Abstract
Introduction
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are precursor lesions that progress to invasive cancer through progressively worsening dysplasia. Although smoking is an established risk factor for pancreatic adenocarcinoma, potential associations with IPMN grade of dysplasia remain unclear.
Methods
Pancreatic resections for IPMN from 1995 to 2013 were retrospectively reviewed. A total of 446 patients in which the smoking status was documented were identified.
Results
Smoking history was positive in 47 % of patients. Of smokers, 50 % had branch-duct, 14 % had main-duct, and 36 % had mixed-type IPMN. Patients with main-duct IPMN were more commonly smokers (65 %), compared to smoking history in 46 % with mixed and 44 % with branch-duct IPMN (p=0.03). High-grade dysplasia occurred in 25 % of smokers and 21 % of nonsmokers (p=0.32), and invasive carcinoma in 25 % of smokers and 25 % nonsmokers (p= 0.95). On multivariate analysis, duct size was independently associated with high-grade dysplasia (OR=3.17, 95 %CI= 1.79–5.64, p<0.001). Presence of mural nodules (OR=3.34, 95 %CI=1.82–6.12, p<0.001), duct size (OR=3.87, 95 %CI=2.21–6.75, p<0.001), and symptoms (OR=7.10, 95 %CI=3.80–13.08, p<0.001), but not smoking history (OR=1.10, 95 %CI=0.64–1.88, p=0.73), were independent predictors of invasive carcinoma. Median overall survival was 70 months for smokers and 88 months for nonsmokers (p=0.68).
Conclusion
Positive smoking history correlated with duct type classification but does not appear to be a risk factor for harboring high-grade dysplasia or invasive carcinoma in IPMNs.
Keywords: Pancreas, IPMN, Intraductal papillary mucinous neoplasm, Smoking, Pancreatectomy
Introduction
Intraductal papillary mucinous neoplasms (IPMNs) are characterized by cystic dilatation of the pancreatic ducts which are lined by mucin-producing epithelial cells with columnar features, with or without papillary projection.1,2 IPMN may arise in the main pancreatic ducts, side branch ducts, or both, known as main-duct type, branch-duct type, and mixed-type IPMNs, respectively.3–5 IPMNs are thought to progress from low-grade dysplasia to high-grade dysplasia to invasive carcinoma, analogous to the adenoma to invasive carcinoma progression of colorectal cancer. IPMNs therefore provide a unique opportunity to intervene before the development of invasive pancreatic cancer.5,6 The frequency of surgically resected IPMNs harboring high-grade dysplasia and/or an associated invasive adenocarcinoma ranges significantly in different studies and is reported to be as low as 6 % in branch-duct IPMNs to as high as 92 % in main-duct IPMNs.4,7–13
Established risk factors for the development of pancreatic adenocarcinoma include family history of pancreatic cancer, tobacco exposure, diabetes mellitus, chronic pancreatitis, and obesity.14,15 Some individuals with a strong family history of pancreatic cancer also appear to have an increased risk of developing an IPMN.1,16 However, evidence regarding environmental risk factors for the development of IPMNs or progression to high-grade dysplasia and invasive carcinoma in existing IPMNs is lacking.17,18
According to the Center for Disease Control and Prevention (CDC), the estimated general population smoking prevalence is reported to be between 18 and 21 %.19 Smoking history has been reported to be responsible for approximately 20 % of pancreatic cancers.15,20 However, the prevalence of smoking in patients with IPMN and the influence of smoking on progression of IPMN to invasive cancer is unclear. Since smoking is a well-established risk factor for the development of pancreatic adenocarcinoma,21 we sought to determine the influence of smoking on IPMN pathology—a precursor to pancreatic adenocarcinoma. Specifically, in this study, we investigate the association of smoking with duct type, with the degree of dysplastic changes, and with surgical outcomes of patients undergoing resection of IPMN.
Materials and Methods
Study Population
This study was approved by the Johns Hopkins Institutional Review Board (IRB). A retrospective review of all patients undergoing surgical resection of IPMN at Johns Hopkins Hospital (JHH) between 1995 and 2013 was performed. We excluded patients without adequate smoking history documentation and only included patients who had at least one preoperative imaging study (CT scan, endoscopic ultra-sound (EUS), or MRI) at JHH. Based on these selection criteria, a total of 446 patients were identified for analysis.
Clinicopathologic data including patient age, gender, race, smoking history, type of surgical resection, and grade of dysplasia were collected. Preoperative imaging findings analyzed were duct type, lesion size, and presence of mural nodules. Smoking history was collected through patients’ records. Complications were scored by the Clavien-Dindo classification scheme with major complications being defined as Clavien grade III or more. Patients were classified as smokers if they reported ever being a habitual smoker, if they ever smoked for 6 months or longer, or if they had a smoking history of >100 cigarettes in their lifetime, and nonsmokers if they denied ever being a smoker. Patients’ survival time was calculated from the time of operation to death or last follow-up. Patients with less than 30 days of follow-up were excluded from the survival analysis.
Intraductal Papillary Mucinous Neoplasms
Based on preoperative imaging performed at JHH, IPMNs were classified as branch-duct type IPMN, in which the cystic lesion was present without the dilation of the main pancreatic duct, as main-duct type, in which the main pancreatic duct was dilated without associated cystic lesion, and as mixed-type IPMN if the cystic lesion was present with a coexisting dilated main duct. Main duct dilation was defined as a main pancreatic duct size of 5 mm or more in any portion of the duct, and cyst size was defined as the maximal size measured on pre-operative imaging. Multifocal IPMN was defined as the presence of more than one grossly separate cyst in more than one region of the pancreas. All pathologic specimens were examined by one expert pancreatic pathologist at JHH. IPMNs were defined as flat or papillary mucin containing epithelial neoplasms with involvement of the main or side branches of pancreatic duct. The highest grade of dysplasia in the lesion was considered as the IPMN histologic grade. Neoplasms were classified as low-, intermediate-, or high-grade dysplasia (formerly known as carcinoma in situ) and as harboring an invasive carcinoma according to WHO guidelines.1,3,6 Margin status was defined as positive if either invasive carcinoma or high-grade dysplasia were present at any surgical margin.
Statistical Analysis
Clinicopathologic characteristics of IPMN patients were compared in the smoker and nonsmokers. We further classified IPMNs into three groups in order to evaluate the effect of smoking and other factors on developing high-grade dysplasia and invasive carcinoma. The three groups were classified as low- or intermediate-grade dysplasia, high-grade dysplasia, and associated invasive carcinoma. Categorical variables were expressed as a percentage and compared using chi-squared or Fisher's exact tests as appropriate. Continuous variables expressed as mean and were compared using a Wilcoxon-Mann-Whitney test or the Kruskal-Wallis test. The impact of smoking status on survival was estimated by using log-rank test and Kaplan-Meier survival curve estimates. Statistical analysis was performed using Stata/MP version 12.1 (StataCorp, College Station, TX).
Results
From 1995 to 2013, 446 patients with adequate smoking history underwent pancreatic resection for an IPMN at our institution. Clinicopathologic characteristics of the entire cohort are shown in Table 1. The study population consisted of 229 (51 %) male patients. The mean age at diagnosis was 69 years (range 19–93 years). A total of 236 (53 %) patients were diagnosed with branch-duct IPMN, 46 (10 %) with main-duct IPMN and 164 (37 %) with mixed-type IPMN. Pancreaticoduodenectomy was performed in 302 (68 %) patients, distal pancreatectomy in 107 (24 %), central pancreatectomy in 3 (1 %), and total pancreatectomy in 34 (7 %) patients.
Table 1.
Characteristics of all IPMNs by smoking history
| Total N (%) | Nonsmoker (%), 238 (53 %) | Smoker (%), 208 (47 %) | p value | |
|---|---|---|---|---|
| Age | ||||
| Mean±sd | 68.0±11.1 | 67.6±11.6 | 68.5±10.4 | 0.59 |
| Median | 69 | 69 | 70 | |
| Gender | ||||
| Male | 229 (51) | 107 (45) | 122 (59) | 0.004 |
| Female | 217 (49) | 131 (55) | 86 (41) | |
| Race (%) | 0.18 | |||
| Caucasian | 391 (88) | 204 (86) | 187 (90) | |
| Others | 55 (12) | 34 (14) | 21 (10) | |
| Duct type | 0.03 | |||
| Branch | 236 (53) | 133 (56) | 103 (50) | |
| Main | 46 (10) | 16 (7) | 30 (14) | |
| Mixed | 164 (37) | 89 (37) | 75 (36) | |
| Multifocal IPMN | 145 (36) | 79 (36) | 66 (36) | 0.91 |
| Surgery type | 0.80 | |||
| Whipple | 302 (68) | 162 (68) | 140 (67) | |
| Distal | 107 (24) | 54 (23) | 53 (25) | |
| Total | 34 (7) | 20 (8) | 14 (7) | |
| Central | 3 (1) | 2 (1) | 1 (1) | |
| Dysplasia | 0.56 | |||
| Low-intermediate | 231 (52) | 128 (54) | 103 (50) | |
| High-grade | 102 (23) | 50 (21) | 52 (25) | |
| Invasive Carcinoma | 113 (25) | 60 (25) | 53 (25) | |
| Positive margin | 45 (10) | 21 (9) | 24 (12) | 0.34 |
| Complications | 242 (54) | 139 (58) | 103 (50) | 0.06 |
| Clavien grade ≥3 | 41 (9) | 23 (10) | 18 (9) | 0.71 |
| PF | 87 (20) | 53 (22) | 34 (16) | 0.12 |
| DGE | 68 (15) | 43 (18) | 25 (12) | 0.08 |
On final pathologic assessment, low-grade dysplasia was observed in 70 (16 %), intermediate dysplasia in 161 (36 %), high-grade dysplasia in 102 (23 %), and IPMN with an associated invasive carcinoma in 113 (25 %) patients.
Smoking History and IPMN
Of 446 patients, 208 (47 %) were smokers and 238 (53 %) were nonsmokers. The mean age of patients was not significantly different in the smoker (mean age 69 years, range 35– 93) versus nonsmoker groups (mean age 68 years, range 19– 90) groups (p=0.59). Male patients were more likely to be smokers (53 vs. 40 %, p=0.004). Interestingly, smoking history was seen more frequently among patients with main-duct type IPMN. Of the 46 patients with main-duct IPMN, 30 (65 %) were smokers and 16 (35 %) were nonsmokers, compared to a positive smoking history in 46 % of patients with mixed-type and 44 % with branch-duct IPMN (p=0.03). There was no difference in the rate of multifocal IPMN in smokers (36 %) versus nonsmokers (36 %) (p=0.91).
Smokers and nonsmokers had no difference in the presence of high-grade dysplasia (25 vs. 21 %, p=0.32) or invasive cancer (25 vs. 25 %, p=0.95). Positive margins (R1 or R2) were observed in 21 (9 %) nonsmokers and 24 (12 %) smokers (p=0.34).
Postoperative complications were observed in 242 (54 %) patients, of which 139 (57 %) were smokers and 103 (43 %) were nonsmokers (p=0.06). Severe postoperative complications occurred in 18 (9 %) smokers and 23 (10 %) nonsmokers (p=0.71). There were no differences amongst smokers and nonsmokers in terms of the rate of pancreatic fistula (PF) (16 vs. 22 %, p=0.12) and delayed gastric emptying (DGE) (12 vs. 18 %, p=0.08) after surgery (Table 1).
On multivariate analysis, main pancreatic duct size was independently associated with the presence of high-grade dysplasia (OR 3.17, 95 % CI 1.79–5.64, p<0.001). The presence of mural nodules (OR 3.34, 95 % CI 1.82–6.12, p<0.001), main pancreatic duct size (OR 3.87, 95 % CI 2.21–6.75, p<0.001), and symptoms (OR 7.05, 95 % CI 3.80–13.08, p<0.001), but not smoking history (OR 1.10, 95 % CI 0.64–1.88, p=0.73), were independent predictors of the presence of an associated invasive carcinoma when compared to patients with low or moderate grade dysplasia (Table 2).
Table 2.
Multivariate analysis of predictors of high-grade dysplasia and invasive carcinoma compared to low-intermediate grade dysplasia in all IPMNs
| High-grade dysplasia |
Invasive carcinoma |
|||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| Age | 1.01 | 0.99, 1.04 | 0.24 | 1.01 | 0.99, 1.04 | 0.40 |
| Gender | 1.19 | 0.72, 1.98 | 0.50 | 1.43 | 0.82, 2.49 | 0.21 |
| Smoker | 1.24 | 0.75, 2.04 | 0.40 | 1.10 | 0.64, 1.88 | 0.73 |
| Cyst size ≥3 cm | 1.64 | 0.98, 2.75 | 0.06 | 1.38 | 0.79, 2.42 | 0.26 |
| Mural nodule | 1.19 | 0.66, 2.14 | 0.57 | 3.34 | 1.82, 6.12 | <0.001 |
| Symptoms | 1.52 | 0.93, 2.51 | 0.10 | 7.05 | 3.80, 13.08 | <0.001 |
| MPD size | 3.17 | 1.79, 5.64 | <0.001 | 3.87 | 2.21, 6.75 | <0.001 |
MPD main pancreatic duct
Branch-Duct IPMN
The characteristics of patients with branch-duct type IPMN are summarized in Table 3. Of branch-duct IPMNs, 122 (52 %) were male. The mean age of this group was 66.8 years (median 69, range 32–90 years).
Table 3.
Characteristics of branch-duct IPMNs by smoking history
| Total N (%) | Nonsmoker (%), 133 (56 %) | Smoker (%), 103 (44 %) | p value | |
|---|---|---|---|---|
| Age | 0.21 | |||
| Mean±sd | 66.8±10.8 | 66.2±10.5 | 67.7±11.2 | |
| Median | 69 | 68 | 69 | |
| Gender | 0.01 | |||
| Male | 122 (52) | 59 (44) | 63 (61) | |
| Female | 114 (48) | 74 (56) | 40 (39) | |
| Race (%) | 0.88 | |||
| Caucasian | 203 (86) | 114 (86) | 89 (86) | |
| Others | 33 (14) | 19 (14) | 14 (14) | |
| Cyst size ≥3 cm | 113 (48) | 57 (43) | 56 (54) | 0.08 |
| Cyst size | 0.09 | |||
| Mean±sd | 3.1±1.6 | 3.1±1.7 | 3.2±1.4 | |
| Median | 3.0 | 2.8 | 3.1 | |
| Multifocal IPMN | 87 (37) | 51 (39) | 36 (35) | 0.56 |
| Mural nodule | 49 (21) | 28 (21) | 21 (20) | 0.90 |
| Surgery type | 0.22 | |||
| Whipple | 150 (64) | 80 (60) | 70 (68) | |
| Distal | 68 (29) | 39 (29) | 29 (28) | |
| Total | 15 (6) | 12 (9) | 3 (3) | |
| Central | 3 (1) | 2 (2) | 1 (1) | |
| Dysplasia | 0.29 | |||
| Low-intermediate | 162 (69) | 92 (69) | 70 (68) | |
| High-grade | 42 (18) | 20 (15) | 22 (21) | |
| Invasive Carcinoma | 32 (13) | 21 (16) | 11 (11) | |
| Positive margin | 17 (7) | 8 (6) | 9 (9) | 0.42 |
| Complications | 139 (59) | 83 (62) | 56 (54) | 0.21 |
| PF | 57 (24) | 38 (29) | 19 (18) | 0.07 |
| DGE | 38 (16) | 22 (17) | 16 (16) | 0.83 |
The mean cyst size of the entire branch-duct group was 3.1 cm (range 0.5–10 cm). On pathologic assessment, low-grade dysplasia was seen in 61 (26 %), intermediate-grade dysplasia in 101 (43 %), high-grade dysplasia in 42 (18 %), and an associated invasive carcinoma in 32 (13 %) patients. Positive surgical margins were found in 17 (7 %) branch-duct IPMN.
Of the 236 patients with branch-duct IPMN, 103 (44 %) were smokers. There was no statistical difference in mean cyst size (3.2 vs. 3.1 cm, p=0.09), presence of mural nodules (20 vs. 21 %, p=0.90), and lesion multifocality (35 vs. 39 %, p=0.56) between smokers and nonsmokers. Among 103 branch-duct IPMN that arose in patients who smoked, 22 (21 %) had high-grade dysplasia and 11 (11 %) had an associated invasive carcinoma. In comparison, patients without a smoking history were found to have high-grade dysplasia in 20 (15 %) and invasive carcinoma in 21 (16 %) of their branch-duct IPMNs. The histologic grade of the lesions did not correlate with smoking history (p=0.29). There was no difference in the presence of positive surgical margins between smokers and nonsmokers (9 vs. 6 %, p= 0.42). The overall complication rate was 54 % in smokers and 62 % in nonsmokers (p=0.21). There was no difference observed in the rates of PF (18 vs. 29 %, p=0.07) or DGE (16 vs. 17 %, p=0.83) amongst smokers and nonsmokers.
Among branch-duct IPMNs, after adjusting for patients’ gender, age, presence of mural nodules, symptoms, and cyst size, smoking history was not found to be a risk factor for high-grade dysplasia (OR=1.22, 95 % CI=0.59–2.48, p= 0.59) or the presence of an associated invasive carcinoma (OR=0.59, 95 % CI=0.23–1.50, p=0.27) based on final pathology (Table 4).
Table 4.
Multivariate analysis of predictors of high-grade dysplasia and invasive carcinoma compared to low-intermediate grade dysplasia in branch-duct IPMN
| High-grade dysplasia |
Invasive carcinoma |
|||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| Age | 1.01 | 0.98, 1.04 | 0.54 | 1.01 | 0.97, 1.06 | 0.67 |
| Gender | 1.26 | 0.61, 2.60 | 0.54 | 1.37 | 0.55, 3.44 | 0.50 |
| Smoker | 1.22 | 0.59, 2.48 | 0.59 | 0.59 | 0.23, 1.50 | 0.27 |
| Cyst size ≥3 cm | 1.94 | 0.93, 4.03 | 0.08 | 1.48 | 0.59, 3.72 | 0.40 |
| Mural nodule | 1.17 | 0.47, 2.89 | 0.73 | 7.80 | 2.92, 20.87 | <0.001 |
| Symptoms | 1.29 | 0.64, 2.62 | 0.48 | 12.25 | 3.94, 38.10 | <0.001 |
Main-Duct IPMN
Forty-six (10 %) patients were diagnosed with a main-duct IPMN, of which 30 (65 %) patients were smokers. Amongst smokers with a main-duct IPMN, low-intermediate grade dysplasia was seen in 30 %, high-grade dysplasia in 17 %, and invasive carcinoma in 53 % of IPMNs. The distribution of dysplasia grades was similar in nonsmokers with main-duct IPMN with low-intermediate grade dysplasia in 31 %, high-grade dysplasia in 38 %, and invasive carcinoma in 31 %. The incidence of complications was similar in the two groups (30 % in smokers and 56 % in nonsmokers, p=0.12). Specifically, smokers and nonsmokers were not statistically different with regard to the development DGE (7 vs. 19 %, p=0.32) and PF (13 vs. 13 %, p=1.00) after resection for a main-duct IPMN (Table 5).
Table 5.
Characteristics of main-duct type IPMNs by smoking history
| Total N (%) | Nonsmoker (%), 16 (35 %) | Smoker (%), 30 (65 %) | p value | |
|---|---|---|---|---|
| Age | 0.64 | |||
| Mean±sd | 66.3±12.5 | 66.1±15.8 | 66.4±10.6 | |
| Median | 67 | 69 | 67 | |
| Gender | 0.85 | |||
| Male | 25 (54) | 9 (56) | 16 (53) | |
| Female | 21 (46) | 7 (44) | 14 (47) | |
| Race (%) | 0.60 | |||
| Caucasian | 42 (91) | 14 (88) | 28 (93) | |
| Others | 4 (9) | 2 (12) | 2 (7) | |
| Surgery type | 1.00 | |||
| Whipple | 34 (74) | 12 (75) | 22 (73) | |
| Distal | 7 (15) | 01 (6) | 6 (20) | |
| Total | 5 (11) | 3 (19) | 2 (7) | |
| Dysplasia | 1.00 | |||
| Low-intermediate | 14 (30) | 5 (31) | 9 (30) | |
| High-grade | 11 (24) | 6 (38) | 5 (17) | |
| Invasive Carcinoma | 21 (46) | 5 (31) | 16 (53) | |
| Positive margin | 7 (15) | 2 (13) | 5 (17) | 1.00 |
| Complications | 18 (39) | 9 (56) | 9 (30) | 0.12 |
| PF (%) | 6 (13) | 2 (13) | 4 (13) | 1.00 |
| DGE | 5 (11) | 3 (19) | 2 (7) | 0.32 |
Mixed-Type IPMN
A total of 164 patients were diagnosed with a mixed-type IPMN on preoperative imaging. Positive smoking history was seen in 46 % (75/164) of patients with mixed-type IPMN (Table 6). The mean size of all resected mixed-type IPMNs was 3.2 cm. The mean cyst size in smokers was similar to nonsmokers (3.4 vs. 3.0 cm, p=0.23), as was the presence of multifocality (40 vs. 31 %, p=0.26). Of smokers, 24 (32 %) had an IPMN with low-intermediate dysplasia, 25 (33 %) had high-grade dysplasia, and 26 (35 %) had an IPMN with an associated invasive carcinoma. These numbers were statistically similar to those observed in nonsmokers, with low-intermediate dysplasia in 31 (35 %), high-grade dysplasia in 24 (27 %), and invasive carcinoma in 34 (38 %) (p=0.67). Smokers and nonsmokers were similar regarding postoperative complication rates (53 vs. 51 %, p=0.78), PF rates (15 vs. 15 %, p=0.99), and DGE rates (20 vs. 9 %, p=0.05).
Table 6.
Characteristics of mixed-type IPMNs by smoking history
| Total N (%) | Nonsmoker (%), 89 (54 %) | Smoker (%), 75 (46 %) | p value | |
|---|---|---|---|---|
| Age | 0.61 | |||
| Mean±sd | 70.2±10.7 | 70.1±12.1 | 70.3±8.9 | |
| Median | 72 | 72 | 71 | |
| Gender | 0.08 | |||
| Male | 82 (50) | 39 (44) | 43 (57) | |
| Female | 82 (50) | 50 (56) | 32 (43) | |
| Race (%) | 0.13 | |||
| Caucasian | 146 (89) | 76 (85) | 70 (93) | |
| Others | 18 (11) | 13 (15) | 5 (7) | |
| Cyst size ≥3 cm | 86 (52) | 47 (53) | 39 (52) | 0.92 |
| Cyst size | 0.23 | |||
| Mean±sd | 3.2±1.6 | 3.0±1.6 | 3.4±1.6 | |
| Median | 3 | 3 | 3 | |
| Multifocal IPMN | 58 (35) | 28 (31) | 30 (40) | 0.26 |
| Mural nodule | 65 (40) | 28 (31) | 37 (49) | 0.02 |
| Surgery type | 0.10 | |||
| Whipple | 118 (72) | 70 (78) | 48 (64) | |
| Distal | 32 (20) | 14 (16) | 18 (24) | |
| Total | 14 (9) | 5 (6) | 9 (12) | |
| Dysplasia | 0.67 | |||
| Low-intermediate | 55 (34) | 31 (35) | 24 (32) | |
| High-grade | 49 (30) | 24 (27) | 25 (33) | |
| Invasive Carcinoma | 60 (37) | 34 (38) | 26 (35) | |
| Complications | 85 (52) | 47 (53) | 38 (51) | 0.78 |
| PF | 24 (15) | 13 (15) | 11 (15) | 0.99 |
| DGE | 25 (15) | 18 (20) | 7 (9) | 0.05 |
| Positive margin | 21 (13) | 11 (12) | 10 (13) | 0.85 |
Survival Outcomes
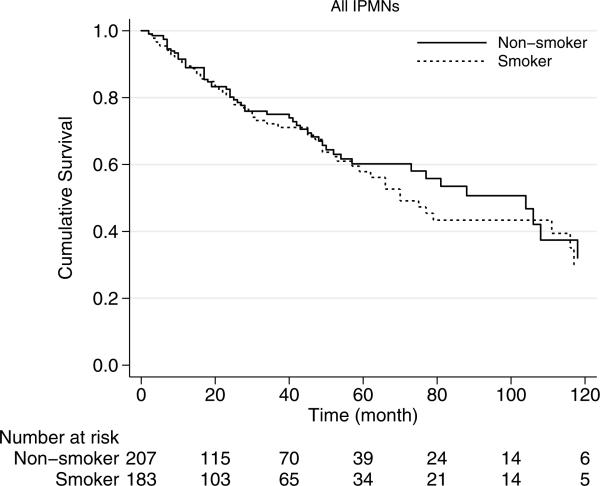
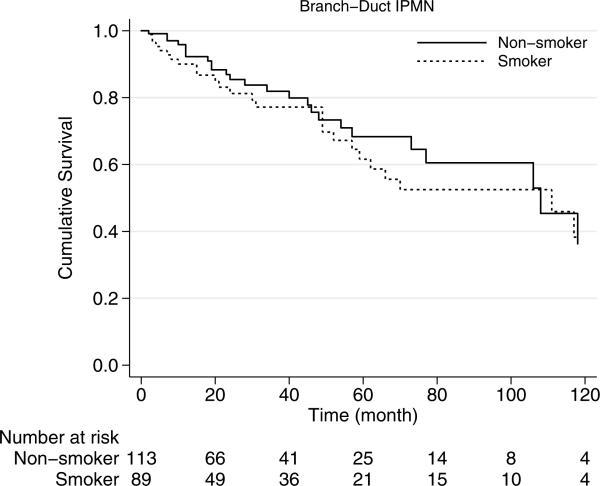
The median overall survival of smokers was 70 months, which was similar to those patients without smoking history who had a median overall survival of 88 months (p=0.68) (Fig. 1). Similarly, among patients with branch-duct IPMN, survival did not correlate with smoking history (p=0.57). Among branch-duct IPMNs, the 5-year survival rate was 62 % for smokers and 68 % for nonsmokers (Fig. 2).
Fig. 1.
Kaplan-Meier survival curves of all the patients with resected IPMN based on smoking history. Median overall survival: smokers, 70 months; nonsmokers, 88 months; p=0.68
Fig. 2.
Kaplan-Meier survival curves of branch-duct type IPMN by smoking history. Survival of the patients with smoking history is similar to those without smoking history (p=0.57)
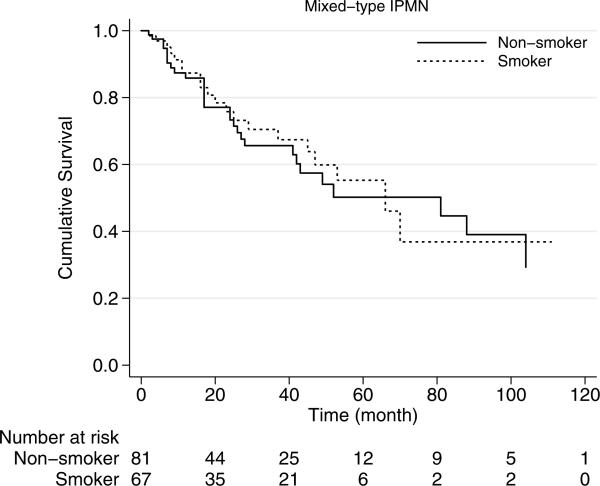
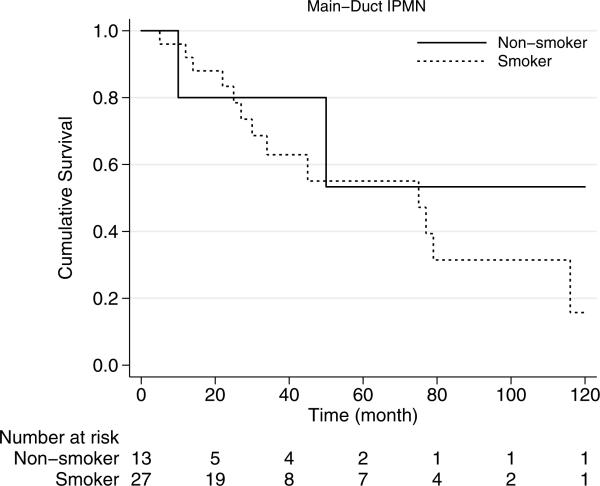
In mixed-type IPMNs, smoking history was associated with median survival of 66 months, which was similar to those without smoking history with median survival of 81 months (p=0.74) (Fig. 3). Median overall survival of main-duct IPMNs was 75 months and was statistically similar in smokers and nonsmokers (p=0.62) (Fig. 4).
Fig. 3.
Kaplan-Meier survival curves of mixed-type IPMN by smoking history. Survival of the patients with smoking history is similar to those without smoking history (p=0.62)
Fig. 4.
Kaplan-Meier survival curves of main-duct type IPMN by smoking history. Survival of the patients with smoking history is similar to those without smoking history (p=0.74)
Discussion
A significant body of evidence supports that IPMNs are precursor lesions of invasive pancreatic adenocarcinoma. Unlike pancreatic intraepithelial neoplasm (PanINs), IPMNs are macroscopic and present an opportunity for early treatment in individuals with this neoplasm. Approximately 70 % of main-duct type and mixed-type IPMNs have either high-grade dysplasia or an associated invasive carcinoma, and recommendations are therefore to resect all lesions with main duct involvement.5,22 In contrast, the prevalence of high-grade dysplasia/associated invasive carcinoma is lower in patients with surgically resected branch-duct IPMN, and as a result, the management of these lesions is still under debate.4,5,7–12,22,23 Since smoking is a well-established risk factor for the development of pancreatic adenocarcinoma,21 we sought to determine the influence of smoking on IPMN pathology. Despite the strong association of smoking with pancreatic cancer, we report that a positive smoking history is not statistically associated with higher incidence of high-grade dysplasia or invasive carcinoma in patients who underwent pancreatic resection for IPMN. To our knowledge, this study is the first to directly evaluate this question in surgically resected IPMNs with pathologic confirmation of the grades of dysplasia. A potential shortcoming of our study is that all subjects were selected for surgical resection and the patients believed to have IPMN that were not selected for resection are difficult to capture in a retrospective study. We found, however, that smoking history was more prevalent among patients who underwent resection for main-duct type IPMN.
Interestingly, while smoking history is an important cause of pancreatic adenocarcinoma, our results do not demonstrate a correlation between smoking history and development of invasive carcinoma or high-grade dysplasia in patients with IPMN lesions. These findings, along with the others investigating IPMN risk factors, indicate that smoking history cannot be used to predict the risk of high-grade dysplasia/associated invasive carcinoma in patients with a known IPMN.
Our results, which uniquely focus on resected patients, are consistent with the work of others on IPMN and smoking. In a case-control study by Baumgaertner et al.24 smoking history was not found to be a risk factor for the development of IPMN. Another case-control study by Capurso et al.17 showed that 51 % of the patients with a histologically proven or presumed diagnosis of IPMN were smokers and demonstrated that there were no difference in the rate of smoking history between the controls and patients with IPMN. Also in support of our finding, they demonstrated that smoking history was associated with the development of main-duct type IPMN. Especially in the subgroup of patients who had a histologically diagnosed IPMN, obtained by EUS or surgical specimens, this association was stronger. This observation could be partly a result of the fact that the majority of the patients with a main-duct IPMN undergo a surgical resection according to the Sendai resection criteria.
Although main-duct IPMNs have a significant risk of progression to invasive carcinoma and despite a higher rate of smoking history we found in patients diagnosed with main-duct type IPMN, our results did not demonstrate any difference in histologic degree of dysplasia in these IPMNs between smokers and nonsmokers. This difference may be a consequence of the different genetic and epigenetic alterations in the two diseases. Although certain driver genes such as KRAS are known to be important in both IPMN associated cancer and non-IPMN-associated cancer, genetic alterations such as GNAS are unique to IPMN tumorigenesis.25–27 In this regard, tobacco carcinogens may differentially alter the unique genetic and epigenetic pathways involved in tumor initiation and progression.
Our study also shows that tobacco consumption is not an independent risk factor for development of high-grade dysplasia or invasive carcinoma in branch-duct IPMN. In a study by Nagai et al., rate of smoking was not significantly associated with malignancy in branch and mixed-type IPMN.28 Sturm et al.29, in their series of 274 surgically resected IPMN, demonstrated that smoking history in obese patients is not associated with malignancy in branch and main-duct type IPMN. While many studies have merged invasive carcinoma and high-grade dysplasia into the term “malignancy,” we separately considered the two conditions in our study. By multivariate analysis, the presence of a mural nodule on preoperative imaging predicted invasive carcinoma, but not high-grade dysplasia.
It should be noted that our study, owing to its retrospective nature, had some limitations, including the lack of quantitative smoking information. In addition, information on the number of tobacco-free years was absent for previous smokers. Although the quantity of tobacco exposure is believed to be associated with the development of pancreatic cancer, a recent study demonstrated that the number of pack-years smoking was not associated with development of IPMN.17 More precise investigation is required to investigate the effect of the duration and amount of smoking exposure on histologic grades of IPMN. Moreover, smoking history is likely to be underreported by patients, which could possibly bias our results. In addition, our study suffers from selection bias because only patients that underwent resection for IPMN were analyzed. Patients being followed for presumed IPMN at our institution obviously lack pathologic confirmation of their diagnosis. Despite these limitations, this study is the largest study that confirms findings from prior, smaller studies that smoking is not associated with an increased risk of high-grade dysplasia or invasive adenocarcinoma in patients with IPMNs.
In conclusion, positive smoking history in patients with IPMN is more common in patients undergoing resection of main-duct IPMN but does not appear to cause a greater risk of harboring high-grade dysplasia or associated invasive carcinoma. Thus, based on our study, smoking history should not alter the management of patients with IPMNs.
Acknowledgments
Funding Supported by NIH SPORE grant CA62924
Contributor Information
Neda Rezaee, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA.
Saami Khalifian, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA.
John L. Cameron, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA
Timothy M. Pawlik, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ralph H. Hruban, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Elliot K. Fishman, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Martin A. Makary, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA
Anne Marie Lennon, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA; Department of Gastroenterology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Christopher L. Wolfgang, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Matthew J. Weiss, Departments of Surgery, Johns Hopkins University School of Medicine, 600 North Wolfe St, Halsted 608, Baltimore, MD 21287, USA Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- 1.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43(1):1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Kloppel G, Luttges J, Memoli VA, Tosteson TD, Yanagisawa A, Wilentz R, Zamboni G. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillarymucinous neoplasms: interobserver agreement. Pancreas. 2005;31(4):344–349. doi: 10.1097/01.mpa.0000186245.35716.18. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–651. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 6.Hruban RH, Pitman MB, Klimstra DS, American Registry of P. Armed Forces Institute of P, Tumors of the Pancreas . Atlas of tumor pathology: Fourth series, Fascicle 6. American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; Washington, DC: 2007. [Google Scholar]
- 7.Crippa S, Fernandez-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Dominguez I, Muzikansky A, Thayer SP, Falconi M, Mino-Kenudson M, Capelli P, Lauwers GY, Partelli S, Pederzoli P, Warshaw AL. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8(2):213–219. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(5):678–685. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SC, Park KT, Lee YJ, Lee SS, Seo DW, Lee SK, Kim MH, Jang SJ, Byun JH, Han DJ. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg. 2008;15(2):183–188. doi: 10.1007/s00534-007-1231-8. [DOI] [PubMed] [Google Scholar]
- 10.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239(6):788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90(10):1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 12.Kanno A, Satoh K, Hirota M, Hamada S, Umino J, Itoh H, Masamune A, Asakura T, Shimosegawa T. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45(9):952–959. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 13.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143(7):639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 14.Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, Castagnini A, Di Francesco V, Frulloni L, Bovo P, Vaona B, Angelini G, Vantini I, Cavallini G, Pederzoli P. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44(7):1303–1311. doi: 10.1023/a:1026670911955. [DOI] [PubMed] [Google Scholar]
- 15.Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, Zheng W, Albanes D, Amundadottir L, Bingham SA, Boffetta P, Boutron-Ruault MC, Chanock SJ, Clipp S, Hoover RN, Jacobs K, Johnson KC, Kooperberg C, Luo J, Messina C, Palli D, Patel AV, Riboli E, Shu XO, Rodriguez Suarez L, Thomas G, Tjonneland A, Tobias GS, Tong E, Trichopoulos D, Virtamo J, Ye W, Yu K, Zeleniuch-Jacquette A, Bueno-de-Mesquita HB, Stolzenberg-Solomon RZ. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170(4):403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142(4):796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capurso G, Boccia S, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, Manta R, Fabbri C, Ventrucci M, Tarantino I, Piciucchi M, Carnuccio A, Boggi U, Leoncini E, Costamagna G, Delle Fave G, Pezzilli R, Bassi C, Larghi A. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case–control study. Am J Gastroenterol. 2013;108(6):1003–1009. doi: 10.1038/ajg.2013.42. [DOI] [PubMed] [Google Scholar]
- 18.Khan S, Sclabas G, Reid-Lombardo KM. Population-based epidemiology, risk factors and screening of intraductal papillary mucinous neoplasm patients. World J Gastrointest Surg. 2010;2(10):314–318. doi: 10.4240/wjgs.v2.i10.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaku IT, King BA, Dube SR. Current Cigarette Smoking Among Adults—United States, 2005–2012. MMWR. Morbidity and mortality weekly report. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 21.Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham E, Bamlet WR, Holly EA, Bertuccio P, Gao YT, Hassan M, Yu H, Kurtz RC, Cotterchio M, Su J, Maisonneuve P, Duell EJ, Boffetta P, La Vecchia C. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case–control Consortium (Panc4). Ann Oncol. 2012;23(7):1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012. 12(3):183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, Razo O, McGrath D, Pederzoli P, Fernandez-Del Castillo C. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133(1):72–79. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgaertner I, Corcos O, Couvelard A, Sauvanet A, Rebours V, Vullierme MP, Hentic O, Hammel P, Levy P, Ruszniewski P. Prevalence of extrapancreatic cancers in patients with histologically proven intraductal papillary mucinous neoplasms of the pancreas: a case–control study. Am J Gastroenterol. 2008;103(11):2878–2882. doi: 10.1111/j.1572-0241.2008.02142.x. [DOI] [PubMed] [Google Scholar]
- 25.House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24(2):193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Chadwick B, Willmore-Payne C, Tripp S, Layfield LJ, Hirschowitz S, Holden J. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl Immunohistochem Mol Morphol. 2009;17(1):31–39. doi: 10.1097/PAI.0b013e31817c02c6. [DOI] [PubMed] [Google Scholar]
- 27.Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C, Goggins MG, Kinzler KW, Vogelstein B, Eshleman JR, Hruban RH, Maitra A. Clinicopathological Correlates of Activating GNAS Mutations in Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann Surg Oncol. 2013;20(12):3802–3808. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai K, Doi R, Ito T, Kida A, Koizumi M, Masui T, Kawaguchi Y, Ogawa K, Uemoto S. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16(3):353–358. doi: 10.1007/s00534-009-0068-8. [DOI] [PubMed] [Google Scholar]
- 29.Sturm EC, Roch AM, Shaffer KM, Schmidt CM, 2nd, Lee SJ, Zyromski NJ, Pitt HA, Dewitt JM, Al-Haddad MA, Waters JA, Schmidt CM. Obesity increases malignant risk in patients with branch-duct intraductal papillary mucinous neoplasm. Surgery. 2013;154(4):803–808. doi: 10.1016/j.surg.2013.07.011. [DOI] [PubMed] [Google Scholar]