parameters. The resulting cohort achieved balance across treatment groups (Supplemental Table 1) where the risk of major bleeding was still associatively higher with dabigatran (RR=1.64; 95% CI 1.27-2.12, p=0.03) and rivaroxaban (RR=1.39; 95% CI 1.00-1.94, p=0.05) referent to warfarin. In our second analysis, we excluded warfarin patients who were potentially under or over anticoagulated by only including patients with at least 60% of their INR readings between 2 and 3. Here, dabigatran (RR=1.46; 95% CI 1.10-1.93, p=0.0001) and rivaroxaban (RR=1.32; 95% CI 0.93-1.87, p=0.03) still associated with an increased risk for major bleeding when compared to warfarin.
Effect of dose and dialysis on dabigatran and rivaroxaban
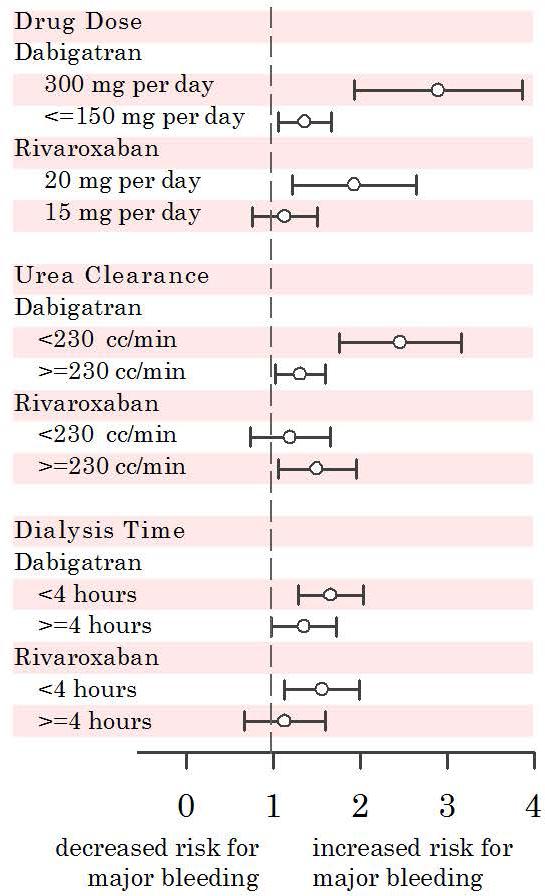
The unadjusted event rate of major bleeding increased with drug dosage: 69.1 events per patient year in dabigatran 150 mg BID, 25.4 in dabigatran 75 mg BID, 42.6 in rivaroxaban 20 mg qD, and 23.9 in rivaroxaban 15 mg qD. Using covariate adjusted Poisson models, we examined the risk of major bleeding by drug dose for dabigatran and rivaroxaban referent to warfarin (Figure 2). Dialysis patients prescribed the full dosage of dabigatran (RR=2.90; 95% CI 1.93-4.34, p<0.0001) or rivaroxaban (RR=1.93; 95% CI 1.22-3.04, p=0.04) had a higher risk of major bleeding than patients who were prescribed a lower drug dose intended for patients with moderate renal impairment (Figure 2).
Figure 2.
Rate ratio of major bleeding with dabigatran and rivaroxaban when compared to warfarin; by drug dose, urea clearance, and dialysis time. All models were adjusted for age, gender, race, diabetes, vintage, catheter vascular access, blood pressure, albumin, hemoglobin, thrombocytopenia, EPO dose, heparin dose, anti-platelet use, Charlson comorbidity score, bleeding index score, recent minor bleeding event, and recent major bleeding event.
We also examined dabigatran (dialyzable) vs. rivaroxaban (non-dialyzable) by dialysis dose and time.13 Patients on dabigatran (dialyzable) who achieved less urea clearance (< 230 cc/min) had more major bleeding than patients whose urea clearance was ≥230 cc/min (Figure 2). The amount of dialysis urea clearance did not decrease the risk of bleeding with rivaroxaban likely because the drug is not affected by dialysis. Shorter dialysis treatment times also associated with an increased risk of bleeding with both dabigatran and rivaroxaban (Figure 2).
DISCUSSION
Dabigatran and rivaroxaban are promising anticoagulants that overcome the inconveniences of frequent serum level monitoring for dosing with warfarin therapy. Both drugs were shown to be at least equivalent to warfarin at stroke prevention without increasing the risk of bleeding; however, these major clinical trials both specifically excluded ESRD patients from their study.4,14 Our study is the first to evaluate these drugs in the dialysis population, and suggests concern given the increasing use of dabigatran and rivaroxaban in the ESRD population despite formal FDA warnings of caution in renal failure. In fact, our secondary analyses suggest excess morbidity and mortality from bleeding are associatively higher with dabigatran and rivaroxaban when compared to warfarin.
Dabigatran exerts its anticoagulant effect through the direct inhibition of thrombin activity by binding to the molecule with high affinity and specificity.15 Approximately 80-85% of the drug is excreted by the kidneys mostly through glomerular filtration. The half-life of the drug is 9 hours, but increases to 25-30 hours in individuals with a creatinine clearance <30 cc/min.13
Dabigatran is also dialyzable and 50-60% of the drug is eliminated during a four hour treatment.16 A dose of 150 mg twice daily was approved for patients with a creatinine clearance >30 cc/min based on the results of the RE-LY trial.4 This study showed dabigatran reduced the stroke risk by 34% with no increase in major bleeding in comparison to warfarin, but excluded patients with severe kidney disease. Of note, a dose of 75 mg twice daily can be considered based only on pharmacokinetic data for patients with a creatinine clearance of 15 to 30 cc/minute; however, the American College of Chest Physicians recommends dabigatran be contraindicated in patients with severe renal impairment (creatinine clearance ≤ 30 cc/minute).6 Rivaroxaban is an oral factor Xa inhibitor where 33% of the drug undergoes renal elimination through proximal tubule secretion. The half-life of the drug is 8 hours and increases minimally to 9.5 hours in patients with severe renal disease (mean creatinine clearance was 22 cc/min).17 Unlike dabigatran, rivaroxaban is not dialyzable as the drug is highly bound to plasma proteins (92-95%). In comparison to warfarin, rivaroxaban was non-inferior at preventing stroke in atrial fibrillation without significant difference in bleeding risk in The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Like RE-LY, patients with a creatinine clearance <30 cc/min were excluded from ROCKET AF. Currently, the use of rivaroxaban at a reduced dose of 15 mg per day may be considered as second line therapy in patients with a creatinine clearance between 15 to 50 cc/minute even though the safety and efficacy of this approach has not been clinically validated; however, rivaroxaban is contraindicated for patients with ESRD or on hemodialysis.6
Our study describes the off-label prescribing patterns that may be expected soon after a new drug is approved and released on the market. When dabigatran and rivaroxaban were approved for use in the United States, there was almost no evidence that these drugs were effective or safe in patients on dialysis because such patients were excluded from randomized controlled trials. Despite the lack of evidence in ESRD, it took only 45 days after the drug's release before dabigatran use began to show up in our dialysis population. The use of these NOAC's were sustained and increased over time. After only four years, almost five percent of anticoagulated patients were taking dabigatran or rivaroxaban.
Our study also highlights the potential for harm when dabigatran is used in dialysis patients, where kidney failure impairs the clearance of the drug. This may lead to bio-accumulation of the drug that places patients at increased risk for serious hemorrhage. The risk may be mitigated by increasing the dialysis dose (Figure 2), but it does not entirely eliminate the concern. The periodic nature of hemodialysis will likely result in fluctuating and unpredictable blood levels of dabigatran which could result in recurring periods of under and over-anticoagulation. It is also well known that dialysis treatments are sometimes shortened, missed, or delayed. This may result in prolonged periods of increased risk for bleeding, especially if the patient continues taking dabigatran in the absence of dialysis. For rivaroxaban, which is not dialyzable, we found little risk reduction when these patients were aggressively dialyzed leaving no means of dealing with excessive exposure to the drug.
We also bring attention to the rate of hemorrhagic stroke (Table 2) in warfarin patients which was four times higher than the rate in dabigatran patients while there were no hemorrhagic strokes among rivaroxaban patients. These findings are consistent with trial results in the general population; however, vascular access was the largest cause of morbidity and mortality from bleeding in the dialysis population.4,5
Finally, comparison of bleeding morbidity and mortality risk between the medications also provided further important information. For dabigatran, the risk of major bleeding increased by 48% and the risk of fatal bleeding increased even more, by 88% referent to warfarin. Similarly, for rivaroxaban, the risk of major bleeding increased 38%, yet increased even higher by 58% for fatal bleeding in comparison to warfarin. Overall, NOAC's may magnify the risk of hemorrhagic death more than the risk of non-fatal bleeding. Perhaps, the lack of reversal agents for dabigatran and rivaroxaban complicate the management of bleeding events where more patients will die from exsanguination. Taken all together, reversal agents are less likely to decrease the risk of bleeding but may have a role in improving the treatment and prognosis of bleeding events in dabigatran and rivaroxaban patients.
The limitations of our study suggest room for further study. We had few stroke and arterial embolic events in the patients on dabigatran and rivaroxaban, such that our cerebrovascular analysis was under powered. The increased risk of major bleeding with dabigatran and rivaroxaban may be acceptable for dialysis patients if these drugs also substantially decrease the risk of stroke more effectively than warfarin; however, the NOAC's did not trend towards lower stroke rates than warfarin in our study. A study with more subjects followed for a longer period of time is needed to completely evaluate the risk-benefit profile of these drugs, and the inclusion of patients with chronic renal insufficiency who are not dialyzed is further worthy of study. Other limitations include the lack of blood transfusion data and the potential for confounding by indication. We mitigated this potential bias from unmeasured factors by performing a matched analysis which supported the main findings of the study.
In conclusion, more dialysis patients are being started on dabigatran and rivaroxaban, even when their use is contraindicated in ESRD. Our data support the current labeling of dabigatran and rivaroxaban, which advise against use in patients on dialysis. Further research is needed into the safety and efficacy of these agents in the ESRD population before they can be recommended.
Supplementary Material
Acknowledgments
KC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources: ERE was supported in part by grants from the NIH (R01 GM 49039).
Footnotes
Disclosures: The authors of the manuscripts have no financial associations with the manufacturers of dabigatran and rivaroxaban. KC and FWM receive salary support from Fresenius Medical Care North America but the company does not purchase or administer dabigatran, rivaroxaban, or warfarin in its facilities. RT is a consultant to Fresenius Medical Care North America.
References
- 1.Gage BF. Can we rely on RE-LY? N Engl J Med. 2009;361:1200–2. doi: 10.1056/NEJMe0906886. [DOI] [PubMed] [Google Scholar]
- 2.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–21. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [2013 Nov 9];FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm [Internet] Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm326654.htm.
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso-Coello P, Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis. Chest. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. pp. e601S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Data on file [2013 Nov 9];Janssen Pharmaceuticals, Inc. Data as of 5/1/13. [Internet] Available from: http://www.xareltohcp.com/
- 8.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–33. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 10.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 11.Kosanke J, Bergstralh E. Gmatch: SAS macro. 2008 http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas.
- 12.Del Zoppo GJ, Eliasziw M. New options in anticoagulation for atrial fibrillation. N Engl J Med. 2011;365:952–3. doi: 10.1056/NEJMe1107516. [DOI] [PubMed] [Google Scholar]
- 13.Knauf F, Chaknos CM, Berns JS, Perazella M a. Dabigatran and Kidney Disease: A Bad Combination. Clin J Am Soc Nephrol. 2013:1–7. doi: 10.2215/CJN.01260213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson S, Troughton R, Richards AM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:2334–5. doi: 10.1056/NEJMc1112233. author reply 2335. [DOI] [PubMed] [Google Scholar]
- 15.Hauel NH, Nar H, Priepke H, Ries U, Stassen J-M, Wienen W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002;45:1757–66. doi: 10.1021/jm0109513. [DOI] [PubMed] [Google Scholar]
- 16.Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, Neumayer H H, Liesenfeld K-H, Lehr T, Härtter S, Friedman J, Peters H, Clemens A. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109:596–605. doi: 10.1160/TH12-08-0573. [DOI] [PubMed] [Google Scholar]
- 17.Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, Lufft V, Wand DD, Philipp T, Bruck H. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70:703–12. doi: 10.1111/j.1365-2125.2010.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.