Abstract
A novel domain, GATE (Glycine-loop And Transducer Element), is identified in the ABC protein DrrA. This domain shows sequence and structural conservation among close homologs of DrrA as well as distantly-related ABC proteins. Among the highly conserved residues in this domain are three glycines, G215, G221 and G231, of which G215 was found to be critical for stable expression of the DrrAB complex. Other conserved residues, including E201, G221, K227 and G231, were found to be critical for the catalytic and transport functions of the DrrAB transporter. Structural analysis of both the previously published crystal structure of the DrrA homolog MalK and the modeled structure of DrrA showed that G215 makes close contacts with residues in and around the Walker A motif, suggesting that these interactions may be critical for maintaining the integrity of the ATP binding pocket as well as the complex. It is also shown that G215A or K227R mutation diminishes some of the atomic interactions essential for ATP catalysis and overall transport function. Therefore, based on both the biochemical and structural analyses, it is proposed that the GATE domain, located outside of the previously identified ATP binding and hydrolysis motifs, is an additional element involved in ATP catalysis.
Keywords: Drug resistance, ABC transporter, DrrAB, Doxorubicin efflux, Nucleotide binding domain, C-terminal domain
INTRODUCTION
ATP-binding cassette (ABC) superfamily of proteins play pivotal roles in multiple biological processes, including transport of various molecules and drugs [1]. ABC proteins typically consist of two nucleotide binding domains (NBDs) and two transmembrane domains (TMDs) which are either found on separate subunits or within the same polypeptide [2]. This investigation focuses on the bacterial ABC transporter DrrAB that carries out efflux of the anticancer antibiotics doxorubicin (Dox) and daunorubicin (Dnr) in the producer organism Streptomyces peucetius [3]. This system belongs to the DRA family of ABC proteins to which eukaryotic proteins of the ABCA sub-family also belong [4]. In this system, DrrA (containing the NBD) and DrrB (the TMD) together form a tetrameric complex in the membrane [5]. Proper association of the two proteins is essential for both proteins to achieve stability and active conformation and therefore the overall function of the transporter complex [5, 6].
ABC proteins typically consist of a 200 amino acid-long ABC cassette normally located within the N-terminal domain (NTD) of the NBD. It contains all the conserved motifs required for ATP binding and hydrolysis, including Walker A, Q-loop, Signature motif, Walker B, and the Switch motif [7] [8, 9] (Fig. 1A). While the function of the ABC cassette has been the subject of intense investigation, the role of the C-terminal domain (CTD) of the NBD has remained largely unexplored. This is possibly due to the fact that the sequence of CTD is highly variable except in closely related ABC proteins. Recent studies have, however, shown that this additional sequence (when present) at the C-terminus of the NBD may be associated with specialized functions [10, 11]. The crystal structures of many of these ABC proteins reveal that despite the diversity present in their amino acid sequence, the CTDs contain a common β-sheet fold indicating that this structure may be critical for the function [10, 11, 12]. Previously developed DrrA homology model using MalK structure as the template showed that the CTD of DrrA also contains a β-sheet-rich structure similar to the one seen in other ABC proteins [13]. Within the CTD of DrrA we identified three novel motifs/domains [13]. Two of these motifs, DEF (previously referred to as LDEVFL, [13]) and CREEM, present in the extreme C terminus of DrrA, are conserved among close prokaryotic and eukaryotic homologs belonging to the DRA family of ABC proteins and were previously shown to be critical for catalytic function and assembly of the DrrAB transporter [13].
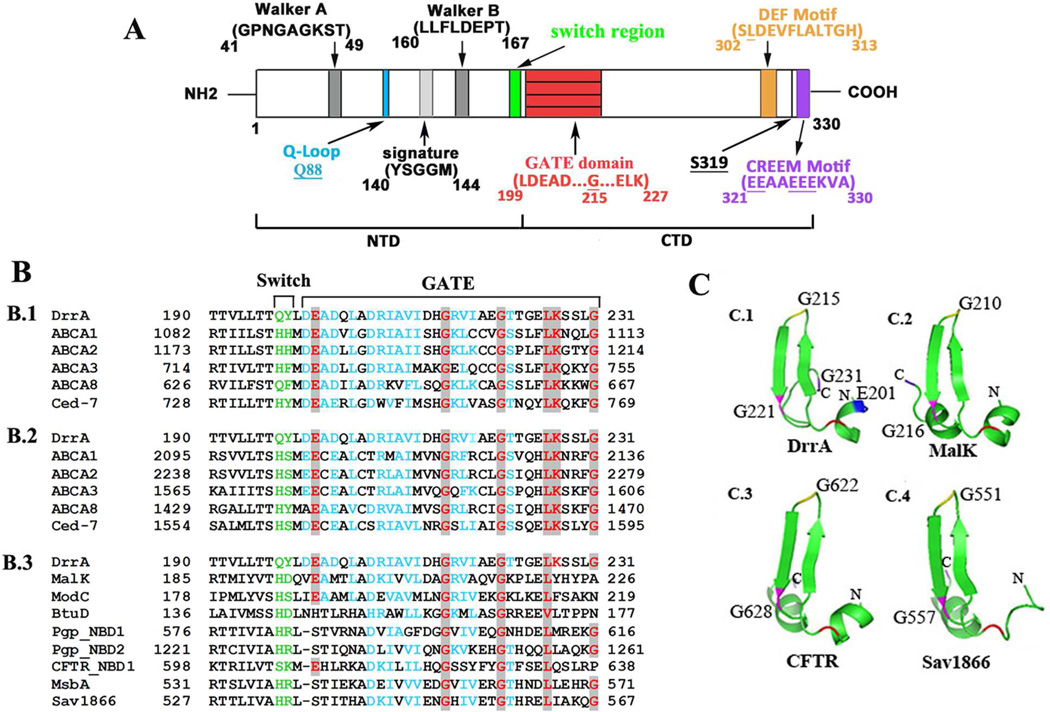
Fig. 1.
Sequence and structure analysis of the GATE domain. A, schematic representation of the conserved domains in DrrA. Numbers indicate the location of specific amino acid residues. B, amino acid alignment of GATE with homologs of the ABC superfamily. B.1 and B.2, alignment of GATE with the corresponding region of NBD 1 and 2 of close homologs of the DRA family. B.3, alignment of GATE with diverse ABC proteins. Switch motif, green; highly conversed residues, red; similar residues, blue. C, tertiary structure of GATE domain. C.1, structure of the GATE domain derived from an established model of DrrA [13]. Key residues E201, G215, G221, and G231 are marked in blue, yellow, pink and purple, respectively. Equivalent residues in the structures of ABC homologs (C.2, C.3 and C.4) have the same labels. The PDB accession numbers and source of each protein are provided in supplementary data.
This study focuses on the third conserved domain, GATE (Glycine-loop And Transducer Element) (previously described as LDEAD, [13]) whose function remains completely unknown. This 33 amino acid region (residues 199–231) is located immediately downstream of the Switch motif and shows high sequence and structural conservation among both close and distant homologs from ABC superfamily. Based on the biochemical and structural analyses shown in this article, we propose that the GATE domain is an additional element that plays a critical role in the catalytic function of the DrrAB complex.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Antibodies
The bacterial strains used in this study include E. coli TG1, N43, LE392ΔuncIC, CC118, and XL1-Blue. The plasmids used in this study were constructed previously in this lab and include pDx101 (drrAB in pSU2718, [5]), pLA330 (drrA::lacZ fusion in pMLB1069, pLAB15 (drrA and the first 45 base pairs of drrB::lacZ fusion in pMLB1069), and pLAB283 (full length drrA-drrB::lacZ fusion in pMLB1069 [5] [14], and the resulted fusion proteins were LA330, LAB15 and LAB283, respectively. For Western blot analysis, rabbit polyclonal antibodies against DrrA and DrrB proteins were used [15]. Chloramphenicol was added to 20 µg/ml for all pDX101-containing cell cultures and ampicillin to 75 µg/ml for all pMLB1069-containing cultures.
Site-directed mutagenesis
Mutations were introduced using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), as described [13]. Plasmids pDX101, pLA330, pLAB15 or pLAB283 were used as templates to generate indicated mutations in GATE domain of DrrA.
Preparation of Inside-Out vesicles (IOVs)
E. coli cells containing indicated plasmids were grown in LB at 37 °C until mid-log phase and induced with 0.25 mM IPTG for 3 h. The cell pellet was resuspended in 1xPBS buffer, pH 7.4, and lysed with French press at 16,000 p.s.i. twice. The membrane fraction was prepared according to the previously published protocol [5] and washed twice with 20 ml of PBS buffer.
Dox Efflux Assay
Dox efflux was analyzed in IOVs prepared from LE392ΔuncIC cells, as described previously [16]. Briefly, 250 µg of IOVs were resuspended in 3 ml of 1× PBS buffer, pH 7.4, with 0.1 mg/ml creatine kinase and 5 mM creatine phosphate. Dox was added to a final concentration of 1.0 µM. The fluorescence spectra were recorded on an Alphascan-2 spectrofluorometer (excitation, 480 nm and emission, 590 nm). After 100 seconds, 1 mM Mg2+ and 1 mM ATP (pH 7.5) were added to start the reaction and detection continued for additional 400 seconds. The rate of transport was determined from the slope of the initial linear range between 100 and 200 seconds.
ATP Binding Assay
E. coli TG1 cells were grown to mid-log phase, induced with IPTG, and membrane fractions prepared as described above. UV-induced photolabeling of DrrA in the membrane fraction was carried out with [a-32P] ATP and 35 µM Dox [7, 15]. The labelled proteins were analyzed by 12% SDS-PAGE and transferred to nitrocellulose membrane. After autoradiography, the same membrane was examined by Western blot using anti-DrrA antibody. ATP-binding was normalized by calculating the ratio of ATP bound/amount of DrrA in each sample. The binding efficiency was calculated as mutant/WT.
ATPase activity assay
7.5 µg IOVs prepared from LE392ΔuncIC cells were incubated in 1 ml reaction containing 50 mM MOPS, pH 7.5, 1 mM dithiothreitol, 10 µL PK/LDH enzyme (Sigma), 5 mM ATP, 0.25 mM NADH, and 1.25 mM phosphor(enol)pyruvic acid at 37 °C for 10 min [16]. The reaction was started by the addition of 2.5mM MgCl2. The optical density at 340 nm was monitored for 10 min using the Shimadzu UV1601 spectrophotometer. The slope of the linear portion of each curve (between 200 and 400 s) was used to calculate ATPase activity. The relative ATPase activity was calculated as activity of mutant/WT.
Modeling analysis
The crystal structure of MalK (PDB: 1Q12 [9]) was used as a template for the modeling analysis of DrrA. The template and restraints for ATP were generated from the structure. The charges for amino acid were generated by using the method of moments atomic charge calculation implemented in AMMP before energy minimization [17, 18]. Structural models of GATE mutants were constructed using the same strategy but target residues were first mutated in the wild type sequence using COOT [19]. PyMOL Molecular Graphics System (http://www.pymol.org/) and Swiss-PdbViewer ([20], http://spdbv.vital-it.ch/) were used to measure the atomic distances and present protein structures.
RESULTS and DISCUSSION
Identification of a conserved domain ‘GATE’
A schematic of DrrA showing the 198 amino acid-long NTD [7] and a 132 amino acid CTD (residues 199–330) is presented in Fig. 1A. In this study, we analyzed a previously uncharacterized domain GATE which is located in a region immediately following the Switch motif (Fig. 1A). This domain is highly conserved not only among close DrrA homologs belonging to the DRA family of ABC proteins (Fig. 1B.1 and 1B.2) but also in diverse ABC proteins, albeit with varying degrees of similarity (Fig.1B.3). Homology in the GATE region extends across the entire length of the 33 residues with E201, G215, G221, L226, K227, and G231 being the most highly conserved among DRA family (Fig 1B, marked in red). Strikingly, three of these conserved residues are glycines (Figs. 1B.1 and 1B.2). Two of these glycines, G215 and G221, are also highly conserved among distant homologs (Fig 1B.3). Although no member of the DRA family has been crystallized so far, the crystal structures of several ABC proteins, including MalK, ModC, Pgp, and Sav1866 are available [9, 10, 21, 22]. A comparison of the different crystal structures interestingly shows a high structural conservation in the region of the GATE domain. Specifically, we found that this region contains two β-sheets sandwiched by an α-helix or a loop on either side (Fig. 1C), which is also seen in the homology model of DrrA [13] (Fig. 1C.1). The highly conserved residue G215 (or the corresponding glycine) is present in the turn region between the two β-sheets in each structure which has been termed the ‘Gly-loop’ (G-loop) in this article.
Highly conserved glycine G215 found in GATE is critical for DrrAB stability
To further understand its function, site-directed mutagenesis of conserved residues in GATE was carried out. Western blot analysis of the membrane fractions generated from these mutants showed that the expression of DrrA and DrrB was affected to varying degrees (Fig. S1). The most striking phenotype was observed with mutations in residue G215 which resulted in complete abolishment of DrrB expression (Figs. S1A and 1B, lanes 6–8). Translation of the drrA or drrAB genes was however unaffected by G215A mutation as determined by β-galactosidase activity of different fusions carrying the G215A allele (Fig S1.C). Therefore, a post-translational effect must be responsible for the observed drastic effect of G215A on DrrA and DrrB stability. Effect of G221A/S and G231A/S mutations on expression of DrrAB was also studied. Both G221A and G221S mutants exhibited lower levels (30–45%) of DrrA and DrrB expression (Fig. S1A, lanes 9–10). However, G231 could be mutated to Alanine or Serine without affecting expression (Fig. S1A, lanes 12–13), indicating that it is not involved in stability but it may play a specific role in function, as shown later. Because of their limited or no expression, G215A/S/P, and G221A were not chosen for further biochemical analysis. G215A was however used for structural analysis, as shown later.
Role of GATE in DrrAB function
Effect of mutations in the conserved GATE residues on Dox resistance and efflux was determined, as described under Materials and Methods. Dox resistance assays showed varying levels of sensitivity to Dox in E. coli cells containing different mutations (Table S1). Dox efflux analysis showed that E201D, G221S, K227R, and G231A/S mutations produce severe reduction in the efficiency of Dox efflux (Fig. 2A), which was found to be comparable to Dox efflux in E165Q mutation [7] that served as a negative control in these assays. These results are largely consistent with the results of the Dox resistance assay, therefore suggesting that these four conserved residues are critical for DrrAB function. Surprisingly, L205V and L226V mutations showed higher Dox efflux efficiency than wild type DrrAB, which is addressed later.
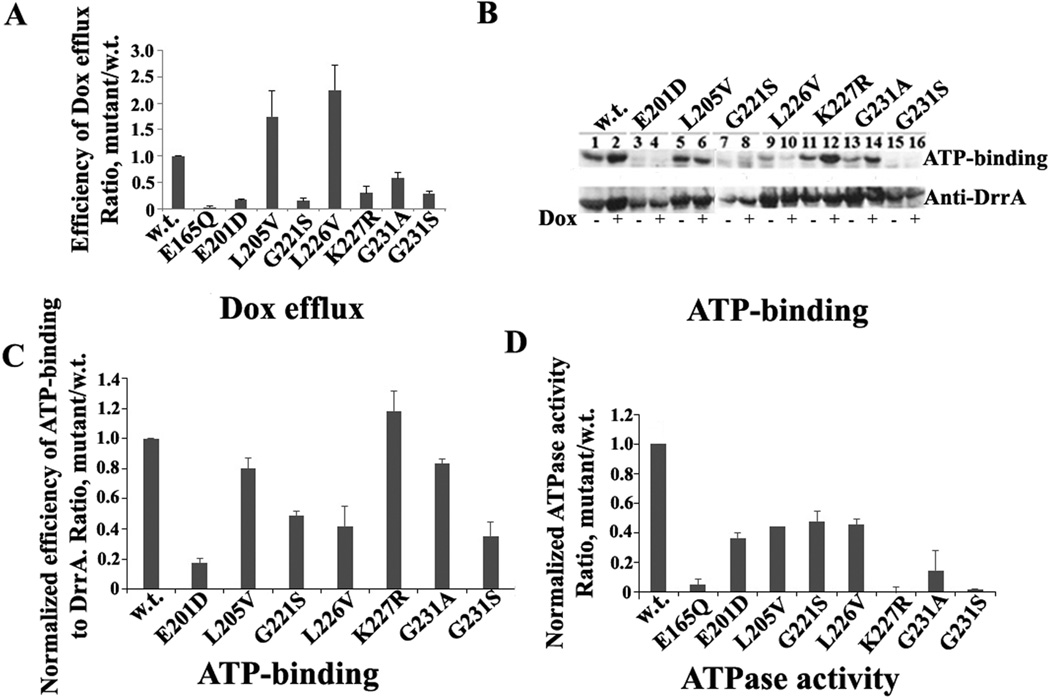
Fig. 2.
Functional analysis of GATE mutants. A, effect of point mutations in GATE on Dox efflux by DrrAB. The background efflux obtained with empty vector was subtracted from WT and mutants. Efflux efficiency was calculated as the mutant slope/WT slope within one set, designating WT efficiency as 1. B, effect of GATE mutations on ATP-binding to DrrA. Top, a representative autoradiogram showing ATP binding to cell membranes containing WT DrrAB and GATE mutants. Bottom, Western blot analysis of samples from above. C, histogram showing ATP binding efficiency to GATE mutants. D, histogram showing the effect of GATE mutations on ATP hydrolysis. Data in Fig. 2A, C, and D represent an average of three independent experiments with error bars showing standard deviation.
To determine if reduction of Dox efflux by the GATE mutants is due to affected ATP-binding, UV-induced [α-32P] ATP binding to DrrA was analyzed (Fig. 2B). E201D and G221S showed reduced ATP binding efficiency (15% and 45%, respectively), again indicating their importance in function. Of special interest however were the mutants that showed normal expression of DrrA and DrrB (Fig. S1) but drastically diminished Dox efflux, for example, K227R, G231A and G231S (Fig. S1 and Fig 2A). G231S mutant showed significantly reduced ATP binding (30%) (Fig. 2C), which may explain the reduced efficiency of Dox efflux by this mutant. Surprisingly, however, both K227R and G231A retained between 90–100% ATP binding efficiency (Fig. 2C), indicating that the function of K227R and G231A may be compromised in a later stage of catalysis, such as hydrolysis of ATP or signal transduction between DrrA and DrrB. The data in Fig. 2D show that the ability of the K227R mutant to hydrolyze ATP is indeed drastically affected despite wild type levels of ATP binding. Interestingly, ATP hydrolysis by K227R was found to be comparable to E165Q mutation [7, 16]. Note that the residue E165 in DrrA corresponds to the well-characterized catalytic base present near the Walker B motif, which is critical for hydrolysis of ATP by ABC proteins [1, 8, 16, 23]. Therefore, a similar phenotype of the K227R and E165Q mutants highlights the importance of the GATE residues in catalysis. Finally, G231A and G231S mutations also showed drastic effect on ATP hydrolysis by DrrAB. Once again, the effect of G231S was more severe than G231A (Fig. 2D), which is in agreement with both ATP binding and Dox efflux data. Since the serine substitution of G231 is expected to make the structure of the region more rigid than alanine, these data indicate that the flexibility conferred by these glycines is critical for function, as shown for glycines found in the ‘turn’ and ‘bend’ regions of other proteins [24]. Interestingly, both L205V and L226V mutations exhibited only about 50% ATP hydrolysis activity (Fig. 2D) although they show higher than wild-type levels of Dox efflux (Fig. 2A). The implication of these findings is not clear, however it may be suggested that the substitution of Leu to Val in these locations may allow better communication between DrrA and DrrB than seen in wild type DrrAB thus resulting in more efficient Dox efflux. Overall, based on the analyses described so far, we conclude that several highly conserved residues in GATE are critical for the integrity and catalytic function of the DrrAB complex.
Structural analysis of GATE
To gain further insights into the mechanism of GATE, atomic analysis of GATE domain was carried out. Since the crystal structure of the DrrA protein is so far not available, the ATP-bound (PDB access: 1Q12) and ATP-free (PDB access: 1Q1E) forms of E. coli MalK [9] were therefore used for analyzing the GATE domain. Our analysis showed that in the ATP-bound conformation of MalK, residue G210 (corresponding to G215 in DrrA, Fig. 1B) lies in close proximity to the Walker A motif within the same chain of MalK (Fig. 3A), and a direct hydrogen bond of the N-H...O type with a distance of 2.9 Å is formed between NH group of G210 and carboxylate oxygen of C40 (Fig. 3A). Notably, the CA (main chain carbon atom) of G210 also forms two unconventional C-H...O interactions with C40 and V18 at the distance of 3.4 Å and 3.3 Å, respectively (Fig. 3A). Both C40 and V18 in MalK were previously shown to make direct contacts with ATP bound to the interface of the MalK dimer [9]. Similar atomic bonding between G210 and the Walker A residues was also observed in the open state of MalK (Fig. 3B), suggesting that the interactions between these residues are maintained in the open and the closed states and probably during the entire catalytic process.
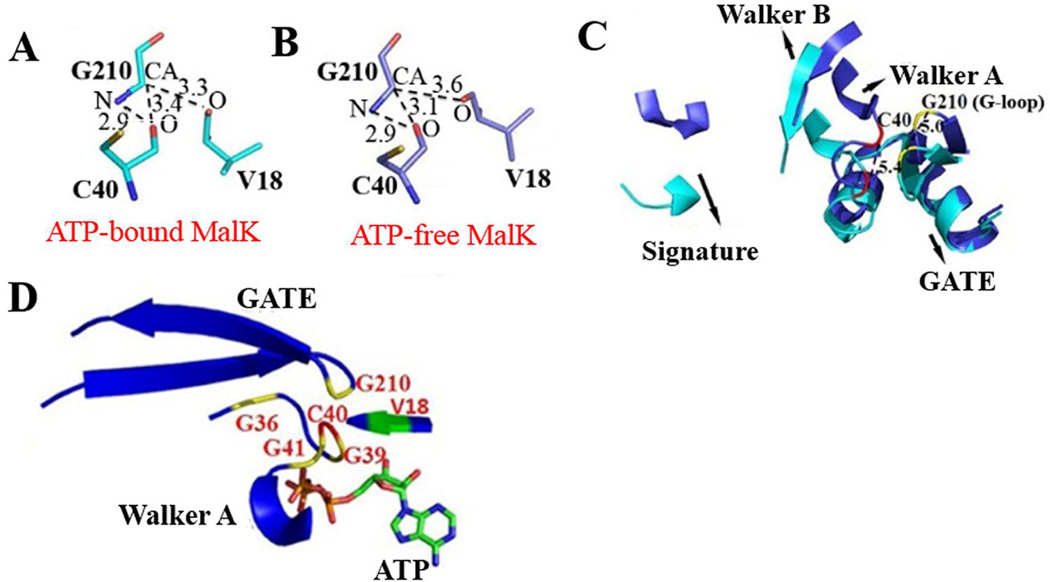
Fig. 3.
Structural analysis of MalK. A and B, hydrogen bond interactions around residue G210 in the crystal structure of the ATP-bound (1Q12, panel A) and ATP-free (1Q1E, panel B) states of MalK [9]. C, superposition of ATP-bound (in cyan) and ATP-free (in slate) forms of MalK. The NBD motifs and GATE domain are shown. Residue C40, red; G210, yellow. D, ATP-bound MalK (1Q12) showing highly conserved glycines from both Walker A and GATE. O, oxygen; N, nitrogen; CA, main chain carbon. Numbers by dashed lines represent the distances between two atoms in Å.
Superimposition of the open and closed states of MalK, interestingly, showed that a change from the open (shown in slate, Fig. 3C) to the closed state (cyan) results in a movement of C40 by 5.4 Å (residue in red, distance was measured between the main chain carbon (CA) atoms) and of V18 by 6.9 Å (not shown). Interestingly, a comparable and parallel movement of G210 in the G-loop (5.0 Å, residue in yellow) was also observed. Conformational changes are also observed in other important motifs of the NBD including the G-loop region and the two surrounding β-sheets (Fig. 3C). No significant changes were observed in other regions between the open and closed structures (Fig. 3C). These observations imply that ATP binding not only produces general conformational changes in the regions involved in ATP-binding, but also in the G-loop of the GATE domain. Interestingly, the Walker A motif itself contains three highly conserved glycines (consensus sequence GxxGxGKS/T; x, any residue), which make hydrogen bonds with β-phosphate of the ATP molecule [9] and affect ATP-binding ability and/or folding of transporters [7, 25]. When viewed together, three glycines of Walker A and G210 in the Gloop lie in close proximity to each other (ranging from 5.4 to 9.4 Å) (Fig. 3D). Therefore it may be reasonable to propose that the conserved G210 in G-loop and the three conserved glycines of Walker A are all important for constituting the ATP binding pocket. On the other hand, a structural explanation for the role of G221 in function of DrrA could not be determined as the corresponding residue G216 in MalK did not show any significant interactions. Moreover, residues G231 and K227 of DrrA are only conserved in the GATE domain of the close DRA family members for which crystal structures are so far not available, therefore their interacting partners could not be identified in the MalK structure.
To determine if the structural features of MalK also apply to the DrrA model, a homology model of ATP-bound DrrA dimer was generated. This model generally supported the findings from the MalK structure in terms of the proximity and interactions between G215 and residues in Walker A. We found that G215 (G210 in MalK) lies in close proximity to the Walker A residues A45 (corresponding to C40 in MalK, [7]) and A23 (corresponding to V18 in MalK) and forms C-H...O interactions with A45 and A23 (Fig. 4A). Moreover, the three conserved glycines (G41, G44 and G46) in the Walker A motif and G215 in the G-loop of GATE were also found to have the same arrangement (Fig. 4E) as seen in MalK (Fig. 3D). Molecular modeling analysis can also allow a comparison of the structures of wild type DrrA and mutants, therefore two of the most interesting mutations uncovered in this study, G215A and K227R, were introduced into DrrA dimer and were analyzed for atomic interactions. Interestingly, G215A mutation resulted in loss of hydrogen bonding between G215 and A45 as well as A23 (Fig. 4B), suggesting that the space around residue 215 can only accommodate glycine but not alanine. Therefore, we propose that the drastic effect of G215 mutations on stability of the DrrAB complex may occur indirectly through the effect of these mutations on the integrity of the ATP-binding pocket.
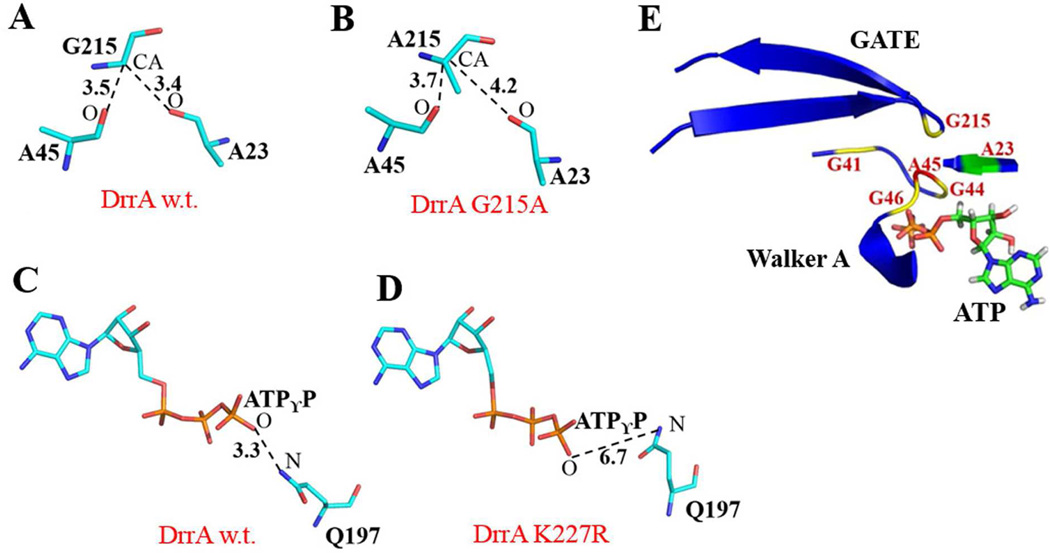
Fig. 4.
Modeling analysis of DrrA. A and B, WT DrrA and G215A allele showing hydrogen bond interactions between G/A215-A23 and G/A215-A45. C and D, WT DrrA and K227R showing the presence or absence of a hydrogen bond between residue Q197 and ATPγP. E, ATP-bound DrrA showing highly conserved glycines from both Walker A and GATE.
Structure modeling of the K227R allele of DrrA showed that this mutation prevents one essential hydrogen bond (required for ATP hydrolysis, [1, 9]) present in wild type DrrA between the Switch residue Q197 and the γ-phosphate of ATP (ATPγP) (Fig. 4C and 4D). In most ABC proteins the Switch motif contains a highly conserved histidine, which is critical for hydrolysis of ATP [1, 9, 23]. In DrrA, this histidine is naturally substituted with a glutamine at position 197 (Fig. 1B). Mutation of this glutamine to histidine completely abolished the ATPase activity and Dox efflux functions of the DrrAB complex [16]. We also showed that the Switch residues Q197Y198 are together critical for signal transduction between DrrA and DrrB [16], indicating the critical role of the Switch region to the functionality and conformation of DrrAB. In this study, we found that the K227R allele in GATE affects the distance of Q197 from γ-ATP and produces a similar effect on the ATPase and Dox efflux activities as seen with Q197H [16] or E165Q (Fig. 2), two critical residues believed to form a catalytic dyad in ABC proteins [16]. Thus it may be concluded that the GATE domain either participates directly in catalysis or regulates the catalytic function of DrrA via its effects on the conserved motifs of the NBD. Future studies will investigate the interaction of the GATE domain with other conserved motifs as well as its participation in the molecular mechanism of energy transduction.
Supplementary Material
Highlights.
A novel domain ‘GATE’ is identified in the ABC protein DrrA
GATE shows high sequence and structural conservation among diverse ABC proteins
GATE is located outside of the previously studied ATP binding and hydrolysis motifs
Conserved GATE residues are critical for stability of DrrAB and for ATP catalysis
Acknowledgements
This research was funded in part by the National institutes of Health grant RO1 GM51981-09 and by the Molecular Basis of Disease Program of Georgia State University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges-Walmsley MI, McKeegan KS, Walmsley AR. Structure and function of efflux pumps that confer resistance to drugs. Biochem J. 2003;376:313–338. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilfoile PG, Hutchinson CR. A bacterial analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci U S A. 1991;88:8553–8557. doi: 10.1073/pnas.88.19.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland IB. ABC proteins : from bacteria to man. London ; San Diego, Calif.: Academic Press; 2003. [Google Scholar]
- 5.Kaur P, Russell J. Biochemical coupling between the DrrA and DrrB proteins of the doxorubicin efflux pump of Streptomyces peucetius. J Biol Chem. 1998;273:17933–17939. doi: 10.1074/jbc.273.28.17933. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan P, Li W, Kaur P. Translational coupling controls expression and function of the DrrAB drug efflux pump. J Mol Biol. 2009;385:831–842. doi: 10.1016/j.jmb.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Rao DK, Kaur P. The Q-Loop of DrrA Is Involved in Producing the Closed Conformation of the Nucleotide Binding Domains and in Transduction of Conformational Changes between DrrA and DrrB. Biochemistry. 2008;47:3038–3050. doi: 10.1021/bi701699a. [DOI] [PubMed] [Google Scholar]
- 8.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Gerber S, Comellas-Bigler M, Goetz BA, Locher KP. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. [DOI] [PubMed] [Google Scholar]
- 11.Kadaba NS, Kaiser JT, Johnson E, Lee A, Rees DC. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science. 2008;321:250–253. doi: 10.1126/science.1157987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbertson L, Kimber MS, Whitfield C. Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proc Natl Acad Sci U S A. 2007;104:19529–19534. doi: 10.1073/pnas.0705709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Pradhan P, Kaur P. The extreme C terminus of the ABC protein DrrA contains unique motifs involved in function and assembly of the DrrAB complex. J Biol Chem. 2010;285:38324–38336. doi: 10.1074/jbc.M110.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon KA, Hsu DK, Brusilow WS. Use of lacZ fusions to measure in vivo expression of the first three genes of the Escherichia coli unc operon. J Bacteriol. 1989;171:3039–3045. doi: 10.1128/jb.171.6.3039-3045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur P. Expression and characterization of DrrA and DrrB proteins of Streptomyces peucetius in Escherichia coli: DrrA is an ATP binding protein. J Bacteriol. 1997;179:569–575. doi: 10.1128/jb.179.3.569-575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Sharma M, Kaur P. The DrrAB efflux system of Streptomyces peucetius is a multidrug transporter of broad substrate specificity. J Biol Chem. 2014;289:12633–12646. doi: 10.1074/jbc.M113.536136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang B, Fu G, Agniswamy J, Harrison RW, Weber IT. Caspase-3 binds diverse P4 residues in peptides as revealed by crystallography and structural modeling. Apoptosis. 2009;14:741–752. doi: 10.1007/s10495-009-0333-y. [DOI] [PubMed] [Google Scholar]
- 18.Fu G, Chumanevich AA, Agniswamy J, Fang B, Harrison RW, Weber IT. Structural basis for executioner caspase recognition of P5 position in substrates. Apoptosis. 2008;13:1291–1302. doi: 10.1007/s10495-008-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 21.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 23.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman MD, Bass RB, Mehan RS, Falke JJ. Conserved glycine residues in the cytoplasmic domain of the aspartate receptor play essential roles in kinase coupling and on-off switching. Biochemistry. 2005;44:7687–7695. doi: 10.1021/bi0501479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saveanu L, Daniel S, van Endert PM. Distinct functions of the ATP binding cassettes of transporters associated with antigen processing: a mutational analysis of Walker A and B sequences. J Biol Chem. 2001;276:22107–22113. doi: 10.1074/jbc.M011221200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.