Abstract
The FOXO1 transcription factor is important for multiple aspects of reproductive function. We previously reported that FOXO1 functions as a repressor of gonadotropin hormone synthesis, but how FOXO1 is regulated in pituitary gonadotropes is unknown. The growth factors, insulin and insulin-like growth factor I (IGF1) function as key regulators of cell proliferation, metabolism and apoptosis in multiple cell types through the PI3K/AKT signaling pathway. In this study, we found that insulin and IGF1 signaling in gonadotropes induced FOXO1 phosphorylation through the PI3K/AKT pathway in immortalized and primary cells, resulting in FOXO1 relocation from the nucleus to the cytoplasm. Furthermore, insulin administration in vivo induced phosphorylation of FOXO1 and AKT in the pituitary. Thus, insulin and IGF1 act as negative regulators of FOXO1 activity and may serve to fine-tune gonadotropin expression.
Keywords: gonadotrope, forkhead box O1, insulin, IGF1, GnRH
1. Introduction
The forkhead box O1 (FOXO1) transcription factor was first recognized as being important for human health when it was identified in a chromosomal translocation in alveolar rhabdomyosarcoma tumors (1); it was later recognized as a tumor suppressor (2). While the FOXO1 null mouse is embryonic lethal at embryonic day 10.5, in vitro studies and tissue specific knockouts have provided insight into the functions of FOXO1 (3–6). Cellular stressors such as nutrient deprivation, oxidative stress, DNA damage, or endoplasmic reticulum stress have been shown to activate FOXO1 (7–9). In response, FOXO1 modulates genes associated with autophagy, cell cycle and DNA repair (9–13). Thus, FOXO1 regulates cell-stress resistance and cell longevity, but can also promote cell apoptosis (14).
Several diverse functions of FOXO1 have been identified and characterized in the reproductive organs (15). In the human uterus during endometrial decidualization, FOXO1 expression is significantly increased leading to FOXO1 upregulation of p57Kip2, a cell cycle inhibitor, and repression of several other genes important for cell cycle progression (16). Based on these findings, FOXO1 is considered to be an important regulator of the decidual process (16). In ovarian granulosa cells, knockdown of FOXO1 had no effect on ovarian morphology, yet the mice were subfertile (17). Further investigation revealed that FOXO1 participates in follicle atresia, likely by enhancing apoptosis (17). FOXO1 is also necessary for spermatogonial stem cell homeostasis and spermatogenesis in the testes (18).
While FOXO1 function in the gonads and uterus has been characterized, little is known about its function in the central portion of the hypothalamic-pituitary-gonadal axis. In the pituitary, the gonadotropin hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH), are produced exclusively in gonadotrope cells (19–21). Both LH and FSH are necessary for human fertility (22–24). LH and FSH are heterodimers composed of a common alpha subunit (CGA) and a unique beta subunit (LHB or FSHB) that provides hormone specificity (25). Gonadotropin synthesis and secretion are primarily regulated by gonadotropin releasing hormone (GnRH), but other hormones such as steroids, activin, follistatin, and inhibin also modulate gonadotropin production (26). GnRH is produced in the hypothalamus and released in a pulsatile pattern. GnRH binds to the GnRH receptor (GnRHR), a G-protein coupled receptor, on the cell surface of gonadotropes (27). GnRHR stimulation drives gonadotropin gene transcription primarily by signaling through protein kinase C (PKC) (28). PKC activates several mitogen activated protein kinase (MAPK) cascades, resulting in the phosphorylation and activation of p38, cJun N-terminal kinases (JNK) and extracellular-signal regulated kinases (ERK) (29). These MAPKs increase the expression or activity of several transcription factors, such as early growth response protein 1 (EGR1), cJUN, cFOS, and activating transcription factor 2 (ATF2), mediating CGA, LHB and FSHB synthesis (28).
FOXO1 was recently reported to be an inhibitor of gonadotropin production, expanding FOXO1’s influence on fertility (30–32). FOXO1 protein has been identified in murine and rat gonadotrope cells (30, 33). While FOXO1 protein expression has not been characterized in human pituitary, FOXO1 mRNA levels were found to be decreased seven fold in human null cell and gonadotrope pituitary tumors (34). FOXO1 was also expressed in immortalized murine gonadotrope-derived cell lines: αT3-1 cells, which only express CGA and represent an immature gonadotrope lineage, and in LβT2 cells, which express CGA, LHB and FSHB (30, 33, 35, 36). In gonadotrope cells, FOXO1 overexpression suppressed transcription of both human and rodent basal and GnRH stimulated LHB and FSHB (30–32). These data suggest that FOXO1 suppression of the gonadotropin promoters may be conserved between rodents and humans. FOXO1 suppression of Fshb and Lhb required an intact FOXO1 DNA binding domain, but electrophoretic mobility shift assays revealed that FOXO1 did not bind to the proximal Lhb or Fshb promoters, although the proximal promoter was sufficient for FOXO1 suppression (30–32). These studies suggest that FOXO1 suppresses gonadotropin synthesis independent of direct DNA binding, likely through protein complex formation with transcription factors important for gonadotropin synthesis, such as paired-like homeodomain transcription factor 1 (PITX1) (30, 31). Protein-protein interaction of FOXO1 with other transcription factors is a known mechanism by which FOXO1 activates or suppresses its gene targets (37). While FOXO1 has been identified as a potential inhibitor of gonadotropin synthesis, the cellular conditions and signaling pathways that regulate FOXO1 function in gonadotropes are unknown.
Previous studies have suggested that insulin-like growth factor I (IGF1) stimulation of αT3-1 cells or insulin treatment of LβT2 cells can result in FOXO1 phosphorylation (30, 38). The growth factors, insulin and IGF1 act as indicators of nutritional status and provide pro-survival signals to cells (39, 40). Insulin and IGF1 have unique receptors, yet stimulation of the insulin receptor or IGF1 receptor results in activation of the phosphatidylinositol-3 kinase (PI3K)/AKT pathway (41). PI3K activation leads to the phosphorylation of AKT, which directly phosphorylates FOXO1 on Ser256, then Ser319 and Thr24 (42, 43). Phosphorylation of these key FOXO1 residues by AKT exposes a nuclear export sequence, permitting export of FOXO1 to the cytoplasm and thereby inhibiting its nuclear activity (44). While the insulin and IGF1 signaling pathways are established regulators of FOXO1 in other tissues, such as adipocytes, pancreatic beta cells and hepatocytes, it is unknown if this type of signaling regulation of FOXO1 occurs in gonadotrope cells (8, 45–47).
Using rat primary pituitary cells and gonadotrope-derived cell lines, multiple studies have demonstrated that the canonical insulin/IGF1 signaling pathway is present in gonadotropes. The insulin receptor, IGF1 receptor, PI3K p85 subunit, and AKT have been identified in gonadotropes (38, 48–51). In addition, insulin has been reported to induce Lhb synthesis in LβT2 cells and LH secretion in rat primary pituitary cells (48, 52–55). Furthermore, insulin enhanced GnRH-induced Lhb synthesis (48, 56). Pharmacologic inhibition of PI3K with LY294002 suppressed basal and GnRH-induced Fshb synthesis in LβT2 cells and cultured rodent pituitary cells, implicating the PI3K pathway in Fshb production (31, 32). A recent report also suggested GnRH may negatively regulate FOXO1 (32). Accordingly, the goal of this study was to investigate how hormone signals such as insulin, IGF1 and GnRH regulate FOXO1 signaling to potentially impact gonadotropin production in the pituitary.
2. Materials and methods
2.1 Reagents
AKT1/2 inhibitor VIII (AKTi), IGF1, and LY294002 were purchased from EMD Chemicals, Inc. (San Diego, CA). Dimethyl sulfoxide (DMSO), insulin and GnRH were purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Cell Culture
LβT2 cells (35) were maintained in 10-cm plates in DMEM (Dulbecco’s modification of Eagle’s medium) from Mediatech Inc. (Mannassas, VA), supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-products, West Sacramento, CA) and penicillin/streptomycin (Life Technologies, Grand Island, NY) (10% FBS DMEM) at 37°C and 5% CO2.
Primary cells were obtained from 10–12 week old male C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) and cultured as previously described (31). Briefly, mice were sacrificed and their pituitaries were collected in ice-cold Dulbecco’s phosphate-buffered saline (PBS). After a PBS rinse, the pituitaries were minced on ice with fine scissors and then placed in dissociation media containing phosphate buffered 0.25% trypsin-EDTA (Life Technologies) and 0.25% collagenase (Life Technologies). The pituitaries were shaken for 30 minutes at 37°C in a water bath, then an equal volume of 10% FBS DMEM was added along with DNaseI (Worthington Biochemical, Lakewood, NJ) at a final concentration of 25 μg/mL and incubated for another 15 minutes at 37°C. After removal of tissue debris, the cells were pelleted by centrifugation and plated at a density of 1×106/2 cm2 well in Primaria plates (BD Biosciences, San Jose, CA). The cells were placed in serum-free media 18 hours prior to hormone treatment. All animal procedures were conducted in accordance with the UCSD Institutional Animal Care and Use Committee requirements.
2.3 Western blot
To harvest cells, the treatment-containing media was removed and the cells were washed twice in cold PBS, then scraped in a lysis buffer [10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, complete protease inhibitor mixture tablet (Roche Applied Science, Indianapolis, IN) and phosphatase inhibitor mixture tablet (Roche Applied Science)] and rotated for 10 minutes at 4°C. Lysates were centrifuged at 16,000 x g at 4°C for 30 minutes. The protein concentration of the supernatant was determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA), and an equal amount of protein per sample was loaded on a 10% SDS-PAGE gel. Proteins were resolved by electrophoresis and transferred for 2 hours at 100 V onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). Blots were blocked overnight in 5% non-fat milk, then probed overnight at 4°C with primary antibody. Primary antibodies used for western were ERK (sc-94; 1:1000), ERK pTyr204 (sc-7383; 1:1000), FOXO1 (sc-11350; 1:1000), FOXO1 pSer319 (sc-101682; 1:1000), AKT (sc-8312; 1:1000), GAPDH (sc-25778; 1:3000), and beta-Tubulin (sc-9104; 1:3000), which were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX). FOXO1 pThr24 (9464; 1:1000), FOXO1 pSer256 (9461; 1:1000), AKT pThr308 (2965; 1:1000), and AKT pSer473 (4051; 1:1000) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Blots were washed and then incubated with anti-rabbit horseradish peroxidase-linked (HRP) (sc-2004; 1:5000) or anti-mouse HRP secondary antibody (sc-2005; 1:3000) as appropriate (Santa Cruz Biotechnology, Inc.). Bands were visualized using the SuperSignal West Dura Substrate (Thermo Scientific, Rockford, IL). Densitometric analysis of band intensity was performed using ImageJ software (National Institute of Health, Bethesda, MD).
2.4 Immunofluorescence of LβT2 cells
LβT2 cells were plated onto poly-L-lysine coverslips (BD Biosciences, Bedford, MA). After 24 hours, the media was changed to serum-free media for overnight serum starvation. The next morning, cells were treated as described for each experiment with hormone, inhibitor or both. After treatments, cells were then washed twice with PBS and fixed with 4% formaldehyde for 10 minutes. Cells were washed twice with PBS and permeabilized for 1 hour with Nonidet P-40 solution (PBS containing 0.2% Nonidet P-40, 20% goat serum, 1% BSA) at room temperature. Cells were washed twice in PBS and incubated with FOXO1 primary antibody (1:100) in blocking buffer (PBS containing 20% goat serum, 1% BSA) for 48 hours at 4 °C. Cells were washed 3 times with PBS for 5 minutes and incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Life Technologies, A-11008; 1:300) in blocking buffer for 1 hour at room temperature. Cells were washed 3 times with PBS for 5 minutes and counterstained with 300 nM 4′,6-diamidino-2-phenylindole, dilactate (DAPI) (Life Technologies) for 4 minutes, then washed 3 times with PBS for 5 minutes each. Coverslips were mounted using Prolong Gold Antifade Reagent (Life Technologies) and cells were viewed using a Nikon Eclipse TE 2000-U inverted fluorescence microscope. Digital images were collected using a CoolSNAP EZ camera (Roper Scientific, Trenton, NJ) and analyzed with the Version 2.3 NIS-elements image analysis system.
2.5 Immunofluorescence of primary pituitary cells
Eight-week old, male C57BL/6 mice (Harlan Laboratories) and heterozygous Foxo1tag/WT mice were used to obtain primary pituitary cells for immunofluorescence imaging. Foxo1tag mice have a FOXO1 allele that encodes a c-terminal in-frame fusion protein tag comprised of green fluorescent protein (GFP), Flag and a biotin labeling peptide, enabling the use of GFP as a reporter of FOXO1 protein expression by microscopy. Foxo1tag mice were generously provided by Dr. Ming O. Li (57). Cells were isolated from animals as described in section 2.2 and plated in 10% FBS containing media at a density of 2.75×105 cells/2 cm2 well with each well containing a poly-L-lysine coverslip (BD Biosciences). After 24 hours, cells were fixed and permeabilized as described in section 2.4, with the exception that formaldehyde concentration was reduced to 3%. Primary antibodies against pituitary hormones were purchased from the NIDDK National Hormone and Peptide Program (Torrance, CA). Cells were incubated with primary antibodies against GFP (Life Technologies, A11222; 1:1200) and either growth hormone (GH) (1:400) or LHB (1:6000) in blocking buffer for 48 hours at 4 °C. After 48 hours, cells were washed, incubated in secondary antibodies Alexa Fluor 594 anti-rabbit (Life Technologies, A-11076; 1:400) and DyLight 488 anti-guinea pig (AbCam, ab96959; 1:400), DAPI, mounted and imaged as described above. All animal procedures were conducted in accordance with the UCSD Institutional Animal Care and Use Committee requirements.
2.6 In vivo insulin challenge
Eight to ten-week old, male C57BL/6 mice (Harlan Laboratories) were housed in the UCSD vivarium for at least one week under standard conditions. The mice were fasted for 6 hours starting at 7:00 AM. After 6 hours, mice were given an intraperitoneal injection of either saline or regular insulin 1.5 U/kg of body weight (Humulin R U-100, MWI Veterinary Supply, Inc., Boise, ID) and euthanized 10 minutes later. Pituitary and liver were immediately harvested from each animal and frozen in liquid nitrogen. Tissues were stored at −80 C until they were processed for western analysis. All animal procedures were conducted in accordance with UCSD Institutional Animal Care and Use Committee requirements.
3. Results
3.1 Insulin and IGF1 induce FOXO1 phosphorylation in a time and concentration dependent manner
To assess the ability of insulin to induce FOXO1 phosphorylation in gonadotropes, we performed concentration and time course experiments. Immortalized gonadotrope-derived LβT2 cells were placed in serum-free media overnight, then treated with media containing vehicle (time 0) or insulin (0.1–100 nM as indicated) for 10 minutes. Cells were lysed and phosphorylation of FOXO1 Ser256 assessed by western (Fig. 1A). An increase in phosphorylation was present at 1 nM, with the greatest amount of phosphorylation occurring with 10 and 100 nM of insulin. Additionally, using serum-free treated cells, a time course was conducted with 10 nM insulin for 0–120 minutes as indicated. FOXO1 Ser256 phosphorylation was significantly increased at 10 minutes and was still present at 120 minutes.
Figure 1. Insulin and IGF1 induce phosphorylation of FOXO1 Ser256 in immortalized gonadotropes.
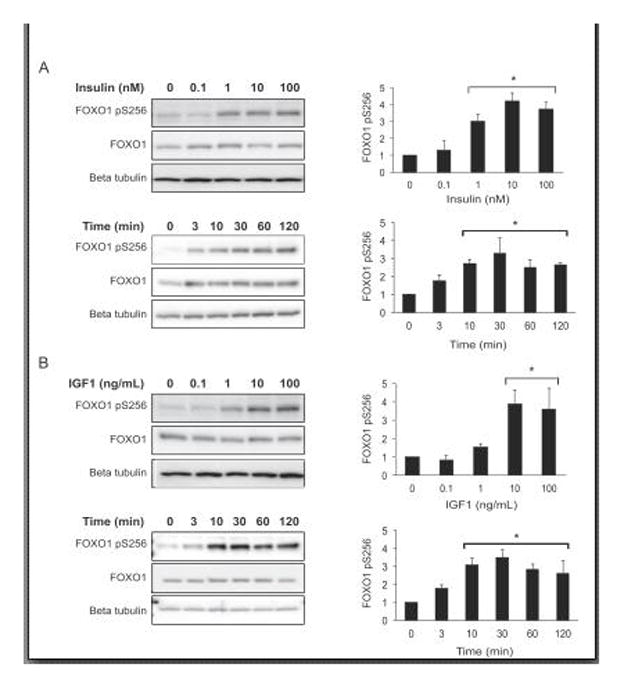
A. Left: westerns of LβT2 cells that were placed in serum-free media overnight, then treated with increasing concentrations of insulin for 10 min or with 10 nM insulin for up to 2 hrs. Right: FOXO1 pSer256 densitometry values were normalized to total FOXO1, then graphed as fold untreated. B. Left: westerns of LβT2 cells that were placed in serum-free media overnight, then treated with increasing concentrations of IGF1 for 10 min or with 10 ng/mL IGF1 for up to 2 hrs. Right: FOXO1 pSer256 densitometry values were normalized to total FOXO1 and expressed as fold untreated. Graphed data represent mean and error bars as SEM, n=3. For statistical analysis, pSer256/total FOXO1 data were analyzed by randomized block one-way analysis of variance (ANOVA) (82) and post-hoc Dunnett’s test using the statistical package JMP 11.0 (SAS, Cary, NC). Significant differences from control were designated as p<0.05 and represented with an asterisk.
Analogous experiments were performed with IGF1 to determine whether this growth factor also had a stimulatory effect on FOXO1 phosphorylation in gonadotropes. Similarly to insulin treatment, IGF1 demonstrated maximal phosphorylation of FOXO1 Ser256 at 10 ng/mL of IGF1 for 10 minutes (Fig. 1B). Using 10 ng/mL IGF1, the time course experiment also demonstrated that FOXO1 Ser256 phosphorylation was significantly induced at 10 minutes and remained elevated at 120 minutes.
3.2 Inhibition of the PI3K/AKT pathway blocks insulin- and IGF-induced phosphorylation and cytoplasmic localization of FOXO1
To identify the signaling pathway mediating the insulin or IGF1 signal to FOXO1 in gonadotropes, we assessed two key kinases associated with the canonical insulin/IGF1 signaling cascade, PI3K and AKT. AKT has two phosphorylation sites, Thr308 and Ser473, that when phosphorylated, are indicative of full kinase activity (58). We pre-treated serum-starved LβT2 cells for 1 hour with DMSO vehicle (V), the PI3K inhibitor LY294002, or the AKT Inhibitor VIII (AKTi). After 1 hour, the cells were treated with vehicle, insulin (10 nM) or IGF1 (10 ng/mL) for 10 minutes. By western, both insulin and IGF1 strongly induced phosphorylation of AKT at Thr308 and Ser473 while FOXO1 was phosphorylated at Thr24, Ser256 and Ser319 (Fig. 2). Phosphorylation of both AKT and FOXO1 was inhibited by LY294002 and AKTi, indicating that insulin- and IGF1-stimulated phosphorylation of FOXO1 is PI3K and AKT dependent (Fig. 2).
Figure 2. Insulin- and IGF1-induced phosphorylation of FOXO1 at Thr24, Ser256 and Ser319 is dependent on PI3K/AKT signaling in LβT2 cells.
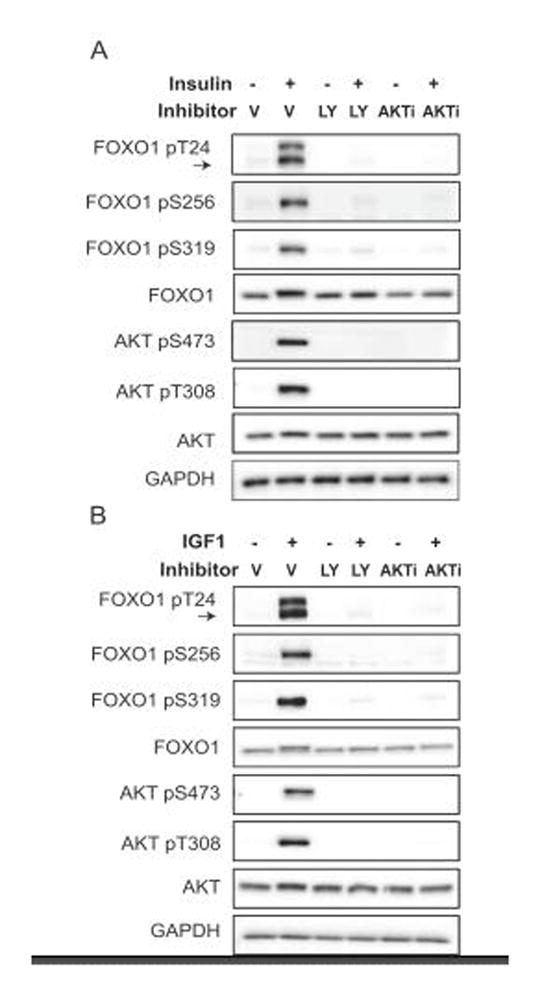
Cells were placed in serum-free media overnight. The next day, cells were pretreated with DMSO (Vehicle, V), LY294002 50 μM (LY) or AKTVIII 3 μM (AKTi) for 1 hr and then treated with insulin (10 nM) or IGF1 (10 ng/mL) for 10 min. FOXO1 and AKT phosphorylation changes were assessed by western. Arrow indicates FOXO1 pT24 band; upper band is FOXO3 pT24. Images are representative of 3 independent experiments.
FOXO1 phosphorylation by AKT induces a conformational change in FOXO1, which exposes a nuclear export signal and results in FOXO1 export to the cytoplasm (59). To determine if insulin or IGF mediated nuclear export of FOXO1 in LβT2 cells, we performed immunofluorescence imaging. We pretreated serum-starved LβT2 cells for 1 hour with DMSO vehicle, 50 μM LY294002 or 3 μM AKTi and then treated the cells with vehicle, insulin (10 nM) or IGF1 (10 ng/mL) for 30 minutes (Fig. 3). Cells were fixed and stained for FOXO1. We determined that both insulin and IGF1 caused a significant increase in cytoplasmic FOXO1 staining compared to the serum-free condition where FOXO1 was present in both the nuclear and cytoplasmic compartments. Pretreatment with LY294002 or AKTi increased the nuclear localization of FOXO1 under serum-free conditions and blocked the nuclear export of FOXO1 in the presence of insulin or IGF1 (Fig. 3).
Figure 3. Insulin- and IGF1-stimulated cytoplasmic localization of FOXO1 is dependent on PI3K/AKT signaling.
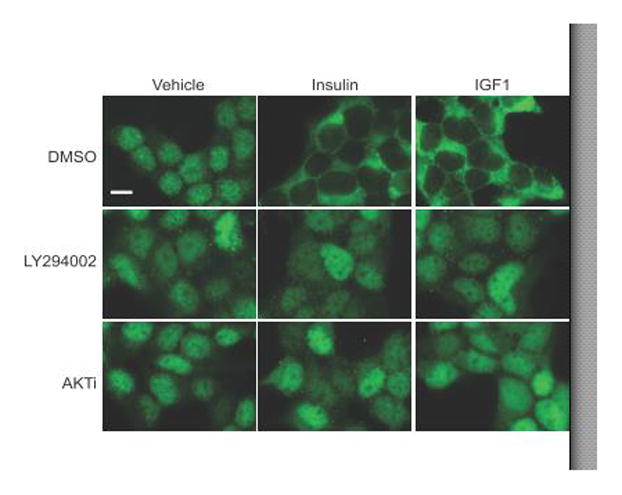
LβT2 cells grown on poly-L-lysine coverslips were placed in serum-free media overnight. The next day, cells were pretreated with DMSO (Vehicle), LY294002 50 μM or AKTVIII 3 μM (AKTi) for 1 hr, then treated with insulin (10 nM) or IGFI (10 ng/mL) for 30 min. Cells were fixed and stained with primary FOXO1 antibody and secondary Alexa Fluor 488 (green) for immunofluorescence microscopy. Images are representative of 3 independent experiments. Scale bar=10 μm.
3.3 Insulin and IGF1 induce phosphorylation of AKT and FOXO1 in primary pituitary cells
To determine if insulin and IGF1 regulate FOXO1 in primary pituitary cells in a similar manner to immortalized gonadotropes, we used primary pituitary cultures from adult male mice. We have previously demonstrated that FOXO1 expression in adult murine paraffin-embedded pituitary is limited to gonadotropes and thyrotropes (30, 33), which comprise ~10% and 5% of the anterior pituitary hormone-producing cell population, respectively (60, 61). For this study, we determined whether FOXO1 was also present in gonadotropes in dispersed primary pituitary cells in culture using mice heterozygous for GFP tagged FOXO1 (FOXO1tag/WT) (57). We also evaluated somatotropes for FOXO1 expression as a previous report had found FOXO1 predominately in GH expressing cells (62). Under culture conditions, FOXO1-GFP expression co-localized with gonadotropes and a subset of somatotropes (Fig. 4A). To determine if insulin or IGF1 could induce FOXO1 phosphorylation in primary cells, dispersed pituitary cells from C57BL/6 mice were cultured for 24 hours in serum-containing media and then changed to serum-free media overnight. Cells were treated with vehicle, insulin (10 nM) or IGF1 (10 ng/mL) for 30 minutes and then lysed. By western, both hormones induced phosphorylation of AKT on Thr308 and Ser473 and FOXO1 on Thr24, Ser256 and Ser319, which is consistent with our results obtained in LβT2 cells (Fig. 4B).
Figure 4. In cultured murine primary pituitary cells, FOXO1 and AKT are phosphorylated in response to insulin and IGF1.
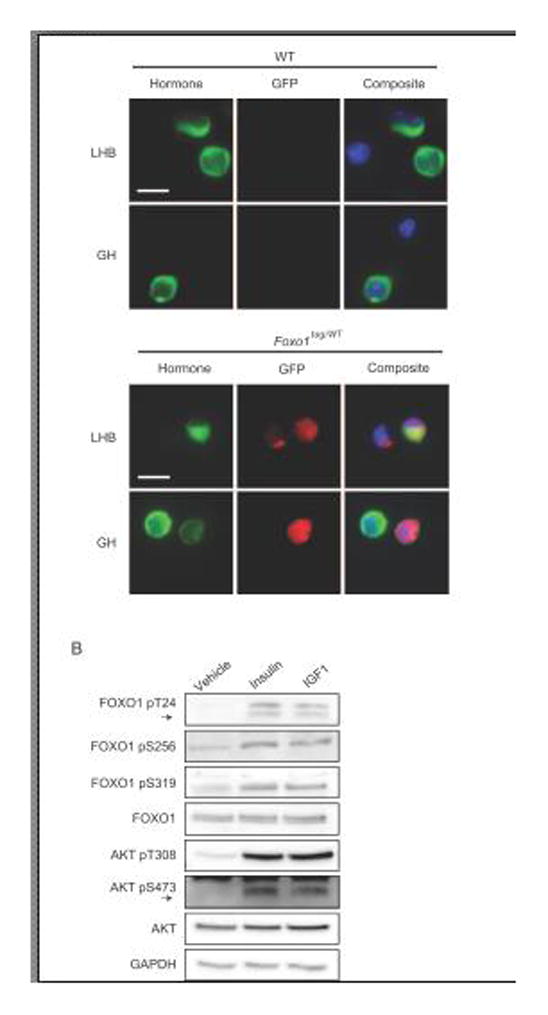
A. Cultured primary pituitary cells from FOXO1tag/WT and WT mice were grown on poly-L-lysine coverslips, fixed and stained with primary GFP antibody along with primary LHB or GH antibody. FOXO1-GFP was detected using secondary Alex Fluor 594 (red), hormones with secondary DyLight 488 (green) and the nuclear compartment with DAPI (blue). Scale bar=10 μm. Images are representative of 3 independent experiments. B. Cultured primary pituitary cells from WT mice were placed in serum-free media for 16 hrs, then treated with insulin (10 nM) or IGF1 (10 ng/mL) for 30 min prior to lysis. Phosphorylation changes in FOXO1 and AKT were assessed by western. Images are representative of 2 independent experiments.
3.4 In vivo insulin challenge increases pituitary FOXO1 and AKT phosphorylation
Because insulin induced phosphorylation of FOXO1 in primary dispersed pituitary cells, we next asked if insulin administered in vivo could also result in FOXO1 phosphorylation. For this experiment, male mice were fasted for 6 hours and then treated with saline or insulin by intraperitoneal injection. Ten minutes later, the mice were killed and tissues were collected. As shown in Figure 5, insulin treatment resulted in phosphorylation of both AKT Thr308 and FOXO1 Ser256 in the pituitary, confirming our results in the primary pituitary cell culture studies. Phosphorylation of FOXO1 Ser256 and AKT Thr308 in the liver were used as positive controls (63).
Figure 5. Insulin increases pituitary FOXO1 phosphorylation in vivo.
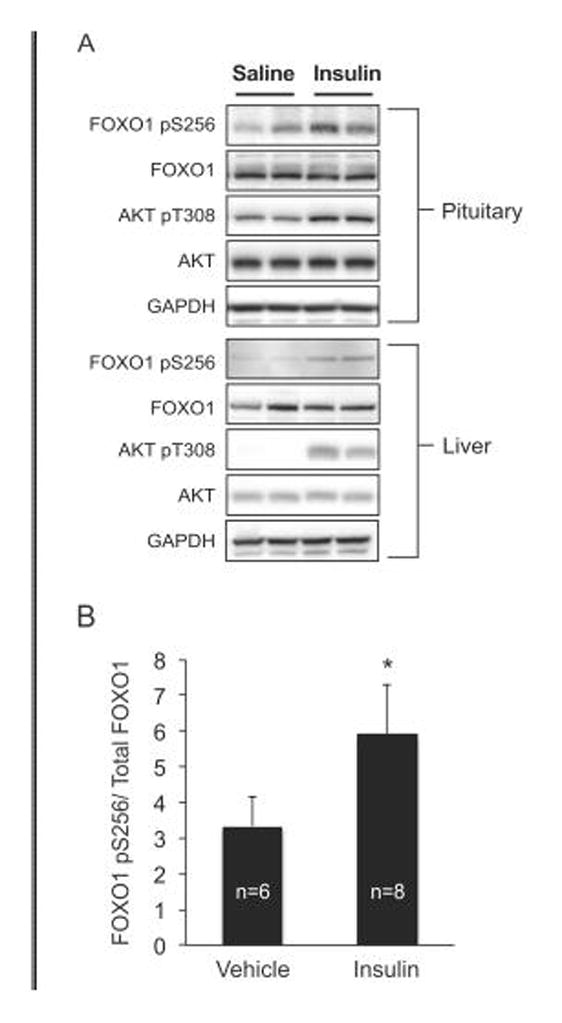
Male mice were fasted for 6 hours, then given either saline or regular insulin (1.5 U/kg) by intraperitoneal injection. After 10 minutes, the mice were sacrificed and their pituitaries and livers harvested and immediately placed in liquid nitrogen. A. Changes in phosphorylation were assessed by western and representative blots are shown for 4 animals, two saline (vehicle) and two insulin. Insulin-induced phosphorylation of FOXO1 Ser256 and AKT Thr308 in the liver were used as positive controls. B. Pituitary FOXO1 pS256 densitometry values were normalized to total FOXO1. Data are presented as the mean and error bars are SEM, n=6 (Vehicle), n=8 (Insulin). The asterisk indicates that insulin significantly increased pituitary FOXO1 Ser256 phosphorylation compared to saline vehicle using Student’s t test, p<0.05.
3.5 GnRH treatment has no effect on FOXO1 phosphorylation or cellular localization
Since FOXO1 has been reported to inhibit gonadotropin synthesis (30–32), and GnRH is the primary regulator of gonadotropin production, we asked if GnRH had any effect on FOXO1 phosphorylation or total protein levels that would inhibit its nuclear activity. LβT2 cells were incubated in serum-free media overnight, then treated with GnRH 10 nM for 0–60 minutes, and then lysed. Phosphorylation of FOXO1 Thr24, Ser256 and Ser319 was assessed by western (Fig. 6A). Stimulation of LβT2 cells with GnRH alone did not significantly alter FOXO1 phosphorylation from the basal state or change total FOXO1 protein levels. ERK phosphorylation at Tyr204, which increased in response to GnRH, was used as a positive control for GnRH stimulation. Furthermore, in contrast to insulin and IGF, treatment of LβT2 cells with GnRH for 30 minutes did not result in a discernable shift in FOXO1 localization from the nucleus to the cytoplasm (Fig. 6B).
Figure 6. GnRH does not regulate FOXO1 phosphorylation.
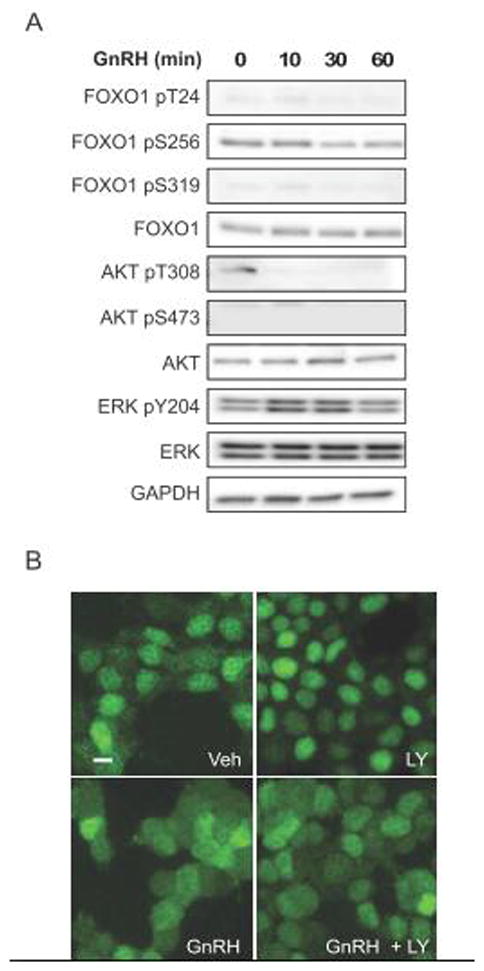
A. LβT2 cells were placed in serum-free media overnight and treated the next day with 10 nM GnRH for up to 2 hrs. Changes in FOXO1 and AKT phosphorylation were assessed by western. Images are representative of 3 independent experiments. B. LβT2 cells were placed in serum-free media overnight. The following day, cells were treated with DMSO (Veh) or LY294002 (LY) 50 μM for 1 hr and then GnRH 10 nM was added for 30 min. Cells were fixed and stained with primary FOXO1 antibody and secondary Alexa Fluor 488 (green) for immunofluorescence microscopy. Images are representative of 5 independent experiments. Scale bar=10 μm
3.6 GnRH blunts insulin and IGF1 activation of AKT
GnRH has been reported to decrease IGF1-induced phosphorylation of AKT Ser473 in αT3-1 cells (38). Another report noted that GnRH attenuated the phosphorylation of AKT Ser473 induced by insulin in LβT2 cells (64). In vivo, gonadotropes are exposed to pulsatile GnRH along with circulating insulin and IGF1. Since our results showed that phosphorylation of FOXO1 at Thr24, Ser256 and Ser319 in response to insulin or IGF1 was AKT dependent, we assessed the effects of GnRH and insulin or IGF1 in a co-treatment paradigm on AKT and FOXO1 phosphorylation. LβT2 cells were treated with vehicle, insulin (10 nM), GnRH (10 nM), or insulin with GnRH for 30 minutes and then lysed (Fig. 7A). The same paradigm was used to investigate IGF1 and GnRH co-treatment (Fig. 7B). By western, FOXO1 phosphorylation at all three AKT dependent sites was induced by insulin and IGF1. In agreement with our previous results, GnRH alone did not alter FOXO1 phosphorylation nor did co-treatment of insulin with GnRH or IGF1 with GnRH. Interestingly, co-treatment of GnRH with insulin or IGF1 substantially decreased AKT phosphorylation at Ser473, which is consistent with previous reports, and also at Thr308 (38, 64). As shown in Figure 7C, co-treatment of insulin or IGF1 with GnRH significantly decreased AKT Thr308 phosphorylation to 22% and 35% respectively of phosphorylation levels with insulin or IGF alone. Since GnRH did not diminish FOXO1 phosphorylation induced by insulin or IGF1, we determined whether GnRH alters the effect of insulin on FOXO1 cellular localization. Using immunofluorescence, we demonstrated that GnRH had no effect on insulin-induced shuttling of FOXO1 from the nucleus to the cytoplasm after 30 minutes of insulin and GnRH co-treatment (Fig. 7D).
Figure 7. GnRH and insulin co-treatment diminishes AKT phosphorylation, but FOXO1 is not affected.
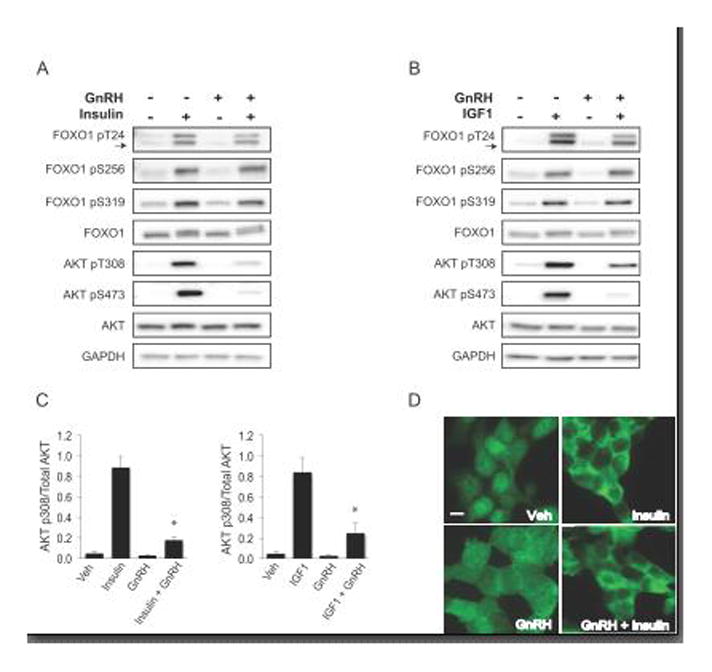
A and B. LβT2 cells were placed in serum-free media overnight. The following day, cells were treated with (A) insulin 10 nM or (B) IGF1 10 ng/mL and GnRH 10 nM co-treatment for 30 min and lysed for western analyses. C. AKT p308 densitometry values were normalized to total AKT and graphed as the mean and error bars as SEM, n=3. Significant interaction was designated by an asterisk as defined by two-way ANOVA from insulin or IGF1 alone, p<0.05. D. Immunofluorescence of LβT2 cells under the same treatment conditions as for westerns in A and B above, then fixed and stained with primary FOXO1 antibody and secondary Alexa Fluor 488 (green). Scale bar=10 μm. All images are representative of 3 independent experiments.
4. Discussion
FOXO1 is an established downstream target of insulin and IGF1 signaling in several tissue types (4, 15), but it was unknown if FOXO1 was regulated by these growth factors in pituitary gonadotrope cells. In the current study, we demonstrated that FOXO1 phosphorylation and cellular localization was regulated by insulin and IGF1 through the canonical PI3K/AKT signaling cascade in gonadotropes. We found that insulin or IGF1 rapidly stimulated FOXO1 phosphorylation in a time- and concentration-dependent manner, without altering total FOXO1 levels (Fig. 1). Moreover, insulin or IGF1 stimulation of LβT2 cells resulted in a significant shift of FOXO1 from the nucleus to the cytoplasm within 30 minutes of hormone treatment (Fig. 3). Pharmacologic inhibition of PI3K or AKT, with LY294002 or AKTi respectively, blocked phosphorylation of FOXO1 at the AKT dependent sites of Thr24, Ser256 and Ser319, identifying PI3K and AKT as two of the major kinases transducing the insulin and IGF1 signals to FOXO1 in gonadotropes (Fig. 2). Furthermore, inhibition of PI3K or AKT activity prevented insulin and IGF1 from shifting FOXO1 from the nucleus to the cytoplasm (Fig. 3). There are three AKT isoforms (AKT1, AKT2 and AKT3), which have isoform-specific signaling (65). It is unknown which isoforms are present in gonadotropes. AKTi selectively inhibits isoforms AKT1 and AKT2 (66, 67), suggesting that AKT3, if present in gonadotropes, is not necessary for insulin or IGF1 signaling to FOXO1. Altogether, these data reveal that the PI3K/AKT1/2 signaling pathway is responsible for transduction of the growth factor signal to FOXO1 and its subsequent redistribution from the nucleus to the cytoplasm in gonadotropes, resulting in inhibition of FOXO1 transcriptional activity.
In order to evaluate the potential for insulin and IGF1 to regulate FOXO1 phosphorylation in vivo, we first used dispersed primary pituitary cells cultured with the same conditions established for LβT2 cells (Figs. 1–3). Since culturing could potentially alter FOXO1 expression in the primary pituitary cells, we assessed FOXO1 expression under these conditions using a GFP antibody to detect FOXO1-GFP expressed in the Foxo1tag/WT mouse. Importantly, we found that FOXO1 was expressed in gonadotrope cells, similar to what we previously observed in paraffin-embedded adult pituitaries using a FOXO1 antibody (Santa Cruz Biotechnology, sc-11350) (30, 33). In contrast to our studies, a previous report by Majumdar et al. using a different FOXO1 antibody (Cell Signaling Technology, 2880) demonstrated that less than 10% of gonadotropes expressed FOXO1 and that FOXO1 was predominately expressed in somatotropes (62). Interestingly, under culture conditions, we observed FOXO1-GFP expression in a small subset of cells producing low levels of GH, whereas cells producing high levels of GH did not appear to express FOXO1. Although more work needs to be done, it is interesting to speculate that these cells may reflect a population of cells that express GH as well as gonadotropins, as has been described in rat pituitary cells (68–70). Altogether, our results provide strong support for the idea that FOXO1 is expressed in a majority of gonadotrope cells and thus, it is likely that our results in primary pituitary culture and in vivo (Figs. 4 and 5) reflect insulin regulation of FOXO1 in gonadotropes in addition to other pituitary cell types that still remain to be fully defined.
It was previously demonstrated that in vivo administration of insulin to mice by intraperitoneal injection induced a two-fold increase in AKT Ser473 phosphorylation in the pituitary of wild-type mice within 10 minutes, but mice in which the insulin receptor was knocked out in the pituitary had no increase in AKT phosphorylation in response to insulin (63). In support of these studies, we found that in cultured murine primary pituitary cells, 30 minutes of insulin or IGF treatment induced AKT phosphorylation at Ser473 as well as Thr308, along with phosphorylation of FOXO1 (Fig. 4B). We also demonstrated that insulin could regulate pituitary AKT Thr308 and FOXO1 Ser256 phosphorylation within 10 minutes in vivo (Fig. 5). These results suggest that ex vivo and in vivo, insulin activation of pituitary insulin receptors results in rapid inhibitory phosphorylation of FOXO1. Taken together, these studies demonstrate that the growth factors, insulin and IGF1, activate AKT through their respective receptor signaling within the pituitary and that FOXO1 is a potential downstream effector of growth factor signaling in gonadotropes and other pituitary cell types such as thyrotropes and a subset of somatotropes.
GnRH signaling is the primary positive regulator of gonadotropin production. Since FOXO1 inhibits gonadotropin transcription, we investigated the potential for GnRH to alter FOXO1 activity. Our investigation found that GnRH did not increase FOXO1 phosphorylation at the PI3K/AKT activated sites of Thr24, Ser256 or Ser319, nor did it increase AKT phosphorylation (Fig. 6A). GnRH also did not result in a detectable nuclear to cytoplasmic shift in FOXO1 localization (Fig. 6B). It should be noted that our findings differ from a recent report by Choi et al., which demonstrated that stimulation of LβT2 or dispersed rat pituitary cells with 100 nM GnRH for 30 minutes resulted in increased FOXO1 Ser256 phosphorylation (32). The authors hypothesized that GnRH signaled to FOXO1 via AKT, since their studies also showed that AKT Ser473 was phosphorylated in response to GnRH treatment, and phosphorylation of both FOXO1 and AKT was blocked by the PI3K inhibitor LY294002. Consistent with our current findings, a previous report in αT3-1 cells demonstrated that IGF1 stimulated AKT Ser473 phosphorylation, but 10 nM GnRH treatment for 30 seconds to 1 hour had no effect on AKT phosphorylation (38). Similarly, LβT2 cells treated with 10 nM GnRH for 15 minutes did not activate AKT, while insulin increased AKT Ser473 phosphorylation 14 fold (64). Furthermore, previous reports have shown that GnRH can decrease growth factor-induced AKT Ser473 phosphorylation, thereby reducing AKT activity (38, 64).
Since cross talk between the GnRH and growth factor signaling pathways might blunt insulin or IGF1 inhibition of FOXO1, we also investigated the effects of GnRH treatment on insulin or IGF1 activation of PI3K/AKT/FOXO1 signaling. When LβT2 cells were co-treated with insulin or IGF1 along with GnRH for 30 minutes, there was significant attenuation of AKT Ser473 phosphorylation, as previously reported (64), and Thr308 phosphorylation compared to insulin or IGF1 treatment alone (Fig. 7). In contrast, there was no significant decrease in FOXO1 phosphorylation at AKT-specific residues during the same time period. It is likely that GnRH inhibition of AKT phosphorylation is rapidly initiated, as studies by Rose and colleagues in αT3-1 cells demonstrated that AKT Ser473 was attenuated within 30 seconds of GnRH and IGF1 co-treatment and a 3-fold decrease in phosphorylation was observed after 5 minutes of co-treatment compared to IGF1 alone. Although it was significantly diminished, GnRH did not eliminate IGF1-induced AKT Ser473 phosphorylation (38). We speculate from our current studies that, while blunted by GnRH signaling, AKT activity is still sufficient for inhibition of FOXO1. This idea is supported by the fact that insulin can still shift FOXO1 cellular localization from the nucleus to the cytoplasm in the presence of GnRH (Fig. 7D), despite decreased AKT phosphorylation (Fig. 7A).
We also explored other possible effects GnRH signaling may have on FOXO1. For instance, FOXO1 is subject to several types of post-translational modification in addition to phosphorylation, such as acetylation, methylation and ubiquitination (71, 72). These modifications are initiated by a variety of stimuli and result in changes in FOXO1 subcellular localization, protein-protein interactions, transcriptional activity, and protein stability (71, 72). For example, GnRH is proposed to activate CREB binding protein (CBP), a histone acetyltranferase, via PKC signaling (73). FOXO1 can be acetylated by CBP at several lysine residues, causing a decrease in FOXO1 DNA binding which permits AKT access to phosphorylate FOXO1 at Ser256 (71, 74). We investigated whether GnRH induced FOXO1 acetylation, which would permit an increase in FOXO1 phosphorylation in the presence of insulin of IGF1 despite blunted AKT activity. Using a 10 nM GnRH time course from 0–6 hours and a FOXO1 acetylation antibody (Santa Cruz sc-49437) (75–79) we found that GnRH had no effect on FOXO1 acetylation in LβT2 cells (data not shown).
In summary, previous studies have established FOXO1 as a potential regulator of Lhb and Fshb synthesis in pituitary gonadotrope cells (30–32). Here, we have identified two extracellular signals that may modulate FOXO1 suppression of Lhb and Fshb transcription. Extracellular signals such as insulin and IGF1 activate cellular survival and metabolic gene programs to maintain homeostasis in nearly all cells (40, 80, 81). We propose that FOXO1 may negatively regulate gonadotropin gene expression when permissive signals such as insulin or IGF1 are reduced due to alterations in metabolic status. As illustrated in Fig. 8, growth factor signaling via PI3K/AKT results in FOXO1 phosphorylation and export to the cytoplasm. FOXO1 sequestration in the cytoplasm prevents FOXO1 suppression of Lhb and Fshb transcription. At this time, it is still unclear what extracellular signals or cellular conditions drive FOXO1 into the nucleus to inhibit gonadotropin promoter activity. For example, does FOXO1 function as a stress-response transcription factor in gonadotropes, as in other cell types (15)? Does FOXO1 respond to cellular conditions such as oxidative stress, DNA damage or endoplasmic reticulum stress to decrease Lhb and Fshb synthesis? Further studies are needed to clarify the role of FOXO1 in gonadotrope physiology. Establishing gonadotrope-specific genetic models lacking FOXO1 or overexpressing a constitutively active FOXO1 will help determine whether FOXO1 is necessary for fertility and how FOXO1 functions in the pituitary to negatively regulate gonadotropin production.
Figure 8. Model of growth factor signaling to FOXO1 in pituitary gonadotrope cells.
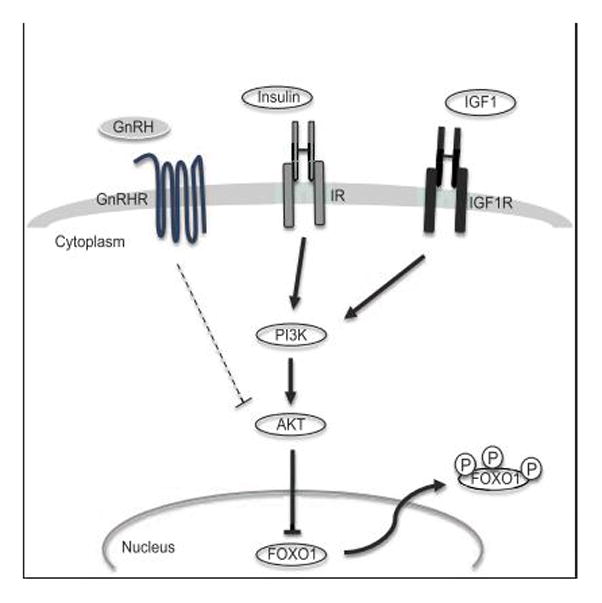
Insulin and IGF1 signal through their cognate receptors to PI3K and AKT, which directly phosphorylates FOXO1. AKT phosphorylation of FOXO1 causes its relocation to the cytoplasm. GnRH receptor activation can also attenuate insulin- or IGF1-induced AKT activity, possibly at the level of AKT, but FOXO1 inhibition by insulin or IGF1 still results in FOXO1 relocation to the cytoplasm. GnRHR, GnRH receptor; IR, insulin receptor; IGF1R, IGF1 receptor.
Highlights.
FOXO1 inhibits Lhb and Fshb transcription in pituitary gonadotropes
How FOXO1 is regulated in gonadotropes is unknown
Insulin and IGF1 regulate FOXO1 via the PI3K/AKT signaling pathway
Insulin and IGF1 are negative regulators of gonadotrope FOXO1 nuclear activity
Acknowledgments
The authors would like to thank Monica Rivera and Alissa Rivera for technical assistance, along with Dr. Kellie Breen-Church and Dr. Nina Grankvist for critical reading of this manuscript. The authors would also like to acknowledge NIDDK’s National Hormone and Peptide Program and Dr. A. F. Parlow for primary antibodies. This work was funded by NICHD/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R01 HD067448 to V.G.T., as well as T32 HD007203, F32 HD074414 and K12 GM068524 to D.V.S., and by NIGHMS through the Endocrine Society Minority Access Program (T36 GM095349) for M.R.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole, dilactate
- ATF2
activating transcription factor 2
- AKTi
AKT inhibitor VIII
- CGA
chorionic gonadotropin alpha subunit
- JNK
cJun N-terminal kinase
- CBP
CREB binding protein
- DMSO
dimethyl sulfoxide
- DMEM
Dulbecco’s modification of Eagle’s medium
- EGR1
early growth response protein 1
- ERK
extracellular-signal regulated kinase
- FBS
fetal bovine serum
- FSHB
follicle stimulating hormone beta
- FOXO1
forkhead box O1
- GnRH
gonadotropin releasing hormone
- GnRHR
GNRH receptor
- HRP
horseradish peroxidase-linked
- IGF1
insulin-like growth factor 1
- LHB
luteinizing hormone beta
- MAPK
mitogen activated protein kinase
- PITX1
paired-like homeodomain transcription factor 1
- PBS
phosphate buffered saline
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKC
protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, 3rd, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50:437–443. doi: 10.1016/j.bone.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress–induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuyama T. Abnormal Angiogenesis in Foxo1 (Fkhr)-deficient Mice. J Biol Chem. 2004:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 7.Goto T, Takano M. Transcriptional role of FOXO1 in drug resistance through antioxidant defense systems. Forkhead Transcription Factors. Adv Exp Med Biol. 2009;665:171–179. doi: 10.1007/978-1-4419-1599-3_13. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 9.Lam EWF, Brosens JJ, Gomes AR, Koo C-Y. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 10.Monsalve M, Olmos Y. The complex biology of FOXO. Curr Drug Targets. 2011;12:1322–1350. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu W-G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 13.Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. BBA - Mol Cell Res. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–241. doi: 10.1007/978-1-4419-1599-3_17. [DOI] [PubMed] [Google Scholar]
- 16.Kajihara T, Brosens JJ, Ishihara O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol. 2013;46:61–68. doi: 10.1007/s00795-013-0018-z. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Castrillon DH, Zhou W, Richards JS. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol. 2013;27:238–252. doi: 10.1210/me.2012-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121:3456–3466. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeburg PH, Mason AJ, Stewart TA, Nikolics K. The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog Horm Res. 1987;43:69–98. doi: 10.1016/b978-0-12-571143-2.50008-3. [DOI] [PubMed] [Google Scholar]
- 20.Vale W, Rivier C, Brown M. Regulatory peptides of the hypothalamus. Ann Rev Physiol. 1977;39:473– 527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- 21.Belchetz PE. Gonadotrophin regulation and clinical applications of GnRH. Clin Endocrinol Meta. 1983;12:619–640. doi: 10.1016/s0300-595x(83)80058-9. [DOI] [PubMed] [Google Scholar]
- 22.Huhtaniemi I. Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol Cell Endocrinol. 2006;254–255:84–90. doi: 10.1016/j.mce.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Huhtaniemi I, Ahtiainen P, Pakarainen T, Rulli SB, Zhang FP, Poutanen M. Genetically modified mouse models in studies of luteinising hormone action. Mol Cell Endocrinol. 2006;252:126–135. doi: 10.1016/j.mce.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSH beta subunit gene is highly conserved. Mol Hum Reprod. 2005;11:601–605. doi: 10.1093/molehr/gah198. [DOI] [PubMed] [Google Scholar]
- 25.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Ann Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 26.Thackray VG, Mellon PL, Coss D. Hormones in synergy: Regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203. doi: 10.1016/j.mce.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16:321–327. doi: 10.1097/MED.0b013e32832d88fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Dobkin-Bekman M, Naidich M, Rahamim L, Przedecki F, Almog T, Lim S, Melamed P, Liu P, Wohland T, Yao Z, Seger R, Naor Z. A Pre-formed Signaling Complex Mediates GnRH- Activated ERK-Phosphorylation of Paxillin and FAK at Focal Adhesions in LbT2 Gonadotrope Cells. Mol Endocrinol. 2009;23:1850–1864. doi: 10.1210/me.2008-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 transcription factor inhibits luteinizing hormone beta gene expression in pituitary gonadotrope cells. J Biol Chem. 2012;287:33424–33435. doi: 10.1074/jbc.M112.362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skarra DV, Arriola DJ, Benson CA, Thackray VG. Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes. Mol Endocrinol. 2013;27:1825–1839. doi: 10.1210/me.2013-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YS, Lee HJ, Ku CR, Cho YH, Seo MR, Lee YJ, Lee EJ. FoxO1 is a Negative Regulator of Follicle Stimulating Hormone-beta Gene Expression in Basal and Gonadotropin-Releasing Hormone-Stimulated Conditions in Female. Endocrinology. 2014;155:2277–2286. doi: 10.1210/en.2013-1177. [DOI] [PubMed] [Google Scholar]
- 33.Thackray VG. Fox tales: Regulation of gonadotropin gene expression by forkhead transcription factors. Mol Cell Endocrinol. 2013;385:62–70. doi: 10.1016/j.mce.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michaelis KA, Knox AJ, Xu M, Kiseljak-Vassiliades K, Edwards MG, Geraci M, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152:3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- 36.Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- 37.van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289–2299. doi: 10.1038/onc.2008.22. [DOI] [PubMed] [Google Scholar]
- 38.Rose A, Froment P, Perrot V, Quon MJ, LeRoith D, Dupont J. The luteinizing hormone-releasing hormone inhibits the anti-apoptotic activity of insulin-like growth factor-1 in pituitary alphaT3 cells by protein kinase Calpha-mediated negative regulation of Akt. J Biol Chem. 2004;279:52500–52516. doi: 10.1074/jbc.M404571200. [DOI] [PubMed] [Google Scholar]
- 39.Katic M, Kahn CR. The role of insulin and IGF-1 signaling in longevity. Cell Mol Life Sci. 2005;62:320–343. doi: 10.1007/s00018-004-4297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers MG, White MF. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 42.Arden KC, Biggs WH. Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch Biochem Biophys. 2002;403:292–298. doi: 10.1016/s0003-9861(02)00207-2. [DOI] [PubMed] [Google Scholar]
- 43.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 44.Nakae J, Barr V, Accili D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 2000;19:989–996. doi: 10.1093/emboj/19.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kousteni S. FoxO1: A molecule for all seasons. J Bone Miner Res. 2011;26:912–917. doi: 10.1002/jbmr.306. [DOI] [PubMed] [Google Scholar]
- 46.Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97:393–403. doi: 10.1093/cvr/cvs426. [DOI] [PubMed] [Google Scholar]
- 47.Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol. 2013;1:125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buggs C, Weinberg F, Kim E, Wolfe A, Radovick S, Wondisford F. Insulin augments GnRH-stimulated LHbeta gene expression by Egr-1. Mol Cell Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C. Activin signaling pathways in ovine pituitary and LbetaT2 gonadotrope cells. Biol Reprod. 2003;68:1877–1887. doi: 10.1095/biolreprod.102.012005. [DOI] [PubMed] [Google Scholar]
- 50.Fowkes RC, Sidhu KK, Sosabowski JK, King P, Burrin JM. Absence of pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein alpha-subunit gene in LbetaT2 gonadotrophs reveals disrupted cAMP-mediated gene transcription. J Mol Endocrinol. 2003;31:263–278. doi: 10.1677/jme.0.0310263. [DOI] [PubMed] [Google Scholar]
- 51.Gutiérrez S, Mukdsi JH, Aoki A, Torres AI, Soler AP, Orgnero EM. Ultrastructural immunolocalization of IGF-1 and insulin receptors in rat pituitary culture: evidence of a functional interaction between gonadotroph and lactotroph cells. Cell Tissue Res. 2007;327:121–132. doi: 10.1007/s00441-006-0283-4. [DOI] [PubMed] [Google Scholar]
- 52.Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- 53.Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- 54.Dorn C, Mouillet JF, Yan X, Ou Q, Sadovsky Y. Insulin enhances the transcription of luteinizing hormone-beta gene. Am J Obstet Gynecol. 2004;191:132–137. doi: 10.1016/j.ajog.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 55.Weiss JM, Polack S, Diedrich K, Ortmann O. Effects of insulin on luteinizing hormone and prolactin secretion and calcium signaling in female rat pituitary cells. Arch Gynecol Obstet. 2003;269:45–50. doi: 10.1007/s00404-003-0506-9. [DOI] [PubMed] [Google Scholar]
- 56.Andrade J, Quinn J, Becker RZ, Shupnik MA. AMP-Activated Protein Kinase Is a Key Intermediary in GnRH-Stimulated LH Gene Transcription. Mol Endocrinol. 2013;27:828–39. doi: 10.1210/me.2012-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354:605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 61.Horvath E, Kovacs K. Fine structural cytology of the adenohypophysis in rat and man. J Electron Microsc Tech. 1988;8:401–432. doi: 10.1002/jemt.1060080410. [DOI] [PubMed] [Google Scholar]
- 62.Majumdar S, Farris CL, Kabat BE, Jung DO, Ellsworth BS. Forkhead Box O1 is present in quiescent pituitary cells during development and is increased in the absence of p27 Kip1. PLoS One. 2012;7:e52136. doi: 10.1371/journal.pone.0052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navratil AM, Song H, Hernandez JB, Cherrington BD, Santos SJ, Low JM, Do MH, Lawson MA. Insulin augments gonadotropin-releasing hormone induction of translation in LbetaT2 cells. Mol Cell Endocrinol. 2009;311:47–54. doi: 10.1016/j.mce.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME, Lindsley CW. Discovery of 2,3,5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorg Med Chem Lett. 2005;15:905–909. doi: 10.1016/j.bmcl.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 68.Childs GV, Unabia G, Rougeau D. Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) beta-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH beta, FSH beta, and/or growth hormone. Endocrinology. 1994;134:990–997. doi: 10.1210/endo.134.2.8299592. [DOI] [PubMed] [Google Scholar]
- 69.Childs GV, Unabia G, Wu P. Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. doi: 10.1210/endo.141.4.7429. [DOI] [PubMed] [Google Scholar]
- 70.Childs GV. Growth hormone cells as co-gonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11:168–175. doi: 10.1016/s1043-2760(00)00252-6. [DOI] [PubMed] [Google Scholar]
- 71.Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813:1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 73.Miller RS, Wolfe A, He L, Radovick S, Wondisford FE. CREB binding protein (CBP) activation is required for luteinizing hormone beta expression and normal fertility in mice. Mol Cell Biol. 2012;32:2349–2358. doi: 10.1128/MCB.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci USA. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30:256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, Yu J, Ng HK, Ling MT, Huang AL, Cai XF, Ko BC. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71:4138–4149. doi: 10.1158/0008-5472.CAN-10-4274. [DOI] [PubMed] [Google Scholar]
- 78.Guido C, Panza S, Santoro M, Avena P, Panno ML, Perrotta I, Giordano F, Casaburi I, Catalano S, De Amicis F, Sotgia F, Lisanti MP, Ando S, Aquila S. Estrogen receptor beta (ERbeta) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle. 2012;11:2911–2921. doi: 10.4161/cc.21336. [DOI] [PubMed] [Google Scholar]
- 79.Yao XH, Nguyen HK, Nyomba BL. Prenatal ethanol exposure causes glucose intolerance with increased hepatic gluconeogenesis and histone deacetylases in adult rat offspring: reversal by tauroursodeoxycholic acid. PLoS One. 2013;8:e59680. doi: 10.1371/journal.pone.0059680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Federici M, Porzio O, Zucaro L, Fusco A, Borboni P, Lauro D, Sesti G. Distribution of insulin/insulin-like growth factor-I hybrid receptors in human tissues. Mol Cell Endocrinol. 1997;15:2099–2111. doi: 10.1016/s0303-7207(97)04050-1. [DOI] [PubMed] [Google Scholar]
- 81.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9:2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lew M. Good statistical practice in pharmacology. Problem 2. Br J Pharmacol. 2007;152:299–303. doi: 10.1038/sj.bjp.0707372. [DOI] [PMC free article] [PubMed] [Google Scholar]


