Abstract
Efficient management of misfolded or aggregated proteins in ASH and NASH is crucial for continued hepatic viability. Cellular protein quality control systems play an important role in the pathogenesis and progression of ASH and NASH. In a recent study, elevated Mca1 expression counteracted aggregation and accumulation of misfolded proteins and extended the life span of the yeast Saccharomyces cerevisiae (Hill, et al, 2014). Mca1 may also associate with Ssa1 and Hsp104 in disaggregation and fragmentation of aggregated proteins and their subsequent degradation through the ER-associated degradation (ERAD) pathway. If degradation is not available, protection of the cellular environment from a misfolded protein is accomplished by its sequestration into two distinct inclusion bodies (Kaganovich et al., 2008) called the JUNQ (JUxta Nuclear Quality control compartment) and the IPOD (Insoluble Protein Deposit). Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97, and p62 all play important roles in protein quality control systems. This study aims to measure the expression of Mca1 and related chaperones involved in protein quality control in alcoholic steatohepatitis (ASH), nonalcoholic steatohepatitis (NASH) compared with normal control livers. Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97, and p62 expressions were measured in three to six formalin-fixed paraffin embedded ASH and NASH liver biopsies and control normal liver specimens by immunofluorescence staining and quantified by immunofluorescence intensity. Mca1, Hsp104, Ydj1 and p62 were significantly up regulated compared to control (p<0.05) in ASH specimens. Ssa1, Hsp40 and VCP/p97 levels did not have significant differences with the control specimens. Although not significantly elevated compared to normal control, Hsp40 and VCP/p97 were significantly elevated in ASH compared to NASH (p<0.05). In NASH, the only significant difference was the increased expression of Hsp104 compared to control (p<0.05). The up regulation of Mca1, Hsp104, Ydj1 and p62 in ASH may be elicited as a response to the chronic exposure of the hepatocytes to the toxicity of alcohol. Recruitment of Mca1, Hsp104, Ydj1 and p62 may indicate that autophagy, ERAD, JUNQ, and IPOD systems are active in ASH. Whereas in NASH, only Hsp104 is significantly elevated compared to control. This may indicate that in NASH, IPOD may be the only active protein quality control system.
Keywords: Protein Quality Control, Alcoholic steatohepatitis, Nonalcoholic steatohepatitis, Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97, p62, ERAD, IPOD, JUNQ
INTRODUCTION
Steatohepatitis frequently includes Mallory-Denk Bodies (MDBs), which are an intracellular deposition of misfolded protein in ballooned hepatocytes. Ballooning of hepatocytes is induced by oxidative stress. MDBs are prevalent in various hepatic diseases including hepatitis B and C viral infections, alcoholic steatohepatitis (ASH), non-alcoholic steatohepatitis (NASH), drug injuries and hepatocellular carcinoma (Zatloukal, French et al. 2007; Basaranoglu, Turhan et al. 2011). Pathological lesions in ASH and NASH are similar. Both can progress to more severe forms of the disease including ballooning of hepatocytes, MDB formation, activation of stellate cells leading to hepatic fibrosis, and ultimately, cirrhosis.
Protein quality control systems play a critical role in the pathogenesis and progression of ASH and NASH. The primary function of protein quality control systems is to detect and efficiently manage misfolded or aggregated proteins in a timely manner for continued cellular function and viability. The process involves recognition of the misfolded protein by chaperones and E3 ligases for ubiquitination and subsequent degradation through various mechanisms. For example, metacaspase 1 (Mca1), with the aid of heat shock protein 104 (Hsp104), counteract the aggregation and accumulation of misfolded proteins (Hill, et. al., 2014). p62 is involved in linking polyubiquitinated protein aggregates to the autophagy machinery (Bjørkøy et al, 2005). The Hsp70/Hsp40 chaperone system also plays an essential role in cell autophagy. Ydj1 is required for ubiquitin-dependent degradation of certain abnormal proteins. Additionally, Ydj1 interacts with Ssa1 and facilitates ER-associated degradation (ERAD) (Lee, 1996). VCP/p97 cooperates with diverse partner proteins to help process ubiquitin-labeled misfolded proteins for recycling or degradation by the 26S proteasome (Bug and Meyer, 2012). However, if such protein degradation mechanisms are unavailable, protection of the cellular environment from a misfolded protein is accomplished by its sequestration into two distinct inclusion bodies (Kaganovich et al., 2008): the JUNQ (JUxta Nuclear Quality control compartment) and the IPOD (Insoluble Protein Deposit).
ASH and NASH may have different protein quality control systems. This study aims to compare the levels of Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97 and p62, all involved in various protein quality control systems. We predicted that ASH and NASH utilize different protein quality control systems.
MATERIALS AND METHODS
Sample Selection
Formalin-fixed paraffin embedded ( FFPE) human liver biopsy blocks from patients with confirmed ASH and NASH diagnoses were first identified from Harbor-UCLA hospital archives. Normal liver biopsies were also obtained and were used as control. Six ASH, five NASH, and three normal control liver biopsies were used in this study.
Slide preparation
Tissue slides were then prepared from the FFPE blocks. The slides were double stained for ubiquitin (Millipore, Temecula, CA) and either Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97, or p62. Ubiquitin was detected using the red fluorescent(Texas Red) antibody, donkey anti-mouse Alexa Fluor 594 (Jackson Labs, West Grove, PA), while Mca1, Hsp104, Hsp40, Ydj1, Ssa1, VCP/p97, and p62 were detected using the green fluorescent(FITC) antibody, donkey anti-rabbit Alexa Fluor 488 or donkey anti-mouse Alexa Fluor 488 (Jackson Labs, West Grove, PA). The nuclei were stained with DAPI blue. The double stain was detected using a tricolor filter. All biopsies were stained at one time to allow accurate comparisons between groups.
Microscopy
The fluorescence staining intensity of the proteins were then measured in 3 different areas on each slide. Using a Nikon 400 fluorescent microscope and the Nikon morphometric system, quantitative measurements were taken with 40× objective magnification and a standard exposure time of 800ms. All photos and measurements were made at the same level of UV light intensity. The results were displayed as a graph attached to the immunofluorescent photography using a screen snip.
Statistical analysis
All data are presented as mean with S.D.. Statistical significance was calculated using one-way ANOVA. Tukey’s multiple comparison test was then used to compare significant differences between normal control versus ASH, normal control versus NASH, and ASH versus NASH measurements. A value of p<0.05 denoted statistical significance.
RESULTS AND DISCUSSION
A. ASH
Constant and prolonged oxidative stress, lipid peroxidation, and acetaldehyde toxicity from chronic consumption of alcohol result in inflammasome activation and Mallory-Denk Body (MDB) formation (Peng et al, 2014). MDBs are intracellular deposition of misfolded proteins in ballooned hepatocytes, and they occur in 80% of biopsies (French 1981, French 1981). All of the ASH liver biopsies showed numerous balloon cells with MBD formation (Fig. 8). In response to oxidative stress and increased lipid peroxidation from chronic alcohol intake, heat shock proteins that identify misfolded or unfolded proteins, and target them for degradation, are expressed. Misfolded and aggregated protein degradation may then proceed via several protein quality control mechanisms, including autophagy and the ERAD, the JUNQ and the IPOD systems.
Figure 8.
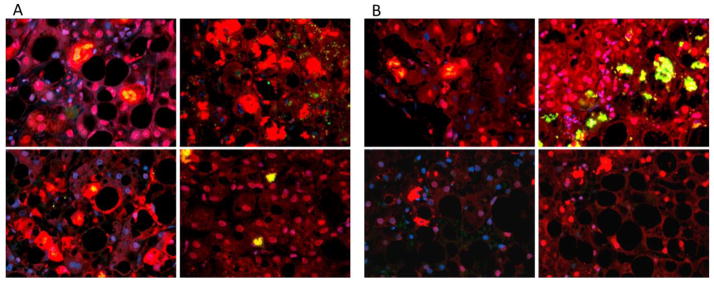
MDBs in ASH(A) and NASH(B). Pictures of the liver biopsies on tricolor filter highlighting the presence of MDBs in both ASH and NASH specimens. MBDs are aggregated proteins and appear as red or bright yellow hyperdense areas.
Levels of Mca1, Hsp104, Ydj1 and p62 were significantly elevated in ASH
Compared to normal control biopsies, ASH showed a significant increase in Mca1, Hsp104, Ydj1 and p62 levels (Fig. 1, Fig. 2, Fig. 4, and Fig. 7). Our data suggests that in ASH, the liver may be actively utilizing autophagy and the ERAD, the JUNQ, and the IPOD systems for protein quality control. Mca1, Hsp104, Ydj1 and p62 have different roles in the systems mentioned.
Figure 1.
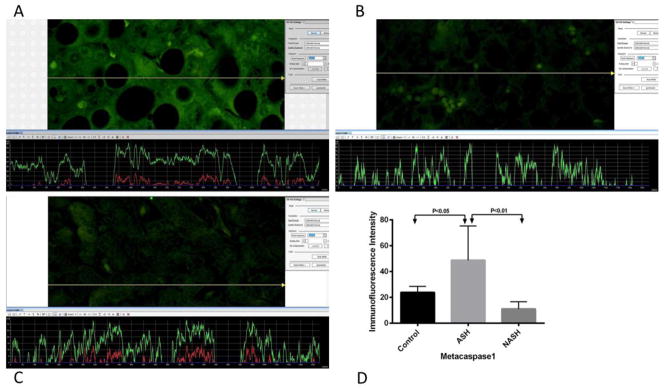
Print screen or screen snip pictures of representative Mca1 measurements from (A) ASH, (B) NASH, and (C) normal control liver samples. The presence of Mca1 was detected by the green immunofluorescence on the liver biopsy picture. A yellow tracer line converted this fluorescence intensity into quantitative figures and represented it as a green line on a graph, shown below each picture. The level of fluorescence intensity is dependent on the amount of Mca1. (A) ASH showed more green fluorescence compared to both (B) NASH, and (C) normal control liver biopsies. More fluorescence corresponded to the increase presence of Mca1. (D) Means with SD of Mca1 in ASH, NASH, and normal control liver. Levels of Mca1 in ASH are significantly elevated (p<0.05) compared to the normal control liver. There is no significant difference in the levels of Mca1 in NASH versus normal control liver. Comparing ASH versus NASH, data showed that Mca1 is significantly elevated in ASH (p<0.01). (×566 magnification)
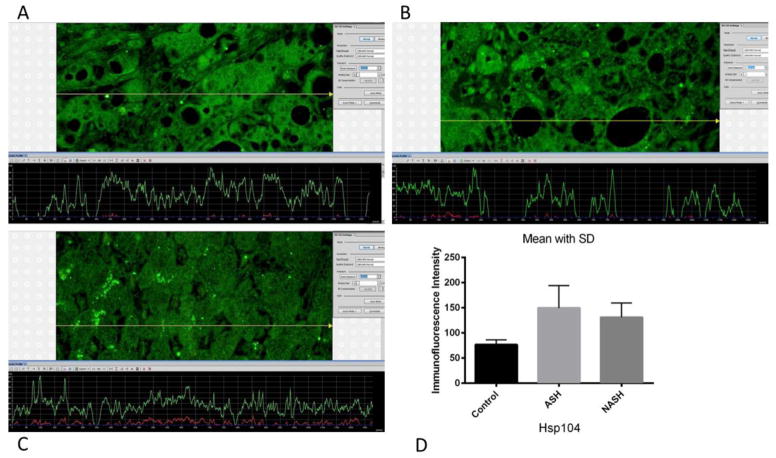
Figure 2.
(A) ASH, (B) NASH and (C) normal control measurements of Hsp104 levels. The presence of Hsp104 was detected by the green immunofluorescence on the liver biopsies. ASH and NASH showed slightly more green fluorescence compared to normal control liver biopsies. More fluorescence corresponded to the increase presence of Hsp104. (D) Means with SD of Hsp104 in ASH, NASH, and normal control liver. Levels of Hsp104 were significantly elevated in ASH (p<0.01) and NASH (p<0.05) compared to normal control. However, ASH and NASH did not show a significant difference from each other in Hsp104 levels. (×566 magnification)
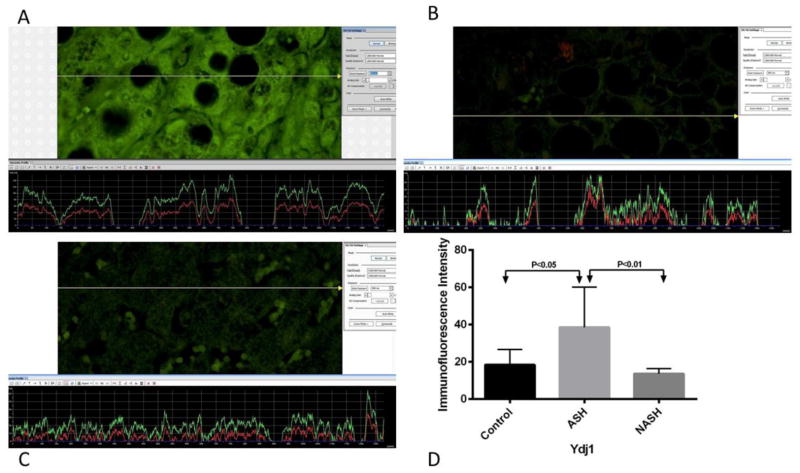
Figure 4.
(A) ASH, (B) NASH and (C) normal control measurements of Ydj1 levels. The presence of Ydj1 was detected by the green immunofluorescence on the liver biopsies. (A) ASH showed brighter green fluorescence compared to (B) NASH and (C) normal control. More fluorescence corresponded to the increase levels of Ydj1. (D) Means with SD of Ydj1 in ASH, NASH, and normal control liver biopsies. Ydj1 was significantly elevated in ASH (p<0.05) compared to normal control liver biopsies. Compared to NASH, Ydj1 was also significantly elevated in ASH (p<0.01). There is no significant difference in the level of Ydj1 between NASH and normal control liver. (×566 magnification)
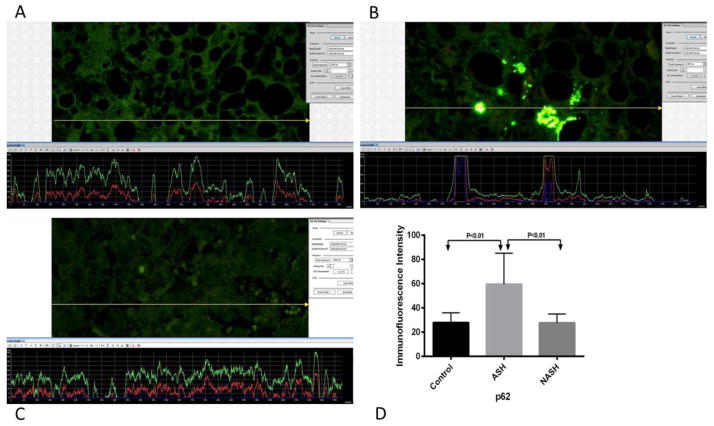
Figure 7.
(A) ASH, (B) NASH and (C) normal control measurements of p62. The presence of p62 was detected by the green immunofluorescence on the liver biopsies. ASH showed more green fluorescence compared to NASH and normal control liver biopsies. More fluorescence corresponded to the increase levels of p62. (D) Means with SD of p62 in ASH, NASH, and normal control liver. Measurements of p62 were significantly elevated in ASH (p<0.01) compared to normal control. Also, compared to NASH, p62 levels were significantly elevated in ASH (p<0.01). There is no difference in p62 levels between NASH and normal control.
Mca1, Hsp104, Ydj1 and p62 are intra-cellular chaperones. They play an important role in protein-protein interactions such as proper protein conformation and prevention of unwanted protein aggregation. p62, which is also named sequestosome1 (SQSTM1), is a common component of protein aggregates that are found in protein aggregation diseases affecting both the brain and the liver. p62 is involved in the mechanism of MDB formation (Nan, et al. 2004). p62 is also involved in linking polyubiquitinated protein aggregates to the autophagy machinery (Bjørkøy et al., 2005) and the protein-degrading complex 26S proteasome (French et al, 1981). Protein inclusions formed by aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by macroautophagy (Kegel et al., 2000; Ravikumar et al., 2002, 2004), which is a bulk degradation pathway in which a double or multimembrane-bound structure called the autophagosome forms to sequester cytoplasm. Subsequently, the autophagosome fuses with the lysosome, and its content and internal membranes are degraded (Levine and Klionsky, 2004; Yoshimori, 2004). Ydj1 is involved in a variety of cellular activities that control polypeptide fate, such as folding and translocation across intracellular membranes. Ydj1 is also required for ubiquitin-dependent degradation of certain abnormal proteins. Ydj1 is a J-domain containing protein that interacts with Ssa1 and facilitates ER-associated degradation (ERAD). ERAD is a cellular pathway which targets misfolded proteins of the endoplasmic reticulum for ubiquitination and subsequent degradation by the 26S proteasome (Ding et al., 2008). Ydj1 may facilitate the recognition of unfolded proteins or may serve as a cofactor for certain ubiquitin-ligating enzymes (Lee et al., 1996). Mca1 may also associate with Ssa1 and Hsp104 in disaggregation and fragmentation of aggregated proteins and their subsequent degradation through ERAD.
Cellular increase of misfolded protein loads, due to constant presence of oxidative stress and alcohol toxicity, may saturate and exhaust the quality control machinery. For example, it has been shown that chronic intragastric tube feeding of alcohol to rats leads to inhibition of the 26S proteasome catalytic activity (Bardag-Gorce, et al., 2005). In these conditions, a second line of active cellular defense is available. Misfolded proteins may be sequestered into two distinct inclusion bodies: the JUNQ and the IPOD. For example, a previous study showed that when there is low expression levels of the proteasome, ubiquitinated misfolded proteins are sorted into the JUNQ (Kaganovich et al., 2008). Delivery of misfolded and aggregated proteins to JUNQ and IPOD require an intact cytoskeleton and specific cellular quality control components, such as heat shock proteins (Specht et al., 2011). During protoestatic stress, Mca1 is recruited to IPOD and JUNQ (Hill, et. al., 2014) to sequester terminally unfolded and aggregated proteins. Mca1 may also be recruited with Hsp104, Ssa1, and Ydj1 for JUNQ sequestration of terminally aggregated proteins. Upon recovery from stress conditions, misfolded proteins that accumulated in the JUNQ are either refolded by the cellular chaperone machinery, or degraded by the 26S proteasome. Therefore, sequestration of a misfolded protein to the JUNQ is reversible.
Elevated Hsp40 and VCP/p97
Although not significantly elevated compared to normal control, Hsp40 and VCP/p97 are significantly elevated in ASH compared to NASH (Fig. 3, Fig. 6). Our data suggests that these two chaperones are also involved in the complex protein quality control machinery in ASH, albeit at low levels. Hsp40, also known as Chaperone DnaJ, is a molecular chaperone protein. The J domain of Hsp40 interacts with Hsp70 heat-shock proteins (Hennessy et al., 2005). Hsp40 heat-shock proteins play a role in regulating the ATPase activity of Hsp70 heat-shock proteins (Fan et al., 2003; Ohtsuka & Hata, 2000). Besides its J-domain stimulating the ATPase activity of the DnaK component of Hsp70, Hsp40 also associates with unfolded polypeptide chains and prevents their aggregation (Christen & Han, 2004).
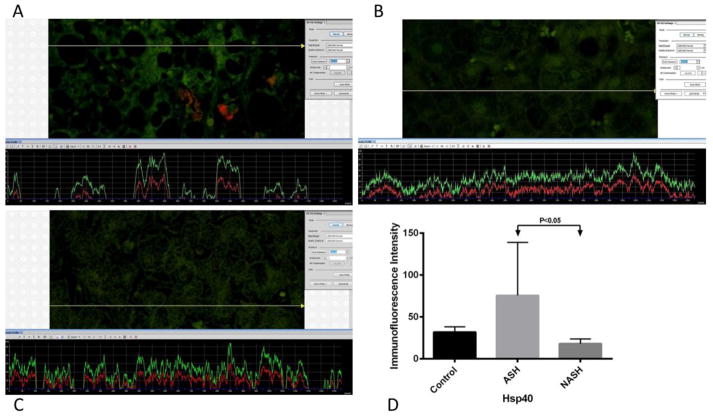
Figure 3.
(A), (B), (C) are representative snip pictures of Hsp40 measurements from ASH, NASH, and normal control, respectively. The presence of Hsp40 was detected by the green immunofluorescence on the liver biopsy. (A) ASH showed a little more green fluorescence compared to both (B) NASH and (C) normal control liver biopsies. More fluorescence corresponded to the increase presence of Hsp40. (D) Means with SD of Hsp40 in ASH, NASH, and normal control liver. Levels of Hsp40 did not show significant difference between control and ASH, as well as, between control and NASH. However, comparing ASH versus NASH, Hsp40 was significantly elevated in ASH (p<0.05). (×566 magnification)
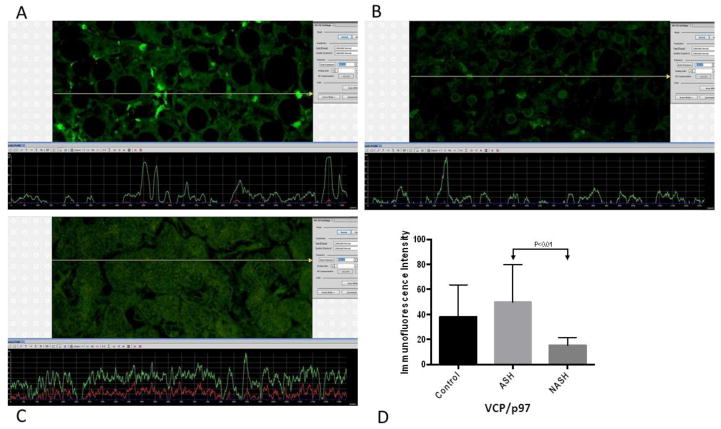
Figure 6.
(A) ASH, (B) NASH and (C) normal control measurements of VCP/p97 levels. The presence of VCP/p97was detected by the green immunofluorescence on the liver snip picture. The level of fluorescence intensity was dependent on the amount of VCP/p97. (A) ASH showed slightly greener fluorescence compared to (B) NASH. More fluorescence corresponded to the elevated levels of VCP/p97. (D) Means with SD of VCP/p97 in ASH, NASH, and normal control liver. VCP/p97levels did not show significant difference between ASH and control, nor between NASH and control. However, comparing ASH versus NASH, VCP/p97is significantly elevated in ASH (p<0.01). (×566 magnification)
The ATP-driven chaperone valosin-containing protein VCP/p97 governs critical steps in ubiquitin-dependent protein quality control and intracellular signaling pathways. It cooperates with diverse partner proteins to help process ubiquitin-labeled proteins for recycling or degradation by the 26S proteasome in many cellular contexts (Meyer and Bug, Feb. 2012). Recently, p97 has been found to be involved in various cellular functions, including autophagy, endosomal sorting, regulation of protein degradation at the outer mitochondrial membrane, and key chromatin-associated processes. These findings extend the functional relevance of p97 to lysosomal degradation and genome stability during proliferation. (Bug and Meyer, Aug. 2012)
B. NASH
NASH is commonly associated with obesity, dyslipidemia and insulin resistance. These metabolic syndromes may lead to accumulation of hepatic fat. Liver inflammation is metabolically induced and may recruit additional inflammatory components (Nseir et al., 2010) leading to NASH. Further progression of the disease is probably caused by chronic inflammation and reactive oxygen species formation. Balloon cell change with MDB formation is a feature of NASH. All the NASH liver biopsies used in this study showed MDB formation in balloon cells (Fig. 8). Few misfolded and aggregated proteins may be present in NASH. Therefore, recruitment of various chaperones may not occur.
In NASH, only Hsp104 is significantly elevated
Compared to normal control biopsies, only Hsp104 was elevated in NASH (Fig. 2). It has been shown that in NASH, Hsp104 localizes to the IPOD upon accumulation of misfolded proteins, whereas in ASH, Hsp104 both localizes to JUNQ and IPOD (Hill, et. al., 2014). Our data suggests that in NASH, the liver may be recruiting other cellular chaperones, or only Hsp104, in refolding misfolded proteins. Additionally, NASH may be utilizing the IPOD system in sequestering terminally aggregated proteins. FRAP and FLIP assays revealed that proteins in the IPOD are tightly packed, insoluble and do not exchange with the cytosol. Interestingly, misfolded proteins in the IPOD are non-ubiquitinated. Ubiquitination of an IPOD substrate results in its sequestration in the JUNQ inclusion. The pre-autophagosomal structure (PAS) is also localized by the IPOD. IPOD substrates may be delivered to the vacuole for autophagy.
Up trending of Ssa1 levels in both ASH and NASH
ASH and NASH both showed a trend for Ssa1 elevation, but not at significant levels (Fig. 5) compared to normal controls. Elevated Ssa1 denoted increase activity of the chaperone to some extent. Ssa1 plays essential functions in protein folding and translocation across the endoplasmic reticulum and mitochondrial membranes in combination with its J-domain partner Ydj1 (McClellan and Brodsky, 2000). Ssa1 also facilitates ER-associated degradation by association with Mca1 and Hsp104 (Hill, et. al., 2014). Ssa1, with Ydj1 and Hsp104, is also required in proteasome-independent disaggregation and refolding of misfolded proteins.
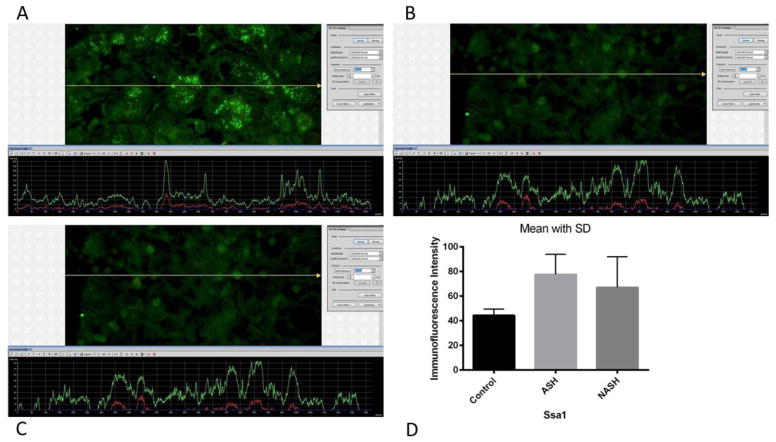
Figure 5.
(A) ASH, (B) NASH and (C) normal control measurements of Ssa1 levels. The presence of Ssa1 was detected by the green immunofluorescence on the liver biopsies. ASH, NASH and normal control liver biopsies showed almost the same green fluorescence intensity. (D) Means with SD of Ssa1 in ASH, NASH, and normal control liver. Measurements of Ssa1 did not show significant difference between the 3 groups. (×566 magnification)
Figure 9.
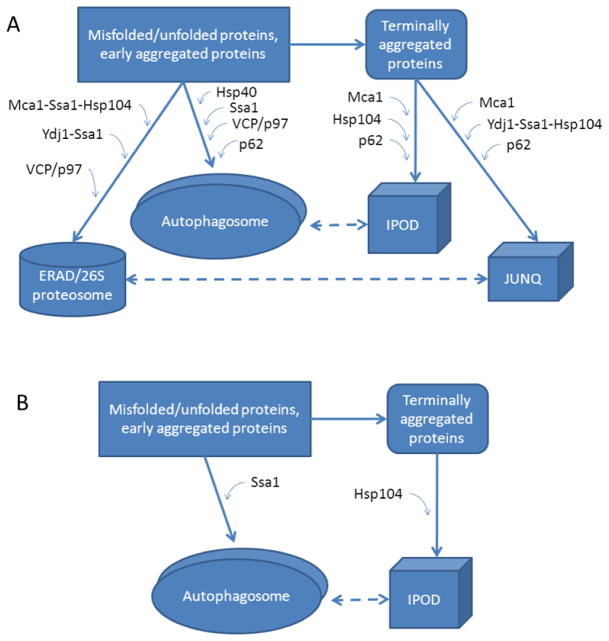
(A) Summary of protein quality control mechanisms in ASH. Levels of Mca1, Hsp104, Hsp40, Ydj1, VCP/p97 and p62 were found to be elevated in ASH. These are involved in all four protein quality control mechanisms discussed: autophagy, the ERAD, the JUNQ and the IPOD systems. (B) Summary of protein quality control mechanisms in NASH. Only Ssa1 and Hsp104, associated with autophagy and the IPOD system, respectively, were found to be elevated in NASH specimens. Our data suggests that different protein quality control mechanisms are utilized in ASH versus NASH.
Acknowledgments
This study was funded by NIH:NIAAA grant # UO1-021898-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardag-Gorce F, Francis T, Nan L, Li J, Lue YH, French BA, French SW. Modifications in P62 occur due to proteasome inhibition in alcoholic liver disease. Life Sciences. 2005;77(20):P2594–2602. doi: 10.1016/j.lfs.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005 Nov;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bug, Meyer Expanding into new markets-VCP/p97 in endocytosis and autophagy. J Struct Biol. 2012 Aug;179(2):78–82. doi: 10.1016/j.jsb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Caldwell S, lkura Y, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen P, Han W. cis-Effect of DnaJ on DnaK in ternary complexes with chimeric DnaK/DnaJ-binding peptides. FEBS Lett. 2004;563(1):146–150. doi: 10.1016/S0014-5793(04)00290-X. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008 Feb;4(2):141–50. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40. Cell stress & chaperones. 2003;8(4):309–16. doi: 10.1379/1466-1268(2003)008<0309:mfrohf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW. The Mallory body: structure, composition, and pathogenesis. Hepatology. 1981;1(1):76–83. doi: 10.1002/hep.1840010113. [DOI] [PubMed] [Google Scholar]
- French SW. Nature, pathogenesis and significance of the Mallory body. Semin Liver Dis. 1981;1(3):217–31. doi: 10.1055/s-2008-1041749. [DOI] [PubMed] [Google Scholar]
- French SW, Bardag-Gorce F, et al. Mallory-Denk body pathogenesis revisited. World J Hepatol. 2010;2(8):295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all J domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions. Protein science : a publication of the Protein Society. 2005 Jul;14(7):1697–709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaganovich Daniel, Kopito Ron, Frydman Judith. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454(7208):1088–95. doi: 10.1038/nature07195. Bibcode:2008Natur.454.1088K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, Di-Figlia M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Sherman MY, Goldberg AL. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4773–4781. doi: 10.1128/mcb.16.9.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Brodsky JL. Mutation of the ATP-binding pocket of Ssa1 indicates that a functional interaction between Ssa1p and Ydj1p is required for post-translational translocation into the yeast endoplasmic reticulum. Genetics. 2000;156:501–512. doi: 10.1093/genetics/156.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012 Feb;14(2):117–23. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- Nan L, Wu Y, Bardag-Gorce F, Li J, French BA, Fu AN, Francis T, Vu J, French SW. p62 is involved in the mechanism of Mallory body formation. Experimental and Molecular Pathology. 2004;77(3):P168–175. doi: 10.1016/j.yexmp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Nseir W, Nassar F, Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World journal of gastroenterology : WJG. 2010;16(21):2579–2588. doi: 10.3748/wjg.v16.i21.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40-a review. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2000;16(3):231–45. doi: 10.1080/026567300285259. [DOI] [PubMed] [Google Scholar]
- Peng Y, French BA, Tillman B, Morgan TR, French SW. The inflammasome in alcoholic hepatitis: Its relationship with Mallory-Denk body formation. Exp Mol Pathol 2014. 2014 Aug;97(2):305–13. doi: 10.1016/j.yexmp.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Specht S, Miller SBM, Mogk A, Bukau B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. The Journal of Cell Biology. 2011;195(4):617–29. doi: 10.1083/jcb.201106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T. Autophagy: a regulated bulk degradation process inside Cells. Biochem Biophys Res Commun. 2004;313:453–458. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- Zatloukal KS, French W, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313(10):2033–49. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]