Abstract
The corpus callosum has been implicated as a region of dysfunctional connectivity in schizophrenia, but the association between age and callosal pathology is unclear. Magnetic resonance imaging (MRI) and diffusion-tensor imaging (DTI) were performed on adults (n=34) and adolescents (n=17) with schizophrenia and adult (n=33) and adolescent (n=15) age- and sex-matched healthy controls. The corpus callosum was manually traced on each participant’s MRI, and the DTI scan was co-registered to the MRI. The corpus callosum was divided into five anteroposterior segments. Area and anisotropy were calculated for each segment. Both patient groups demonstrated reduced callosal anisotropy; however, the adolescents exhibited reductions mostly in anterior regions while the reductions were more prominent in posterior regions of the adults. The adolescent patients showed greater decreases in absolute area as compared with the adult patients, particularly in the anterior segments. However, the adults showed greater reductions when area was considered relative to whole brain white matter volume. Our results suggest that the initial stages of the illness are characterized by deficiencies in frontal connections, and the chronic phase is characterized by deficits in the posterior corpus callosum; or, alternatively, adolescent-onset schizophrenia may represent a different or more severe form of the illness.
Keywords: diffusion tensor imaging, white matter, schizophrenia spectrum, MRI, magnetic resonance imaging
1. Introduction
Schizophrenia has been described as a ‘disconnection syndrome,’ characterized by dysfunctional cortical integration and abnormal functional connectivity. Defective interhemispheric communication of language, somatosensory and attentional information (Endrass et al., 2002; Mohr et al., 2000; Phillips et al., 1996; Rushe et al., 2007), and decreased language lateralization (Spironelli et al., 2008) have been demonstrated in this disorder. As the largest white matter tract in the human brain, the corpus callosum has been implicated as an important region of interest in the neuropathology of schizophrenia (Crow, 1998).
Indeed, structural abnormalities in the corpus callosum have been widely reported in schizophrenia patients. Post-mortem studies initially revealed increased callosal thickness in the brains of schizophrenia patients (Rosenthal and Bigelow, 1972), particularly chronic patients with an early-onset of illness (Bigelow et al., 1983). More recently, magnetic resonance imaging (MRI) studies of schizophrenia patients have largely reported reduced size of the corpus callosum, both in chronic (Downhill et al., 2000; Mitelman et al., 2009; Rotarska-Jagiela et al., 2008) and first-episode patient groups (Arnone et al., 2008; Walterfang et al., 2008a). More recently, diffusion tensor imaging (DTI) methods for examining white matter tracts have revealed reduced anisotropy, which may be indicative of poor alignment of axon bundles and unhealthy or unmyelinated axons (Kantarci et al., 2001), in the corpus callosum in both first-episode (Cheung et al., 2008; Federspiel et al., 2006; Gasparotti et al., 2009; Kyriakopoulos and Frangou, 2009; Perez-Iglesias et al., 2010; Price et al., 2007) and chronic adult patients (Friedman et al., 2008; Kong et al., 2011; Mitelman et al., 2009), as well as first-episode adolescent patients (Davenport et al., 2010; Douaud et al., 2007; Henze et al., 2012; Kyriakopoulos et al., 2008; White et al., 2009). Furthermore, lower values of anisotropy in chronic patients are associated with greater symptom severity (Mitelman et al., 2009). The genu and splenium have been examined and cited most often as sub-regions of the corpus callosum with reduced area (Downhill et al., 2000; Rotarska-Jagiela et al., 2008; Walterfang et al., 2008a) and anisotropy (e.g., Ellison-Wright et al., 2014; Federspiel et al., 2006; Friedman et al., 2008; Perez-Iglesias et al., 2010), perhaps due to their relationship to the frontal and temporal lobes, two areas strongly implicated in the neuropathology of schizophrenia. Dense small diameter myelinated fibers in the anterior corpus callosum reciprocally interconnect the prefrontal cortex in each of the hemispheres while those in the posterior corpus callosum interconnect the parietal, temporal and occipital lobes (Hofer and Frahm, 2006).
Although corpus callosum pathology has consistently been shown to be a feature of schizophrenia, investigations into the association between corpus callosum pathology and illness duration have yielded inconsistent results. Disturbances in the size and structural integrity of white matter tracts of the corpus callosum appear to be present at, and even before, illness onset (Henze et al., 2012; Walterfang et al., 2008a; Walterfang et al., 2008b). Cross-sectional studies report more severe corpus callosum size and anisotropy reductions in chronic versus first-episode patients (Collinson et al., 2014; Downhill et al., 2000; Friedman et al., 2008; Kong et al., 2011), suggesting a degenerative pattern. However, a longitudinal approach revealed anisotropy abnormalities become less marked over time in chronic patients (Mitelman et al., 2009), and a meta-analysis reported that first-episode patients, compared with chronic patients, exhibit a greater effect of reduced absolute area (Arnone et al., 2008).
The aim of the current study was to examine both area and anisotropy of the corpus callosum in adult and antipsychotic drug-naïve adolescent schizophrenia patients and age- and sex-matched healthy controls to evaluate (1) whether differences in these measures are present in adolescent patients prior to the use of medication and (2) how alterations in the corpus callosum, albeit in a cross-sectional sample, may vary with age. Studying antipsychotic drug-naïve adolescent patients affords the opportunity to examine potential neurological abnormalities in this patient population without the influence of prolonged exposure to antipsychotic medication and/or hospitalization. To date, only one study has examined corpus callosum abnormalities in antipsychotic drug-naïve adolescent patients using stereotaxically-located regions of interest and reported no differences in anisotropy (Schneiderman et al., 2009). The current study improves on this research by using manually-traced regions-of-interest (the gold standard) and examining the corpus callosum in its near entirety. The following hypotheses were tested: (1) the patient groups will demonstrate reductions in anisotropy and area of the corpus callosum, particularly in the genu and splenium, as these areas have been most commonly cited as areas of abnormality and are related to the frontal and temporal lobes—areas strongly implicated in the pathology of schizophrenia (Buchsbaum et al., 1990; Hazlett et al., 2000); (2) the adult patients would demonstrate more severe reductions in anisotropy and area as we propose that age is associated with more severe reductions, assuming degenerative processes are at work. We also tested two exploratory hypotheses: (3) lateral-medial effects were explored as much of the research to date has only examined more medial portions of the corpus callosum; and (4) correlations were conducted to explore the hypothesis that lower anisotropy and size of the corpus callosum would be associated with greater clinical symptom severity.
The current study reports novel data from manually-traced corpus callosum regions-of-interest in the adolescent samples and examines diagnostic group by age group effects and symptom correlates that have not been previously published. White matter anisotropy data from the participants were described in earlier reports which utilized investigatory methods of stereotaxically-located regions of interest to examine normal aging effects (Schneiderman et al., 2007) and aging effects in the patient groups (Schneiderman et al., 2009). Volume of cortical and subcortical structures and cortical and corpus callosum anisotropy in the adult patients and adult healthy controls have also been previously published by our group (Mitelman et al., 2005a; Mitelman et al., 2005b; Mitelman et al., 2005c; Mitelman et al., 2006; Mitelman et al., 2009; Mitelman et al., 2005d). Neurocognitive functioning of the adolescent patients has been described by Brickman et al. (2004). All participants received the PANSS on the day of their scan to assess clinical symptom severity.
2. Methods
2.1. Participants
Adolescent (n=17) and adult schizophrenia patients (n=34) and age- and sex-matched healthy controls (n=15 and n=33, respectively) were recruited as described elsewhere (Schneiderman et al., 2009; see Table-1 for demographic information). Only those adolescents who ultimately received a diagnosis of schizophrenia were included in the current study. All adolescents were 21 years old or younger, and the adult over 21 years of age. The adolescent patients were experiencing their first psychotic episode. The adult patients had a mean illness duration of 21.2 years. One participant’s scan was unable to be traced and was excluded from the analysis.
Table 1.
Sample Characteristics.
| Variable | Adults | Adolescents | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Patients (n=34) | Healthy Controls (n=33) | Statistic | P | Patients (n=17) | Healthy Controls (n=15) | Statistic | P | |
| Age, M(SD) | 43.7 (10.2) | 42.2 (11.5) | t (65)=0.58 | 0.57 | 15.9 (1.7) | 17.1 (2.1) | t (30)=1.73 | 0.44 |
| Sex, n (%) | X2(1)=1.89 | 0.17 | X2(1)=0.098 | 0.75 | ||||
| Male | 25 (74%) | 19 (58%) | 10 (59%) | 8 (53%) | ||||
| Female | 9 (26%) | 14 (42%) | 7 (41%) | 7 (47%) | ||||
| Handedness, n (%) | X2(1)=3.24 | 0.08 | X2(1)=0.098 | 0.68 | ||||
| Right | 34 (100%)a | 30 (91%) | 14 (82%) | 13 (87%) | ||||
| Left | 0 (0%) | 3 (9%) | 3 (18%) | 2 (13%) | ||||
| PANSS Scores, M(SD) | ||||||||
| Positive | 18.7 (6.9) | - | 21.2 (5.0)b | - | t (47)=1.25 | 0.22 | ||
| Negative | 17.1 (6.4) | - | 25.3 (8.2)b | - | t (47)=3.81 | <0.001 | ||
| Total | 71.9 (17.4) | - | 90.8 (14.7) | - | t (49)=3.84 | <0.001 | ||
Includes 1 mixed-handed participant.
Scores unavailable for 2 participants.
2.2 Image acquisition and processing
T1-weighted MR images were acquired using a 1.5 T Signa 5× scanner (GE Medical Systems) with a 3D-SPGR sequence (TR=24ms, TE=5 ms, flip angle=40°, matrix size 256×256, field of view23 cm, NEX=1, slice thickness 1.2 mm, total slices 128). The diffusion tensor sequence acquired fourteen 7.5-mm thick slices TR=10 s, TE=99 ms, TI=2.2 s, b=750 s/mm2, Δ =31ms, Δ =73ms, NEX=5, voxel size 1.8×1.8×7.5 mm, FOV=230, no gaps). In order to solve for the components of the diffusion tensor, seven diffusion EPI images were obtained: six with different non-collinear gradient weightings and one with no diffusion gradient applied. The diffusion tensor for every voxel in a slice was then computed by solving the seven simultaneous signal equations relating the measured signal intensity to the diffusion tensor. Anatomical SPGR MR images were resectioned to standard Talairach–Tournoux position using the algorithm of Woods et al. (1993), a 6-parameter rigid body transformation, and the standard MNI brain. The anisotropy images from each subject were then aligned to subject’s own standard-position anatomical images using the 12-parameter transformation.
2.3. Corpus callosum region of interest
The corpus callosum was manually traced on twelve sagittal slices, 1.2 mm thick. The mid-sagittal slice was identified by locating the x-coordinate of the midline of the axial slice with the most visible splenium. The corpus callosum was traced in its full extent on six sagittal slices on either side of the mid-sagittal slice using our standard Sobel-gradient filter which enhances gray/white matter edges (Figure-1; Gonzales and Wintz, 1987). To determine inter-rater reliability, ten subjects were randomly chosen and traced by two independent researchers, and area measures were compared between the two. The intraclass correlation was greater than 0.90, indicating good inter-rater reliability. The medial axis of the traced outline was then found by skeletonization, smoothed, and extended to the front and back of the corpus callosum using the slope and the end of the medial axis. Its length was divided into 5 equal segments and perpendiculars to the line formed and extended to the edges of the corpus callosum. Thus, each outline was divided into five anteroposterior areas corresponding to the rostrum and genu, anterior body, midbody, posterior body, and splenium (Figure-2). The five sectors are approximations of the functional segmentation of the corpus callosum proposed by Hofer and Frahm (2006). Area and anisotropy values were averaged for the three most lateral slices and the three most medial slices in the left and right hemisphere, resulting in twenty segments for the area and anisotropy analysis (5 (anteroposterior segments) × 2 (lateral, medial) × 2 (left, right hemisphere). Thus, the lateral slice mean Talairach coordinate would be x=5. Anisotropy is expressed relative to mean whole-brain anisotropy, as we have previously done (e.g., Buchsbaum et al. 2006; Mitelman et al., 2007, 2009; Schneiderman et al., 2007, 2009). This approach is analogous to correcting BOLD or FDG PET values to whole-brain activity as is widely reported. Anisotropy, area, and relative area data for each of the corpus callosum segments are provided in Supplementary Tables 1 and 2.
Figure 1.
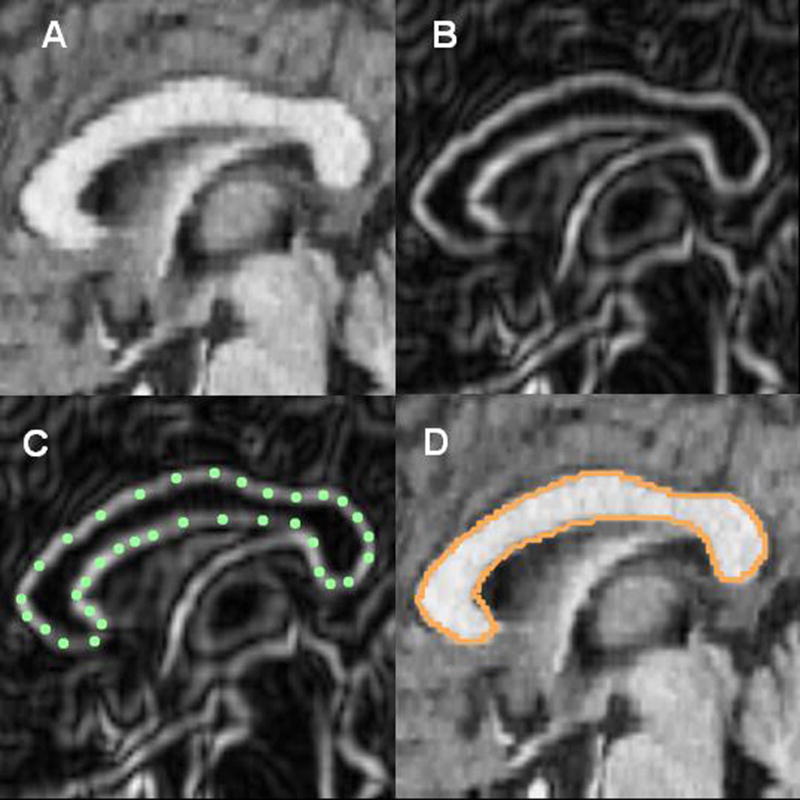
Tracing the corpus callosum. (A) Magnetic resonance image showing a sagittal cross-section through the corpus callosum. (B) Using an edge contrast-enhancing technique (Sobel-gradient filter), gray/white boundaries of the corpus callosum are enhanced. (C) the corpus callosum is outlined; and (D) the spline curve is fit.
Figure 2.
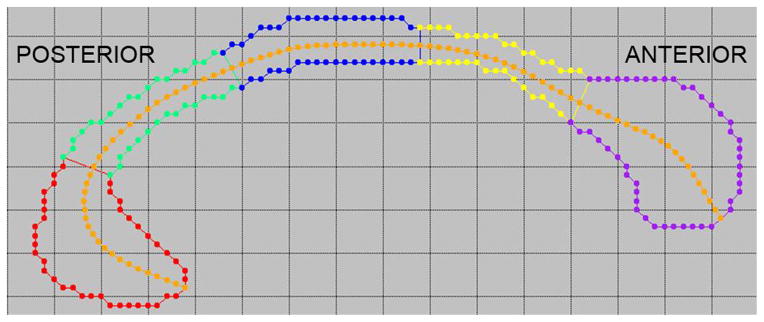
Segmentation of the corpus callosum. The medial axis (in orange) is divided into five segments of equal length, and perpendicular cuts are used to create the boundaries of the anteroposterior divisions. The segments are labeled (from anterior to posterior): the genu (purple), anterior body (yellow), midbody (blue), posterior body (green), and splenium (red).
For three-dimensional examination, the callosal outlines were warped to the average outline at the corresponding lateral position to provide voxel x,y,z locations common to all participants. T-tests were performed on these voxel values and displayed in 3D by VTK routines. The midline was displayed as a semi-transparent sheet of spheres for orientation.
2.4. Whole brain volume
Total brain volume was determined by summing the volumes of gray and white matter for all 39 Brodmann areas using our standard Brodmann program described elsewhere (Hazlett et al., 1998).
2.5. Statistical analysis
To examine diagnostic differences in anisotropy and area (absolute and relative to whole brain white matter volume), we used a series of mixed-model multivariate analysis of variance (MANOVA) designs with diagnosis (schizophrenia patients vs. healthy controls) and age (adolescent vs. adult) as between-group factors and lateromedial section (lateral, medial), hemisphere (left, right) and anteroposterior segment (1 (most anterior) to 5 (most posterior) as repeated measures. We also conducted a mixed-model MANOVA to investigate group differences in whole brain volume.
All significant main effects and interactions involving diagnosis are reported. All analyses were conducted with Statistica (StatSoft, 2013). For all MANOVAs, we report the multivariate F (Wilks’ Lambda). Significant interactions with group were followed up with Fisher’s Least Significant Difference tests to determine the direction of the effect. We minimized Type II error by using nested repeated measures to reduce the number of statistical tests. Pearson’s correlation coefficients were used to examine associations between corpus callosum values (anisotropy and area) and negative and positive symptom severity.
3. Results
3.1. Diagnostic effects
3.1.1. Whole brain volume
Combining adults and adolescents, the schizophrenia patients had significantly smaller whole brain volume (1137 ± 128 cm3) compared with the healthy controls (1203 ± 134cm3; F(1,95)=8.78, p<0.01).
3.1.2. Anisotropy
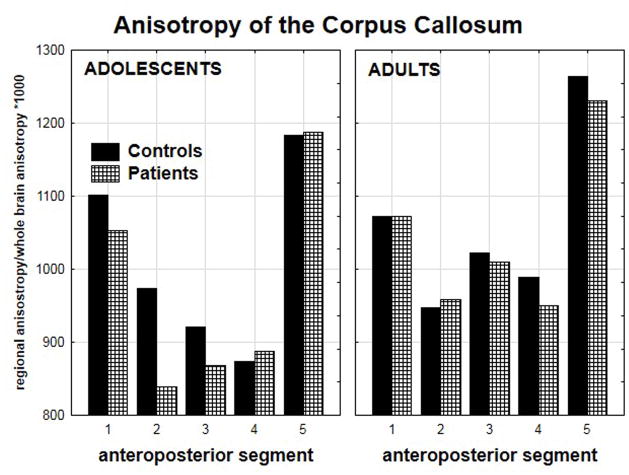
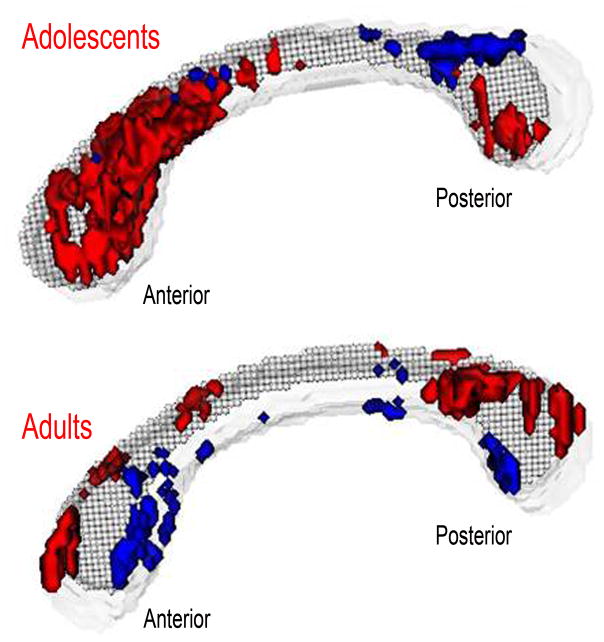
Both patient groups demonstrated reductions in anisotropy. The adolescent patient group demonstrated anisotropy reductions in the most anterior portions of the corpus callosum while the adult patient group demonstrated anisotropy reductions in the posterior portions (diagnostic group × age group × anteroposterior segment, F(4,92)=2.50, p<0.05; Figure-3). Despite the significant interactions, none of the post-hoc tests were significant. However, there was a non-significant trend towards lower anisotropy in the anterior body of the corpus callosum in the adolescent patients compared with the adolescent healthy controls (p=0.08). Pixel-by-pixel t-tests illustrate the pattern of reduced anterior anisotropy in the adolescent patients and reduced posterior anisotropy in the adult patients (Figure-4).
Figure 3.
Mean anisotropy for the anteroposterior segments of the corpus callosum is shown for the four groups (diagnosis × age × anteroposterior segment, F(4,92)=2.50, p<0.05). The adolescents showed reduced anisotropy in the anterior segments whereas the adults showed reduced in the posterior segments. Note that anisotropy values are standardized as a ratio of the mean regional value to mean whole-brain value (×1000).
Figure 4.
ROI pixel-by-pixel t-tests. Red indicates significantly higher anisotropy in the healthy controls. Blue indicates significantly higher anisotropy in the patients (p<0.05, t-test, corrected for multiple tests). Top: The adolescent patients showed a marked decrease in anisotropy in the anterior section of the corpus callosum. Bottom: The adult patients demonstrated greater reductions in the posterior section of the corpus callosum.
Averaged over age, the schizophrenia patients demonstrated greater anisotropy reductions in the lateral corpus callosum compared with the medial (diagnostic group × lateromedial section, F(1,95)=4.86, p<0.03). None of the post-hoc tests were significant.
3.1.3. Area
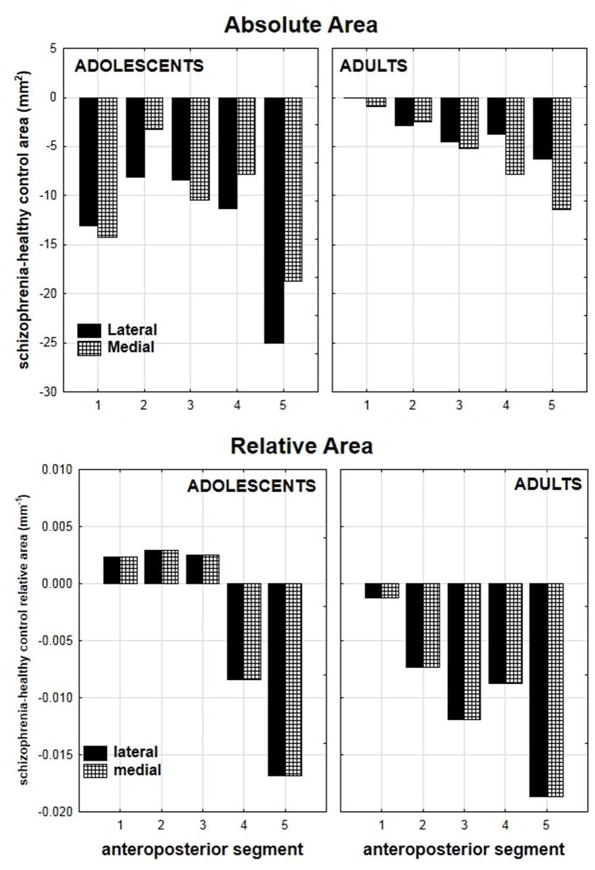
Combining adults and adolescents, the patients exhibited smaller absolute area in the corpus callosum (F(1, 95)=5.92, p<0.03). The differences between the healthy controls and adolescent schizophrenia patients was most marked in the genu and the splenium, while in adult patients, only the splenium area reduction was observed (diagnostic group × age group × lateromedial section × anteroposterior segment, F(4,92)=3.07, p<0.03; Figure-5). Post-hoc tests revealed significantly smaller area in the lateral (p<0.01) and medial (p<0.04) splenium in the adolescent patients. There was also a trend towards smaller area in the medial splenium of the adult patients (p=0.06).
Figure 5.
Mean absolute and relative area values for the five corpus callosum segments are shown for the four groups. Top: The adolescent and adult patient groups showed reduced absolute area in nearly all the callosal segments, with the adolescent patients showing more marked reductions (diagnosis × age × lateromedial section × anteroposterior segment, F(4,92)=3.07, p<0.03). Bottom: The adult patient group showed decreased relative area in all the segments while the adolescents showed a less consistent pattern, with increases in the anterior portions but clear decreases in the posterior regions, including the body and splenium (diagnostic group × age × lateromedial section × anteroposterior segment, F(4,92)=2.42, p=0.06).
There were no significant effects for the relative area analysis. However, the diagnostic group × age × lateromedial section × anteroposterior segment interaction approached significance (F(4,92)=2.42, p=0.06). The adult patients demonstrated decreased relative area across the entire corpus callosum while the adolescents demonstrated a mixed pattern of results, with increased relative area in the anterior portions of the corpus callosum and decreased relative area in the posterior portions (Figure-5). Post-hoc tests revealed significantly smaller relative area in the medial splenium of the adult patients (p<0.05).
3.2 Symptom correlates
Greater negative symptom severity, as indexed by the negative scale score of the PANSS, was associated with lower anisotropy in the right, lateral posterior body of the adult patients (r=−0.35, df=32, p<0.05) and the left, medial anterior body and midbody of the adolescent patients (r=−0.63, df=13, p<0.02; r=−0.58, df=13, p<0.03).
4. Discussion
4.1. Anisotropy
To our knowledge, this is the first study to report anisotropy reductions in the corpus callosum in antipsychotic drug-naïve adolescent patients. The anisotropy results were somewhat unexpected as we anticipated reductions across the corpus callosum in both adolescent and adult patient groups, particularly in the genu and splenium. However, we found genu abnormalities in anisotropy more prominent in adolescents with schizophrenia and posterior abnormalities more prominent in adults with schizophrenia. In another study of adolescent patients with schizophrenia (Henze et al., 2012), they similarly observed lower anisotropy in the anterior segments with the smallest magnitude of difference in the third segment rather than in the fourth segment as in our data. Interestingly and unexpectedly, the anisotropy pathology of the adolescent and adult patient groups differed qualitatively; that is, the adult group’s pathology did not simply represent a more severe version of the adolescent group, as would be predicted by a degenerative hypothesis. Furthermore, anisotropy was inversely related to negative symptoms in posterior regions in the adult patients and anterior regions in the adolescents. Our results suggest that deficiencies in the structural integrity of the corpus callosum are present at illness onset in adolescents as well as in the chronic phase of the illness, although each stage may be characterized by differing regions of deficiency. The differences in the patient age groups may represent a qualitative change over time that occurs in the corpus callosum, or signify that adolescent-onset schizophrenia represents a different or more severe form of the illness. Development of the frontal lobe continues into young adulthood (Sowell et al., 1999); aberrant frontal connectivity in the adolescents, therefore, could disrupt normal development of the frontal lobe, potentially contributing to executive dysfunction and hypofrontality characteristic of this population (Hazlett et al., 2000; Hutton et al., 1998). The genu is one of the later regions to be myelinated (Rakic and Yakovlev, 1968); reduced anisotropy of the anterior corpus callosum in the adolescent patients may represent a malfunction of this process. Adolescent-onset schizophrenia is associated with a poorer prognosis (Hollis, 2000), and early disturbances or developmental failure in frontal connectivity could conceivably contribute to such outcomes. In contrast, our adult sample was characterized by reductions in posterior callosal anisotropy, suggesting a distinctive disturbance in the temporal region in the chronic stage of illness. Mitelman et al. (2009) reported that reduced anisotropy in the splenium and body was specific to patients with poor outcomes, suggesting that more posterior callosal involvement is a function of chronicity or severity of illness. Disturbances in the temporal lobe, for which the posterior segments provides connections, have been shown to progress over the course of the illness (Vita et al., 2012), and poor outcome has also been associated with reduced temporal volume (Mitelman et al., 2003).
It is important to note that while our age comparisons are adolescents vs. adults, most diagnosis by age interactions tested in the literature are examining the effect of aging in adults only. Consistent with our results, Voineskos et al. (2010) found younger adult patients to have reduced anisotropy in the genu and older adult patients to have reduced anisotropy only in the splenium; a patient group by age group statistical contrast was not presented. Kochunov et al. (2014) found anisotropy decreasing faster with age in adult patients than heathy volunteers. For comparison, we examined the age regression line slopes between age and anisotropy in our adults only and similarly found greater rates of decrease in patients with schizophrenia (slope −5.75 ×10−3 anisotropy/year) than volunteers, (−4.83×10−3), but the difference was not statistically significant. Our data suggests that adolescent onset patients may be different from adult onset patients and that linear regression across the entire lifespan may oversimplify effects.
4.2 Area
In our examination of area measures, we found reduced absolute area in all sub-regions of the corpus callosum in the adolescent patient group. However, when using our relative area measure, these reductions decreased or were even reversed, with the exception of the posterior segments. This discrepancy indicates that the reduced absolute area in the adolescent group may be representative of a general white matter volume reduction not specific to the corpus callosum and stresses the importance of considering corpus callosum area relative to white matter volume. We did not find evidence of reduced relative area of the anterior portions where anisotropy was found to be reduced in the adolescent sample. Therefore, these axons may not be smaller or less in number as would be captured in area measures but simply deficient in their myelination and/or organization. In the adult sample, absolute and relative area findings were similar to each other and demonstrated slight reductions in the entirety of the corpus callosum, most markedly in the splenium. We did expect to see reduced area in the genu of the adult patients as has been demonstrated previously (Downhill et al., 2000; Walterfang et al., 2008a); however, the area pattern demonstrated in our adult group is consistent with a posterior pathology associated with the chronic phase, similar to the anisotropy findings. Our absolute area findings were consistent with previous work reporting larger reductions in corpus callosum area in first-episode versus chronic patients (Arnone et al., 2008). However, this pattern did not hold when we examined relative area.
4.3. Lateral-medial effects
In our examination of lateral-medial effects, we found greater anisotropy reductions in the lateral portions of the corpus callosum. These results are similar to the results of Ellison-Wright (2014) who noted that the genu and body locations of significant cluster centers from voxel mapping were x=−13 and 13 for the left and right genu. The correlation between anisotropy in the corpus callosum of schizophrenia patients and an empathy measure (The Interpersonal Reactivity Index) also had a cluster center at a lateral x=11 (Fujino et al., 2014). Since the effect was lower medially, this is consistent with the speculation that fibers diverge more rapidly in anteroposterior and dorsoventral directions in patients, or that the topographical organization of their paths is less coherent. This lateral to medial effect does not appear clearly related to volume as our results did not yield a lateral/medial interaction with group, and the volume effect is maximum for a midline rather than lateral sagittal section in close examination of Figure-1 in the sample of Salgado-Pineda et al. (2014).
4.4. Limitations
A few limitations of the current study should be noted. This is a cross-sectional study rather than a longitudinal study, and, therefore the effects of age may be subject to pathophysiological differences due to age of onset, illness duration, exposure to and duration of antipsychotic treatment, and cohort effects such as diagnostic thresholds and biases in recruitment characteristics. Also, our adolescent patients had significantly higher scores on the PANSS indicating higher symptom severity in this group compared with the adult patients. The adolescent patients were unmedicated and exhibiting psychotic symptoms at the time of the study while the adult patients were medicated, possibly accounting for the discrepancy in symptom severity. However, it should be noted that our results may be confounded by illness severity or medication effects. Lastly, there is evidence of sexual dimorphism in both area and anisotropy of the corpus callosum (Ardekani et al., 2013; Menzler et al., 2011) as well as sex by diagnosis interactions for callosal thickness in particular subregions (Nasrallah et al., 1986); however, because we did not have enough females in the age groups, we were unable to use sex as an additional factor in our analyses.
Our study is the first to report reduced corpus callosum anisotropy in antipsychotic-naïve adolescent schizophrenia patients. Our results suggest that the initial stages of schizophrenia are characterized by deficiencies in frontal connections which recede as the illness progresses, and the chronic phase is characterized by deficits in the posterior corpus callosum; alternatively, adolescent-onset schizophrenia may represent a different and/or more severe form of the illness.”
Supplementary Material
Highlights.
We examined the corpus callosum in adult and adolescent schizophrenia patients.
Area and anisotropy were measured in the corpus callosum.
Adolescents had reduced anisotropy and area, most markedly in the anterior region.
Reductions in anisotropy were more prominent in posterior regions in the adults.
Acknowledgments
Funding for this study was provided in part by grants from the National Center for Advancing Translational Sciences (NCATS)(UL1TR000067), a component of the National Institutes of Health (NIH), NIH grants (R01MH60023, R01MH56489, R01MH60384S to MSB), Eli Lilly company (adolescent MRI and clinical assessment), and the Veteran’s Administration (VA Merit Award I01CX00026 to EAH).
Footnotes
Contributors
ECB worked on image processing, statistical analysis, and manuscript writing. Data were drawn from several separate projects on adult and adolescent patients designed by EAH, MMH and MSB. EAH and MSB also supervised the writing, image and statistical analysis. MMH, JA, and RB recruited, assessed and tested the patients. WB helped with interpreting the statistical analysis and editing the manuscript. JSS, KC, REN, EW, and CYT helped with the image and statistical analysis. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardekani BA, Figarsky K, Sidtis JJ. Sexual dimorphism in the human corpus callosum: an MRI study using the OASIS brain database. Cerebral Cortex. 2013;23:2514–2520. doi: 10.1093/cercor/bhs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2008;101:124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bigelow LB, Nasrallah HA, Rauscher FP. Corpus callosum thickness in chronic schizophrenia. The British Journal of Psychiatry. 1983;142:284–287. doi: 10.1192/bjp.142.3.284. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Bloom R, Bokhoven P, Paul-Odouard R, Haznedar MM, Dahlman KL, Hazlett EA, Aronowitz J, Heath D, Shihabuddin L. Neuropsychological functioning in first-break, never-medicated adolescents with psychosis. The Journal of Nervous and Mental Disease. 2004;192:615–622. doi: 10.1097/01.nmd.0000138229.29157.3e. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nuechterlein KH, Haier RJ, Wu J, Sicotte N, Hazlett E, Asarnow R, Potkin S, Guich S. Glucose metabolic rate in normals and schizophrenics during the Continuous Performance Test assessed by positron emission tomography. The British Journal of Psychiatry. 1990;156:216–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, Ahmed S, Tang C. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Annals of General Psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological Medicine. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Collinson SL, Gan SC, Woon PS, Kuswanto C, Sum MY, Yang GL, Lui JM, Sitoh YY, Nowinski WL, Sim K. Corpus callosum morphology in first-episode and chronic schizophrenia: combined magnetic resonance and diffusion tensor imaging study of Chinese Singaporean patients. The British Journal of Psychiatry. 2014;204:55–60. doi: 10.1192/bjp.bp.113.127886. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as a transcallosal misconnection syndrome. Schizophrenia Research. 1998;30:111–114. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research. 2010;181:193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Jr, Buchsbaum MS, Wei T, Spiegel-Cohen J, Hazlett EA, Haznedar MM, Silverman J, Siever LJ. Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophrenia Research. 2000;42:193–208. doi: 10.1016/s0920-9964(99)00123-1. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Nathan PJ, Bullmore ET, Zaman R, Dudas RB, Agius M, Fernandez-Egea E, Muller U, Dodds CM, Forde NJ, Scanlon C, Leemans A, McDonald C, Cannon DM. Distribution of tract deficits in schizophrenia. BMC Psychiatry. 2014;14:99. doi: 10.1186/1471-244X-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Mohr B, Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neuroscience Letters. 2002;320:57–60. doi: 10.1016/s0304-3940(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Federspiel A, Begre S, Kiefer C, Schroth G, Strik WK, Dierks T. Alterations of white matter connectivity in first episode schizophrenia. Neurobiology of Disease. 2006;22:702–709. doi: 10.1016/j.nbd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. The American Journal of Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, Sasamoto A, Fujiwara H, Aso T, Fukuyama H, Murai T. Impaired empathic abilities and reduced white matter integrity in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:117–123. doi: 10.1016/j.pnpbp.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B, Sacchetti E. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophrenia Research. 2009;108:41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Wintz P. Digital Image Processing. Addison-Wesley; Boston, MA: 1987. [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, Jimenez EA, Buchsbaum BR, Troyer BT. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Jeu LA, Nenadic I, Fleischman MB, Shihabuddin L, Haznedar MM, Harvey PD. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophrenia Research. 2000;43:33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- Henze R, Brunner R, Thiemann U, Parzer P, Klein J, Resch F, Stieltjes B. White matter alterations in the corpus callosum of adolescents with first-admission schizophrenia. Neuroscience Letters. 2012;513:178–182. doi: 10.1016/j.neulet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hollis C. Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. The American Journal of Psychiatry. 2000;157:1652–1659. doi: 10.1176/appi.ajp.157.10.1652. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-episode schizophrenia. Psychological Medicine. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219:101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA, Nugent K, McMahon RP, Carpenter WT, Muellerklein F, Sampath H, Hong LE. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Research. 2014;223:148–156. doi: 10.1016/j.pscychresns.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Ouyang X, Tao H, Liu H, Li L, Zhao J, Xue Z, Wang F, Jiang S, Shan B, Liu Z. Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. Journal of Psychiatry and Neuroscience. 2011;36:120–125. doi: 10.1503/jpn.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M, Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Current Opinion in Psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biological Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage. 2011;54:2557–2562. doi: 10.1016/j.neuroimage.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophrenia Research. 2005a;75:265–281. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005b;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann’s areas of the left hemisphere in schizophrenia. The American Journal of Psychiatry. 2005c;162:1733–1735. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. White matter fractional anisotropy and outcome in schizophrenia. Schizophrenia Research. 2006 doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Nikiforova YK, Canfield EL, Hazlett EA, Brickman AM, Shihabuddin L, Buchsbaum MS. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophrenia Research. 2009;114:144–153. doi: 10.1016/j.schres.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman S, Shihabuddin L, Brickman A, Hazlett E, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann areas of the cortex in patients with schizophrenia with good and poor outcomes. The American Journal of Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophrenia Research. 2005d;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mohr B, Pulvermuller F, Cohen R, Rockstroh B. Interhemispheric cooperation during word processing: evidence for callosal transfer dysfunction in schizophrenic patients. Schizophrenia Research. 2000;46:231–239. doi: 10.1016/s0920-9964(00)00020-7. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Andreasen NC, Coffman JA, Olson SC, Dunn VD, Ehrhardt JC, Chapman SM. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biological Psychiatry. 1986;21:274–282. doi: 10.1016/0006-3223(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, McGuire PK, Roiz-Santianez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010;49:199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Woodruff PW, David AS. Stroop interference and facilitation in the cerebral hemispheres in schizophrenia. Schizophrenia Research. 1996;20:57–68. doi: 10.1016/0920-9964(95)00088-7. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35:458–466. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. The Journal of Comparative Neurology. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Bigelow LB. Quantitative brain measurements in chronic schizophrenia. The British Journal of Psychiatry. 1972;121:259–264. doi: 10.1192/bjp.121.3.259. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Rushe TM, O’Neill FA, Mulholland C. Language and crossed finger localization in patients with schizophrenia. Journal of the International Neuropsychological Society. 2007;13:893–897. doi: 10.1017/S1355617707071123. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Landin-Romero R, Fakra E, Delaveau P, Amann BL, Blin O. Structural abnormalities in schizophrenia: further evidence on the key role of the anterior cingulate cortex. Neuropsychobiology. 2014;69:52–58. doi: 10.1159/000356972. [DOI] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Canfield EL, Tang C, Aronowitz J, Paul-Odouard R, Hof PR. Age and diffusion tensor anisotropy in adolescent and adult patients with schizophrenia. Neuroimage. 2009;45:662–671. doi: 10.1016/j.neuroimage.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Tang C, Aronowitz J, Paul-Odouard R, Byne W, Hof PR. Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology. 2007;55:96–111. doi: 10.1159/000104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A, Stegagno L. Failure of language lateralization in schizophrenia patients: an ERP study on early linguistic components. Journal of Psychiatry and Neuroscience. 2008;33:235–243. [PMC free article] [PubMed] [Google Scholar]
- StatSoft, Inc. STATISTICA. 12 2013. [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain : a journal of neurology. 2010;133:1494–1504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Translational Psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first-episode and chronic illness. The British Journal of Psychiatry. 2008a;192:429–434. doi: 10.1192/bjp.bp.107.041251. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophrenia Research. 2008b;103:1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C. White matter ‘potholes’ in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry Research. 2009;174:110–115. doi: 10.1016/j.pscychresns.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.