Abstract
Objective
Mutations of ATP1A3 have been associated with Rapid Onset Dystonia-Parkinsonism and more recently with Alternating Hemiplegia of Childhood. Here we report one child with catastrophic early life epilepsy and shortened survival, and another with epilepsy, episodic prolonged apnea, postnatal microcephaly, and severe developmental disability. Novel heterozygous mutations (p.Gly358Val and p.Ile363Asn) were identified in ATP1A3 in these children.
Methods
Subjects underwent next-generation sequencing under a research protocol. Clinical data were collected retrospectively. The biochemical effects of the mutations on ATP1A3 protein function were investigated. Post-mortem neuropathologic specimens from control and affected subjects were studied.
Results
The mutations localized to the P domain of the Na,K-ATPase α3 protein, and resulted in significant reduction of Na,K-ATPase activity in vitro. We demonstrate in both control human brain tissue and that from the subject with the p.Gly358Val mutation that ATP1A3 immunofluorescence is prominently associated with interneurons in the cortex, which may provide some insight into the pathogenesis of the disease.
Significance
The findings indicate these mutations cause severe phenotypes of ATP1A3-related disorder spectrum that include catastrophic early life epilepsy, episodic apnea, and postnatal microcephaly.
Keywords: ATP1A3, early life epilepsy, apnea, postnatal microcephaly, Na, K-ATPase, next-generation sequencing
Introduction
Mutations of ATP1A3 have been associated with Rapid Onset Dystonia-Parkinsonism (RDP), Alternating Hemiplegia of Childhood (AHC)1–3, and more recently with Cerebellar ataxia, Areflexia, Pes cavus, Optic atrophy, and Sensorineural hearing loss (CAPOS)4. The onset of RDP usually occurs between 10 and 30 years of age (range 8–55 years)5, although a recent report described early onset at 9 and 14 months in two children6. Dystonic spells are often triggered by external factors such as emotional stress, exercise, fatigue, hyperthermia, or illnesses, and the dystonia usually stabilizes within a month5,7. The dystonia in RDP has a clear rostrocaudal topographical gradient (face > arm > leg) with prominent bulbar involvement, and minimal overall improvement. Affected individuals often have mild cognitive abnormalities8. Some have had psychosis9, and a few have had seizures10.
Children with AHC have recurrent episodes of hemiplegia and hemidystonia that alternate between sides, with onset usually by 6 months and episodes occurring daily to monthly3. AHC attacks can be brought on by specific triggers such as emotional stress, fatigue, and bathing, and typically disappear with sleep11. Affected children usually have developmental delay, variable intellectual disability, seizures (50–80%), ataxia, and dysarthria. The hemiplegic episodes persist throughout life, although they usually become shorter and less frequent as the individual gets older, while dystonia and choreoathetosis increase12–14. Both RDP-causing and AHC-causing mutations in ATP1A3 occur predominantly in the protein’s membrane domain. RDP-causing mutations often reduce ATP1A3 protein expression significantly1, while AHC-causing mutations are reported to reduce ATPase activity without greatly affecting protein expression2. At least one mutation, Asp923Asn, can manifest as either RDP or AHC in the same family15.
Intractable neonatal seizures, early life epilepsy, and status epilepticus have been reported in children with clinically-defined AHC3,16,17, and an association has been made with the Glu815Lys mutations and neonatal-onset seizures18. Seizures have also been reported at 1–2 months of age in infants with ATP1A3 mutations later diagnosed with AHC3. Unlike in AHC, seizures are rare in RDP, and usually easily controlled7.
We report two children, not meeting diagnostic criteria for AHC, one with catastrophic neonatal onset epilepsy and the other with epilepsy and episodic prolonged apnea who were found through next-generation sequencing to have novel heterozygous mutations in ATP1A3. In one child, the seizures were frequent and intractable, while in the other recurrent episodes of prolonged apnea required tracheostomy and ventilator support for many months. The observed mutations resulted in significant reduction of ATP1A3 activity in vitro. We also demonstrate that ATP1A3 protein, the α3 subunit of the Na,K-ATPase, is prominently expressed in GABAergic neurons of human cortex and basal ganglia. These data demonstrate the severe phenotypes that can be seen with ATP1A3 mutations include catastrophic early life epilepsy, apnea and severe neurodevelopmental disability.
Methods
Patient ascertainment
Subjects were ascertained through the Molecular Genetic Studies of Developmental Disorders research program (approved by the IRB of Seattle Children’s Hospital) and selected for next-generation sequencing after available clinical testing failed to uncover an etiology for their disorder. Retrospective clinical records, EEG tracings, patient video, and brain MRI scans were reviewed. DNA was extracted from peripheral blood and/or postmortem brain using the Gentra PureGene DNA isolation kit.
Massively parallel sequencing
Whole exome sequencing (WES) was performed on peripheral blood DNA from subject LR11-328 and both parents. The Agilent SureSelect 50 Mb whole exome capture kit was used. Targeted exon capture massively parallel sequencing was performed on peripheral blood DNA from subject LR11-147. The exons of a total of 1461 genes were targeted for various neurological conditions, including seizures, brain development, intellectual disability, and movement disorders (SureSelect, Agilent). For both LR11-328 and LR11-147 sequence was generated on an Illumina HiSeq 2000 platform. Sequence was aligned to hg19 using BWA 0.5.7, and single nucleotide variants and indels were called using GATK (version 1.3 for LR11-328 and version 1.6.5 for LR11-147). Mean read depth of the targeted exome was calculated with the GATK DepthOfCoverage walker. Annotation of variants was performed with SeattleSeq Annotation 134. Common variants were identified by filtering against the NHLBI Exome Variant Server. For subject LR11-328, de novo, autosomal recessive, and X-linked variants were identified using SOLVE-Brain 1.0.1. See Web Resources for all tools used.
Sanger sequencing
Variants identified as possibly disease causing were confirmed by standard polymerase-chain-reaction (PCR) combined with bidirectional Sanger sequencing using standard methods. For subject LR11-328 the forward primer CTCGTGTCGCTCATCCAAC and reverse primer CAGAACAGGGACAGCTGAGG were used. For subject LR11-147 the forward primer GCTGCCTCATTCTTTTCCAG and reverse primer AGGTGAGGCCAGTAGCTGAA were used. Sequences were compared with normal control samples and the reference sequences for ATP1A3 (NCBI reference number NG_008015.1).
ATP1A3 protein structure analysis
The crystal structure of Na,K-ATPase in the E2 major protein conformation (K+-bound, 2ZXE)19 was displayed in the Swiss PDB viewer DeepView v.4.1, and the mutated residues were identified by their conserved sequence homology1.
ATP1A3 functional activity and expression
A pCVM6-XL5 plasmid containing full-length human ATP1A3 cDNA was previously mutated at two extracellular residues to make it ouabain-resistant1. The mutations Gly358Val and Ile363Asn were individually introduced into the plasmid by site-directed mutagenesis (Mutagenex, Piscataway, NJ), and verified by full sequencing. Transfection of HEK-293 cells (ATCC) was performed with Lipofectamine 2000 (Invitrogen/Life Technologies) according to the manufacturer’s protocol. As a positive control for Na,K-ATPase α3 subunit expression, a stably transfected line with the original ouabain-resistant plasmid without additional mutations was selected over a period of weeks by ability to grow in 0.5 μM ouabain. Transient transfections of each of the plasmids were separately used to test the survival of cells in 0.5 μM ouabain, which was added 48 h after transfection, and the results were assessed at 72 and 96 hours by phase contrast microscopy. Separate transient transfections without ouabain treatment were used to assess ATP1A3 α3 protein expression on Western blots stained with Santa Cruz Biotechnology sc-16052 (C16) antibody. Detection was with ThermoScientific West Dura luminol method and a LAS 4000 imager with ImageQuant.
Immunohistochemistry
Formalin fixed paraffin-embedded 4 μm human brain sections were obtained from the Seattle Children’s Hospital Pathology Department. Specimens were deparaffinized by incubation in xylene, followed by rehydration through graded ethanol/water solutions and equilibration in PBS. Antigen retrieval was performed by heating in 0.01M sodium citrate (pH 6.0) in a 90° C water bath for 45 minutes. Specimens were then blocked in PBS with 10% donkey serum, 0.1% Triton X-100 and 2% BSA for 30 minutes and incubated overnight at 4° C in primary antibodies diluted in blocking solution. Primary antibodies used in these studies included ATP1A3 (F-1) (Santa Cruz Biotechnology). This is a mouse monoclonal IgG3 antibody directed against an epitope mapping between amino acids 419–446 of human ATP1A3, which is part of the intracellular N-domain well separated from the detected mutation in subject LR11-328. Other primary antibodies included mouse monoclonal MAP2 (Millipore), rabbit polyclonal GAD65/67 (Millipore), rabbit polyclonal calbindin (Millipore), goat polyclonal calretinin (Millipore), and goat polyclonal GFAP (Santa Cruz). Slides were then incubated in fluorescent secondary antibodies at room temperature for 2 hours. Following incubation in DAPI nuclear counterstain and Sudan Black to block endogenous fluorescence, sections were examined and photographed using a Nikon Eclipse Ni-U epifluorescence microscope.
Results
Clinical reports
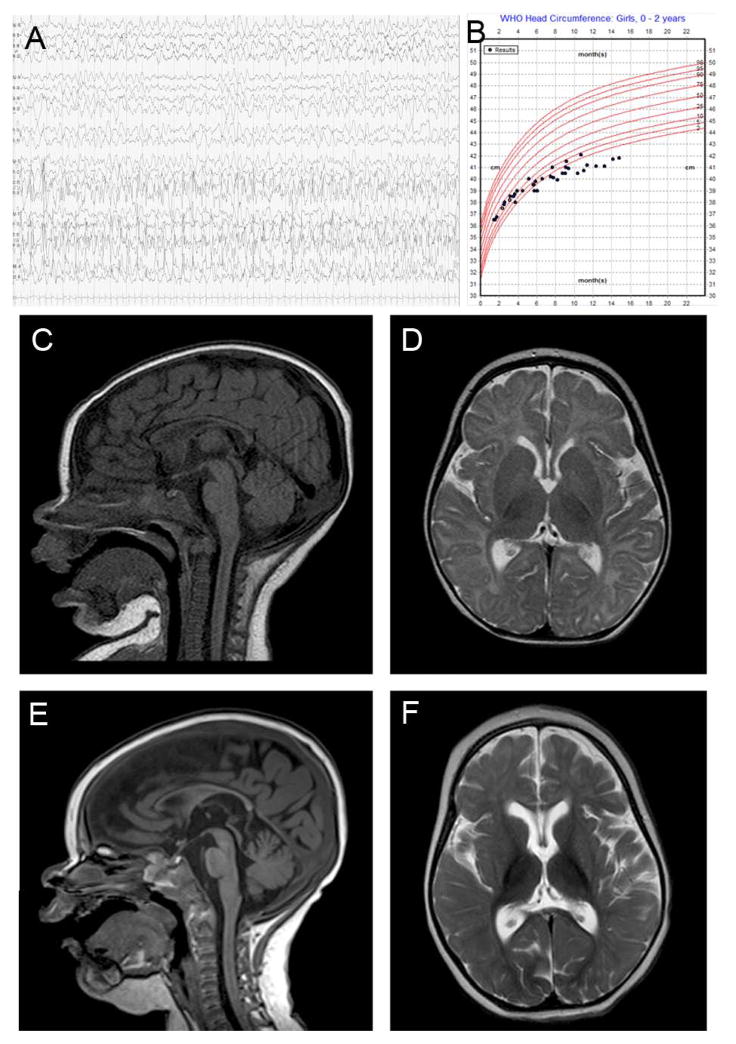
Subject LR11-328 was a term infant girl born after an uncomplicated pregnancy who had her first seizure at four hours after birth. She continued to have seizures after initiation of phenobarbital, and multiple anti-epileptic drugs were tried in succession without effect. At 2 months of age her seizures increased in frequency and duration, resulting in multiple hospitalizations for recurrent status epilepticus, and EEG studies demonstrated multifocal seizures (Fig. 1A). Some seizures were accompanied by post-ictal hemiparesis. She also had episodes of nystagmus, disconjugate gaze, mouth and tongue movements and reduced responsiveness that had no electrographic correlate. By 11 months of age she developed continuous athetotic movements of her extremities at rest. Head growth decelerated, and she developed postnatal microcephaly (Fig. 1B). Brain MRI showed progressive atrophy (Fig. 1C–F). Her epilepsy continued to be intractable with recurrent episodes of status epilepticus, and she was transitioned to hospice care. She died at home after a respiratory illness at 16 months of age, and an autopsy was performed.
Figure 1.
Representative EEG tracing from subject LR11-328 at 2 months showing left hemispheric electrographic seizure (A). Head size measurements showed postnatal onset microcephaly (B). Brain MRI of subject LR11-328 showing progressive cortical and subcortical atrophy at 5 months on sagittal (C) and axial views (D), compared to sagittal (E) and axial views (F) at age 12 months. The corpus callosum remained thin, with cortical and anterior cerebellar vermis volume loss.
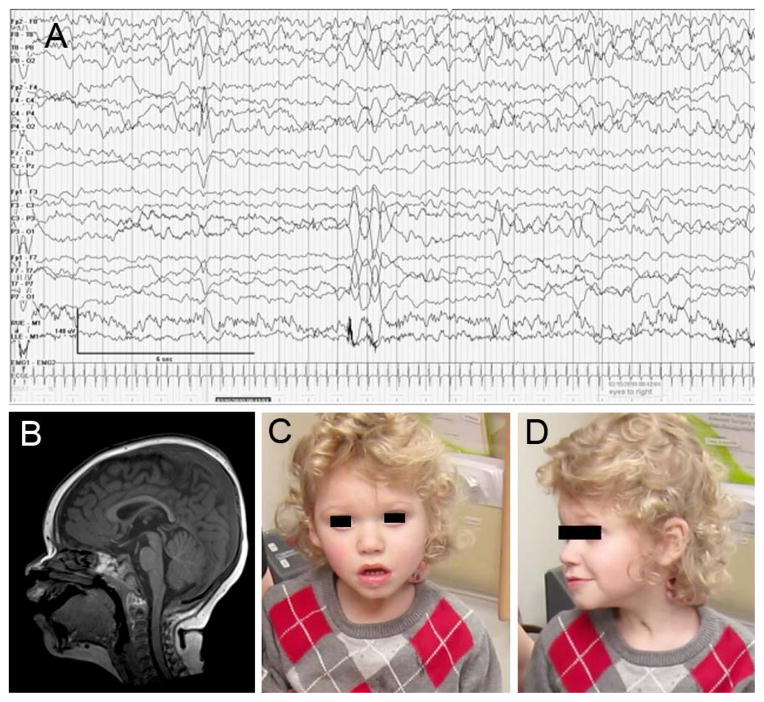
Subject LR11-147 was a term boy born after a pregnancy complicated by maternal tachycardia requiring beta-blocker therapy. Brief positive pressure ventilation was required at delivery. At 6 weeks he had onset of seizures characterized by apnea, gaze deviation, and decreased responsiveness, with electrographic correlate (Fig 2A). Levetiracetam did not control the seizures, and lamotrigine was added. At 6 weeks he had onset of apnea spells with no electrographic correlate that consisted of gradually decreasing respirations and movements until he became completely apneic, akinetic, and unresponsive during longer spells. Most occurred during sleep, but when awake they were preceded by lethargy for 5–15 minutes. The spells lasted from 15 minutes to several hours, and were accompanied by hypoxia. Brain MRI was normal (Fig. 2B). He underwent tracheostomy at 5 months and was discharged with a home ventilator at 8 months after a 5-month inpatient stay. By 16 months apneic episodes had stopped, and the home ventilator was discontinued. The apnea spells gradually transitioned to spells consisting of panting respirations, gaze deviation, bruxism, and stiffness with flexion and fisting of his arms and legs lasting 10 minutes about once a week. An EEG showed diffuse slowing but no epileptiform discharges. These episodes, consistent with dystonic spells, were partly responsive to lorazepam. At 4 years he had a prolonged spell characterized by marked lethargy with decreased use of his right arm and right gaze deviation, the first spell that resembled alternating hemiplegia. The weakness resolved over the following 4 days. An EEG showed diffuse slowing but no epileptiform discharges. At last follow-up at 4 years of age (Fig. 2C,D), he had severe developmental disability, made only brief eye contact, occasional vocalizations, was nonambulatory, and entirely gastrostomy tube fed due to dysphagia. He had postnatal microcephaly, with head circumference measuring at the 2nd percentile, a deceleration from the 75th percentile at birth.
Figure 2.
EEG tracing from subject LR11-147 with a right hemispheric electrographic seizure associated with eye deviation and apnea (A). Brain MRI at 1 year in subject LR11-147 (B) was unremarkable. At 3.5 years of age he was encephalopathic, with nondysmorphic facies (C,D).
Next-generation sequencing
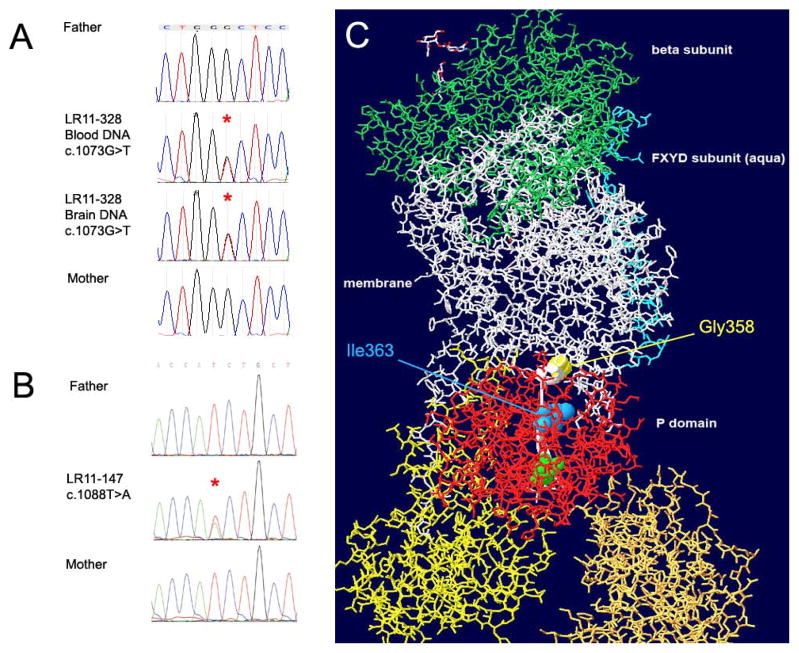
Analysis of NGS data identified the de novo heterozygous sequence variants in ATP1A3 consisting of chr19:g.42486179C>A, c.1073G>T, p.Gly358Val in subject LR11-328 and chr19:g.42486164A>T, c.1088T>A, p.Ile363Asn in subject LR11-147. Both amino acid substitutions occurred in a highly conserved region of the protein, were predicted to be deleterious by PolyPhen and SIFT, and were absent from 6500 control exomes in the NHLBI Exome Variant Server. Next generation sequencing metrics are summarized in Supplementary Table 1. Both mutations were confirmed to be de novo by bidirectional Sanger sequencing in probands and parents (Fig.3 A,B). In LR11-328 we showed the c.1073T>G mutation to be present in both blood and brain derived DNA, with no evidence of mosaicism.
Figure 3.
Chromatographs of Sanger sequencing in subject LR11-328 (A) and LR11-147 (B) showing de novo status of mutations c.1073G>T and c.1088T>A in ATP1A3. The resulting amino acid substitutions Gly358Val (yellow spacefill) and Ile363Asn (blue spacefill) localize to the P domain of the ATP1A3 protein (red stick representation). A beta-strand (thin ribbon backbone highlighted white) connects the mutated residues to the active site phosphorylatable Asp366 (green spacefill). The structure is tilted to reveal the linear relationship between the mutations and the active site aspartate. The β subunit (green stick representation) is at the extracellular surface, and the yellow and gold A and N domains, like the red P domain, are cytoplasmic.
ATP1A3 protein structure
Both mutations are located in the cytoplasmic P domain that is transiently phosphorylated during enzyme turnover, on an extended beta-sheet strand that connects them to the active site aspartate, Asp366 (Fig. 3C). Gly358 is located at a critical interface between the P domain and the longest transmembrane span, M5. Valine is bulkier and may disrupt this interaction. Ile363 is buried deeper in the P domain, contacting the distal half of the P domain and a more distal position of the M5 transmembrane span. Asparagine is both more polar and slightly bulkier than isoleucine, and also likely to disrupt those interactions. The active site Asp366 caps the end of the beta strand and is exposed at the surface where it can contact the terminal phosphate of bound ATP. If the mutations displace this residue it would directly affect enzyme activity.
ATP1A3 functional activity and expression
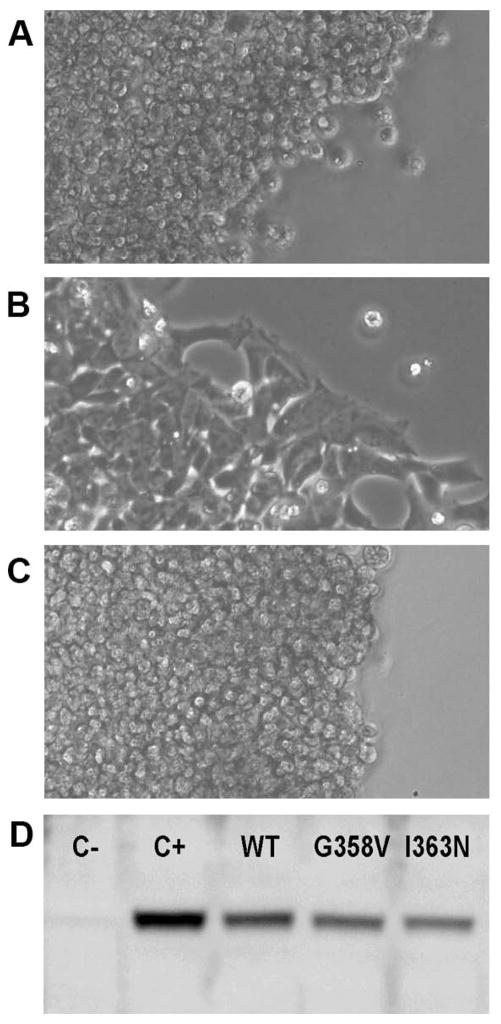
Ouabain is a specific inhibitor of Na,K-ATPase, and 0.5–1.0 μM ouabain kills HEK-293 cells within 24 hours. We used ouabain as a tool to suppress the activity of the ATP1A1 (α1) isoform that is endogenously expressed in HEK-293 cells, making a transfected, ouabain-resistant ATP1A3 (α3) cDNA vector essential for cell survival. Transient transfection with ouabain-resistant α3 bearing either Gly358Val or Ile363Asn mutations resulted in massive cell death within 24 hours of ouabain addition, and no cells survived past 48 hours (Figure 4A–C). This indicates that both mutations cause either substantial inactivation or loss of protein stability. Western blots of protein expression (Figure 4D) showed that expression of either mutation resulted in levels of intact protein similar to the WT control.
Figure 4.
Deficiency of Na,K-ATPase function in HEK-293 cell transfectants. HEK-293 cells were transfected with lipofectamine alone as a procedural control (A); ouabain-resistant human ATP1A3 cDNA vector without mutation (B); or ouabain-resistant vector with Gly358Val mutation (C). Twenty-four hours after transfection, 0.5 μM ouabain was added to inhibit the endogenous ATP1A1 (α1). The images show that after an additional 3 days in culture, both the control cells and the Gly358Val cells were dead. A few dead cells were present in cells transfected with wild type-ouabain resistant vector (B), but a majority were alive and migrating out from the edge. Identical complete cell death was obtained with the Ile363Asn mutation (data not shown). Mutant protein expression (D). Lysates of cells were analyzed with α3-specific antibody. C- is lysate from control HEK-293 cells, and the faint band is presumed to be slight crossreactivity of the antibody with endogenous α1. C+ is lysate of stably-transfected cells expressing ouabain-resistant WT α3 at a level that supports cell growth in the presence of 0.5 μM ouabain selection at the same rate as the parent cell line. WT, G358V, and I363N are lysates of transient transfections 48 hours after addition of plasmid, with no ouabain present. Data are representative of 3 experiments.
Neuropathological findings
On neuropathological examination, the brain of subject LR11-328 was normally formed but small, with a weight of 618 gm as compared with an expected weight of 1010 gm. Widening of the cerebral sulci was noted, consistent with atrophy. On microscopic examination, patchy gray matter and subpial gliosis, atrophy and gliosis of the white matter, and increased microglia were seen (Supplementary Figure 1). However, myelination was normal. The hippocampus had loss of CA1 neurons with relative preservation of CA3 and the hippocampal dentate gyrus had mild thinning (3–4 cell layers thick) and gliosis. In the basal ganglia, severe atrophy and gliosis of the caudate along with a patchy, irregular pattern of gliosis and atrophy of the putamen and globus pallidus were found. In the cerebellum, extensive gliosis of the dentate nucleus, mild Purkinje cell drop-out, and Bergmann gliosis were noted. White matter gliosis was also present in the brainstem and spinal cord.
ATP1A3 immunoreactivity in human brain
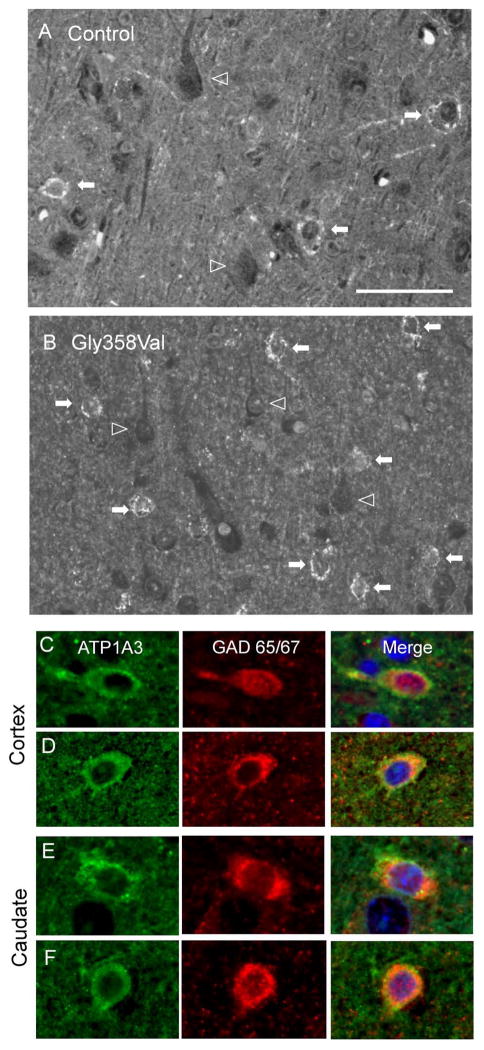
The distribution of ATP1A3 immunofluorescence (IF) in brain was determined in subject LR11-328 (p.Gly358Val) and three control pediatric postmortem specimens, including two children with epilepsy from other causes. In all 4 brains, prominent ATP1A3 IF was identified in the perisomatic and dendritic regions of small neurons with interneuronal morphology, most abundantly in cortical layers 2/3 (Figure 5 A,B). Although fainter ATP1A3 IF was also present diffusely throughout the neuropil, similar concentrated labeling was not found associated with pyramidal neurons. The pattern of expression was identical between ATP1A3 p.Gly358Val and control cortices. Identification of the ATP1A3-associated cells as GABAergic was confirmed by co-labeling for GAD 65/67 (Figure 5C, D). ATP1A3-associated GABAergic cells were also seen in the basal ganglia in the caudate nucleus (Figure 5E, F), putamen, and globus pallidus. Again, the pattern of ATP1A3 expression was identical between ATP1A3 p.Gly358Val and control basal ganglia specimens. Given the limitations of light microscopy, we were unable to definitively identify interneuronal ATP1A3 localization as membranous or due to dense presynaptic innervation.
Figure 5.
ATP1A3 immunofluorescence was localized to GABAergic cells in human temporal cortex and basal ganglia. A,B) Control (A) and ATP1A3 Gly358Val (B) cortex. Filled arrows indicate cells with interneuron morphology and perisomatic ATP1A3 IF; open arrowheads indicate outlines of cells with pyramidal morphology and absence of ATP1A3 IF. C,D) ATP1A3 (green) co-localizes with the interneuron marker GAD 65/67 (red) in control (C) and Gly358Val (D) human cortex. E,F) ATP1A3 colocalization with GAD 65/67 in human caudate nucleus. Scale bars: 50 μM
Discussion
We report two patients with de novo loss-of-function mutations in the P domain of ATP1A3, one of whom had catastrophic early life epilepsy, and the other with epilepsy and life-threatening recurrent apnea leading to a severely impaired neurodevelopmental outcome. While LR11-328 had hemiplegic episodes, these were interpreted as post-ictal Todd paralysis. LR11-147 had no hemiplegic episodes until many months after the mutation was discovered. In neither child was AHC considered in the initial differential diagnosis. These cases represent the most severe end of the spectrum of phenotypes reported with ATP1A3 mutations.
The Gly358Val and Ile363Asn mutations localize to the P domain of ATP1A3. Other ATP1A3 mutations in this domain include the RDP mutation Thr370Asn20,21, the AHC mutation Leu371Pro3, the most recurrent RDP mutation Thr613Met1, and RDP mutation Ser684Phe22. The mutations reported here resulted in ATP1A3 loss-of-function with severe reduction in protein activity and cell death when endogenous α1 activity was inhibited in culture. Like all of the AHC mutations tested in culture to date, and some of the RDP mutations, the mutated proteins were substantially stable in the cellular expression system. This implies that they fold without major impairment and may associate with β and FXYD subunits, whereas some of the RDP mutations are poorly expressed. While variable dominant-negative effects on protein synthesis or folding of the normal allele are possible, it is not clear why the Gly358, Thr370, Ile363, and Leu371 mutations produce three such different phenotypes. In fact, Thr370Asn has manifested both as AHC and RDP20,21.
Our studies of the neuropathologic changes and ATP1A3 protein distribution in the child with severe epileptic encephalopathy due to the Gly358Val mutation as well as in epileptic and non-epileptic control human brain provide possible insights into the pathophysiology, of ATP1A3-related diseases. Although gross and microscopic brain structure was normal, we found marked atrophy and astrogliosis of the cortex, basal ganglia, and cerebellum. Some of the neuropathologic changes, such as hippocampal sclerosis and cortical subpial gliosis, were likely secondary to intractable epilepsy. However, the presence of white matter gliosis in the brainstem and spinal cord are more suggestive of a primary neurodegenerative disorder. While reported brain MRI studies in most children with AHC have been reported as normal, a few children have had cerebral and cerebellar atrophy or hippocampal sclerosis, similar to that seen in our subject13. The neuropathology of a child with AHC prior to discovery of ATP1A3 mutations was previously described23. That child had minimal EEG abnormalities but extensive neuronal loss in the hippocampus and astrogliosis in the hippocampus and thalamus. Neuropathology of adult brains in patients with RDP found changes in the dentaorubral, pallidoluysian, olivocerebellar, and dentato-olivary pathways24. These findings are suggestive of a neurodegenerative process.
In rodents, the α3 subunit containing Na, K-ATPase is expressed widely in neurons of the cerebral cortex, basal ganglia, hippocampus, cerebellum, red nucleus and pons25–28. While ATP1A1 subunits are expressed in both neurons and glia, ATP1A3 subunits are exclusively present in neurons, where they are thought to be responsible for restoration of basal intracellular Na+ concentrations after sustained activity29–31. In rodent cortex, hippocampus, and cerebellum, Atp1a3 mRNA has been identified in both pyramidal cells and interneurons, while Atp1a3 immunofluorescence is found preferentially localized to GABAergic neuronal cell bodies and terminals26–28,32. Electrophysiological recordings have shown higher density of Na+/K+ pump currents in cortical and hippocampal interneurons than in pyramidal cells33. Impairment of Na+/K+ homeostasis in cortical and hippocampal interneurons might be expected to contribute to seizure generation. Indeed, Myshkin mice, which possess a heterozygous loss of function Atp1a3 mutation, exhibited spontaneous seizures and hippocampal hyperexcitability after high-frequency synaptic activity, and showed histological sequelae including hippocampal gliosis34. In addition, impairment of striatal interneuron function has been hypothesized to promote dystonia and hyperkinetic movement disorders35,36. These data suggest that further anatomical and neuropathologic studies of ATP1A3-related disease are warranted.
In summary, we report two interesting patients found to have novel deleterious mutations in ATP1A3. Their presentation had atypical features, even when cases of AHC with epilepsy are considered. Nonepileptic apnea was striking in the second patient. Our neuropathologic studies suggest a possible contribution of neuronal degeneration and interneuron dysfunction in the mechanism of disease. These patients illustrate the severe end of the spectrum in ATP1A3-related disorders, and indicate that mutations in this gene should be considered in patients presenting with catastrophic epilepsy, unusual apnea spells, and postnatal microcephaly.
Supplementary Material
Neuropathologic findings in ATP1A3 Gly358Val brain. A) Subpial gliosis and astrocytosis of entorhinal cortex shown by GFAP immunofluorescence. B) Severe gliosis of temporal lobe white matter. C,D) Marked neuronal loss in hippocampal layer CA1 (C) as compared with CA3 (D). E) Thinning and astrocytosis of the dentate gyrus (DG) of the hippocampus. F) Decreased neuronal density and astrocytosis of the caudate nucleus. Green = MAP2; Red = GFAP; Blue = DAPI.
Acknowledgments
The authors would like to thank the two children and their parents for participation in this study, and the parents of LR11-328 who provided funding for the studies of ATP1A3 activity. We also wish to acknowledge Dr. Raj Kapur, Seattle Children’s Hospital, for his assistance with the clinical neuropathology data. Research reported in this work was supported by the National Institute of Neurologic Disorders and Stroke (NINDS) of the National Institutes of Health under award numbers K08NS078054 (A.R.P), K02NS072162 (L.A.J.), R21NS081558 (K.J.S.), R01NS046616 (W.B.D.).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Web Resources
BWA: http://bio-bwa.sourceforge.net/bwa.shtml
GATK: http://www.broadinstitute.org/gatk/
SeattleSeq Annotation 134: http://snp.gs.washington.edu/SeattleSeqAnnotation134/
NHLBI Exome Variant Server: http://evs.gs.washington.edu/EVS/
SOLVE-Brain: https://paciorkowski-lab.urmc.rochester.edu/solve_brain
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.De Carvalho Aguiar P, Sweadner KJ, Penniston JT, et al. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Heinzen EL, Swoboda KJ, Hitomi Y, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet. 2012;44:1030–1034. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosewich H, Thiele H, Ohlenbusch A, et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 2012;11:764–773. doi: 10.1016/S1474-4422(12)70182-5. [DOI] [PubMed] [Google Scholar]
- 4.Demos MK, van Karnebeek C, Ross C, et al. A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J Rare Dis. 2014;9:15. doi: 10.1186/1750-1172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobyns WB, Ozelius LJ, Kramer PL, et al. Rapid-onset dystonia-parkinsonism. Neurology. 1993;43:2596–2602. doi: 10.1212/wnl.43.12.2596. [DOI] [PubMed] [Google Scholar]
- 6.Brashear A, Mink JW, Hill DF, et al. ATP1A3 mutations in infants: a new rapid-onset dystonia-Parkinsonism phenotype characterized by motor delay and ataxia. Dev Med Child Neurol. 2012;54:1065–1067. doi: 10.1111/j.1469-8749.2012.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brashear A, Dobyns WB, de Cavalho Aguiar P, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- 8.Cook JF, Hill DF, Snively BM, et al. Cognitive impairment in rapid-onset dystonia-parkinsonism. Mov Disord. 2014;29:344–350. doi: 10.1002/mds.25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brashear A, Cook JF, Hill DF, et al. Psychiatric disorders in rapid-onset dystonia-parkinsonism. Neurology. 2012;79:1168–1173. doi: 10.1212/WNL.0b013e3182698d6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brashear A, DeLeon D, Bressman SB, et al. Rapid-onset dystonia-parkinsonism in a second family. Neurology. 1997;48:1066–1069. doi: 10.1212/wnl.48.4.1066. [DOI] [PubMed] [Google Scholar]
- 11.Ozelius LJ. Clinical spectrum of disease associated with ATP1A3 mutations. Lancet Neurol. 2012;11:741–743. doi: 10.1016/S1474-4422(12)70185-0. [DOI] [PubMed] [Google Scholar]
- 12.Mikati MA, Kramer U, Zupanc ML, Shanahan RJ. Alternating hemiplegia of childhood: clinical manifestations and long-term outcome. Pediatr Neurol. 2000;23:134–141. doi: 10.1016/s0887-8994(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 13.Sweney MT, Silver K, Gerard-Blanluet M, et al. Alternating hemiplegia of childhood: early characteristics and evolution of a neurodevelopmental syndrome. Pediatrics. 2009;123:e534–541. doi: 10.1542/peds.2008-2027. [DOI] [PubMed] [Google Scholar]
- 14.Panagiotakaki E, Gobbi G, Neville B, et al. Evidence of a non-progressive course of alternating hemiplegia of childhood: study of a large cohort of children and adults. Brain. 2010;133:3598–3610. doi: 10.1093/brain/awq295. [DOI] [PubMed] [Google Scholar]
- 15.Roubergue A, Roze E, Vuillaumier-Barrot S, et al. The multiple faces of the ATP1A3-related dystonic movement disorder. Mov Disord. 2013;28:1457–1459. doi: 10.1002/mds.25396. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Inui T, Sakakibara T, et al. Evolution of hemiplegic attacks and epileptic seizures in alternating hemiplegia of childhood. Epilepsy Res. 2010;90:248–258. doi: 10.1016/j.eplepsyres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Ishii A, Saito Y, Mitsui J, et al. Identification of ATP1A3 mutations by exome sequencing as the cause of alternating hemiplegia of childhood in Japanese patients. PLoS ONE. 2013;8:e56120. doi: 10.1371/journal.pone.0056120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki M, Ishii A, Saito Y, et al. Genotype-phenotype correlations in alternating hemiplegia of childhood. Neurology. 2014;82:482–490. doi: 10.1212/WNL.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 19.Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2. 4 A resolution. Nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- 20.Rosewich H, Ohlenbusch A, Huppke P, et al. The expanding clinical and genetic spectrum of ATP1A3-related disorders. Neurology. 2014;82:945–955. doi: 10.1212/WNL.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Gao H, Zhang J, Xu X, et al. ATP1A3 mutations and genotype-phenotype correlation of alternating hemiplegia of childhood in Chinese patients. PLoS ONE. 2014;9:e97274. doi: 10.1371/journal.pone.0097274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svetel M, Ozelius LJ, Buckley A, et al. Rapid-onset dystonia-parkinsonism: case report. J Neurol. 2010;257:472–474. doi: 10.1007/s00415-009-5385-y. [DOI] [PubMed] [Google Scholar]
- 23.Becker LE. Alternating hemiplegia of childhood. Raven Press; 1995. pp. 57–65. [Google Scholar]
- 24.Oblak AL, Hagen MC, Sweadner KJ, et al. Rapid-onset dystonia-parkinsonism associated with the I758S mutation of the ATP1A3 gene: a neuropathologic and neuroanatomical study of four siblings. Acta Neuropathol. 2014;128:81–98. doi: 10.1007/s00401-014-1279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11:381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards KS, Bommert K, Szabo G, Miles R. Differential expression of Na+/K+-ATPase alpha-subunits in mouse hippocampal interneurones and pyramidal cells. J Physiol (Lond) 2007;585:491–505. doi: 10.1113/jphysiol.2007.144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu Y, Parada I, Prince DA. Temporal and topographic alterations in expression of the alpha3 isoform of Na+, K(+)-ATPase in the rat freeze lesion model of microgyria and epileptogenesis. Neuroscience. 2009;162:339–348. doi: 10.1016/j.neuroscience.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bøttger P, Tracz Z, Heuck A, et al. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol. 2011;519:376–404. doi: 10.1002/cne.22524. [DOI] [PubMed] [Google Scholar]
- 29.Azarias G, Kruusmagi M, Connor S, et al. A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3. J Biol Chem. 2013;288:2734–2743. doi: 10.1074/jbc.M112.425785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci. 2005;10:2373–2396. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Satake S, Onaka T, et al. Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. J Physiol (Lond) 2013;591:3433–3449. doi: 10.1113/jphysiol.2012.247817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson TR, Huguenard JR, Prince DA. Differential effects of Na+-K+ ATPase blockade on cortical layer V neurons. J Physiol (Lond) 2010;588:4401–4414. doi: 10.1113/jphysiol.2010.191858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clapcote SJ, Duffy S, Xie G, et al. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci US A. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gittis AH, Leventhal DK, Fensterhelm BA, et al. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci. 2011;31:15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calabresi P, Di Filippo M. A pathophysiological link between dystonia, striatal interneurons and neuropeptide Y. Brain. 2013;136:1341–1344. doi: 10.1093/brain/awt096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropathologic findings in ATP1A3 Gly358Val brain. A) Subpial gliosis and astrocytosis of entorhinal cortex shown by GFAP immunofluorescence. B) Severe gliosis of temporal lobe white matter. C,D) Marked neuronal loss in hippocampal layer CA1 (C) as compared with CA3 (D). E) Thinning and astrocytosis of the dentate gyrus (DG) of the hippocampus. F) Decreased neuronal density and astrocytosis of the caudate nucleus. Green = MAP2; Red = GFAP; Blue = DAPI.