Abstract
Copper (Cu) is essential mineral, but its toxicity necessitates existence of powerful machinery responsible for the extraction of excess Cu from the cell. Cu exposure was recently shown to induce the translocation of Cu pump ATP7B to the lysosomes followed by lysosomal exocytosis. Here we sought to investigate the mechanisms underlying the effect of Cu on lysosomal exocytosis. We found that brief exposure to Cu activates lysosomal exocytosis, which was measured as a release of the lysosomal digestive enzyme β-hexosaminidase (β-hex) into the extracellular medium and by the presence lysosomal protein LAMP1 at the plasma membrane. Such release depends on calcium (Ca) and on the lysosomal SNARE VAMP7. ATP7B knockdown using RNAi suppressed the basal lysosomal exocytosis, but did not affect the ability of Cu to activate it. ATP7B knockdown was associated with sustained oxidative stress. The removal of Ca from the extracellular medium suppressed the Cu-dependent component of the lysosomal exocytosis. We propose that Cu promotes lysosomal exocytosis by facilitating a Ca-dependent step of the lysosomal exocytosis.
Keywords: Lysosomes, exocytosis, copper, calcium
Introduction
Lysosomal exocytosis has been originally described as a means of repairing plasma membrane via recruitment of the lysosomal membrane to a place of membrane damage [1]. Lysosomal fusion with the plasma membrane depends on a specific set of SNARE components [2], suggesting a regulated process. Therefore, the significance of lysosomal exocytosis likely extends beyond pathological conditions of membrane rupture, possibly including response, adaptation, or signaling involvement. The latter idea finds support in the recent series of evidence on the role of lysosomes in transition metal extraction from cells [3, 4].
Transition metals such as Fe, Zn and Cu enter cells via plasma membrane transporters or via endocytosis followed by absorption through lysosomal/endosomal transporters [5–7]. While all cells require some levels of transition metals, an excessive exposure to transition metals is toxic, necessitating their tight regulation. In the cytoplasm, transition metals are bound to chelating proteins, exported via plasma membrane transporters or absorbed into organelles, which is followed by exocytosis or metal-filled organelles. Among the transporters implicated into transition metals absorption into lysosomes are Zn transporters ZnT2 and ZnT4 (SLC30A2 and SLC30A4), and a Cu transporter ATP7B. Suppression of these transporters was shown to significantly affect Zn and Cu handling [3, 4].
Lysosomal transition metal importers are regulated in a variety of ways. ATP7B, the Cu transporter whose loss is responsible for Wilson’s disease [8], responds to Cu exposure by moving from trans-Golgi to the lysosomes [3, 9]. Cu absorption by the lysosomes is followed by its extraction from the cells via SNARE-dependent lysosomal exocytosis [3]. Thus, the main mechanism of ATP7B regulation appears to be translocation to the lysosomes or perhaps formation of the new population of ATP7B-bearing lysosomes. In addition, ATP7B interacts with p62 subunit of dynactin facilitating lysosomal transport towards the apical pole of hepatic cell where Cu is released [3]. ZnT transporters, especially ZnT2, have been shown to translocate to the lysosomes in response or in parallel to Zn exposure, and structural determinants of such translocation have been proposed [10]. At the same time, transcription of genes coding for several ZnTs is regulated by the transcription factor MTF-1, which responds to Zn and other transition metals [11]. These data show that lysosomal metal uptake capacity is regulated by the cytoplasmic transition metals. Whether or not Cu regulates the rate of the lysosomal metal extraction has not been consistently explored. It is the main question of the present studies.
Materials and methods
Cell Culture and treatments
HeLa cells were maintained in DMEM (Dulbecco’s modified Eagle’s medium; Lonza) supplemented with 10% FBS (Atlanta Biologicals) (growth medium) at 37°C in the presence of 5% CO2. For Cu treatments, cells were incubated with 100 µM CuCl2 for the indicated time in growth medium or regular buffer (10 mM Hepes pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 2 g/L glucose), as indicated for each experiment. For TBHP treatments, cells were incubated for 1 hour with 400 µM TBHP (Invitrogen, Carlsbag, CA) in regular buffer. For LaCl3 experiments, cells were pre-treated for 5 min with the indicated concentration of LaCl3 in regular buffer. LaCl3 was removed, added back and kept in the medium for the length of the assay.
siRNA transfection
Control (universal negative control #1), VAMP7, and ATP7B siRNAs were acquired from Sigma-Aldrich (St Louis, MO). Cells were transfected using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbag, CA). Transfections were performed as described by manufacturer’s protocol. Briefly, cells were seeded at subconfluency and transfected next day either with control siRNA, or siRNA for VAMP7 or ATP7B. Media was changed 16 to 24 hours later. siRNA efficiency was measured by qPCR.
β-Hexosamindase activity assay
HeLa cells on 12-well plates were washed once with regular buffer and 250 µl of buffer was added to each well. For Cu treatments, cells were incubated with 250 µL of CuCl2 diluted in regular buffer. Buffer was collected at the indicated times and incubated with 300 µl of 3 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide (N9376, Sigma-Aldrich, St Louis, MO) for 30 minutes at 37°C in 0.1 M citrate buffer (0.1 M sodium citrate, 0.1 M citric acid, pH 4.5). Reactions were stopped by adding 650 µl borate buffer (100 mM boric acid, 75 mM NaCl, 25 mM sodium borate, pH 9.8) and the absorbance was measured in a spectrophotometer at 405 nm. To determine total cellular content of β-hexosamindase, cells were lysed with 250 µl of 1% Triton X-100 in PBS and after a 10,000×g spin for 5 minutes at 4°C, 25 µl of the cell extracts were used for the enzyme activity reaction. Enzyme activity was determined as the amount of 4-nitrophenol produced. Absorbance was calibrated with different amounts of 4-nitrophenol (N7660, Sigma-Aldrich, St Louis, MO) in 0.1 M citrate buffer.
Flow Cytometric surface LAMP1 assay
HeLa cells plated on 6-well plates were incubated with 100 µM CuCl2 in regular buffer for 1 hour at 37°C or left untreated (control). Cells were trypsinized and washed in PBS before fixing in 1% paraformaldehyde (PFA) for 30 minutes. To detect surface LAMP1, fixed cells were incubated with CD107a LAMP1 antibody APC conjugated (Life Technologies, Carlsbag, CA) in 5% BSA in PBS for 30 minutes. Cells were washed and resuspended in PBS prior to analysis in BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Reverse Transcriptase and Quantitative PCR (qPCR)
For qPCR assays, cell were seeded in 12-well plates, transfected, and treated as indicated. Total RNA was isolated from HeLa using TRIzol (Invitrogen) according to manufacturer’s protocol. cDNA was synthesized with MuLV Reverse Transcriptase (Applied Biosystems, Foster City, CA) using 2 µg of total RNA and 0.5 µg of oligo(dT)18 (IDT, Cralville, IA) as primer. qPCR was carried out using 1:500 dilutions of cDNA, 2X SYBR Green (Fermentas, Glen Burnie, MD), and 4 µM primer mix per 10 µl reaction. For gene expression analysis, the following primers (IDT, Cralville, IA) were used: HMOX1, forward 5’-GAGACGGCTTCAAGCTGGTGAT-3’ and reverse 5’- CCGTACCAGAAGGCCAGGTC-3’; ATP7B, forward 5’- GTGGGCAATGACACCACTTT-3’ and reverse 5’- TGGGTGCCTTTGACATCTGA-3’, and RPL32, forward 5’- CAACATTGGTTATGGAAGCAACA-3’ and reverse 5’-TGACGTTGTGGACCAGGAACT-3’. To ensure amplification of cDNA only, all primers were designed to span exons and negative RT reactions were performed as control. The Relative Quantification method on the 7300 Real Time System (Applied Biosystems, Foster City, CA) was used to perform qPCR. Samples were amplified with the following program: 2 min at 50°C, 10 min at 95°C, and 40 cycles at 95°C for 15 sec followed by 60°C for 1 min. Samples were run in triplicates. At least 3 biological replicates were performed per condition. Relative gene expression was calculated using the ΔΔCt method, where Ct represents the cycle threshold. ΔCt values were calculated as the difference between the target genes and the expression of the endogenous gene RPL32 and ΔΔCt values were calculated relative to untreated controls. Data are presented as fold increase.
Statistical significance was calculated using a one-tailed, unpaired t-test with p<0.05 considered significant. Data are presented as mean ± S.E.M
Results and discussion
To analyze the dependence of lysosomal exocytosis on Cu we exposed HeLa cells to fresh buffer containing normal levels of free Ca (1 mM) and measured β-hex levels in the extracellular medium, followed by measuring β-hex content in the total cellular lysate. Fig 1A shows that cells gradually released β-hex, and at the 1-hour time point, cells released about 20% of their β-hex content, which is in line with the previously published data [4]. The addition of 100 µM CuCl2 to the extracellular medium significantly increased the β-hex exocytosis rate (Fig 1A). At the 1-hour mark, the amount of β-hex released by Cu-treated cells was 41.9% higher than in control cells (n=3, p<0.05). The effect was concentration-dependent, as exposure to 1 and 10 µM CuCl2 had no effect on β-Hex release (Fig 1D). Flow cytometry analysis revealed that the plasma membrane levels of lysosomal protein LAMP1 were increased when cells were treated with 100 µM CuCl2 for 1 hour (Fig 1B), which is in agreement with the β-Hex data. In addition, lysosomal exocytosis was increased in retinal pigment epithelium cells (RPE1) exposed to 100 µM CuCl2 for 1 hour (Fig 1C, 367.7% increase, n=3, p<0.05). Together, these data indicate that Cu stimulate lysosomal exocytosis.
Fig. 1. Cu stimulates lysosomal exocytosis in HeLa and RPE1 cells.
A. HeLa cells were exposed to 100 µM CuCl2 in regular buffer for 5, 15, 60, and 180 min or left untreated (control). β-hex activity was measured in the extracellular medium at the specified times. β-hex activity in the medium is normalized to the total cellular β-hex activity content, which was evaluated by lysing cells with 1% Triton as described in the Methods section. Cu significantly increased lysosomal secretion at 60 min. B. Flow cytometry analysis of LAMP1 at the plasma membrane. HeLa cells were treated with 100 µM CuCl2 in regular buffer for 1 hour, fixed, and incubated with anti-LAMP1 antibody APC-conjugated. Graphs represent APC fluorescence intensity at the plasma membrane, which is increased by Cu. C. Cu stimulates the release of β-hex in RPE1 cells treated for 1 hour with 100 µM CuCl2 in regular buffer. D. The effect of Cu on lysosomal exocytosis is dose dependent. HeLa cells were treated with 1, 10, and 100 µM CuCl2 in regular buffer for 1 hour. Values represented as mean ± SEM percent of total β-hex activity; three independent experiments; statistical significance was calculated using a two-tailed, unpaired t-test with p<0.05 (*).
In the previously published studies we showed that in agreement with the SNARE/Ca-dependent model of lysosomal exocytosis, β-hex release is enhanced by intracellular Ca and suppressed by the removal of SNARE components [4]. Accordingly, we find that the stimulatory effect of Cu on lysosomal exocytosis is mediated by a SNARE-dependent process, since VAMP7 knockdown reduced the basal and Cu-induced lysosomal exocytosis by 13 and 30%, respectively (Fig 2A, n=3, p<0.05).
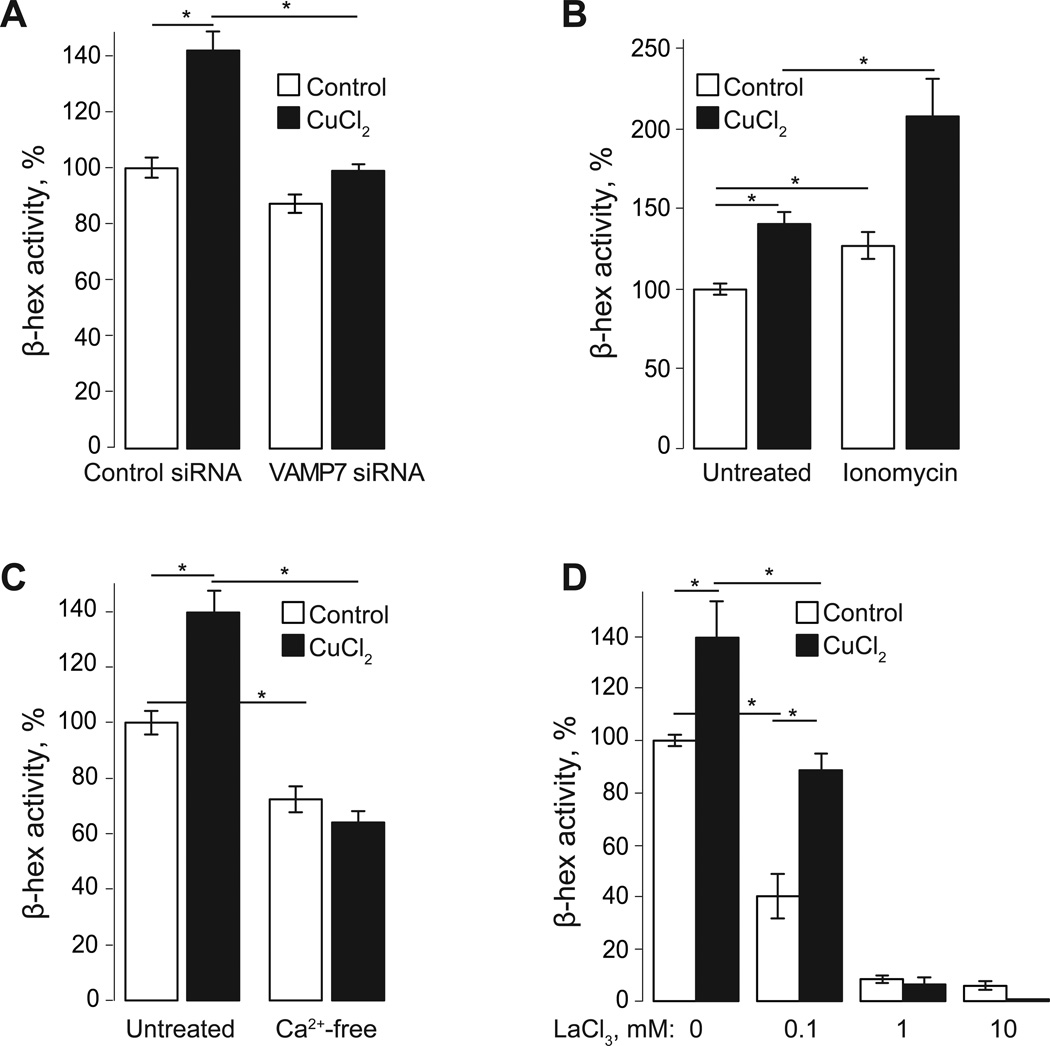
Fig. 2. Cu increases VAMP7 and Ca-dependent lysosomal exocytosis.
A. HeLa cells were transfected with VAMP7 or control siRNA 48 hours before the experiment was performed. Cells were treated for 1 hour with 100 µM CuCl2 in regular buffer or left untreated. β-hex activity was measured in extracellular medium at 1 hour. VAMP7 siRNA reduces both basal and Cu-induced lysosomal exocytosis. B. Addition of the Ca ionophore, ionomycin, for 15 min increased both basal and Cu-induced lysosomal exocytosis, observed as a decrease in β-hex activity in extracellular buffer at 1hour. C, D. Extracellular Ca is required for lysosomal exocytosis. Incubation of cells in Ca-free buffer reduced β-hex activity in extracellular medium and prevented Cu-induced exocytosis after 1hour (C). Addition of LaCl3 reduced lysosomal exocytosis in a dose-dependent manner. Cells were exposed to 0.1, 1, and 10 mM LaCl3 for 1 hour in the presence or absence of Cu (D). Values represented as mean ± SEM percent of β-hex activity in control cells (untreated); three independent experiments; statistical significance was calculated using a two-tailed, unpaired t-test with p<0.05 (*).
The dependence of lysosomal exocytosis on Ca was analyzed by increasing intracellular Ca levels with ionomycin and by removing extracellular Ca. Fig 2B shows that Ca ionophore ionomycin increased both basal and Cu-induced lysosomal exocytosis by 27% and 49% respectively (n=3, p<0.05). The fact that ionomycin was more effective in stimulating lysosomal exocytosis when Cu was present suggests that Cu facilitates a Ca-dependent step of the lysosomal exocytosis.
Incubation of cells with a Ca-free buffer reduced the basal lysosomal exocytosis by 28% (n=3, p<0.05) and prevented the stimulation of lysosomal exocytosis by Cu (Fig 2C). To further explore this outcome we used a broad plasma membrane Ca channel blocker Lanthanum (La) [12]. Pre-incubation of cells with 0.1 mM LaCl3 suppressed the basal lysosomal exocytosis, however, its effect on the basal exocytosis was higher than on the Cu-stimulated exocytosis (60% vs 36% reduction, n=3, p<0.05, Fig 2D).
High levels (1–10 mM) of La inhibited both basal and Cu-dependent aspects of the lysosomal exocytosis (Fig 2D). Taken together, these data confirm the dependence of lysosomal exocytosis on extracellular Ca. The different effects of Ca-free buffer and La on the ability of Cu to activate lysosomal exocytosis suggest two possibilities regarding the mechanism of its effect on lysosomal exocytosis. First, Cu may activate a plasma membrane channel whose sensitivity to La is low. Although our experiments using Ca dye Fura-2,am did not show any measureable Ca influx in response to Cu addition (not shown), it is possible that such influx is very small and localized. Second, Cu may facilitate a Ca-dependent step in the lysosomal exocytosis making it possible to happen under the conditions of suppressed extracellular Ca influx, at cytoplasmic Ca levels slightly below normal.
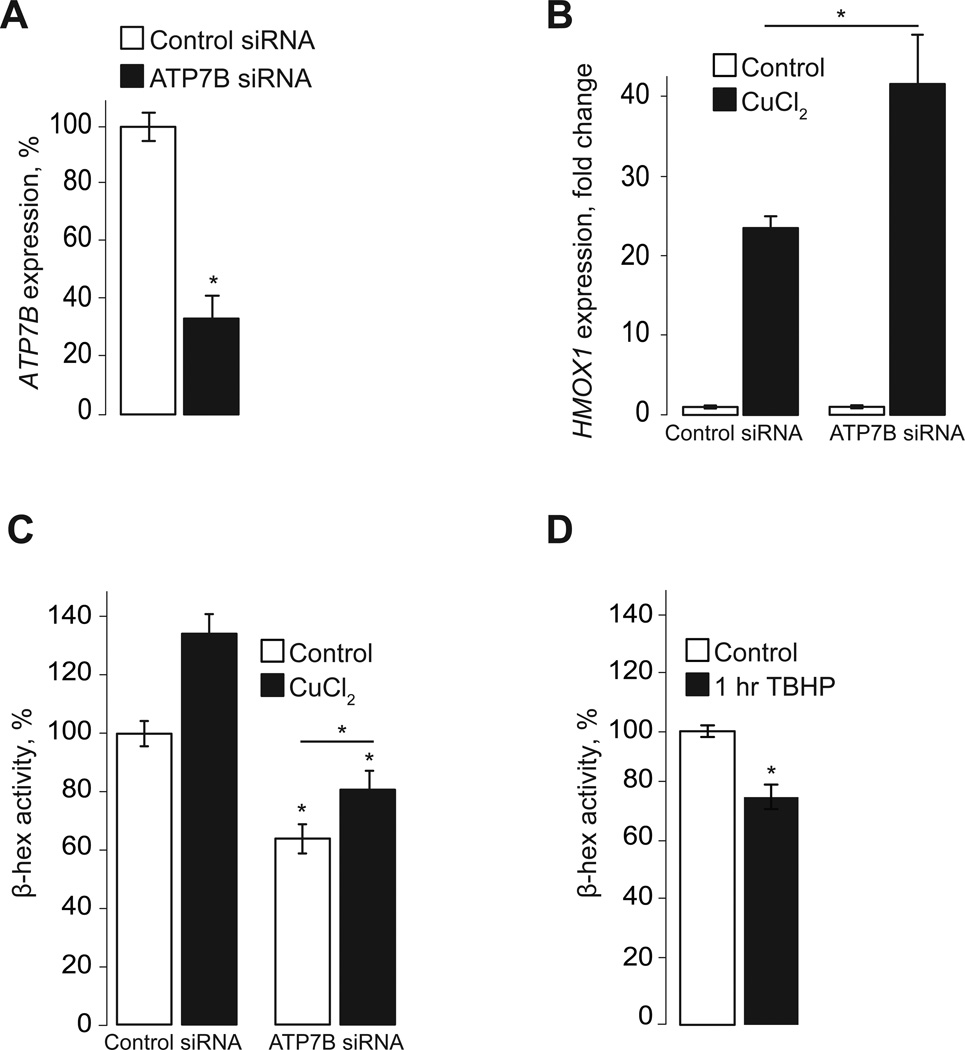
In order to document the physiological impact of lysosomal Cu uptake and exocytosis we knocked down the lysosomal Cu transporter ATP7B using siRNA (Fig 3A). Cu catalyzes the production of reactive oxygen species [7, 13, 14], which are toxic. Evacuation of Cu and Zn via lysosomal exocytosis was proposed to be a key component of transition metal detoxification [3, 4]. Thus, we reasoned that reducing the evacuation of Cu, by preventing lysosomal Cu uptake, would induce oxidative stress. Fig 3B shows that a 48-hour-long ATP7B knockdown is associated with a measurable increase in the ability of Cu to induce oxidative stress, as indicated by increased heme oxygenase 1 expression (HMOX1 gene), in response to Cu (23.5 fold increase in control siRNA cells vs 42.6 fold increase in ATP7B siRNA cells; n=3, p<0.05). HMOX1 expression is a reliable tool to measure oxidative stress [15, 16] and it is associated with increased lipid peroxidation in cells treated with Cu (Fig S1). Further analysis of ATP7B-knockdown cells suggests that Cu affects lysosomal exocytosis from the cytoplasm. Fig 3C shows that ATP7B knockdown noticeably suppressed basal lysosomal exocytosis, but did not eliminate the stimulatory effect of Cu on lysosomal exocytosis: in ATP7B-knockdown cells, the gain of β-hex release in response to Cu was indistinguishable from that in cells transfected with a control siRNA (26.3% and 34.4% increase, respectively; n=3). Therefore, Cu affects lysosomal exocytosis by acting on a component of the fusion machinery and not by affecting the lysosomal lumen.
Fig. 3. Preventing Cu evacuation through lysosomal exocytosis increases oxidative stress in HeLa cells.
A, B. qPCR analysis shows that transfection of HeLa cells with ATP7B siRNA for 48 hours significantly reduced VAMP7 mRNA levels (A) and increased the expression of HMOX1, a ROS-responsive gene, in cells treated for 4 hours with 100 µM CuCl2 in growth medium, compared to cells transfected with a control siRNA (B). Values represented as mean ± SEM percent expression of control siRNA (A) or fold change (B). C. Both basal and Cu-induced lysosomal exocytosis was significantly reduced in ATP7B knockdown cells. D. Induction of oxidative stress with TBHP decreased β-hex activity in extracellular medium of HeLa cells, indicating that oxidative stress reduced lysosomal exocytosis. Values represented as mean ± SEM percent of β-hex activity in control cells (untreated); three independent experiments; statistical significance was calculated using a two-tailed, unpaired t-test with p<0.05 (*).
The inhibitory effect of ATP7B knockdown on lysosomal exocytosis is likely mediated by the resulting oxidative stress induced by Cu, as exposure to oxidative stress induced by tert-Butyl hydroperoxide (TBHP) inhibited β-hex exocytosis as well, indicating that oxidative stress inhibits lysosomal exocytosis (Fig 3D). Based on these results we propose that Cu stimulates lysosomal exocytosis to accelerate Cu extraction and prevent oxidative damage.
Lysosomes have emerged as key determinants of transition metal detoxification as lysosomal exocytosis was shown to be indispensable for removal of Cu and Zn from cells. Although the molecular determinants of metal excretion via lysosomal exocytosis have been delineated [3, 4], the functional relations between transition metals and exocytosis are not well understood. The active response of lysosomal metal transporters to transition metal exposure (increased transcription and translocation) suggests sophisticated relationship between transporters and lysosomal exocytosis.
We show that exposure to Cu stimulates lysosomal exocytosis. The aspects of the lysosomal exocytosis stimulated by Cu require Ca. While Ca release through the lysosomal ion channel TRPML1 was suggested to drive lysosomal exocytosis, it is unlikely to contribute to the Cu-dependent lysosomal exocytosis. First, TRPML1 does not conduct Cu and does not seem to be activated by Cu [17]. Second, siRNA-driven TRPML1 knockdown described in our previous studies did not affect the Cu-dependent component of the lysosomal exocytosis (not shown). Finally, stimulation of the lysosomal exocytosis by Cu is inhibited by removal of extracellular Ca (Fig 2C) or by addition of extracellular LaCl3 (Fig 2D), suggesting involvement of a plasma membrane Ca channel activated by Cu and poorly sensitive to La. Information on the effect of Cu on plasma membrane channels is limited (see summary in a recent review [12]). TRPA1 and some members of the TRPM family are among candidates for the role of such a channel.
On the other hand, the fact that we were unable to detect any measurable spike in cytoplasmic Ca in response to the extracellular Cu application suggests a possibility of Cu inducing a very local Ca influx, or an effect on La-insensitive Ca transporter. Finally, it is possible that Cu sensitizes the machinery responsible for the lysosomal fusion with the plasma membrane to Ca. We find that the Cu-dependent component of the lysosomal exocytosis persists in ATP7B-deficient cells (Fig 3B), making it unlikely that Cu exerts its effect via ATP7B-dynactin interaction only. However, the stimulation of lysosomal exocytosis by Cu was absent in VAMP7-depeleted cells (Fig 2A), again underscoring the possible role of Cu in Ca-dependent aspects of the lysosomal exocytosis. Whether or not Cu regulates the Ca-dependence of other components of the membrane fusion machinery remains to be answered.
The effect of ATP7B knockdown on lysosomal exocytosis is intriguing. ATP7B not only mediates lysosomal Cu uptake; it also facilitates the transport of lysosomes to the plasma membrane [3]. The reduction of lysosomal exocytosis in control cells transfected with ATP7B siRNA (Fig 1C) can be a consequence of the latter. Furthermore, reducing ATP7B protein levels affects Cu homeostasis in two ways: lysosomal Cu uptake is reduced and evacuation of lysosomal Cu is prevented. Under these circumstances even low levels of Cu present in growth medium can induce oxidative stress and further affect lysosomal exocytosis. Taken together with the increased oxidative stress in ATP7B-knockdown cells (Fig 3B) and with the suppression of the lysosomal exocytosis by oxidative stress, it provides more evidence for a dynamically regulated cytoprotective system driven by lysosomal uptake of transition metal followed by their exocytosis. Beyond their role in digestion, this redefines lysosomes as cytoprotective organelles.
Supplementary Material
Highlights.
-
-
Brief exposure to copper activates lysosomal exocytosis
-
-
Cytoplasmic calcium and a cytoplasmic step are involved in the copper effects.
-
-
ATP7B is essential for copper-dependent lysosomal exocytosis.
Acknowledgements
This work was supported by the National Institutes of Health grants HD058577 and ES01678 to Kirill Kiselyov. We thank Sreeram Ravi for technical support. We also thank Mike Myers at the University of Pittsburgh Cancer Institute Flow Cytometry Facility.
Abbreviations
- β-hex
β-hexosaminidase
- RNAi
RNA interference
- siRNA
short interfering RNA
- SNARE
SNAP (Soluble NSF Attachment Protein) Receptor
- TBHP
tert-Butyl hydroperoxide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106(2):157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 2.Rao SK, et al. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279(19):20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 3.Polishchuk EV, et al. Wilson Disease Protein ATP7B Utilizes Lysosomal Exocytosis to Maintain Copper Homeostasis. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kukic I, Kelleher SL, Kiselyov K. Zinc efflux through lysosomal exocytosis prevents zinc-induced toxicity. J Cell Sci. 2014 doi: 10.1242/jcs.145318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleackley MR, Macgillivray RT. Transition metal homeostasis: from yeast to human disease. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2011;24(5):785–809. doi: 10.1007/s10534-011-9451-4. [DOI] [PubMed] [Google Scholar]
- 6.Graper ML, et al. Introduction to Human Disorders of Copper Metabolism. Ann N Y Acad Sci. 2014;1314:v–vi. doi: 10.1111/nyas.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegde ML, et al. Oxidative genome damage and its repair in neurodegenerative diseases: function of transition metals as a double-edged sword. J Alzheimers Dis. 2011;24(Suppl 2):183–198. doi: 10.3233/JAD-2011-110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi H, et al. Compound overload of copper and iron in patients with Wilson's disease. Med Mol Morphol. 2006;39(3):121–126. doi: 10.1007/s00795-006-0326-7. [DOI] [PubMed] [Google Scholar]
- 9.Harada M, et al. The Wilson disease protein ATP7B resides in the late endosomes with Rab7 and the Niemann-Pick C1 protein. Am J Pathol. 2005;166(2):499–510. doi: 10.1016/S0002-9440(10)62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick NH, et al. The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution. J Mammary Gland Biol Neoplasia. 2014;19(1):59–71. doi: 10.1007/s10911-013-9314-4. [DOI] [PubMed] [Google Scholar]
- 11.Andrews GK. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2001;14(3–4):223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 12.Bouron A, Kiselyov K, Oberwinkler J. Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 14.Scheiber IF, Mercer JF, Dringen R. Metabolism and functions of copper in brain. Prog Neurobiol. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Coblentz J, St Croix C, Kiselyov K. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem J. 2014;457(2):361–368. doi: 10.1042/BJ20130647. [DOI] [PubMed] [Google Scholar]
- 16.Alam J, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275(36):27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 17.Dong X, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455(7215):992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.