An in vitro study examining factors produced by human mesenchymal stem cells on spine implant materials. Mesenchymal stem cells undergo differentiation to osteoblasts and secrete anti-inflammatory factors on micro-/nano-textured Ti alloy surfaces. Mesenchymal stem cells fail to differentiate on PEEK and produce high levels of proinflammatory factors.
Keywords: mesenchymal stem cells, PEEK, Ti6Al4V, interbody spine cage, inflammatory mediators, implant surface, osteogenesis, fibrosis, mRNA array
Abstract
Study Design.
An in vitro study examining factors produced by human mesenchymal stem cells on spine implant materials.
Objective.
The aim of this study was to examine whether the inflammatory microenvironment generated by cells on titanium-aluminum-vanadium (Ti-alloy, TiAlV) surfaces is affected by surface microtexture and whether it differs from that generated on poly-ether-ether-ketone (PEEK).
Summary of Background Data.
Histologically, implants fabricated from PEEK have a fibrous connective tissue surface interface whereas Ti-alloy implants demonstrate close approximation with surrounding bone. Ti-alloy surfaces with complex micron/submicron scale roughness promote osteoblastic differentiation and foster a specific cellular environment that favors bone formation whereas PEEK favors fibrous tissue formation.
Methods.
Human mesenchymal stem cells were cultured on tissue culture polystyrene, PEEK, smooth TiAlV, or macro-/micro-/nano-textured rough TiAlV (mmnTiAlV) disks. Osteoblastic differentiation and secreted inflammatory interleukins were assessed after 7 days. Fold changes in mRNAs for inflammation, necrosis, DNA damage, or apoptosis with respect to tissue culture polystyrene were measured by low-density polymerase chain reaction array. Data were analyzed by analysis of variance, followed by Bonferroni's correction of Student's t-test.
Results.
Cells on PEEK upregulated mRNAs for chemokine ligand-2, interleukin (IL) 1β, IL6, IL8, and tumor necrosis factor. Cells grown on the mmnTiAlV had an 8-fold reduction in mRNAs for toll-like receptor-4. Cells grown on mmnTiAlV had reduced levels of proinflammatory interleukins. Cells on PEEK had higher mRNAs for factors strongly associated with cell death/apoptosis, whereas cells on mmnTiAlV exhibited reduced cytokine factor levels. All results were significant (P < 0.05).
Conclusion.
These results suggest that fibrous tissue around PEEK implants may be due to several factors: reduced osteoblastic differentiation of progenitor cells and production of an inflammatory environment that favors cell death via apoptosis and necrosis. Ti alloy surfaces with complex macro/micro/nanoscale roughness promote osteoblastic differentiation and foster a specific cellular environment that favors bone formation.
Level of Evidence: N/A
Materials such as titanium-aluminum-vanadium alloy (Ti-6Al-4V, TiAlV) and poly-ether-ether-ketone (PEEK) are commonly used in spinal interbody fusion surgical procedures. These 2 materials, while used for similar clinical applications, have substantially different surface characteristics, especially on a micron scale. Poly-ether-ether-ketone is popular because its modulus of 3 to 4 GPa1,2 is close to that of native cortical bone, 14 to 18 GPa. In addition, PEEK is radiolucent, allowing surgeons to examine whether bone fills the intervertebral space. However, it is often encapsulated by fibrous tissue. The lack of bone integration can ultimately result in implant subsidence and nonunion.
Ti alloys have higher elastic moduli than bone but have yielded successful results clinically.3,4 Studies in animal models show greater bone apposition to Ti and Ti alloy surfaces, particularly when the surfaces have a rough microtopography.5–7In vitro studies indicate that microtextured Ti and Ti alloy surfaces promote osteoblast differentiation and production of factors that favor bone formation in vivo, whereas PEEK does not.8–10
After a material is implanted into the body, the immune system initiates an immune response sequence.11 The inflammatory response to the biomaterial is mediated in large part by the local inflammatory microenvironment, which results in a cascade triggering migration of other cells to the vicinity. A high level of inflammation creates a longer resolution period. Fibroblasts initially produce extracellular matrix in an effort to support the damaged tissue; however, extended activation of macrophages and other immune cells leads to reduction in matrix remodeling and the fibrotic scar tissue that was formed in the support stage of wound healing, which remains.
The persistence of fibrosis around PEEK implants in contrast to peri-implant bone formation around Ti alloy suggests that PEEK may stimulate formation of microenvironment consisting of specific inflammatory cytokines that enhance fibrous tissue formation, whereas micron-scale–roughened Ti alloy surfaces reduce production of these factors. To test this hypothesis, we cultured human mesenchymal stem cells (MSCs) on disks consisting of machined PEEK, machined Ti6Al4V, and microtextured Ti6Al4V, and examined their production of factors associated with inflammation, apoptosis, and necrosis.
MATERIALS AND METHODS
Material Fabrication
Fifteen-millimeter diameter disks of PEEK, smooth TiAlV (sTiAlV), and macro-/micro-/nano-rough (mmnTiAlV) were provided by Titan Spine (Mequon, WI). Processing of these disks resulted in varying surface topographies with average roughness (Sa) for sTiAlV of 0.27 ± 0.01 μm or 2.74 ± 0.18 μm for mmnTiAlV. PEEK substrates were machined, resulting in a Sa of 0.43 ± 0.07 μm. Surface topography was visualized using scanning electron microscopy (SEM, Ultra 60 FEG-SEM; Carl Zeiss SMT Ltd., Cambridge, United Kingdom) recorded using a 5 kV accelerating voltage and 30-μm aperture. All disks were ultrasonically cleaned in ultrapure water (Millipore, Billerica, MA) and autoclave sterilized (Tuttnauer, Hauppauge, NY) before use in cell culture studies.9
Cell Culture
Human MSCs (Lonza Biosciences, Walkersville, MD) were seeded on PEEK, sTiAlV, or mmnTiAlV at an initial density of 10,000 cells/cm2 and cultured in MSC growth media (MSCGM, Lonza Biosciences) at 5% CO2 and 100% humidity. Cells cultured on tissue culture polystyrene (TCPS) served as an internal control.
Osteoblast Phenotype
When cells reached confluence on TCPS, the media were changed and cells were incubated for 24 hours. Cells were lysed in 0.05% Triton X-100 and homogenized using a sonic dismembrator. Alkaline phosphatase activity, an early marker of osteoblast differentiation that reaches a peak just before matrix mineralization, was assayed in lysates by measuring the release of p-nitrophenol from p-nitrophenyl phosphate at pH 10.2. Activity was normalized to total protein content (Thermo Fisher Pierce BCA Protein Assay, Rockford, IL) of the cell lysates. Secreted osteocalcin, a later marker of osteoblast differentiation important in modulating hydroxyapatite crystallization, was measured using a radioimmunoassay (Biomedical Technologies, Stoughton, MA) and normalized to DNA content (Quant-iT Assay Kit, Life Technologies, Carlsbad, CA) in the cell lysate.
Interleukin Protein Production
Cells were cultured as described previously and at confluence on TCPS, cells on all surfaces were incubated with fresh medium for 24 hours. Levels of secreted cytokines IL1β, IL6, IL8, and IL10 were assayed in the conditioned medium (R&D Systems DuoSet ELISA, Minneapolis, MN) and normalized to DNA in the cell lysate.
Polymerase Chain Reaction Array
Cells were cultured on TCPS, PEEK, sTiAlV, or mmnTiAlV substrates. Cells were incubated with fresh medium for 12 hours after reaching confluence on TCPS. RNA was harvested using a TRIzol (Life Technology) extraction method following manufacturer's protocol and was quantified (NanoDrop 1000, Thermo Scientific, Waltham, MA). RNA (500 ng) was amplified by reverse transcription (RT2 First Strand Kit, Qiagen, Valencia, CA). mRNA was measured for 39 genes using PathwayFinder RT2 Profiler PCR Array (polymerase chain reaction array; Qiagen) and fold change to TCPS was normalized to 3 housekeeping genes in the array using the Web-based PCR Array Data Analysis Software (Qiagen).
Statistical Analysis
PCR array experiments were performed on n = 3 samples per variable. Statistical differences were determined using Qiagen software, and changes greater than 2-fold was considered significant. All other experiments involved 6 independent cultures for each variable. Data from each experiment were analyzed separately by analysis of variance and significant differences between groups were determined using Bonferroni's modification of Student t-test in GraphPad Prism Version 5.04. P value of less than 0.05 was considered to be significant.
RESULTS
SEM imaging qualitatively demonstrated differences in surface structures. PEEK disks had relatively smooth surfaces and had only minor parallel grooves because of processing (Figure 1). Likewise, sTiAlV surfaces were mostly smooth, with superficial grooves from machining (Figure 1). Rough mmnTiAlV surfaces featured large pits and craters with superimposed micron- and submicron-scale features (Figure 1).
Figure 1.

Scanning electron microscopy images of PEEK (left panel), sTiAlV (middle panel), and mmnTiAlV (right panel) surfaces obtained at 1k× magnification. PEEK indicates poly-ether-ether-ketone; sTiAlV, smooth titanium alloy; mmnTiAlV, micro-textured rough titanium alloy.
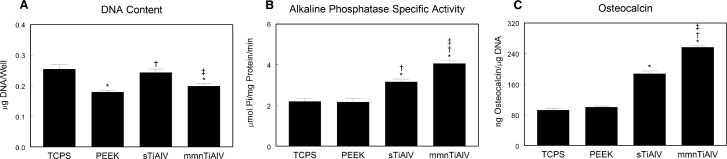
DNA content was significantly lower in cultures on PEEK and mmnTiAlV, but not different on sTiAlV, in comparison with TCPS (Figure 2A). Alkaline phosphatase activity was the same in MSCs cultured on TCPS or PEEK (Figure 2B) and was significantly higher on TiAlV surfaces in comparison with both TCPS and PEEK. Levels were significantly higher on mmnTiAlV than activity on the sTiAlV surface. Likewise, osteocalcin production was increased only on the Ti alloy surfaces, with the effect being greater on mmnTiAlV (Figure 2C).
Figure 2.
DNA content (A), alkaline phosphatase–specific activity (B), and osteocalcin production (C) in mesenchymal stem cells cultured on TCPS, PEEK, sTiAlV, or mmnTiAlV. *P < 0.05 versus TCPS; †P < 0.05 versus PEEK; ‡P < 0.05 versus sTiAlV. TCPS indicates tissue culture polystyrene; PEEK, poly-ether-ether-ketone; sTiAlV, smooth titanium-aluminum-vanadium alloy; mmnTiAlV, macro-/micro-/nano-textured rough TiAlV.
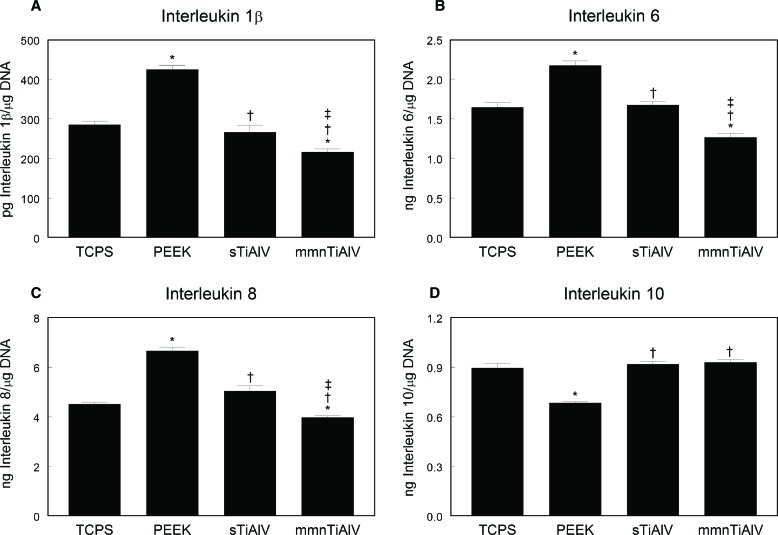
Production of proinflammatory interleukins IL1β, IL6, and IL8 by MSCs was highest on PEEK compared with all other materials (Figure 3A–C). Conversely, production was lowest on the mmnTiAlV surface and was even lower than on TCPS. These were consistent observations, regardless of the protein analyzed. Levels of anti-inflammatory IL10 were comparable in conditioned media of cultures grown on TCPS and the TiAlV surfaces (Figure 3D). Moreover, in cultures grown on the Ti alloy substrates, levels of IL10 were significantly greater than on PEEK.
Figure 3.
Levels of IL1β (A), IL6 (B), IL8 (C), and IL10 (D) in the conditioned media of mesenchymal stem cells cultured on TCPS, PEEK, sTiAlV, or mmnTiAlV. *P < 0.05 versus TCPS; †P < 0.05 versus PEEK; ‡P < 0.05 versus sTiAlV. TCPS indicates tissue culture polystyrene; PEEK, poly-ether-ether-ketone; sTiAlV, smooth titanium-aluminum-vanadium alloy; mmnTiAlV, macro-/micro-/nano-textured rough TiAlV.
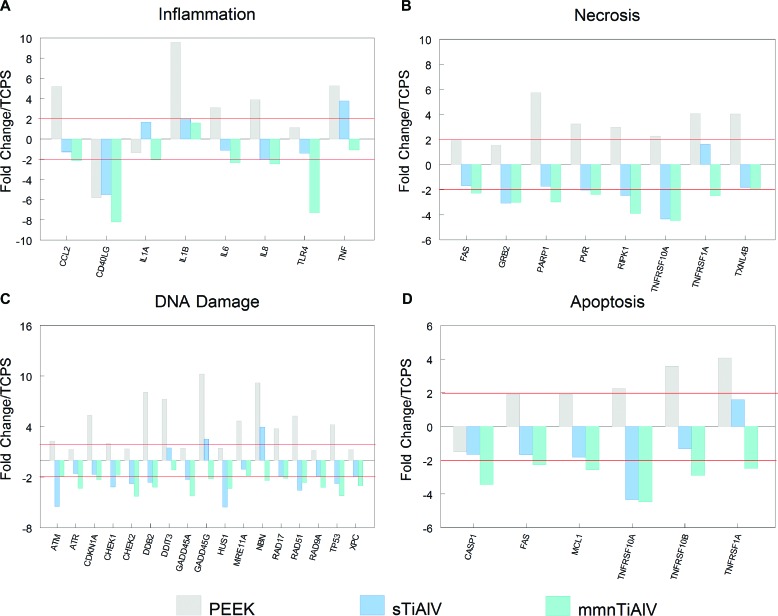
The PCR array (Figure 4) demonstrated that cells cultured on mmnTiAlV exhibited the lowest levels of mRNAs for proinflammatory proteins (Figure 4A) and for proteins associated with necrosis (Figure 4B), DNA damage (Figure 4C), and apoptosis (Figure 4D). In contrast, fold changes in these mRNAs on PEEK were the highest in comparison with cells on TCPS.
Figure 4.
Analysis of inflammatory (A), necrotic (B), DNA damage (C), and apoptotic (D) factors by real-time qPCR array of mesenchymal stem cells cultured on PEEK, sTiAlV, or mmnTiAlV surfaces. Data are presented as fold change to TCPS (2-fold change indicated by solid horizontal line). TCPS, tissue culture polystyrene; PEEK, poly-ether-ether-ketone; sTiAlV, smooth titanium-aluminum-vanadium alloy; mmnTiAlV, macro-/micro-/nano-textured rough TiAlV.
DISCUSSION
Spine surgeons traditionally augment interbody fusion implants with bone graft or bone graft substitutes of varying biologic potency. It is, therefore, challenging to discern meaningful differences between Ti alloy and PEEK implant materials in a clinical study. An in vitro model can identify cellular response differences between materials without use of additives in the medium to promote osteogenesis.
Previous in vitro studies showed that osteoblast differentiation of human MSCs12 and osteoblasts13 is influenced by implant surface properties. When MSCs are cultured on PEEK, cells fail to exhibit known markers of bone formation such as increased alkaline phosphatase activity or osteocalcin production compared with cells cultured on TCPS. In contrast, MSCs cultured on rough Ti and Ti alloy do exhibit increased levels of these markers as well as production of proteins that favor osteoblast differentiation (BMP-2, BMP-4, VEGF), even in the absence of media supplements used to stimulate expression of an osteoblast phenotype.12 These in vitro studies are supported by in vivo results examining peri-implant bone formation in sheep spine, where Ti alloy pedicle screws with micron scale and submicron scale roughness exhibited 2-fold increases in pullout strength.14
Histologically, Ti alloy implants demonstrate close apposition with surrounding bone; however, implants fabricated from PEEK develop a fibrous connective tissue interface.1,14,15 Differences in the chemical and physical properties of an implant surface can directly affect immune cell response. Studies examining dendritic cell maturation show that it is sensitive to both chemistry and shape of a biomaterial,16–18 including surface microstructure.19 When immature dendritic cells were cultured on microtextured Ti surfaces compared with smooth surface Ti surfaces, the expression of a mature dendritic cell phenotype was reduced.
Our results suggest that differences in biological response to Ti alloy and PEEK may be due to differences in the inflammatory microenvironment generated by cells on the implant surface. Increase of proinflammatory cytokines, specifically high levels of IL1β, is associated with fibrous tissue formation,20 and IL1β, IL6, and IL8 are increased in chronic inflammation. We observed the lowest levels of these inflammatory factors in MSC cultures grown on mmnTiAlV. In contrast, the cultures grown on PEEK resulted in the highest levels, suggesting a profibrosis, inflammatory response.
The opposite was true with respect to the anti-inflammatory factor IL10. Reduced levels of this mediator favor a proinflammatory state, and PEEK was associated with reduced levels of IL10 compared with Ti. Taken together, our results showed that mmnTiAlV reduced the local inflammatory environment, decreasing the proinflammatory cytokines but also increasing the levels of the anti-inflammatory cytokine IL10.
Particularly interesting was the observation that expression of factors associated with DNA damage and necrosis was upregulated on PEEK but either unchanged or reduced on Ti alloy. Similarly, PEEK consistently upregulated factors for apoptosis whereas the mmnTiAlV reduced these factors more than smooth Ti. Our results suggest that cells grown on PEEK are exposed to cellular stress and increase expression of genes that lead to DNA damage, apoptosis, and necrosis. All results together demonstrate that cells grown on PEEK produce a proinflammatory environment, but it is not clear whether PEEK can induce apoptosis and necrosis by direct contact or as a result of the high proinflammatory environment.
The question remains as to whether our findings were due to PEEK's chemistry or to its surface structure. PEEK surface topography varies with processing, and rougher PEEK surfaces do support greater osteoblast differentiation of human osteoblasts than smooth surfaced PEEK.21 Recent studies have shown that bone formation is improved around PEEK implants that have been blasted using biphasic calcium phosphate22; however, residual mineral may contribute to the outcome. PEEK that has been treated by oxygen plasma exhibits improved osseointegration,23 supporting the hypothesis that surface topography is an important variable. In vitro studies also indicate that adipose-derived stem cells exhibit improved osteoblast differentiation when grown on PEEK treated by oxygen plasma, but the surface modifications lead to changes in contact angle and electrochemical properties in addition to altered nanostructure.24 Another modification of the PEEK surface has been generated using a porogen filler, polymer extrusion, and removal of the filler.25 Bone marrow stromal cells cultured on these surfaces exhibit osteoblast differentiation, but the contribution of surface chemistry is not known. Well-controlled experiments in which porosity on the PEEK surface is produced using various chemical methods show that small differences in resulting surface properties can alter osteoblast growth and differentiation as well as osseointegration.26 Although these studies demonstrate the value of surface roughness in osteogenic effects of PEEK materials, few reports have directly compared responses to PEEK with responses to Ti6Al4V. Even those studies that have examined responses to PEEK and Ti6Al4V have not assessed effects on immune modulation.
Our study did not address the contribution of substrate stiffness to the outcomes measured. PEEK and Ti6Al4V have different moduli, both of which differ from that of the bone surface. Stiffness of a substrate does influence MSC differentiation, but it is very difficult to separate effects of stiffness from those of chemistry. We have attempted to investigate this very question using photopolymerized networks.27 Our results indicated that chemistry was the primary regulator of osteoblast differentiation, and the effect of stiffness was secondary to the effect of surface chemistry. Although the greatest degree of osteoblast differentiation was on the stiffest polymers in 1 copolymer system, when grown on a different copolymer system, cells became more differentiated on the less stiff surface. When cells were grown on substrates with identical stiffness and surface topography but differing chemistry, chemistry proved to be a critical variable.28,29 Thus, even if PEEK and Ti6Al4V could be fabricated to have comparable stiffness and surface microstructure, differences in biological response would be likely.
In conclusion, this study found that MSCs are compatible with the mmnTiAlV surface, and when cultured on it, reduce production of inflammatory mediators and enhance production of anti-inflammatory mediators compared with PEEK. Although we did not address fibrosis specifically, our results suggest that the fibrous tissue interface seen with PEEK implants may be due to increased inflammatory cytokines and decreased cell viability. In addition, the macro-/micro-/nano-scale–roughened Ti alloy surface is more effective than smooth Ti alloy in promoting an osteogenic environment with low inflammation and robust cell viability.
Key Points
MSCs differentiate into osteoblasts on micro-/nano-textured Ti alloy surfaces but not on PEEK surfaces.
MSCs produce anti-inflammatory factors on micro-/nano-textured Ti alloy surfaces but not on PEEK surfaces.
Factors produced by MSCs on PEEK surfaces may favor fibrosis whereas factors produced on Ti alloy surfaces favor osteogenesis.
Acknowledgment
Ti6Al4V and PEEK disks were provided as a gift to the Georgia Institute of Technology by Titan Spine LLC (Mequon, WI).
Footnotes
Acknowledgment date: September 22, 2014. Revision date: December 16, 2014. Acceptance date: December 17, 2014.
The manuscript submitted does not contain information about medical device(s)/drug(s).
National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR052102 funds were received to support this work.
Relevant financial activities outside the submitted work: board membership, consultancy, employment, grants, travel/accommodations/meeting expenses, royalties, stocks.
References
- 1.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007;28:4845–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res 1988;235:224–36. [PubMed] [Google Scholar]
- 3.Thome C, Krauss JK, Zevgaridis D. A prospective clinical comparison of rectangular titanium cages and iliac crest autografts in anterior cervical discectomy and fusion. Neurosurg Rev 2004;27:34–41. [DOI] [PubMed] [Google Scholar]
- 4.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, et al. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine 2006;4:447–53. [DOI] [PubMed] [Google Scholar]
- 5.Stenport VF, Johansson CB. Evaluations of bone tissue integration to pure and alloyed titanium implants. Clin Implant Dent Relat Res 2008;10:191–9. [DOI] [PubMed] [Google Scholar]
- 6.Linder L. Osseointegration of metallic implants. I. light microscopy in the rabbit. Acta Orthop Scand 1989;60:129–34. [DOI] [PubMed] [Google Scholar]
- 7.De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 minimatic implants. Int J Oral Maxillofac Implants 1999;14:384–91. [PubMed] [Google Scholar]
- 8.Gittens RA, Olivares-Navarrete R, Schwartz Z, et al. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater 2014;10:3363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivares-Navarrete R, Hyzy SL, Gittens RA, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J 2013;13:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J 2012;12:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz S, Rammelt S, Scharnweber D, et al. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011;32:6692–709. [DOI] [PubMed] [Google Scholar]
- 12.Olivares-Navarrete R, Hyzy SL, Hutton DL, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 2010;31:2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao G, Zinger O, Schwartz Z, et al. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res 2006;17:258–64. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am 2008;90:2485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster TJ, Patel AA, Rahaman MN, et al. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater 2012;8:4447–54. [DOI] [PubMed] [Google Scholar]
- 16.Kou PM, Pallassana N, Bowden R, et al. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 2012;33:1699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A 2011;96:239–60. [DOI] [PubMed] [Google Scholar]
- 18.Kou PM, Babensee JE. Validation of a high-throughput methodology to assess the effects of biomaterials on dendritic cell phenotype. Acta Biomater 2010;6:2621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou PM, Schwartz Z, Boyan BD, et al. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater 2011;7:1354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahdavian Delavary B, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology 2011;216:753–62. [DOI] [PubMed] [Google Scholar]
- 21.Sagomonyants KB, Jarman-Smith ML, Devine JN, et al. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008;29:1563–72. [DOI] [PubMed] [Google Scholar]
- 22.Daculsi G, Goyenvalle E, Aguado E. Improvement of bone ingrowth on PEEK surface implant. Key Eng Mat 2011;493–4:795–9. [Google Scholar]
- 23.Poulsson AH, Eglin D, Zeiter S, et al. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 2014;35:3717–28. [DOI] [PubMed] [Google Scholar]
- 24.Waser-Althaus J, Salamon A, Waser M, et al. Differentiation of human mesenchymal stem cells on plasma-treated polyetheretherketone. J Mater Sci Mater Med 2014;25:515–25. [DOI] [PubMed] [Google Scholar]
- 25.Landy BC, Vangordon SB, McFetridge PS, et al. Mechanical and in vitro investigation of a porous PEEK foam for medical device implants. J Appl Biomater Funct Mater 2013;11:e35–44. [DOI] [PubMed] [Google Scholar]
- 26.Zavalloni D, De Benedictis M, Pagnotta P, et al. New CoreValve Evolut 23 mm technology for treatment of degenerated bioprosthesis. Heart Lung Circ 2014;23:183–5. [DOI] [PubMed] [Google Scholar]
- 27.Smith KE, Hyzy SL, Sunwoo M, et al. The dependence of MG63 osteoblast responses to (meth)acrylate-based networks on chemical structure and stiffness. Biomaterials 2010;31:6131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Wasilewski CE, Almodovar N, et al. The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors. Biomaterials 2012;33:7386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Olivares-Navarrete R, Wasilewski CE, et al. Use of polyelectrolyte thin films to modulate osteoblast response to microstructured titanium surfaces. Biomaterials 2012;33:5267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]