CONSPECTUS
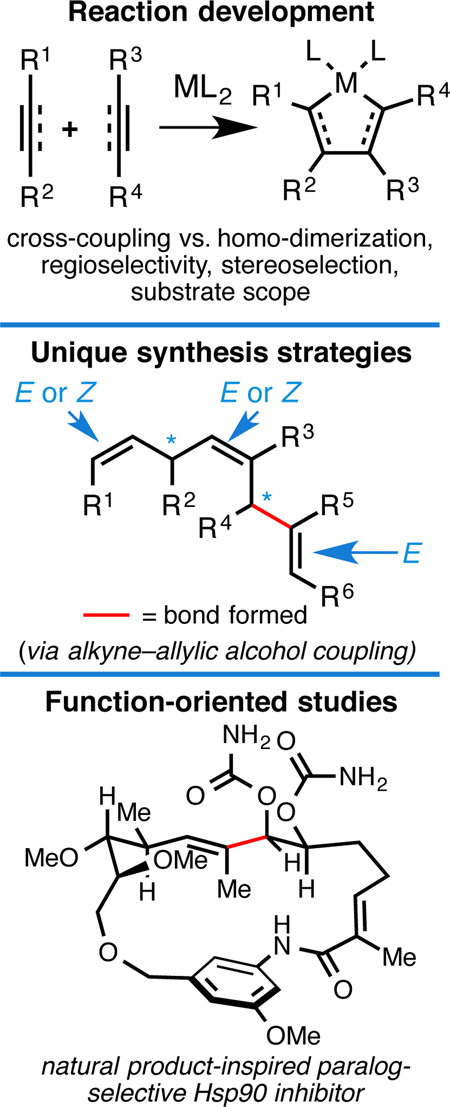
Convergent C–C bond-forming reactions define the fabric of organic synthesis and, when applied in complex molecule synthesis, can have a profound impact on efficiency by decreasing the longest linear sequence of transformations required to convert simple starting materials to complex targets. Despite their well-appreciated strategic significance, campaigns in natural product synthesis typically embrace only a small suite of reactivity to achieve such bond construction (i.e. nucleophilic addition to polarized π-bonds, nucleophilic substitution, cycloaddition, and metal-catalyzed “cross-coupling”), therefore limiting the sites at which convergent coupling chemistry can be strategically employed. In our opinion, it is far too often that triumphs in the field are defined by chemical sequences that do not address the challenges associated with discovery, development and/or production of natural product-inspired agents. We speculated that advancing an area of chemical reactivity not represented in the few well-established strategies for convergent C–C bond formation may lead to powerful new retrosynthetic relationships that could simplify approaches to the syntheses of a variety of different classes of natural products. Our studies ultimately embraced the pursuit of strategies to control the course of metallacycle-mediated “cross-coupling” between substrates containing sites of simple π-unsaturation (ubiquitous functionality in organic chemistry including alkenes, alkynes, allenes, aldehydes and imines, among others). In just eight years since our initial publication in this area, we have defined over twenty stereoselective intermolecular C–C bond-forming reactions that provide access to structural motifs of relevance for the synthesis of polyketides, fatty acids, alkaloids, and terpenes, while doing so in a direct and stereoselective fashion. These achievements continue to serve as the foundation of my group’s activity in natural product and function-oriented synthesis, where our achievements in reaction development are challenged in the context of complex targets. Among our early efforts, we achieved the most concise synthesis of a benzoquinone ansamycin ever described (macbecin I), and moved beyond this achievement to explore the role of our chemistry in function-oriented synthesis targeting the discovery of natural product-inspired Hsp90 inhibitors. These later efforts have led to the discovery of a uniquely selective benzoquinone ansamycin-inspired Hsp90 inhibitor that lacks the problematic quinone present in the natural series. This achievement was made possible by a concise chemical synthesis pathway that had at its core the application of metallacycle-mediated cross-coupling chemistry.
1. INTRODUCTION
Natural products continue to serve a central role in the discovery and development of pharmaceutical agents as well as the elucidation of new biological targets of therapeutic relevance.1 Despite this, efforts to advance natural products as therapeutics are often confronted by either or both of the following: (1) Challenges accessing substantial quantities of the natural product (from isolation or by synthetic means), and (2) difficulties associated with producing closely related synthetic analogs that may offer superior biological and physical properties in comparison to the natural material. While natural product isolation, engineered biosynthesis, and synthetic organic chemistry all have the potential to address these issues, it is the latter of these that is least constrained as a tool. That said, modern triumphs of organic chemistry in natural product synthesis, while typically demonstrating the utility/limitations of chemical methods and sometimes leading to the discovery of new reactivity, often proceed with levels of efficiency that make function-oriented pursuits or large-scale production cost prohibitive. It is this perspective that molded our early pursuits, as we envisioned offering contributions to help realize a future where the efficiency of chemical synthesis more frequently addresses/overcomes these limitations.
With such a lofty goal in mind that intentionally lacked a precise molecular focus (i.e. no specific target structure would be used to guide our program), early attention was directed at more clearly specifying the chemical focus of our program. We recognized that convergency is among the most important strategic considerations in complex molecule synthesis, providing a means to decrease the number of chemical steps required in sequence to convert simple starting materials into intricate structures. Because yields of organic reactions are typically less than quantitative, substantial contraction in the longest linear sequence of reactions employed in a synthesis campaign can have a profound impact on efficiency (i.e. overall yield and time). Also of substantial interest in function-oriented studies, points of convergency offer opportunities to install structural variation in a concise manner. Therefore, we moved forward with an analysis of methods typically employed for convergent coupling chemistry in the context of natural product synthesis.
While carbon–heteroatom bond-forming reactions are of paramount importance for the synthesis of natural product oligomers like carboyhydrates and peptides, carbon–carbon bond-forming processes are arguably more relevant for “small molecule” natural products whose backbones are often defined by an intricately organized collection of carbon atoms. Despite their importance, we recognized that only a small suite of chemical reactivity is routinely employed for convergent C–C bond formation in modern chemical synthesis: (1) nucleophilic addition to polarized π-bonds, (2) cycloaddition, and (3) metal-catalyzed “cross-coupling” (i.e. Pd- and Ni-catalyzed crosscoupling) and to a lesser extent, (4) nucleophilic substitution. While many other modes of reactivity are employed regularly to access molecular complexity (i.e. cation–olefin cyclization, radical cyclization, metal-catalyzed cycloisomerization, etc…) those that require an intramolecular setting to address reactivity and selectivity issues are, by definition, not convergent. Also, while carbometalation chemistry (i.e. Heck reaction)2 and olefin metathesis3 are playing an increasingly important role in synthesis, these areas of reactivity have not yet grown to the prominence of other methods for convergent coupling due to issues concerning substrate scope and stoichiometry, functional group compatibility, and/or stereoselectivity.
We wondered whether a different mode of chemical reactivity could be advanced to realize a collection of new convergent coupling reactions that would offer complementary retrosynthetic relationships to those stemming from the four basic modes of chemical reactivity discussed above. If the transformations pursued within this area of reactivity embraced commonly encountered functional groups, and delivered complex and otherwise difficult to prepare natural product motifs (or intermediates of utility to prepare such motifs), we expected that studies in this area could have a substantial impact on synthetic organic chemistry and natural product-based function-oriented synthesis.
Defining the Methodological Challenge
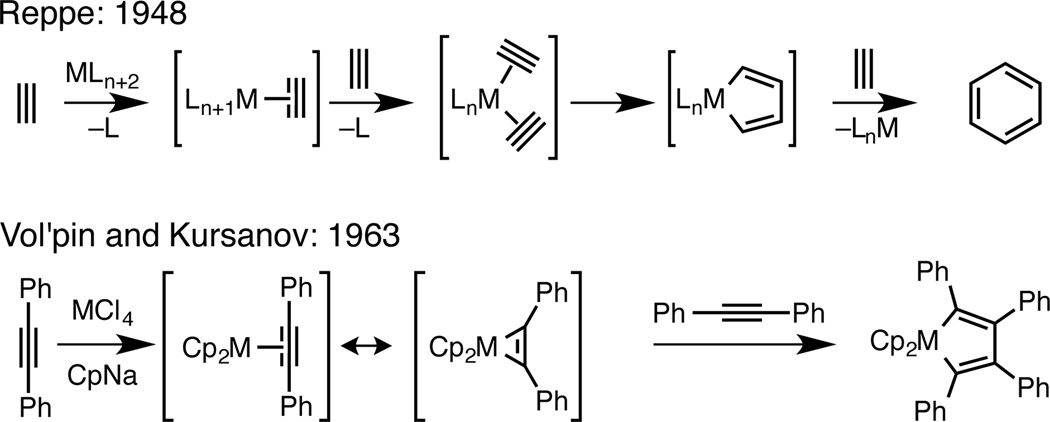
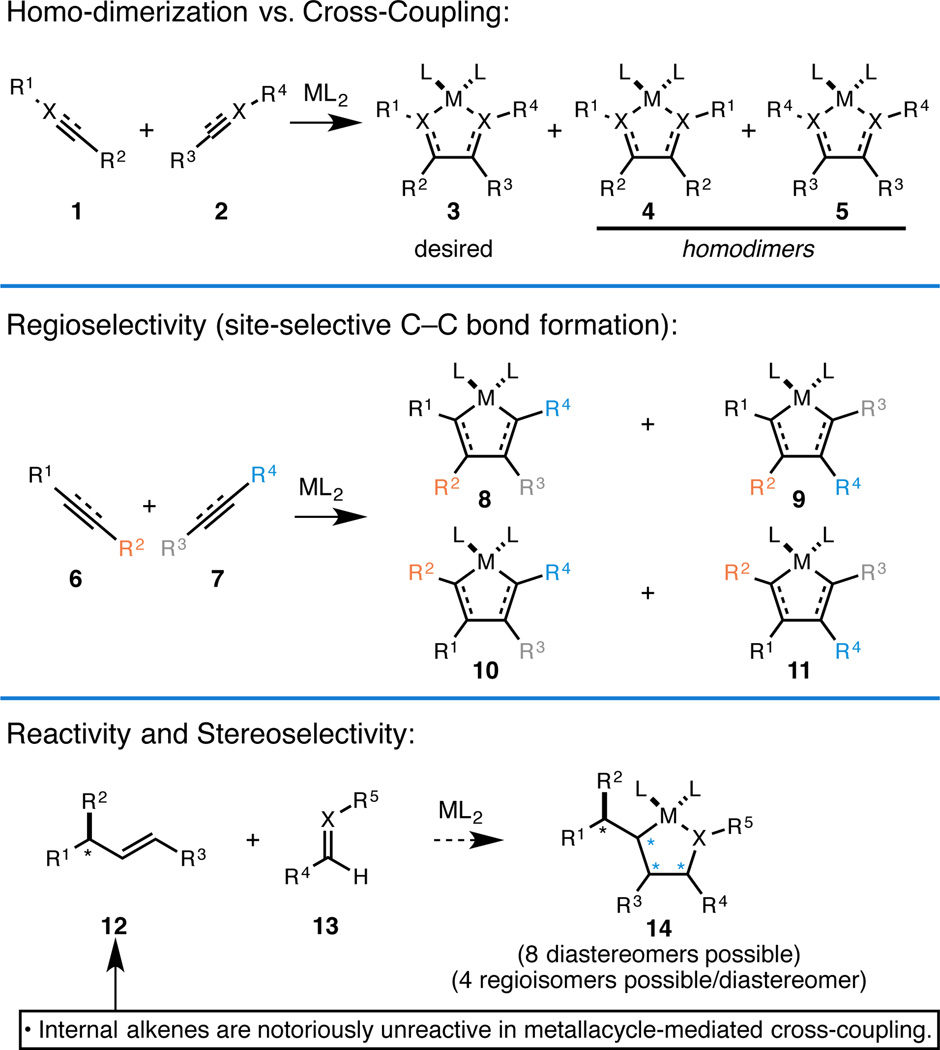
With the aforementioned design criteria in mind, we quickly gravitated toward chemistry associated with the synthesis and functionalization of metallacyclopentanes, formed from [2+2+1] annulation between low-valent metals and two unsaturated functional groups, the earliest examples of which date back to the Ni-catalyzed trimerization of acetylenes for the synthesis of substituted benzenes (Figure 1).4 While numerous advances in this area have been made over the last sixty years most complex demonstrartions remain constrained to intramolecular settings.5 In fact, independent of the nature or stoichiometry of the metal employed, intermolecular metallacycle-mediated bond-forming reactions have been broadly limited in scope due to challenges associated with: (1) favoring cross-coupling over homo-dimerization, (2) controlling site-selectivity (regioselection), (3) controlling stereoselection, and (4) overcoming generally low levels of reactivity (i.e. substituted alkenes) (Figure 2).
Figure 1.
Selected history of metallacycle-mediated cross-coupling.
Figure 2.
Ever-present challenges associated with metallacycle-mediated cross-coupling reactions.
Until recently most reports of metallacycle-mediated cross-coupling have addressed only a boutique collection of substrates, where the coupling partners have suitable properties to allow for successful/selective intermolecular bond formation (i.e. alkyne + aldehyde, and internal alkyne + terminal alkyne or minimally substituted alkene). Despite elegant modern contributions that realize catalysis in metallacycle-mediated bond construction, the utility of metal-centered [2+2+1] chemistry remains broadly limited by the aforementioned factors. By inventing chemical strategies to overcome these barriers, we aimed to define a broad class of chemical reactivity capable of delivering a range of new retrosynthetic relationships in organic chemistry while making available a plethora of new convergent C–C bond forming reactions.
Our Approach and Summary of Accomplishments
Our general strategy to control metallacycle-mediated cross-coupling chemistry was to seek a means to render this mode of reactivity “substrate directed”6 and, in particular, employ a common functional group encountered in natural product synthesis to direct such transformations. For this reason, the hydroxy group (R–OH) arose as an ideal candidate. For many C–C bond-forming reactions hydroxy groups are liabilities, requiring carefully orchestrated masking of their inherent reactivity to successfully execute the desired C–C bond construction.7 We thought it ideal to consider using an unprotected hydroxy group as a key organizational element to control what would emerge as a new class of C–C bond-forming convergent coupling reactions.
Additional attention was focused on designing a general means to favor cross-coupling over homo-dimerization across a large substrate scope. Because substoichiometric use of a low-valent metal establishes an ever-present competition between homo- and cross-coupling, at the outset of our program we were dissuaded from considering catalysis with respect to the central metal. We settled on a general strategy based on stoichiometric activation of one coupling partner, with coupling being achieved upon addition of the second π-component.
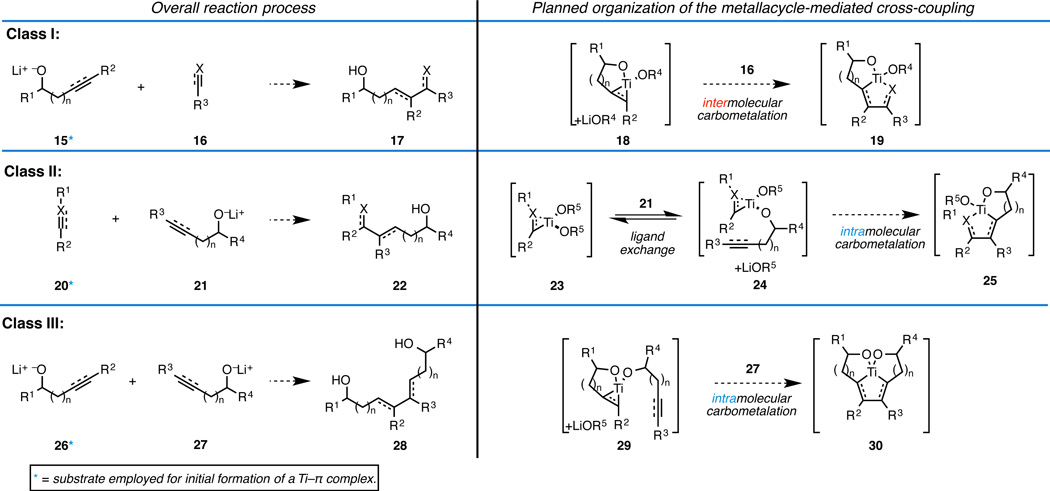
For the reasons articulated above, we decided to employ Ti(IV)-alkoxides as the central metal component in our program [Ti(Oi-Pr)4 currently costs < $20/mole, and on aqueous work up delivers byproducts that are both non-toxic and easily removed from reaction products of interest: TiO2 and i-PrOH].8 We conceived a few simple reaction designs for “hydroxyl- directed metallacycle-mediated cross-coupling,” where a metal alkoxide would play a central role in orchestrating the bimolecular C–C bond forming event (Figure 3). We distinguished between these designs by the order of bond-forming events planned and the nature of the carbometalation event (inter/intramolecular), naming them Class I, Class II, and Class III alkoxide-directed processes.
Figure 3.
Design of alkoxide-directed metallacycle-mediated cross-coupling reactions.
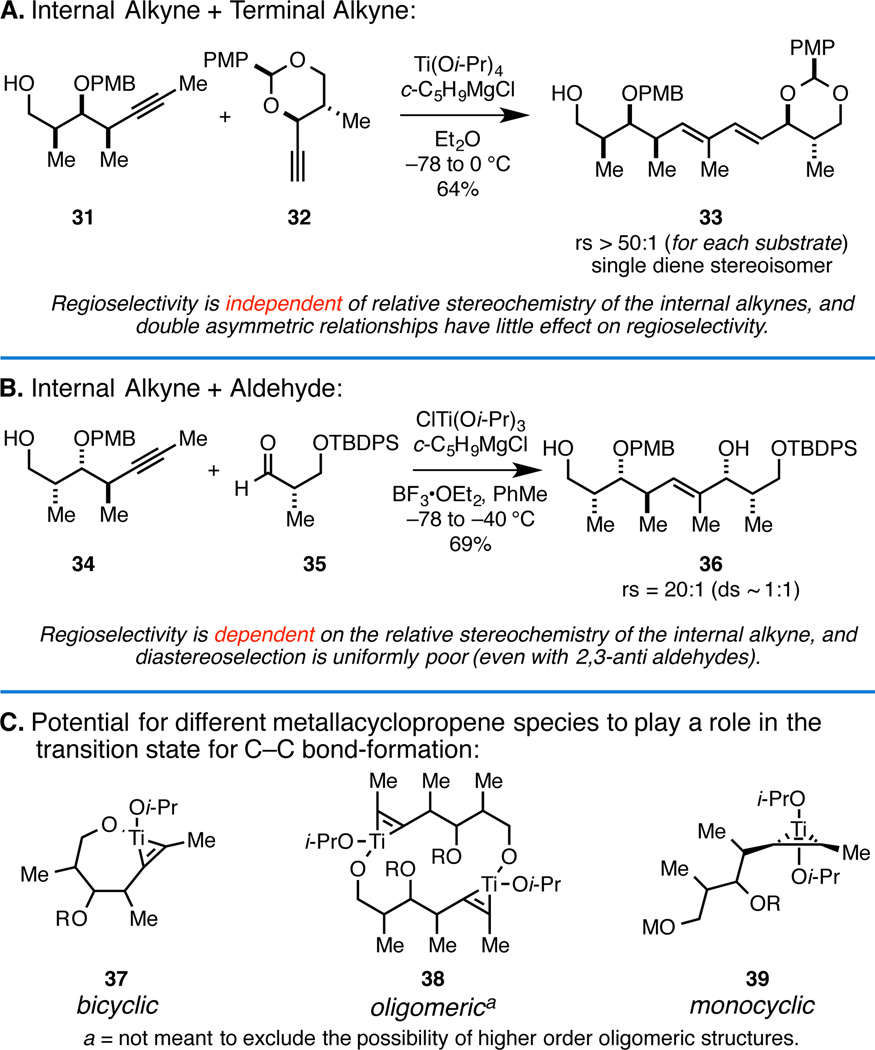
Our initial studies investigated the Class I design and led to the elucidation of regioselective coupling reactions of utility for the synthesis of polyketide-derived natural products (Figure 4A/B).9 While successes in this area led to the establishment of regioselective versions of known coupling reactions (i.e. alkyne + aldehyde or terminal alkyne) and defined an early foundation of our program, controlling these processes proved to be non-trivial, likely due to uncertainty regarding the nature of the reactive metallacyclic species that would participate in the transition state (i.e. 37–39; Figure 4).
Figure 4.
Class I alkoxide-directed metallacycle-mediated cross-coupling.
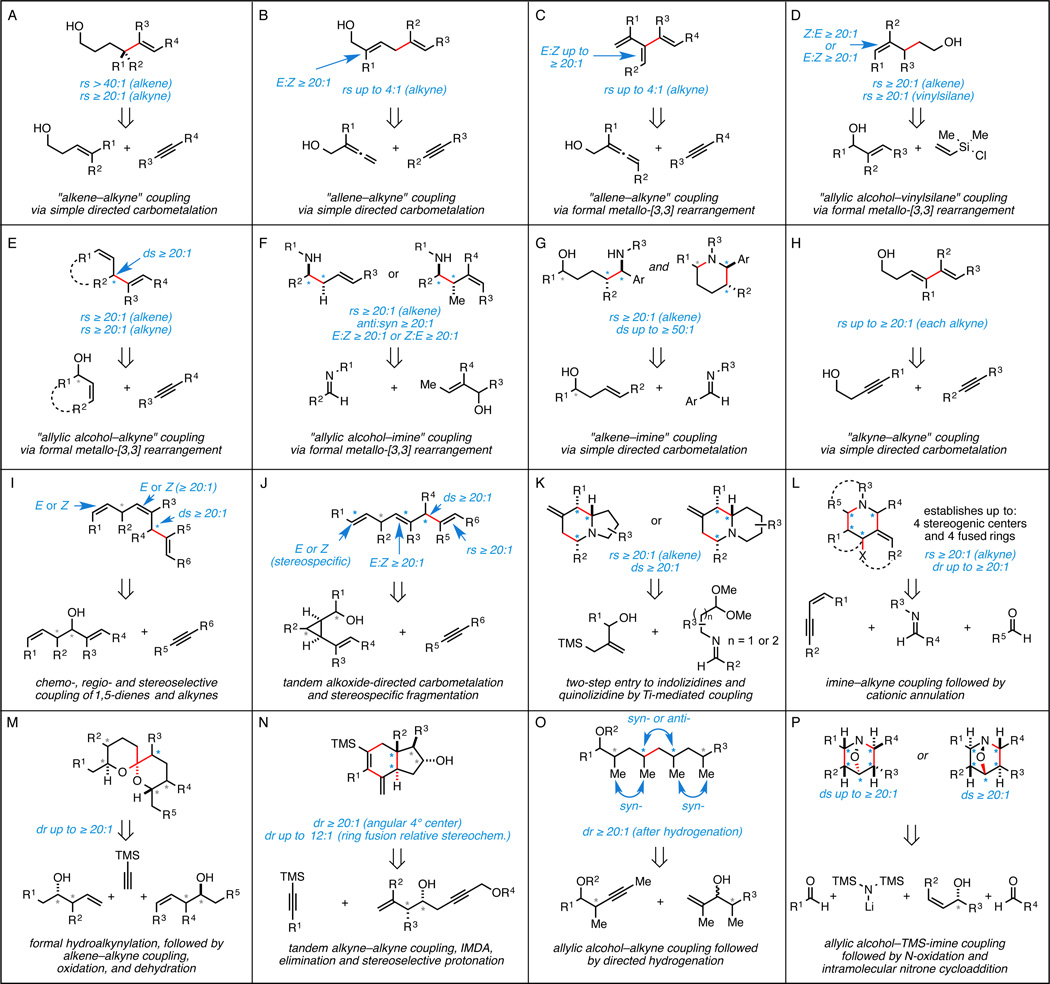
In contrast to these early pursuits, our investigation of Class II hydroxyl-directed coupling reactions has resulted in a large suite of novel and highly selective C–C bond-forming processes (Figure 5). Our initial forays were focused on simple alkoxide directed metallacycle-mediated cross-coupling (Figure 5A,B,G,H),10 while more complex coupling reactions soon emerged that were based on initial formation of a metallacyclopentane intermediate and subsequent elimination (such transformations were central to the contributions summarized in Figure 5C, 5D, 5E, 5F).11
Figure 5.
Examples of stereoselective convergent coupling reactions that proceed by Class II alkoxide-directed metallacycle-mediated coupling.
While we continue to pursue the development of novel coupling reactions, much of our recent effort has focused on moving forward with our initial suite as central components of strategies to access complex stereodefined motifs of potential utility in the synthesis of polyketides, fatty acids, alkaloids, and terpenes (i.e. Figure 5I–5P).12
Thorough study of the Class III design has not yet been accomplished, but some reports have appeared that support the feasibility of the approach for controlling convergent C–C bond formation.13
2. FUNCTION-ORIENTED STUDIES
After achieving success with our methods development program, the natural progression of our science was to explore how metallacycle-mediated coupling chemistry would perform in natural product synthesis. In particular, we aimed to identify targets of significant therapeutic potential, where state-of-the-art science had not yet been successful as a tool to drive large-scale production and/or function-oriented studies. Members of the benzoquinone ansamycin family of natural products rose as among the first targets that we pursued in this vein.
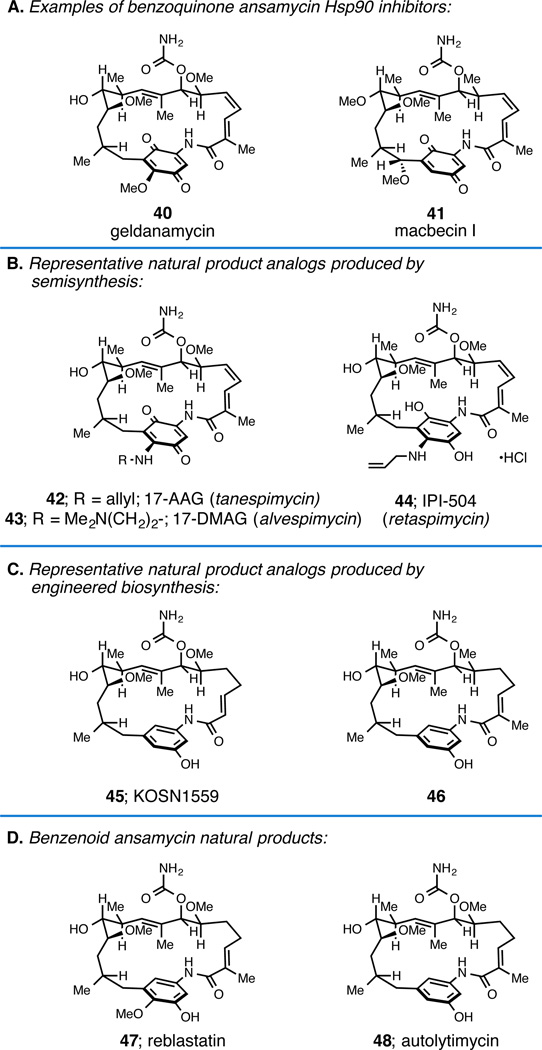
Nearly twenty years ago, geldanamycin (40) – the prototypical member of the benzoquinone ansamycins (Figure 6A) was found to inhibit Hsp90-pp60V-SRC heterocomplex formation.14 While this discovery provided evidence for the essential role that stress proteins play in oncogenic transformation, a growing body of literature contributed to significant enthusiasm for the development of clinically relevant Hsp90 inhibitors, as they could accomplish what many molecularly targeted anticancer agents do not: the simultaneous disruption of multiple signaling pathways critical to tumor cell growth and survival.
Figure 6.
Representative benzoquinone ansamycins, geldanamycin analogs and benzenoid ansamycins.
Due to the availability of geldanamycin from fermentation, and its historical position as the first known inhibitor of Hsp90, it was placed at the center of early drug development efforts. Unfortunately, geldanamycin proved to be an unsuitable therapeutic agent due to its poor “drug-like” properties that include an undesirable solubility profile and insufficient stability in vivo. These issues were compounded by the observation that administration of geldanamycin in animals results in significant hepatotoxicity. This latter observation is thought to be due, in part, to the presence of a quinone in the geldanamycin structure.14e Further, geldanamycin has nearly equipotent activity for inhibition of Grp94 — an Hsp90-like protein localized to the endoplasmic reticulum that chaperones secreted and membrane proteins.15 While known clients of Grp94 include Toll-like receptors, integrins, and bile salt-dependent lipase (BSDL), Grp94 plays an important role in lymphocyte development.16
The pursuit of a clinically relevant natural product-like Hsp90 inhibitor has been firmly rooted in natural product derivatization (semisynthesis). To date, the most well studied analogs offer decreased electrophilic character of the quinone and a more favorable solubility profile vs. the natural product (17-AAG (42), 17-DMAG (43), and IPI-504 (44); Figure 6B). The most studied analog, 17-AAG still has a relatively poor physical profile that requires formulation with DMSO or Cremophor. More soluble analogs have surfaced, but have not yet been demonstrated to be suitable as therapeutics (17-DMAG, IPI-504 – a hydroquinone analog of 17-AAG that is known to exist in equilibrium with 17-AAG in vivo).18
Given the molecular limitations inherent to semisynthesis, it is not surprising that analogs surfacing from early explorations typically retain the benzoquinone toxicophore — a structural motif that is not required for Hsp90 inhibition and that serves as a locus of redox cycling.17
Engineered biosynthesis has delivered natural product-like Hsp90 inhibitors that contain molecular motifs not seen in analogs derived from semisynthesis, two examples of which are illustrated in Figure 6C: (1) KOSN1559 (45) (Hsp90 Kd = 160 nM),19 and (2) macbecin analog 46 (Hsp90 Kd = 3 nM).20 These agents contain a non-benzoquinone aromatic core reminiscent to that observed in the potent benzenoid ansamycin natural products reblastatin (47) and autolytimycin (48) (Figure 6D).
While total synthesis could be viewed as a potential tool to produce novel compositions of matter in this class, the modest molecular complexity of this family has presented a substantial barrier to efficient synthesis.21 Structurally, they contain a functionalized benzoquinone/aromatic core that is bridged by a stereochemically dense fifteen-carbon ansa-chain. A number of total syntheses have been described, the first of which appeared nearly twenty five years ago and delivered macbecin I through a pathway that required over 40 sequential transformations. Notable improvements in efficiency were seen in later syntheses of macbecin I, herbimycin A, and geldanamcyin (some between 22–30 linear steps). Despite these significant achievements and the continued interest in developing Hsp90 inhibitors as clinically useful anticancer agents, chemical synthesis has yet to drive programs aimed at the discovery/ development of benzoquinone ansamycin-inspired Hsp90 inhibitors.
Our total synthesis of macbecin and a look forward to function-oriented studies:21n
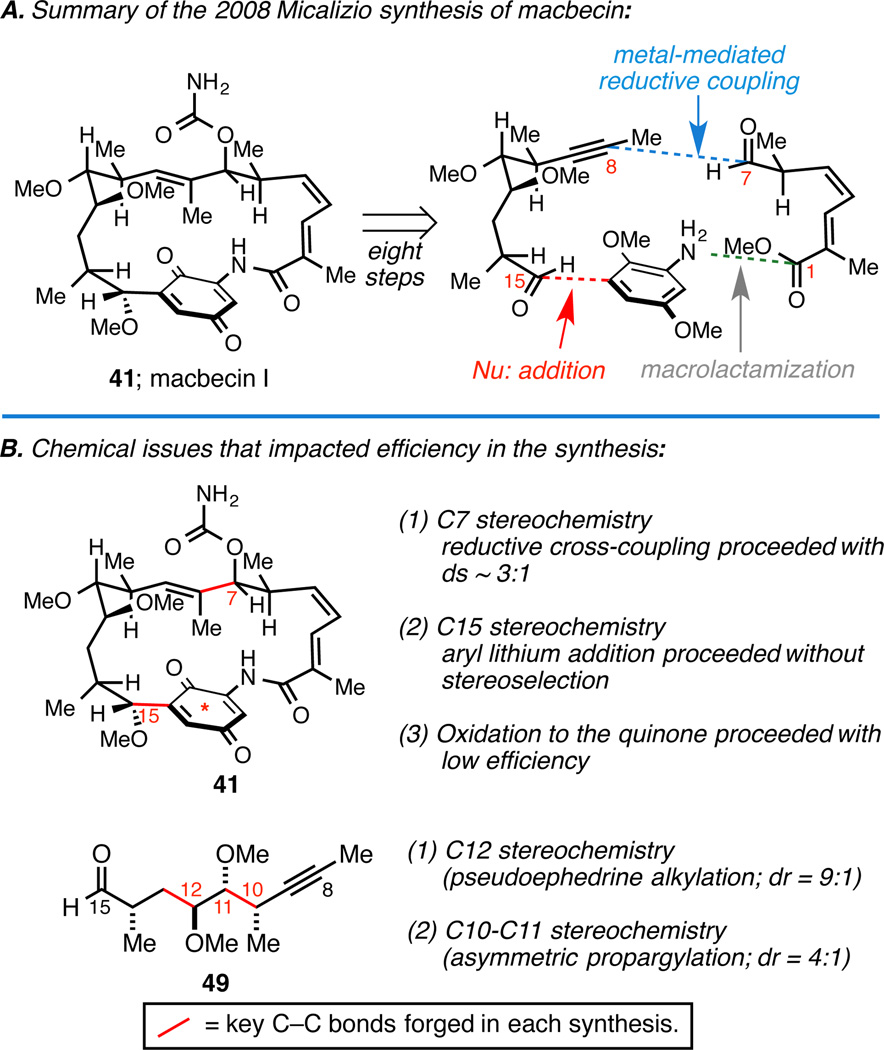
With industrial efforts continuing to pursue semisynthesis (alongside small molecule pursuits unrelated to the benzoquinone ansamycins), we initiated a program aimed at accomplishing a modern laboratory synthesis of macbecin I (41) that was meant to explore the potential utility of metallacycle-mediated cross-coupling in this class of natural products (Figure 7A). Our efforts wound up showcasing the mild reaction conditions and functional group compatibility of Ti(IV)-alkoxide-mediated reductive cross-coupling between internal alkynes and aldehydes, and was employed to establish the C7–C8 bond. The natural product was assembled in just eight steps from the coupling partners illustrated (longest linear sequence of 18 steps from commercially available material), and the route defined the shortest chemical sequence to any benzoquinone ansamycin ever reported.
Figure 7.
Summary and challenges encountered in our macbecin synthesis.
While the synthesis was considered a triumph, stereoselection in key convergent coupling reactions was moderate at best (particularly with respect to C7 and C15; ds ≤ 3:1; Figure 7B), late stage oxidation to the quinone proceeded with poor efficiency (as previously noted in the first total synthesis of macbecin I), and synthesis of the C8–C15 coupling partner suffered from two moderately selective reactions: (1) an alkylation to establish the stereochemistry at C12 (dr = 9:1), and (2) propargylation to set the C10 and C11 stereocenters (dr = 4:1).
As we moved forward, our goals shifted to defining a concise and efficient entry to benzoquinone ansamycin-inspired macrocyclic lactams that may offer unique Hsp90-binding profiles and superb physical properties in comparison to analogs derived from semisynthesis. These efforts required careful consideration of the molecular features required for Hsp90 inhibition and judicious selection of chemistry to maximize efficiency, selectivity, and flexibility of the synthetic route. While the aforementioned issues with stereoselection did not thwart our efforts to achieve a concise synthesis of the natural product, they greatly complicated our efforts to employ this chemical route in function-oriented synthesis studies.
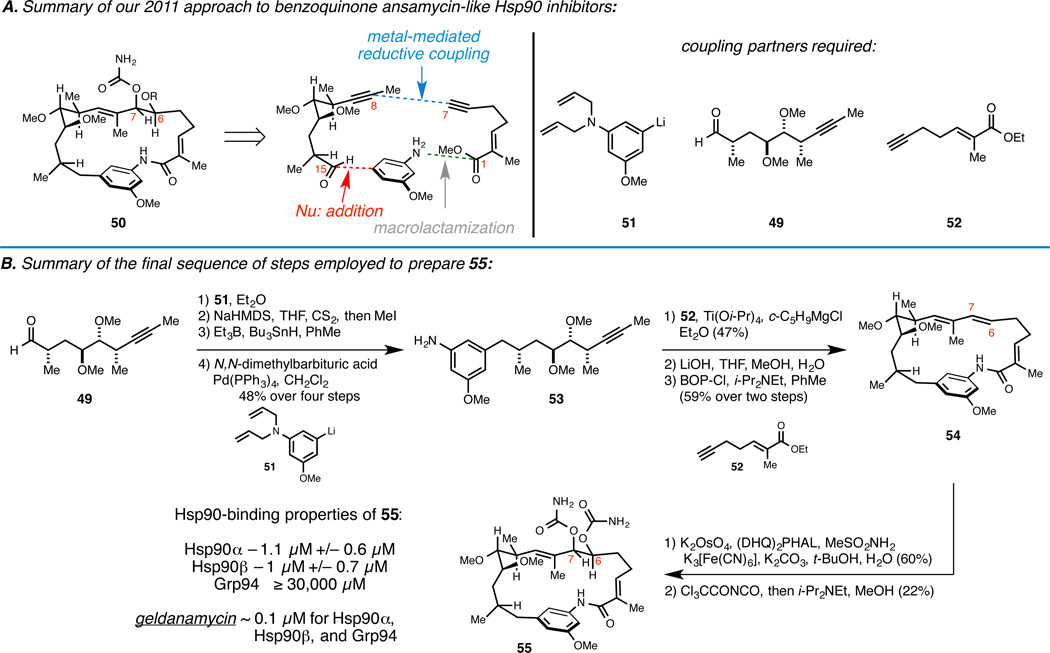
First-generation route to benzoquinone ansamycin-inspired Hsp90 inhibitors: Addressing C7 and C15.22
To address C7 in a concise fashion, we decided to alter the central metallacycle-mediated coupling process (Figure 8A). Not only was the aldehyde–alkyne coupling not stereoselective, it required a rather unstable coupling partner (the β,γ-unsaturated aldehyde). We thought that an alkyne–alkyne coupling process, to deliver a stereodefined 1,3-diene spanning C6–C9, could set the stage for subsequent site- and stereoselective Sharpless asymmetric dihydroxylation as an alternative means of establishing the C6 and C7 stereocenters. Also recognizing that a variety of natural products in this class lack the C15 – OMe substitution seen in macbecin, we moved forward with the design of a synthesis pathway to analogs that would lack substitution at this position.
Figure 8.
First–generation chemical approach for the synthesis of natural product-inspired Hsp90 inhibitors.
Aldehyde 49 (a substrate that was employed in our macbecin synthesis) was advanced to the alkyne-containing coupling partner 53 by a simple four-step sequence (Figure 8B). Next, Ti-mediated reductive cross-coupling with terminal alkyne 52 was followed by saponification of the ethyl ester and cyclization to deliver macrocyclic lactam 54. While site-selective Sharpless asymmetric dihydroxylation successfully delivered a stereodefined diol intermediate with the appropriate stereochemistry at C6 and C7, we were unable to differentiate this diol. Knowing that the C7 carbamate functionality is an essential structural feature for Hsp90-inhibition, we decided to convert both C6 and C7 hydroxy groups to carbamates, leading to the preparation of analog 55.
Interestingly, 55 had significant affinity to Hsp90, and possessed a unique selectivity in comparison to natural product or natural product-like members of this class. In fact, affinity for Hsp90α and Hsp90β was over three orders of magnitude greater than for Grp94 – a serendipitous discovery that we believe emerged from our inability to selectively functionalize the C6–C7 diol produced by Sharpless asymmetric dihydroxylation of macrocyclic triene 54.
Second-generation route to benzoquinone ansamycin-inspired Hsp90 inhibitors:23
The unique biological profile of 55 led to enthusiasm to continue our pursuit of natural product- inspired Hsp90 inhibitors that have unique selectivity profiles. That said, this optimism was justly dampened by (1) the decreased propensity of 55 to displace fluorescently labeled geldanamycin from Hsp90, and (2) our dependence on the C8–C15 aldehyde 49 – a subunit whose synthesis was plagued by moderate stereoselection, laborious chromatographic steps, and the requirement of using a chiral allenylstannane reagent.
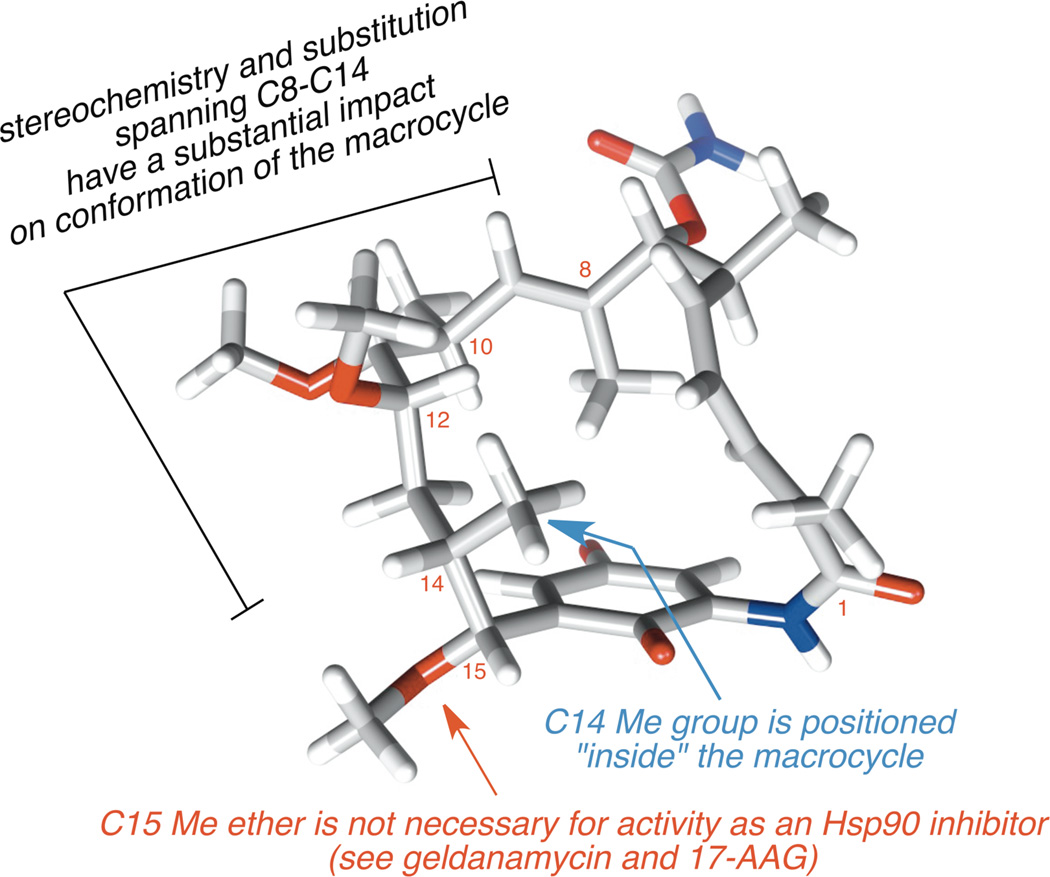
Moving forward, we recognized that the C8–C15 subunit of the benzoquinone ansamycins is a critical feature of their macrocycle, providing a substantial bias to organize the conformation of the ansa-chain (Figure 9).24 While some variation is seen at C11 (free hydroxyl group or methyl ether), and C15 [(R)-CHOMe or CH2], the stereotetrad between C10-C14 is a consistent structural motif. Further inspection led to recognizing that the C14 Me group occupies a position on the inside of the macrocyclic skeleton and likely does not play an important role in molecular recognition with Hsp90.
Figure 9.
Hsp90-relevant conformation of macbecin.24
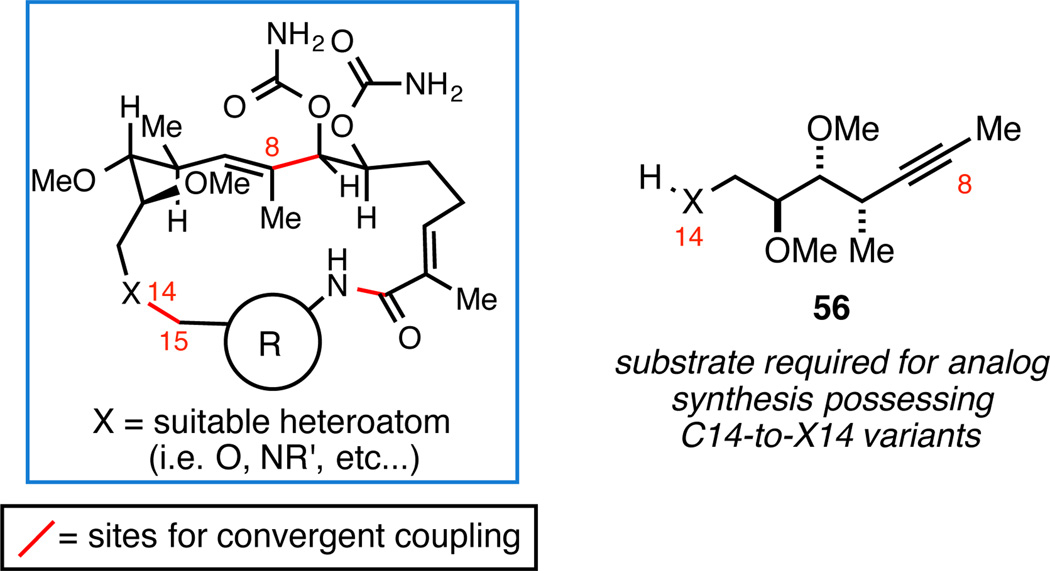
With these considerations in mind, we designed an alternate molecular composition to replace the C8–C15 natural product substructure. Speculating that the most important stereochemical features associated with this fragment derive from interactions that span C8–C12, and assuming that the C14 substitution is non-essential, we targeted the synthesis of macrocyclic analogs where the C14 carbon was replaced with a suitable heteroatom (Figure 10). Such a plan called for the use of an alkyne of general structure 56.
Figure 10.
Plan for heteroatom variants of benzoquinone ansamycins.
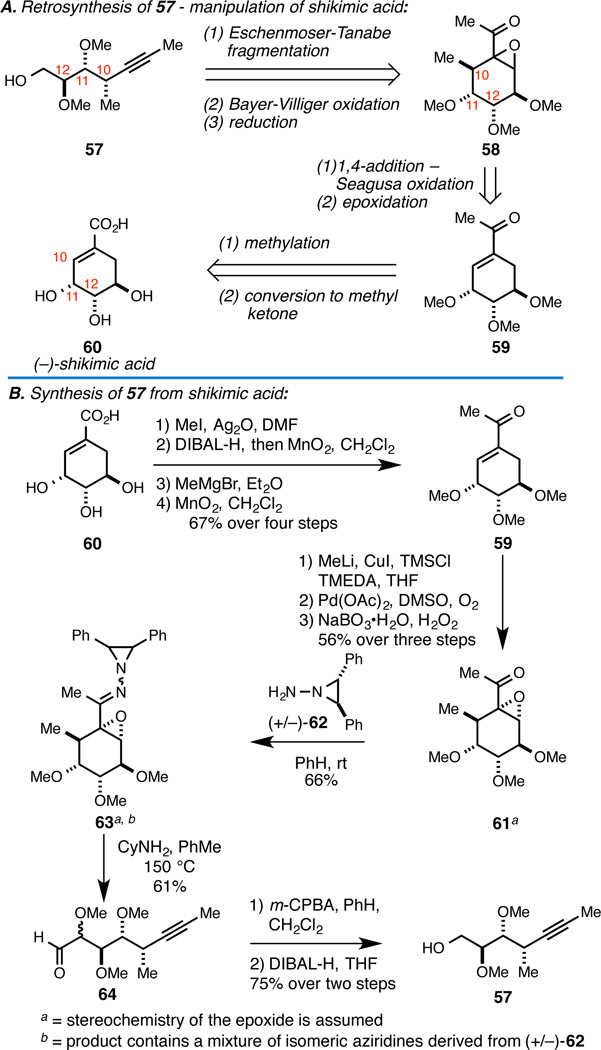
Instead of embracing modern methods for acyclic stereocontrol, where varying levels of stereoselectivity complicated our earlier efforts, we designed a new route to subunit 57 via stepwise functionalization of shikimic acid (60; Figure 11A). Here, two stereocenters of 60 would wind up as central features of 57, while the C10-stereocenter would be introduced by stereoselective 1,4-addition to enone 59. Finally, we expected that the carbocyclic intermediate 58 could be converted to the desired acyclic product by Eschenmoser-Tanabe fragmentation, Bayer-Villiger oxidation, and carbonyl reduction.
Figure 11.
Synthesis of coupling partner 57.
The successful execution of this strategy is illustrated in Figure 11B. While requiring eleven steps from shikimic acid, the route was tailored to employ only seven chromatographic operations – a fact that contributed to the ease with which gram scale quantities of 57 were accessible with this sequence.
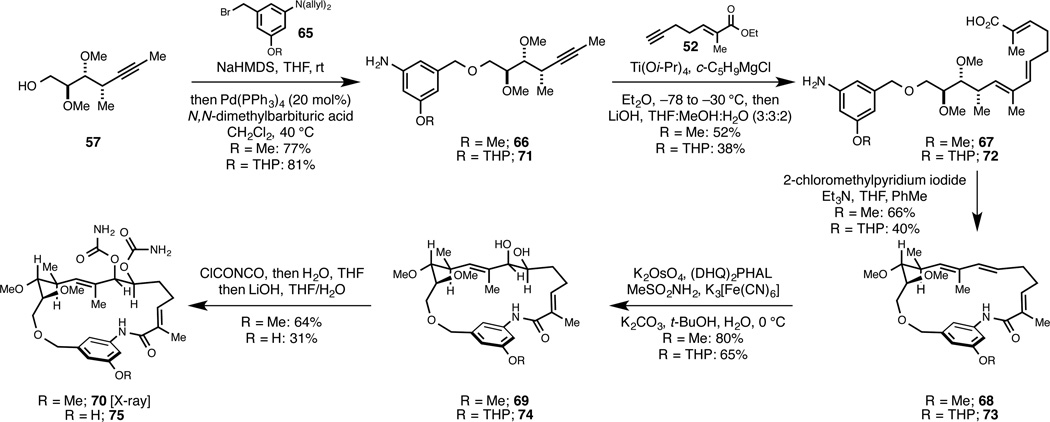
As illustrated in Figure 12, synthesis of macrocyclic benzoquinone ansamycin-like ethers proceeds in just six steps from 57 (benzylation, metallacycle-mediated cross-coupling, saponification, macrolamization, Sharpless asymmetric dihydroxylation, and carbamate formation).
Figure 12.
Preparation of macrocyclic ether, non-benzoquinone analogs of geldanamycin by metallacycle-mediated alkyne–alkyne coupling.
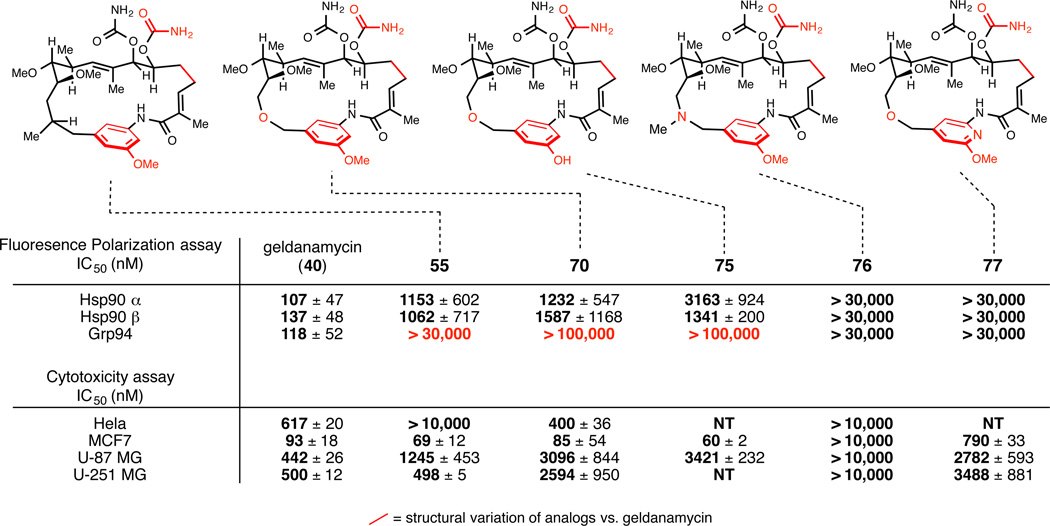
As summarized in Figure 13, analogs 70 and 75 had low μM activity vs. Hsp90α and Hsp90β and markedly decreased binding capacity toward Grp94 (up to ≥ 104). Interestingly, an analog bearing a tertiary amine at position 14 of the macrocycle (76) and one that replaced the quinone of the natural product with a susbstituted pyridine (77) were inactive (IC50 > 30,000 nM).
Figure 13.
Binding affinity of analogs to Hsp90α, Hsp90β and Grp94 and their cytotoxicity in different cancer cell lines. IC50 values are from an average of four fluorescence polarization and three cytotoxicity experiments, each of which were read in triplicate. NT; not tested. Evaluation of synthetic macrolactams in vitro was accomplished via a cell titer glo assay.
While biochemical assay revealed the unique paralog selectivity of a subset of our analogs, cytotoxicity assays demonstrated the potential value of these compounds as anticancer agents. In particular, analog 70 had geldanamycin-like potency in Hela (cervical cancer) and MCF7 cells (breast cancer), and showed comparable activity to geldanamycin in two glioblastoma cell lines (U-87 MG and U-251 MG; within one order of magnitude). This cytotoxicity was later correlated to Hsp90 inhibition by confirming the compound’s effects on Hsp90 client protein degradation in MCF-7 cells (Her-2 and Akt; Western blot).
The ability of this natural product-inspired paralog-selective Hsp90α/β inhibitor to effect cell viability is noteworthy, as a recently described Grp94-selective inhibitor prevents intracellular trafficking of the Toll receptor, inhibits secretion of IGFII, suppresses Drosophila larval growth and has little effect on cell viability at concentrations up to 25 μM.25 As such, selective targeting of Hsp90α and β over Grp94 with a natural product-inspired agent may represent a viable approach to the development of unique Hsp90-targeted anticancer therapeutics.
Regarding the pharmacokinetic properties of analog 70, C57Bl6 mice were dosed with the synthetic macrolactam and 17-AAG [orally (5 mg/kg) and IV (1 and 5 mg/kg)] and plasma and tissue concentration levels were assessed at 1 and 6 h post dosing. At 1 h, mice dosed orally with 70 had plasma concentration levels 5-fold higher than those dosed with 17-AAG (100 ng/ml vs 20 ng/ml; n=3). Similarly, after 1 h lung concentrations were also elevated (1.5-fold) in mice treated with 70 in comparison to those treated with 17-AAG (861 ng/ml vs. 581 ng/ml; n=3). Overall, analog 70 had comparable pharmacokinetic properties to 17AAG in vivo.23
3. CONCLUSION AND FUTURE CHALLENGES
Every year the community bears witness to substantial achievements in target-oriented synthesis that speak to the great power of modern organic chemistry. That said, these triumphs are sometimes achieved through chemical sequences that are of limited utility in function-oriented pursuits.26 With an eye toward efficiency in complex molecule synthesis, and the desire to offer new retrosynthetic relationships for streamlining efforts in natural product synthesis, my laboratory has been focused on the development of a suite of new metallacycle-mediated cross-coupling reactions. Over the last eight years, we have described a great diversity of intermolecular C–C bond forming reactions that address complex structural motifs encountered in fatty acids, polyketides, alkaloids, and terpenes. These contributions define the foundation of our program, and efforts in natural product synthesis serve as the testing ground for these new chemical reactions. With the bar set to define chemistry necessary to fuel function-oriented studies, success in target-oriented synthesis serves as the starting point for these latter and more demanding pursuits. Our study of the benzoquinone ansamycins and the discovery of a unique paralog selective natural product-inspired Hsp90 inhibitor represent an early success in this program. We hope to continue science in this vein, offering new reactions and synthesis strategies suitable for complex molecule construction and the discovery of natural product-inspired agents with potential clinical significance.
While we remain enthusiastic about our progress with developing and applying metallacycle-mediated cross-coupling chemistry in organic synthesis, a great variety of challenges lie ahead. These include, but are certainly not limited to: 1) Broadening substrate scope, particularly for the formation of metal-π complexes suitable for intermolecular coupling (our studies have only employed titanacycles generated from internal alkynes, imines, and minimally substituted vinylsilanes), 2) altering regio- and stereochemistry in every subset of coupling reaction, 3) popularizing new retrosynthetic relationships in stereoselective synthesis that are based on metallacycle-mediated cross-coupling, 4) developing complex variants of reactions in this class that embrace the penultimate organometallic intermediate from cross-coupling for subsequent C–C and C–X bond-forming processes, and 5) defining areas where reactivity and selectivity from this subset of chemistry can address long-standing problems in chemical synthesis. We hope that the broader scientific community is beginning to see the unique power and potential of metallacycle-mediated cross-coupling chemistry and look forward to witnessing this area grow to address as yet undefined problems in organic synthesis, including target- and function-oriented pursuits.
ACKNOWLEDGMENT
We gratefully acknowledge financial support of our program, from methods development to total synthesis to function- oriented synthesis, by the NIH (GM080266), the James and Esther King Biomedical Research Program (10KG-09), and the American Cancer Society (RSG-06-117-01). The author also acknowledges the significant collaborative efforts of Professor Philip LoGrasso in the Department of Molecular Therapeutics at The Scripps Research Institute for all efforts to understand the Hsp90 activity of analogs made from our program.
Biographies
Sarah B. Hale received her B.A. in chemistry and history from Colgate University (NY) in 2008, and a M.S. in chemistry from the University of Connecticut under the supervision of Professor Nicholas E. Leadbeater. Currently, she is a graduate student in the Department of Chemistry at Dartmouth College (NH), where her research in the laboratory of Professor Glenn C. Micalizio is focused on developing new metallacycle-centered chemistry for acyclic stereocontrol.
Glenn C. Micalizio obtained a Ph.D. at the University of Michigan in 2001 under the supervision of Professor William R. Roush. After postdoctoral study as a Fellow of the Helen Hay Whitney Foundation at Harvard University in the laboratory of Professor Stuart L. Schreiber, he begain his independent academic career at Yale University as an Assistant Professor in the Department of Chemistry (2003). In 2008, he was recruited to the Department of Chemistry at The Scripps Research Institute as an Associate Professor and in 2013 relocated to the Department of Chemistry at Dartmouth College where he is currently the New Hampshire Professor of Chemistry. His research is focused on the development of new synthetic methods and application of these methods to complex molecule synthesis.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oestreich M, editor. The Mizoroki-Heck Reaction. New York: Wiley; 2009. p. 608. For recent new directions, see: Mei T-S, Werner EW, Burckle AJ, Sigman MS. Enantio-selective Redox-Relay Oxidative Heck Arylations of Acyclic Alkenyl Alcohols Using Boronic Acids. J. Am. Chem. Soc. 2013;135:6830–6833. doi: 10.1021/ja402916z.
- 3.Grela K, editor. Olefin Metathesis: Theory and Practice. Hoboken, New Jersey: Wiley; 2014. p. 608. [Google Scholar]
- 4.(a) Reppe W, Schweckendick WJ. Cyclisierende Polymerisation von Acetylen. III Benzol, Benzolderivate und hydroaromatische Verbindungen. Justus Liebigs Ann. Chem. 1948;560:104–116. [Google Scholar]; (b) Vol’pin ME, Dubovitskii VA, Nogina OV, Kursanov DN. Titanocene Compounds with Tolanoe. Dokl. Akad. Nauk SSSR. 1963;151:1100. [Google Scholar]
- 5.For recent reviews, see: Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Regioselective Reductive Cross-Coupling Reactions of Unsymmetrical Alkynes. Eur. J. Org. Chem. 2010:391–409. doi: 10.1002/ejoc.200901094. Reichard HA, Micalizio GC. Metallacycle-mediated cross-coupling with substituted and electronically unactivated alkenes. Chem. Sci. 2011;2:573–589. doi: 10.1039/C0SC00394H.
- 6.For an early review on substrate-directed chemical reactions, see: Hoveyda AH, Evans DA, Fu GC. Substrate-Directable Chemical Reactions. Chem. Rev. 1993;93:1307–1370.
- 7.Wuts PG, Greene TW. Greene’s Protective Groups in Organic Synthesis. 4th edition. Hoboken, New Jersey: Wiley; 2007. p. 1082. [Google Scholar]
- 8.For a history regarding the use of Ti(Oi-Pr)4 for the formation of metal–alkene, metal–alkyne, and metal–imine complexes, see: Kulinkovich OG, de Meijere A. 1,n-Dicarbanionic Titanium Intermediates from Monocarbanionic Organometallics and Their Application in Organic Synthesis. Chem. Rev. 2000;100:2789–2834. doi: 10.1021/cr980046z. Sato F, Urabe H, Okamoto S. Synthesis of Organotitanium Complexes from Alkenes and Alkynes and Their Synthetic Applications. Chem. Rev. 2000;100:2835–2886. doi: 10.1021/cr990277l.
- 9.(a) Perez LJ, Shimp HL, Micalizio GC. Stereoselective Synthesis of Trisubstituted (E,E)-1,3-Dienes by the Site-Selective Reductive Cross-Coupling of Internal Alkynes with Terminal Alkynes: A Fragment Coupling Reaction for Natural Product Synthesis. J. Org. Chem. 2009;74:7211–7219. doi: 10.1021/jo901451c. [DOI] [PubMed] [Google Scholar]; (b) Bahadoor AB, Micalizio GC. A Studies in macrolide antibiotic synthesis: The role of tethered alkoxides in titanium alkoxide-mediated regioselective reductive coupling reactions. Org. Lett. 2006;8:1181–1184. doi: 10.1021/ol0600786. [DOI] [PubMed] [Google Scholar]
- 10.(a) Ryan J, Micalizio GC. An alkoxide-directed carbometalation of internal alkynes. J. Am. Chem. Soc. 2006;128:2764–2765. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]; (b) Reichard HA, Micalizio GC. A site- and stereo-selective intermolecular alkene-alkyne coupling process. Angew. Chem. Int. Ed. 2007;46:1440–1443. doi: 10.1002/anie.200603515. [DOI] [PubMed] [Google Scholar]; (c) McLaughlin M, Takahashi M, Micalizio GC. An alkoxide-directed intermolecular [2+2+1] annulation: A three-component coupling reaction for the synthesis of tetrasubstituted α,β-unsaturated γ-lactams. Angew. Chem. Int. Ed. 2007;46:3912–3914. doi: 10.1002/anie.200605060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shimp HL, Micalizio GC. An alkoxide-directed alkyne-allene cross-coupling for stereoselective synthesis of 1,4-dienes. Chem. Commun. 2007:4531–4533. doi: 10.1039/b708256h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Takahashi M, Micalizio GC. Regio- and stereoselective cross-coupling of substituted olefins and Imines. A convergent stereoselective synthesis of saturated 1,5-aminoalcohols and substituted piperidines. J. Am. Chem. Soc. 2007;129:7514–7516. doi: 10.1021/ja071974v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Kolundzic F, Micalizio GC. Synthesis of substituted 1,4-dienes by direct alkylation of allylic alcohols. J. Am. Chem. Soc. 2007;129:15112–15113. doi: 10.1021/ja075678u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McLaughlin M, Shimp HL, Navarro R, Micalizio GC. Regio- and stereoselective direct cross-coupling of aromatic imines with allenic alcohols. Synlett. 2008:735–738. doi: 10.1055/s-2008-1042808. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shimp HL, Hare A, McLaughlin M, Micalizio GC. Allene-alkyne cross-coupling for stereoselective synthesis of substituted 1,4-dienes and cross-conjugated trienes. Tetrahedron. 2008;64:6831–6837. doi: 10.1016/j.tet.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Belardi JK, Micalizio GC. Conversion of Allylic Alcohols to Stereodefined Trisubstituted Alkenes: A Complementary Process to the Claisen Rearrangement. J. Am. Chem. Soc. 2008;130:16870–16872. doi: 10.1021/ja8074242. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Takahashi M, McLaughlin M, Micalizio GC. Complex Allylation by the Direct Cross-Coupling of Imines with Unactivated Allylic Alcohols. Angew. Chem. Int. Ed. 2009;48:3648–3652. doi: 10.1002/anie.200900236. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Barlan AU, Micalizio GC. The regio- and stereo-chemical course of reductive cross-coupling reactions between 1,3-disubstituted allenes and vinylsilanes: synthesis of (Z)-dienes. Tetrahedron. 2010;66:4775–4783. doi: 10.1016/j.tet.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Macklin TK, Micalizio GC. Convergent and stereospecific synthesis of complex skipped polyenes and polyunsaturated fatty acids. Nature Chem. 2010;2:638–643. doi: 10.1038/nchem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Chen MZ, McLaughlin M, Takahashi M, Tarselli MA, Yang D, Umemura S, Micalizio GC. Preparation of Stereodefined Homoallylic Amines from the Reductive Cross-Coupling of Allylic Alcohols with Imines. J. Org. Chem. 2010;75:8048–8059. doi: 10.1021/jo101535d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yang D, Belardi JK, Micalizio GC. Generation of quaternary centers by reductive cross-coupling: shifting of regioselectivity in a subset of allylic alcohol-based coupling reactions. Tetrahedron Lett. 2011;52:2144–2147. doi: 10.1016/j.tetlet.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Chen MZ, Micalizio GC. Convergent Synthesis of Piperidines by the Union of Conjugated Alkynes with Imines: A Unique Regioselective Bond Construction for Heterocycle Synthesis. Org. Lett. 2009;11:4982–4985. doi: 10.1021/ol902169k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Umemura S, McLaughlin M, Micalizio GC. Convergent Synthesis of Stereo-defined exo-Alkylidene-γ-Lactams from β-Halo Allylic Alcohols. Org. Lett. 2009;11:5402–5405. doi: 10.1021/ol9022134. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yang D, Micalizio GC. A Convergent Stereoselective Synthesis of Quinolizidines and Indolizidines: Chemoselective Coupling of 2-Hydroxymethyl-Substituted Allylic Silanes with Imines. J. Am. Chem. Soc. 2009;131:17548–17549. doi: 10.1021/ja908504z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Canterbury DP, Micalizio GC. Polyketide Assembly by Alkene-Alkyne Redutive Cross-Coupling: Spiroketals through the Union of Homoallylic Alcohols. J. Am. Chem. Soc. 2010;132:7602–7604. doi: 10.1021/ja102888f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Diez PS, Micalizio GC. Chemoselective Reductive Cross-Coupling of 1,5-Diene-3-ols with Alkynes: A Facile Entry to Stereo-defined Skipped Trienes. J. Am. Chem. Soc. 2010;132:9576–9578. doi: 10.1021/ja103836h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yang D, Micalizio GC. Convergent and Stereodivergent Synthesis of Complex 1-Aza-7-oxabicyclo[2.2.1]heptanes. J. Am. Chem. Soc. 2011;133:9216–9219. doi: 10.1021/ja202900h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen MZ, Micalizio GC. Three-Component Coupling Sequence for the Regio-specific Synthesis of Substituted Pyridines. J. Am. Chem. Soc. 2012;134:1352–1356. doi: 10.1021/ja2105703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Greszler SN, Reichard HA, Micalizio GC. Asymmetric Synthesis of Dihydroindanes by Convergent Alkoxide-Directed Metallacycle-Mediated Bond Formation. J. Am. Chem. Soc. 2012;134:2766–2774. doi: 10.1021/ja2105043. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Diez PS, Micalizio GC. Convergent Synthesis of Deoxypropionates. Angew. Chem. Int. Ed. 2012;51:5152–5156. doi: 10.1002/anie.201200035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Jeso V, Aquino C, Cheng X, Mizoguchi H, Nakashige M, Micalizio GC. Synthesis of Angularly Substituted Trans-Fused Hydorindanes by Convergent Coupling of Acyclic Precursors. J. Am. Chem. Soc. 2014;136:8209–8212. doi: 10.1021/ja504374j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Perez LJ, Micalizio GC. Titanium-mediated fragment union processes in complex molecule synthesis: Development of a branched reaction pathway of high step economy for the synthesis of complex and diverse polyketides. Synthesis. 2008:627–648. [Google Scholar]; (b) Wu J, Pu Y, Panek JS. Divergent Synthesis of Functionalized Carbocycles through Organosilane-Directed Asymmetric Alkyne–Alkene Reductive Coupling and Annulation Sequence. J. Am. Chem. Soc. 2012;134:18440–18446. doi: 10.1021/ja3083945. [DOI] [PubMed] [Google Scholar]
- 14.(a) Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chiosis G, Vilenchik M, Kim J, Solit D. Hsp90: the vulnerable chaperone. Drug Discovery Today. 2004;9:881–888. doi: 10.1016/S1359-6446(04)03245-3. [DOI] [PubMed] [Google Scholar]; (c) Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic Hsp90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Whitesell L, Lin NU. Hsp90 as a platform for the assembly of more effective cancer chemotherapy. Biochim. Biophys. Acta, Mol. Cell Res. 2012;1823:756–766. doi: 10.1016/j.bbamcr.2011.12.006. [DOI] [PubMed] [Google Scholar]; (e) Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta, Mol. Cell Res. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee W-C. Heat Shock Protein 90: Inhibitors in Clinical Trials. J. Med. Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 15.Marzec M, Eletto D, Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta, Mol. Cell Res. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staron M, Yang Y, Liu B, Li J, Shen YK, Zuniga-Pflucker JC, Aguila HL, Goldschneider I, Li ZH. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lyphopoiesis. Blood. 2010;115:2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cysyk RL, Parker RJ, Barchi JJ, Steeg PS, Hartman NR, Strong JA. Reaction of Geldanamycin and C17-Substituted Analogues with Glutathione: Product Identifications and Pharmacological Implications. Chem. Res. Toxicol. 2006;19:376–381. doi: 10.1021/tx050237e. [DOI] [PubMed] [Google Scholar]
- 18.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, Patterson J, Penders C, Pink M, Read MA, Sang J, Woodward C, Zhang YL, Grayzel DS, Wright J, Barrett JA, Palombella VJ, Adams J, Tong JK. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel K, Piagentini M, Rascher A, Tian Z-Q, Buchanan GO, Regentin R, Hu ZH, Hutchinson CR, McDaniel R. Engineered Biosynthesis of Geldanamycin Analogs for Hsp90 Inhibition. Chem. Biol. 2004;11:1625–1633. doi: 10.1016/j.chembiol.2004.09.012. Patel K, Piagentini M, Rascher A, Tian Z-Q, Buchanan GO, Regentin R, Hu Z, Hutchinson CR, McDaniel R. Engineered Biosynthesis of Geldanamcyin Analogs for Hsp90 Inhibition. Chem. Biol. 2006;13:341. doi: 10.1016/j.chembiol.2004.09.012. For other analogs, see: Manzella HG, Tran TT, Carney JR, Lau-Wee J, Galazzo J, Reeves CD, Carreras C, Mukadam S, Eng S, Zhong Z, Timmermans PBMWM, Murli S, Ashley GW. Potent non-benzoquinone ansamycin heat shock protein 90 inhibitors from genetic engineering of Streptomyces hygroscopicus. J. Med. Chem. 2009;52:1518–1521. doi: 10.1021/jm900012a.
- 20.Zhang M-Q, Gaisser S, Nur-E-Alam M, Sheehan LS, Vousden WA, Gaitatzis N, Peck G, Coates NJ, Moso SJ, Radzom M, Foster TA, Sheridan RM, Gregory MA, Roe SM, Prodromou C, Pearl L, Boyd SM, Wilkinson B, Martin CJ. Optimizing Natural Products by Biosynthetic Engineering: Discovery of Nonquinone Hsp90 Inhibitors. J. Med. Chem. 2008;51:5494–5497. doi: 10.1021/jm8006068. [DOI] [PubMed] [Google Scholar]
- 21.(a) Baker R, Castro JL. The total synthesis of (+)-macbecin I. J. Chem. Soc. Chem. Commun. 1989:378–381. [Google Scholar]; (b) Baker R, Castro JL. Total synthesis of (+)-macbecin I. J. Chem. Soc. Perkin Trans. 1. 1990:47–65. [Google Scholar]; (c) Nakata M, Osumi T, Ueno A, Kimura T, Tamai T, Tatsuta K. Total Synthesis of Herbimycin A. Tetrahedron Lett. 1991;32:6015–6018. [Google Scholar]; (d) Evans DA, Miller SJ, Ennis MD, Ornstein PL. Asymmetric Synthesis of Macbecin I. J. Org. Chem. 1992;57:1067–1069. [Google Scholar]; (e) Evans DA, Miller SJ, Ennis MD. Asymmetric Synthesis of the Benzoquinoid Ansamycin Antitumor Antibiotics: Total Synthesis of (+)-Macbecin. J. Org. Chem. 1993;58:471–485. [Google Scholar]; (f) Panek JS, Xu F. Total Synthesis of (+)-Macbecin I. J. Am. Chem. Soc. 1995;117:10587–10588. [Google Scholar]; (g) Panek JS, Xu F, Rondon AC. Chiral Crotylsilane-Based Approach to Benzoquinoid Ansamycins: Total Synthesis of (+)-Macbecin I. J. Am. Chem. Soc. 1998;120:4113–4122. [Google Scholar]; (h) Andrus MB, Meredith EL, Simmons BL, Sekhar BBVS, Hicken EJ. Total Synthesis of (+)-Geldanamycin and (−)-o-Quinogeldanamycin with Use of Asymmetric Anti- and Syn-Glycolate Aldol Reactions. Org. Lett. 2002;4:3549–3552. doi: 10.1021/ol0267432. [DOI] [PubMed] [Google Scholar]; (i) Andrus MB, Meredith EL, Hicken EJ, Simmons BL, Glancey RR, Ma W. Total Synthesis of (+)-Geldanamycin and (−)-o-Quinogeldanamycin: Asymmetric Glycolate Aldol Reactions and Biological Evaluation. J. Org. Chem. 2003;68:8162–8169. doi: 10.1021/jo034870l. [DOI] [PubMed] [Google Scholar]; (j) Carter KD, Panek JS. Total Synthesis of Herbimycin A. Org. Lett. 2004;6:55–57. doi: 10.1021/ol036092p. [DOI] [PubMed] [Google Scholar]; (k) Wrona IE, Garbada AE, Evano G, Panek JS. Total Synthesis of Reblastatin. J. Am. Chem. Soc. 2005;127:15026–15027. doi: 10.1021/ja055384d. [DOI] [PubMed] [Google Scholar]; (l) Canova S, Bellosta V, Bigot A, Mailliet P, Mignani S, Cossy J. Total Synthesis of Herbimycin A. Org. Lett. 2007;9:145–148. doi: 10.1021/ol062642i. [DOI] [PubMed] [Google Scholar]; (m) Qin H-L, Panek JS. Total Synthesis of the Hsp90 Inhibitor Geldanamycin. Org. Lett. 2008;10:2477–2479. doi: 10.1021/ol800749w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Belardi JK, Micalizio GC. Total Synthesis of Macbecin I. Angew. Chem. Int. Ed. 2008;47:4005–4008. doi: 10.1002/anie.200800400. [DOI] [PubMed] [Google Scholar]
- 22.Jeso V, Cherry L, Macklin TK, Pan SC, LoGrasso PV, Micalizio GC. Convergent Synthesis and Discovery of a Natural Product-Inspired Paralog-Selective Hsp90 Inhibitor. Org. Lett. 2011;13:5108–5111. doi: 10.1021/ol2019828. [DOI] [PubMed] [Google Scholar]
- 23.Jeso V, Iqbal S, Hernandez P, Cameron MD, Park H, LoGrasso PV, Micalizio GC. Synthesis of Benzoquinone Ansamycin-Inspired Macrocyclic Lactams from Shikimic Acid. Angew. Chem. Int. Ed. 2013;52:4800–4804. doi: 10.1002/anie.201301323. [DOI] [PubMed] [Google Scholar]
- 24.Martin CJ, Gaisser S, Challis IR, Carletti I, Wilkinson B, Gregory M, Prodromou C, Roe SM, Pearl LH, Boyd SM, Zhang M-Q. Molecular characterization of macbecin as an Hsp90 inhibitor. J. Med. Chem. 2008;51:2853–2857. doi: 10.1021/jm701558c. [DOI] [PubMed] [Google Scholar]
- 25.Duerfeldt AS, Peterson LB, Maynard JC, Ng CL, Eletto D, Ostrovsky O, Shinogle HE, Moore DS, Argon Y, Nicchitta CV, Blagg BSJ. Development of a Grp94 inhibitor. J. Am. Chem. Soc. 2012;134:9796–9804. doi: 10.1021/ja303477g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]