Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder characterized by the progressive growth of renal cysts that, over time, destroy the architecture of the renal parenchyma and typically lead to kidney failure by the 6th decade of life. ADPKD is common and represents a leading cause of renal failure worldwide. Currently, there are no FDA approved treatment for the disease, and the existing standard of care is primarily supportive in nature. However, significant advances in the understanding of the molecular biology of the disease have inspired investigation into potential new therapies. Several drugs designed to slow or arrest the progression of ADPKD have shown promise in pre-clinical models and clinical trials, including vasopressin receptor antagonists and somatostatin analogs. This article examines literature underlying the rationale for molecular therapies for ADPKD and reviews the existing clinical evidence for their indication for human patients with the disease.
Keywords: Autosomal Dominant Polycystic Kidney Disease (ADPKD), Polycystin-1 (PC1), Polycystin-2 (PC2), Cyclic adenosine 3’-5’ monophosphate (cAMP), End stage renal disease (ESRD), Vasopressin V2 receptor (V2R) antagonists, Somatostatin analogues, AMP-activated protein kinase (AMPK) activators, Cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, Src inhibitors
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the fourth leading cause of renal failure in adults and affects approximately 1:500 to 1:1000 live births1. Individuals with the disease are born with normal renal function that declines progressively as renal cysts grow and expand, eventually leading to end stage renal disease (ESRD) in many patients by the 6 th decade of life. ADPKD is a multisystem disease characterized by the progressive development of bilateral renal cysts, and can additionally present with a wide variety of extrarrenal manifestations that include hepatocystic disease, intracranial aneurysms and cardiac defects among others2. Currently there exists no FDA-approved therapy for the disease outside of supportive care, including renal replacement therapy for those who develop ESRD.
ADPKD is caused by mutations in the genes PKD1 or PKD2, which encode the proteins polycystin-1 (PC1) and polycystin-2 (PC2), respectively. Whether a third gene may explain a small number of unlinked families is still unknown. While the exact mechanisms of pathogenesis remain to be elucidated, the dysregulation of intracellular Ca++ signaling observed to follow the loss of functional PC1 and PC2 is thought to induce important changes in cyclic nucleotide metabolism that drive the hypersecretory and hyperproliferative cellular phenotype characteristic of ADPKD3,4,5.
In the last decade, advances in the understanding of the molecular biology of ADPKD have prompted the study of a number of pharmacological therapies designed to slow or arrest the progression of the disease. Most therapies recently or currently being tested are aimed at 1) reducing cyclic adenosine 3’-5’ monophosphate (cAMP) levels, 2) inhibiting tubular cell proliferation and 3) reducing fluid secretion by cystic epithelium. This article focuses on new therapies that have recently completed or are currently being tested in clinical trials of ADPKD
CLINICAL COURSE, CRISP and HALT TRIALS in PATIENTS WITH ADPKD
A significant barrier to the development of an effective therapy for ADPKD has been the lack of a sensitive means by which to measure the progression of the disease over time. Serum creatinine is not typically elevated in patients until late in the course of disease progression, by which time significant and irreversible damage to the renal parenchyma has already occurred. New markers of disease progression were needed to provide better prognosis and assess the efficacy of candidate therapies in clinical trials for patients with ADPKD. For this reason, the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) was founded to investigate the use of magnetic resonance imaging (MRI) to quantify renal expansion as a means of non-invasive surveillance of patients with ADPKD. CRISP has produced valuable information, identifying baseline predictors of disease severity and renal function decline.
CRISP I enrolled 241 patients with ADPKD between the ages of 15 to 45 with a minimum creatinine clearance of 70 mL per minute, of whom sixty percent were also comorbid with hypertension. Patients were assessed annually from 2001 to 2005, to investigate the correlation between total kidney volume (TKV) and glomerular filtration rate (GFR), as measured by MRI and iothalamate clearance, respectively. Two years after the completion of CRISP I, 201 participants re-enrolled into an additional 5-year follow-up study (CRISP II). Participants had TKV and GFR measurements as in CRISP I, but this time were evaluated every two years. The main findings from the initial study (241 participants) were that TKV increased steadily in all over time, but at a uniform rate unique to each patient. The mean baseline TKV was 1076 mL with a mean increase of 63.4 mL (5.27%) per year, a rate significantly correlated with the measured decline of GFR. However, the rate of TKV increase and the corresponding rate of decline in GFR varied widely among patients. When grouped by baseline TKV, only patients with an initial value greater than 1500 mL showed a significant average decrease in GFR slopes (−4.3 mL/min/year)6. Despite heterogeneity, the strong correlation between baseline TKV and the rate of decline in GFR, provided evidence that TKV might effectively forecast the age of progression to ESRD. From the 201 re-enrollees (CRISP II), 194 completed an average 8 years of follow-up. The primary endpoint from this phase of the study was renal insufficiency, defined as stage 3 CKD or a GFR <60 ml/min per 1.73 m2. The overall annual rate of increase in TKV was similar to the initial period of follow-up (5.5% ± 3.8%/yr versus 5.3% ± 4.0%/yr). In addition, this extension in follow-up period confirmed the exponential relationship between TKV and age observed after 3 years. By receiver operator characteristic (ROC), an htTKV value of 600mL/m or greater forecasted the onset of renal insufficiency within 8 years. Further analysis showed that each 100mL increment of baseline htTKV increased the odds of developing renal insufficiency within 8 years by nearly 150 percent7. This confirmed the findings of CRISP I and implied that just a single determination of htTKV may be sufficient for prognosis.
Cardiovascular disease, particularly hypertension and its sequelae, is a leading cause of morbidity and mortality in patients with ADPKD8. However, to date, an optimal antihypertensive therapy for ADPKD has yet to be defined. The HALT-PKD is an ongoing clinical trial designed to evaluate whether there is a difference in the progression, or in slowing down the decline of renal function between those given dual angiotensin converting enzyme (ACEi) inhibitor and angiotensin-II receptor blocker (ARB) therapy versus an ACEi alone, in patients with GFR > 60 ml/min per 1.73 m2 (study A), and in patients with GFR 25 to 60 ml/min per 1.73 m2 (study B) respectively. In those with GFR > 60 ml/min per 1.73 m2, the study will further investigate the effect of medicating to a standard (120-130/70-80 mmHg) versus low (95-110/60-75 mmHg) target blood pressure on renal function over time9. Initial analysis of baseline parameters have shown that estimated GFR and higher urine albumin were independently associated with a higher baseline htTKV, confirming the findings of the CRISP consortium10.
Together, CRISP and HALT-PKD trials have provided important insight into the progression of ADPKD that will prove beneficial for both patient management and the evaluation of candidate therapeutics in clinical trials
GENETICS & PATHOPHYSIOLOGY
The majority of the patients with ADPKD present mutations in the PKD1 gene; approximately 85%, which is associated with a more severe renal cystic disease than mutations in PKD211. On average, patients with mutations in PKD1 develop ESRD at younger ages compared to patients with PKD2 mutations (54.3 vs 74 years of age), but extrarenal phenotypes are known to affect patients with mutations on both genes12. Extensive genetic analysis of patients with ADPKD has revealed that a wide variety of mutations can cause the disease. To date, 1272 and 202 confirmed pathogenic mutations have been described in PKD1 and PKD2, respectively.http://pkdb.mayo.edu/index.html
It has been held that the position of the mutation in PKD1 or PKD2 plays a role in determining the severity of disease13, 14, but recent clinical evidence suggests that the type of mutation may be more important, as patients with truncating mutations have been shown to present with more severe kidney disease than those with non-truncating mutations15, 16. Hypomorphic mutations and incompletely penetrant PKD1 or PKD2 alleles have been described. A hypomorphic allele alone may result in mild cystic disease while two such alleles can cause typical to severe disease, and one hypomorphic allele in combination with an inactivating allele is associated with early onset severe disease.
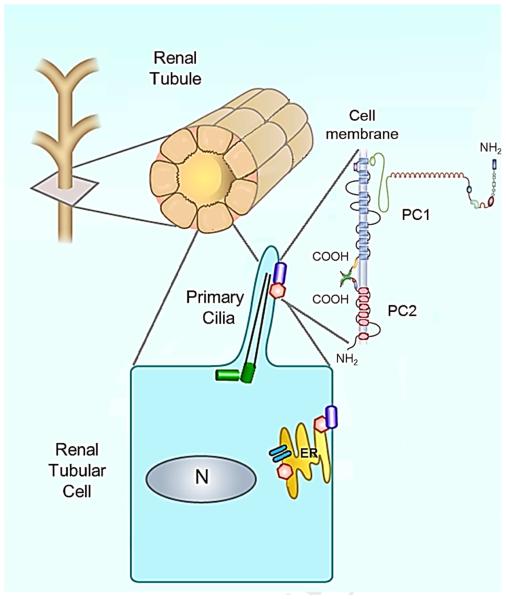
The pathophysiology of ADPKD originates from the loss of functional polycystin-1 (PC1) and polycystin-2 (PC2), the protein products of PKD1 and PKD2 respectively. These proteins are known to form a complex that is believed to function as a transient receptor potential (TRP) channel involved in the regulation of intracellular calcium homeostasis17, 18. Research indicates this complex to be localized to the primary cilium19 as well as the endoplasmic reticulum (ER)20, where it exerts specific influence on calcium concentrations in several subcellular compartments21, 22. In the primary cilium, it is believed that the PC1-PC2 complex functions as a mechanoreceptor to induce the influx of extracellular calcium in response to fluid shear stress23-25, while in the ER it associates with the ryanodine receptor (RyR) and functions as a calcium release channel (Figure 1) 20, 26. Recent evidence suggests that ciliary calcium signaling is isolated from the cytoplasm, and that any influence it may exert on the rest of the cell is not due to bulk entry of calcium via the primary cilium27, 28.
Figure 1. Schematic representation of the PKD1 protein, polycystin-1 (PC1) and the PKD2 protein, polycystin-2 (PC2), and their interaction in a normal tubular cell.
PC1 and PC2 are found on the primary cilium but also in the plasma membrane (PC1) and the endoplasmic reticulum (ER) (PC2). The PC1/PC2 complex in primary cilia senses flow and translates it into Ca2+ entry and ER Ca2+ release. Disruption of PC1/PC2 in ADPKD lowers intracellular Ca2+ and results in up regulation of cAMP signaling and increased fluid secretion and cell proliferation.
While the mechanisms are poorly understood, loss of the functional PC1-PC2 complex results in phenotypic changes which include the inability to maintain planar cell polarity, an increase in proliferation and apoptosis, the expression of a secretory phenotype, and remodeling of the extracellular matrix. The major signaling pathways implicated in these phenotypic changes include the intracellular deregulation of calcium homeostasis, cAMP accumulation and activation of protein kinase A (PKA), activation of mitogen activated protein (MAPK) and mammalian target of rapamycin (mTOR) kinases, canonical Wnt signaling and other intracellular signaling mechanisms. 5, 29, 30.
ROLE of cAMP in PKD
Many animal models of PKD have increased levels of intracellular cAMP not only in the kidney but also in other tissues affected by the disease, including cholangiocytes31, vascular smooth muscle32 and choroid plexus33, which has been shown to be of central importance to the pathogenesis of ADPKD.
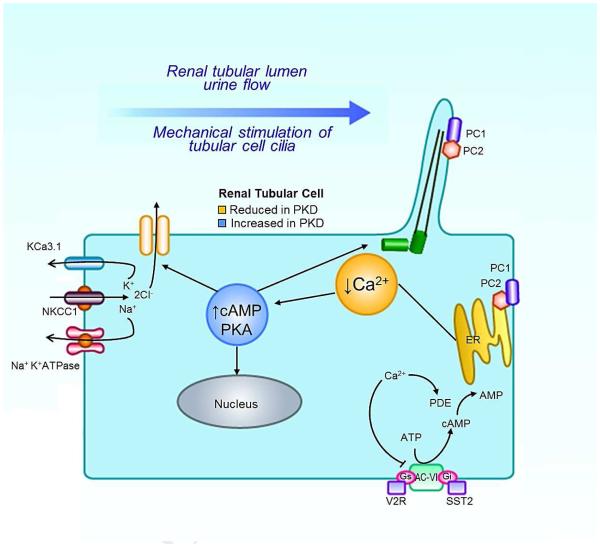
Cyclic AMP levels are normally regulated by the balanced activity of soluble and G-protein coupled receptor (GPCR) associated adenylyl cyclases (ACs) and phosphodiesterases (PDEs). Increased levels of cAMP have been attributed to a variety of mechanisms, most of which are related to altered calcium signaling. Reduced calcium is known to inhibit directly or indirectly the activity of PDE1 and PDE3 respectively, and to activate membrane-bound AC6, thus producing a net increase in cAMP concentration (Figure 2). Likewise, depletion of calcium in the ER (perhaps due to leakage of PC222) induces the assembly of stromal interaction molecule 1 (STIM1) and the concomitant activation of AC634. The compartmentalized nature of cAMP signaling35 suggests that certain ACs and PDEs may be more important than others to the pathogenesis of ADPKD due to their influence on specific subcellular pools of cAMP. The specific importance of AC6 was underlined in a recent study in which the collecting duct specific ablation of Pkd1 induced a renal cystic phenotype that was rescued by the concomitant knockout of AC636. Similarly, recent studies suggest that specific PDEs, including PDEs 1, 3, and 4, may be more important than others in the pathogenesis of ADPKD37, 38. The compartmentalization of cAMP regulatory enzymes may present a unique future opportunity for specifically targeted therapies.
FIG 2.
PKO SIGNALING DIAGRAM w/ TARGETS OF INTERVENTION
cAMP exerts its effects via PKA, an intermediate identified as contributing to several distinct elements of ADPKD pathophysiology. It is possible that PKA signaling may induce dysfunctional renal development via the aberrant activation of Wnt/β-catenin signaling39, 40, a pathway known to be disrupted in ADPKD41. This sustained PKA-dependent activation of canonical Wnt signaling interferes with nephrogenesis and results in disorganized epithelial clusters and dilated renal tubules42. Furthermore, cAMP signaling has been demonstrated to be involved with the hypersecretory cellular behavior that drives cyst expansion. Forskolin, a drug that upregulates cAMP synthesis, was shown early on to induce fluid secretion in intact cysts excised from patients with ADPKD43. The bulk of this fluid secretion is driven by the active transport of chloride into the lumen of the cyst44, 45, which is drawn across the basolateral membrane epithelium by the combined activity of the Na+K+ATPase and the Na+K+Cl− cotransporter. PKA-mediated phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) induces the opening of its channel and permits chloride ions to flow down the electrochemical gradient and into the cyst46. This is supported by the observation that pathogenic mutations of the CFTR in the context of PKD function to attenuate cystogenesis both in laboratory studies47 and in patients with ADPKD.48, 49.
PKA-mediated cAMP signaling is also believed to be responsible for much of the hyperproliferative cellular phenotype observed in ADPKD. Treatment with cAMP induces proliferation in primary cells derived from polycystic renal tissue in an extracellular signal- regulated kinase (ERK) dependent manner, but inhibits proliferation in cells derived from healthy human kidneys50, 51. PKA-mediated ERK signaling has been shown to activate mTOR52, 53, a protein that forms two distinct multimeric complexes (mTORC1 and mTORC2) that regulate a variety of cellular processes involved with growth, proliferation, and autophagy54. The upregulation of a number of other pro-proliferative transcription factors have been linked to PKA-mediated cAMP signaling in PKD, including cAMP response element-binding protein (CREB)55, paired box gene 2 (Pax2)56, and signal transducer and activator of transcription 3 (STAT3)5, 57. STAT3 is of particular interest due to its role in the development and maintenance of a pro-inflammatory cellular environment, a characteristic of cystic tissue in PKD58. STAT3 activation is known to induce the secretion of a number of pro-inflammatory soluble factors which, in turn, activate tumor-associated macrophages in an alternative pathway (M2) dependent manner59. Research indicates that macrophages undergo alternative activation in response to unidentified elements secreted by the tubular epithelium of PKD model animals. Alternatively activated macrophages have been shown to induce cellular proliferation and fibrogenesis in the tissues of orthologous models for PKD, and depletion of these macrophages via introperitoneal clodronate injections has been shown to attenuate the severity of PKD in vivo60, 61.
THERAPIES TARGETING cAMP SIGNALING
The importance of cyclic nucleotide signaling in ADPKD, specifically cAMP, provides rationale for therapies towards decreasing its levels (i.e. vasopressin V2 receptor antagonists, somatostatin analogs) and have been used in clinical trial with promising results.
Vasopressin V2 receptor (V2R) antagonists
Vasopressin is a small peptide hormone secreted by the pituitary gland in response to increased plasma osmolality, and functions to induce the reabsorption of water in the collecting duct by binding to the G-protein coupled arginine vasopressin (AVP) V2 receptor. In doing so, AVP also stimulates the production of cAMP via membrane bound adenylate cyclase 6. Circulating AVP levels and renal V2R expression are increased in rodent PKD, possibly due to AVP resistance manifested by a urinary concentrating defect. As the expression of the V2 receptor is almost exclusive to the late nephron; the site of renal cystogenesis in ADPKD,62 V2R are an attractive target to modulate cAMP with minimal side effects.. Several preclinical trials have shown that treatment with AVP V2 receptor antagonists successfully slow disease progression in orthologous animal models of both ARPKD and ADPKD, including those with mutations on Pkd2 63, 64. The importance of circulating AVP in the natural progression of the disease was demonstrated by the finding that increased water intake reduced the progression of renal cystic disease in an ARPKD model (PCK rat)65. This was confirmed when the same animals were bred with rats lacking circulating AVP (AVP−/−, so-called Brattleboro rats66), producing double-homozygous offspring (AVP−/− Pkhd1−/−) that were shown to have almost normal renal structure and function, but could be induced to develop renal cystic disease through the administration of synthetic 1-deamino-8-D-arginine vasopressin (DDAVP), a synthetic V2 receptor agonist67.
Tolvaptan is a highly potent and selective AVP V2 receptor antagonist and has been shown to slow the progression of PKD in preclinical trials68 In the US, it is currently approved for the treatment of hypervolemic and euvolemic hyponatremia. In Japan, it has now been approved as a therapy to slow down the progression of ADPKD in patients with rapidly growing kidneys. Open-label Phase 2 clinical trials in the US69 and Japan70 have demonstrated the long-term safety and tolerability of tolvaptan and generated pilot efficacy data..
The Tolvaptan Efficacy and Safety in Management of ADPKD and its Outcomes (TEMPO) 3:4 trial was a phase 3, multicenter, randomized, double blinded, placebo controlled, parallel-group clinical study, designed to assess the impact of tolvaptan therapy on the progression of ADPKD71. One thousand, four hundred and forty five ADPKD patients aged 18-50 years, with a TKV >750 mL and CrCL>60 mL/min, across 129 sites worldwide were enrolled in the study between January 2007 and January 2009. Of these, 961 initiated treatment with tolvaptan and the other 483 (one withdrawal pre-treatment) with placebo. One thousand, one hundred and fifty seven patients completed all three years of the trial, corresponding to 77% and 86.2% of the tolvaptan and placebo group, respectively. All patients with at least one follow-up MRI, 1307 of the original 1445 patients, were included in the primary efficacy analysis and all 1445 in the secondary safety analysis. In the tolvaptan group, therapy consisted of either a high dose (120mg/day, n=404), medium dose (90mg/day, n=157), or low dose (60mg/day, n=179), with high rates of self-reported adherence in the majority of patients. The primary end point for the study was the annual rate of percent change in TKV as measured by MRI, with several secondary endpoints corresponding to a measurable decline renal function, including a composite endpoint of investigator-assessed disease progression.
The results showed that during the 3-year trial tolvaptan reduced the rate of kidney growth by nearly 49%. Total kidney volume increased an average of 2.8% per year in patients treated with tolvaptan versus 5.5% per year growth rate in the placebo group (p<0.001). This corresponded to the observed difference in eGFR slope (as measured by the reciprocal of serum creatinine level) between the two groups: −2.61 mg/mL per year versus −3.81 mg/mL per year, for tolvaptan and placebo respectively (p<0.001), and the average increase in serum creatinine from 1.05 to 1.21 mg/dL versus 1.04 to 1.27 mg/dL in the tolvaptan and placebo respectively (p<0.001). Furthermore, analysis of the key secondary composite endpoint of time to clinical progression showed fewer ADPKD-related events per 100 person-years of follow-up with tolvaptan than placebo (hazard ratio 0.87, 95% C. 0.78 to 0.97, P=0.01). The protective effect was observed across all prespecified subgroups, including stratification by sex, age, diagnosis of hypertension, baseline TKV and baseline creatinine clearance. However, this effect was nominally greater in patients 35 years of age or older and those with hypertension and/or a TKV of greater or equal to 1500mL. It was also observed that the effect of tolvaptan on renal volume expansion was larger in the first year than in the second and third years. The frequency of adverse events (AEs) experienced by tolvaptan and placebo group were roughly equivalent; 97.9% vs 97.1% respectively. Increased aquaresis related AEs (thirst, polyuria, nocturia and polydipsia) were higher in frequency in the tolvaptan group and ADPKD-related AEs (kidney pain, hematuria, urinary tract infection, and back pain) were higher in the placebo group. Serious AEs occurred in at least 0.5% of the patients in either group. The most frequent SAEs occurring in the tolvaptan treatment group were: alanine aminotransferase elevation 0.9%, aspartate aminotransferase elevation 0.9 %, chest pain 0.8% and headache 0.5%. In the placebo group, most frequent SAEs included: pyelonephritis 1.0%, renal-cyst infection 0.8%, renal-cyst hemorrhage 0.8%, renal pain 0.8%, appendicitis 0.8%, nephrolithiasis 0.6%, urinary tract infection 0.6%, and hypertension 0.6%.
Currently, tolvaptan is not approved for the treatment of ADPKD in the United States.
Somatostatin Analogs
Somatostatin (SST) is a peptide hormone involved in the endocrine regulation of cellular metabolism. Interaction with the G-protein coupled somatostatin receptors (SSTRs 1-5) inhibits adenylate cyclase mediated cAMP synthesis, which functions to inhibit proliferation and secretion in a variety of tissues. Treatment with somatostatin has been shown to inhibit collecting duct specific cAMP synthesis in vitro72 and in vivo73, and has been shown to have similar effects in cholangiocytes as well74, 75. While the anti-proliferative and secretory effects of somatostatin have long been of interest to medical science, the three-minute serum half-life of somatostatin makes it impractical for therapeutic use. For this reason, the synthetic analogs octreotide, lanreotide and pasireotide were developed as stable alternatives.These analogs differ in stability and receptor selectivity; octreotide and lanreotide have a half-life of 2 hours in circulation and bind with high affinity to SSTRs 2&3 and moderate affinity to SSTR 5. Pasireotide, however, binds with high affinity to all SSTRs except SSTR4 and has a serum half- life of 12 hours76.Preclinical studies demonstrated that octreotide and pasireotide could successfully reduce proliferation and intracellular cAMP concentrations in cholangiocytes, both in vitro and in vivo31, 77, indicating that somatostatin analogs may be an effective therapy for ADPKD patients with cystic liver disease.
Three small, randomized, placebo-controlled clinical studies (NCT00309283, NCT00426153, NCT00565097) provided initial evaluation of octreotide or lanreotide treatment in ADPKD and polycystic liver disease (PLD). A 6 months cross-over study of octreotide treatment (40 mg i.m. once every 28 days) was well tolerated and reduced the rate of TKV growth by approximately 56% (71mL and 162mL for treatment and placebo respectively) in 14 patients with ADPKD (mean baseline GFR 57.1 mL/min, range 24.4 – 95.3 mL/min). GFR, measured as iohexol clearance, did not change significantly during both treatment periods.78 In a secondary post hoc analysis, significant changes in liver volumes between the two treatment periods were found. Liver volumes significantly (p< 0.05) decreased by 71±57 mL during octreotide versus 14±85 mL increase while no treatment.79
Another 6-month study compared treatment with Lanreotide (120 mg once every 28 days subcutaneously) to placebo in 54 patients with PLD,( of which 32 had ADPKD) Total liver volume decreased significantly (p<0.01) by 2.9% (4606mL to 4471mL) in Lanreotide versus a 1.6% increase in the placebo group (4689mL to 4895mL). A post hoc stratification for ADPKD/PLD revealed similar changes in liver volume between both disease groups, concluding that the change in liver volume was independent of cause of disease and for both, the difference between placebo and lanreotide treatment was significantly different (p<0.01).80. A 6 month open label extension, where 41 patients (25 ADPKD) re-enrolled to complete a total 12 months of lanreotide treatment, showed median decrease in liver volume of 4% (p<0.05) in the overall study population. The greatest effect was observed during the first 6 months of treatment and liver volume remained unchanged during the following 6 months. In 22 patients that had a CT scan 6 months after stopping 12 months of lanreotide treatment, a 4% rebound growth in liver volume was observed.81
Another octreotide study (40 mg i.m every 28 days) in 42 patients with PLD (of which 34 had ADPKD) showed a reduction in liver volume of 4.95% in the octreotide group compared with an increase of 0.92% in the placebo group. In addition, TKV remained practically unchanged in octreotide treated patients but increased significantly (p=0.045) in non-treated patients (+0.25% vs 8.61%) over the course of a year82. This study was also continued as open-label for an additional 12 months. In the initial octreotide treated group, the change in liver volume was maintained, but no significant change was observed during the following 12 months (−0.77% year 1 vs. year 2 p=0.57). Patients that crossed to octreotide experienced a significant 7.66% reduction in liver volume by the end of the octreotide treatment period (year 1 to year 2 p=0.01) compared to the initial 0.92% increase during placebo (Baseline to year 1).83
Another study that included 44 patients (29 PCLD, 15 ADPKD) comparing the effects of octreotide alone (40 mg i.m. once every 28 days) vs octreotide plus everolimus (40 mg i.m. once every 28 days + everolimus 2.5 mg daily) (NCT01157858) found no significant decrease in liver volume among the two groups (3.5% vs 3.8%; p = 0.73) over the course of 48 weeks.84
Most recently, a three-year multicenter, randomized, single-blind, placebo-controlled, parallel-group study of octreotide treatment in Italy published results supporting the use of octreotide in ADPKD85. Seventy-nine adult (>18 years old) patients with an eGFR greater than 40mL/min/1.73m2 were enrolled in either a treatment (two 20mg i.m. octreotide-LAR injections every 28 days) or placebo group. Of these, 37 octreotide-LAR and 36 placebo treated patients completed the 3 year study. After year one of follow up, it was found that octreotide reduced the average increase in TKV by nearly two-thirds that of the control group (46.2mL vs. 143.7mL, respectively p=0.032). However, at three year follow-up, even though TKV increase in the octreotide-LAR group was smaller compared to placebo group, the difference in TKV growth was not significant between the two groups (p=0.25).
Several larger clinical trials of somatostatin analogs for ADPKD and PLD are currently underway. A European study designed to examine the renoprotective efficacy of lanreotide in ADPKD, DIPAK, is ongoing. This multicenter clinical trial will enroll 300 ADPKD patients with an eGFR between 30 and 60mL/min/1.73m2 and measure the effect of lanreotide treatment (120mg q.28 days via subcutaneous injection) over the course of two and a half years86. The primary study outcome is the slope of eGFR measurements taken between weeks 12 and 120, with secondary outcome parameters of changes in kidney volume, liver volume, and self-reported quality of life.
THERAPIES TARGETING mTOR SIGNALING
It had been observed that contiguous germline deletions affecting PKD1 and the immediately adjacent TSC2, a gene associated with tuberous sclerosis, led to abnormally severe disease in patients87-89. This gave rise to the hypothesis that the two genes may function as part of a common pathway. PC1 physically interacts and retains tuberin at the plasma membrane thus preventing it phosphorylation by AKT (protein kinase B) and its ability to inhibit the mTOR pathway90. Other studies have shown that ERK activation, downstream from PKA activation, leads to phosphorylation of tuberin preventing its association with hamartin and the inhibition of Rheb and mTOR by the tuberin-hamartin complex.52, 53
Early preclinical studies in rodent models of PKD demonstrated that mTOR was highly upregulated in the cystic epithelium 91, 92 and that sirolimus93, 94 treatment decreased proliferation in cystic and non-cystic tubules, markedly inhibited renal enlargement and cystogenesis and prevented the loss of kidney function giving rise to the hope they would be effective in humans as well. Further differences between rat and mouse studies raised questions on whether doses of mTOR inhibitors necessary for effectiveness in PKD were feasible in the clinical setting. At doses and blood levels achievable in humans, sirolimus and everolimus were effective in a rat model of PKD affecting the proximal tubules but not in an orthologous model affecting the distal nephron and collecting duct.95 Lower dose treatment in an orthologous mouse model similarly demonstrated no effect 96.
Clinical trials for mTOR inhibitors in patients with ADPKD have been largely disappointing, possibly because blood levels capable to inhibit mTOR activity in peripheral blood mononuclear cells are not sufficient to inhibit mTOR activity in the kidney.97 The use of sirolimus in the treatment of patients with ADPKD showed only a small benefit (SIRENA)98, (RAPYD)99 or no benefit (SUISSE)100 on kidney volume or GFR. Additionally, one clinical trial using everolimus showed inhibition of kidney growth but more decline in eGFR.101
Results from a new pilot trial of rapamycin in 30 adult patients with ADPKD102 have been recently published. Patients were randomly assigned to low-dose (n=10, 2–5 ng/ml rapamycin), standard-dose (n=10, 5–8 ng/ml rapamycin) or standard care (n=10). Braun et al. hypothesized that a more accurate measure of change in GFR using 125I-iothalamate (iGFR) as compared by eGFR (CKD-EPI) (primary endpoint) would reveal a treatment effect with better tolerated low-dose rapamycin treatment for 12 months. The results show that low dose rapamycin was associated with improved short-term (6 and 12 months) renal function as measured by change in iGFR without significant change in TKV or eGFR.
Other therapeutic targets currently in clinical trials for ADPKD
Botusimib
The Src inhibitor SKI-606 retarded cyst growth in PCK rats and bpk and Pkd1 heterozygous mice.103, 104 A phase II, multicenter, randomized, double blind, placebo control clinical trial with bosutinib (a Src/Abl inhibitor) is currently ongoing (NCT01233869).
Statins
A randomized double-blind placebo-controlled phase III clinical trial (from 2007 to 2012) to assess the effect of pravastatin on HtTKV and left ventricular mass index (LVMI) by MRI and urine microalbumin excretion (UAE) in children and young adults with ADPKD was recently completed and results are now available. The trial enrolled 110 patients (8–22 years of age) of whom 91 completed the trial. Those patients randomized to pravastatin (n=56) demonstrated significantly less increase in HtTKV over 3 years compared to patient randomized to placebo (n=54,23%±3% for statin versus 31%±3% for placebo per 3 years; P=0.02). There was no significant change in creatinine clearance, urine protein excretion or LDL cholesterol between groups. Pravastatin was well tolerated with no apparent adversity. The findings support a role for early intervention with pravastatin in ADPKD.105
SUMMARY.
ADPKD is a disease of remarkably complex genetic and molecular etiology. Advances in the understanding of its pathogenesis, in particular the role of cAMP, have led to the development of candidate therapies that have demonstrated significant promise in clinical trials. Unlike the existing standard of care, these treatments are designed to attenuate the progression of the disease in target tissues and to prevent the onset of organ failure. Unfortunately, the limitations of clinical study design hamper accurate evaluation of their efficacy in patients. Traditional surrogate markers of renal function are poorly correlated to the progression of ADPKD, making it difficult to assess the impact of a given therapy over the short timespan of a clinical trial; Total kidney volume may provide a more accurate alternative. Vasopressin V2 receptor antagonists and somatostatin analogs have been shown to safely slow kidney growth and protect renal function in patients with ADPKD, and represent the most well-characterized and promising candidate therapies to date. While the search for a cure is far from over, it is seems increasingly possible that ADPKD will one day be a treatable disease. For this to occur, advances at the laboratory benchtop must continue for us to provide relief at the patient bedside.
Acknowledgments
Dr. Torres discloses that he has received research funding from Otsuka Pharmaceuticals. There is no funding to report. The authors have not received any editorial support for preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal’s policy on disclosure of potential conflicts of interest.
References
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Wu G, Markowitz GS, Li L, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–78. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 3.Somlo S, Ehrlich B. Human disease: calcium signaling in poylcystic kidney disease. Curr Biol. 2001;11:R356–360. doi: 10.1016/s0960-9822(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta. 2011;1812:1291–1300. doi: 10.1016/j.bbadis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres VE, Harris PC. Strategies Targeting cAMP Signaling in the Treatment of Polycystic Kidney Disease. J Am Soc Nephrol. 2014;25 doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. The New England journal of medicine. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 7.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9:2–11. doi: 10.2174/1573402111309010002. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: design and implementation. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres VE, Chapman AB, Perrone RD, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–585. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 12.Hateboer N, v Dijk MA, Bogdanova N, et al. European PKD1-PKD2 Study Group Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol. 2002;13:1230–1237. doi: 10.1097/01.asn.0000013300.11876.37. [DOI] [PubMed] [Google Scholar]
- 14.Rossetti S, Harris PC. The genetics of vascular complications in autosomal dominant polycystic kidney disease (ADPKD) Current hypertension reviews. 2013;9:37–43. doi: 10.2174/1573402111309010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei Y, Lan Z, Wang K, et al. A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int. 2012;81:412–417. doi: 10.1038/ki.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 Mutation Influences Renal Outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassilev PM, Guo L, Chen XZ, et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochemical and biophysical research communications. 2001;282:341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 18.Anyatonwu GI, Ehrlich BE. Organic cation permeation through the channel formed by polycystin-2. The Journal of biological chemistry. 2005;280:29488–29493. doi: 10.1074/jbc.M504359200. [DOI] [PubMed] [Google Scholar]
- 19.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 20.Koulen P, Cai Y, Geng L, et al. Polycystin-2 is an intracellular calcium release channel. Nature cell biology. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 21.Foggensteiner L, Bevan AP, Thomas R, et al. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- 22.Santoso NG, Cebotaru L, Guggino WB. Polycystin-1, 2, and STIM1 interact with IP(3)R to modulate ER Ca release through the PI3K/Akt pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;27:715–726. doi: 10.1159/000330080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 24.Nauli SM, Kawanabe Y, Kaminski JJ, et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masyuk AI, Masyuk TV, Splinter PL, et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anyatonwu GI, Estrada M, Tian X, et al. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A. 2007;104:6454–6459. doi: 10.1073/pnas.0610324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCaen PG, Delling M, Vien TN, et al. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delling M, DeCaen PG, Doerner JF, et al. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 30.Paavola J, Schliffke S, Rossetti S, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. Journal of molecular and cellular cardiology. 2013;58:199–208. doi: 10.1016/j.yjmcc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Kip SN, Hunter LW, Ren Q, et al. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circulation research. 2005;96:873–880. doi: 10.1161/01.RES.0000163278.68142.8a. [DOI] [PubMed] [Google Scholar]
- 33.Banizs B, Komlosi P, Bevensee MO, et al. Altered pH(i) regulation and Na(+)/HCO3(−) transporter activity in choroid plexus of cilia-defective Tg737(orpk) mutant mouse. American journal of physiology Cell physiology. 2007;292:C1409–1416. doi: 10.1152/ajpcell.00408.2006. [DOI] [PubMed] [Google Scholar]
- 34.Spirli C, Locatelli L, Fiorotto R, et al. Altered store operated calcium entry increases cyclic 3',5'-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology. 2012;55:856–868. doi: 10.1002/hep.24723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 35.Flores J, Witkum PA, Beckman B, et al. Stimulation of osmotic water flow in toad bladder by prostaglandin E1. Evidence for different compartments of cyclic AMP. The Journal of clinical investigation. 1975;56:256–262. doi: 10.1172/JCI108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees S, Kittikulsuth W, Roos K, et al. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol. 2014;25:232–237. doi: 10.1681/ASN.2013010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sussman C, Ward CJ, Leightner A, et al. Regulation of renal cyst formation by phosphodiesterase 1a in zebrafish [Abstract] J Am Soc Nephrol. 2012;1A [Google Scholar]
- 38.Ye H, Wang X, et al. Genetic Approach to Evaluate the Role of PDE3 Subfamilies in Polycystic Kidney Disease. J Am Soc Nephrol. 2012;23 [Google Scholar]
- 39.Wuebken A, Schmidt-Ott KM. WNT/beta-catenin signaling in polycystic kidney disease. Kidney Int. 2011;80:135–138. doi: 10.1038/ki.2011.87. [DOI] [PubMed] [Google Scholar]
- 40.Taurin S, Sandbo N, Qin Y, et al. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. The Journal of biological chemistry. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 41.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends in molecular medicine. 2010;16:349–360. doi: 10.1016/j.molmed.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallegos TF, Kouznetsova V, Kudlicka K, et al. A protein kinase A and Wnt-dependent network regulating an intermediate stage in epithelial tubulogenesis during kidney development. Developmental biology. 2012;364:11–21. doi: 10.1016/j.ydbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye M, Grantham JJ. The Secretion of Fluid by Renal Cysts from Patients with Autosomal Dominant Polycystic Kidney Disease. New England Journal of Medicine. 1993;329:310–313. doi: 10.1056/NEJM199307293290503. [DOI] [PubMed] [Google Scholar]
- 44.Veizis IE, Cotton CU. Role of kidney chloride channels in health and disease. Pediatric nephrology (Berlin, Germany) 2007;22:770–777. doi: 10.1007/s00467-006-0355-4. [DOI] [PubMed] [Google Scholar]
- 45.Grantham JJ. Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl. 1997;63:S93–97. [PubMed] [Google Scholar]
- 46.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Yang W, Mendes F, et al. Impact of the cystic fibrosis mutation F508del-CFTR on renal cyst formation and growth. American journal of physiology Renal physiology. 2012;303:F1176–1186. doi: 10.1152/ajprenal.00130.2012. [DOI] [PubMed] [Google Scholar]
- 48.O'Sullivan DA, Torres VE, Gabow PA, et al. Cystic fibrosis and the phenotypic expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:976–983. doi: 10.1016/s0272-6386(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 49.Xu N, Glockner JF, Rossetti S, et al. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J Nephrol. 2006;19:529–534. [PubMed] [Google Scholar]
- 50.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi T, Pelling JC, Ramaswamy NT, et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000;57:1460–1471. doi: 10.1046/j.1523-1755.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 52.Distefano G, Boca M, Rowe I, et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29:2359–2371. doi: 10.1128/MCB.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spirli C, Okolicsanyi S, Fiorotto R, et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360–371. doi: 10.1053/j.gastro.2009.09.005. e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betz C, Hall MN. Where is mTOR and what is it doing there? The Journal of Cell Biology. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aguiari G, Bizzarri F, Bonon A, et al. Polycystin-1 regulates amphiregulin expression through CREB and AP1 signalling: implications in ADPKD cell proliferation. Journal of molecular medicine (Berlin, Germany) 2012;90:1267–1282. doi: 10.1007/s00109-012-0902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin S, Taglienti M, Cai L, et al. c-Met and NF-kappaB-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol. 2012;23:1309–1318. doi: 10.1681/ASN.2011030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talbot JJ, Song X, Wang X, et al. The Cleaved Cytoplasmic Tail of Polycystin-1 Regulates Src-Dependent STAT3 Activation. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013091026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. 1995;41:693–765. doi: 10.1016/s0011-5029(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 59.Bai B, Horlad H, Saito Y, et al. Role of Stat3 activation in cell-cell interaction between B- cell lymphoma and macrophages: the in vitro study. Journal of clinical and experimental hematopathology : JCEH. 2013;53:127–133. doi: 10.3960/jslrt.53.127. [DOI] [PubMed] [Google Scholar]
- 60.Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2011;22:1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swenson-Fields KI, Vivian CJ, Salah SM, et al. Macrophages promote polycystic kidney disease progression. Kidney Int. 2013;83:855–864. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verani RR, Silva FG. Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod Pathol. 1988;1:457–463. [PubMed] [Google Scholar]
- 63.Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 64.Gattone VH, 2nd, Wang X, Harris PC, et al. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 65.Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17:2220–2227. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 66.Valtin H. Hereditary hypothalamic diabetes insipidus in rats (Brattleboro strain). A useful experimental model. The American journal of medicine. 1967;42:814–827. doi: 10.1016/0002-9343(67)90098-8. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Wu Y, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. Journal of the American Society of Nephrology. 2008;19:102–108. doi: 10.1681/ASN.2007060688. WC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Gattone V, 2nd, Harris PC, et al. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–851. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 69.Torres VE, Winklhofer FT, Chapman AB, et al. Phase 2 Open-Label study to determine long-term safety, tolerability and efficacy of split-dose tolvaptan in ADPKD. J Am Soc Nephrol. 2009;20 [Google Scholar]
- 70.Higashihaha E, Nutahara K, Horie S, et al. An open-label long term administration study of tolvaptan in patients with ADPKD in Japan. J Am Soc Nephrol. 2009;20 [Google Scholar]
- 71.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. New England Journal of Medicine. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedlander G, Amiel C. Somatostatin and alpha 2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS letters. 1986;198:38–42. doi: 10.1016/0014-5793(86)81180-2. [DOI] [PubMed] [Google Scholar]
- 73.Winkler SN, Torikai S, Levine BS, et al. Effect of somatostatin on vasopressin-induced antidiuresis and renal cyclic AMP of rats. Mineral and electrolyte metabolism. 1982;7:8–14. [PubMed] [Google Scholar]
- 74.Tan CK, Podila PV, Taylor JE, et al. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908–1916. doi: 10.1016/0016-5085(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 75.Tracy TF, Jr., Tector AJ, Goerke ME, et al. Somatostatin analogue (octreotide) inhibits bile duct epithelial cell proliferation and fibrosis after extrahepatic biliary obstruction. Am J Pathol. 1993;143:1574–1578. [PMC free article] [PubMed] [Google Scholar]
- 76.Irazabal MV, Torres VE. Experimental therapies and ongoing clinical trials to slow down progression of ADPKD. Current hypertension reviews. 2013;9:44–59. doi: 10.2174/1573402111309010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masyuk TV, Radtke BN, Stroope AJ, et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 79.Caroli A, Antiga L, Cafaro M, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5:783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Keimpema L, Nevens F, Vanslembrouck R, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. e1661-1662. [DOI] [PubMed] [Google Scholar]
- 81.Chrispijn M, Nevens F, Gevers TJ, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35:266–274. doi: 10.1111/j.1365-2036.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 82.Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hogan MC, Masyuk TV, Page L, et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chrispijn M, Gevers TJ, Hol JC, et al. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: Results from a randomized controlled trial. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Caroli A, Perico N, Perna A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 86.Meijer E, Drenth JP, d'Agnolo H, et al. Rationale and Design of the DIPAK 1 Study: A Randomized Controlled Clinical Trial Assessing the Efficacy of Lanreotide to Halt Disease Progression in Autosomal Dominant Polycystic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:446–455. doi: 10.1053/j.ajkd.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ong AC, Harris PC, Davies DR, et al. Polycystin-1 expression in PKD1, early-onset PKD1, and TSC2/PKD1 cystic tissue. Kidney Int. 1999;56:1324–1333. doi: 10.1046/j.1523-1755.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 88.Harris PC. Autosomal dominant polycystic kidney disease: clues to pathogenesis. Hum Mol Genet. 1999;8:1861–1866. doi: 10.1093/hmg/8.10.1861. [DOI] [PubMed] [Google Scholar]
- 89.Longa L, Scolari F, Brusco A, et al. A large TSC2 and PKD1 gene deletion is associated with renal and extrarenal signs of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1997;12:1900–1907. doi: 10.1093/ndt/12.9.1900. [DOI] [PubMed] [Google Scholar]
- 90.Dere R, Wilson PD, Sandford RN, et al. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS One. 2010;5:e9239. doi: 10.1371/journal.pone.0009239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zafar I, Belibi FA, He Z, et al. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD) Nephrol Dial Transplant. 2009;24:2349–2353. doi: 10.1093/ndt/gfp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian Q, Du H, King BF, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–638. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wahl PR, Serra AL, Le Hir M, et al. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 95.Renken C, Fischer DC, Kundt G, et al. Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant. 2011;26:92–100. doi: 10.1093/ndt/gfq384. [DOI] [PubMed] [Google Scholar]
- 96.Novalic Z, van der Wal AM, Leonhard WN, et al. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol. 2012;23:842–853. doi: 10.1681/ASN.2011040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canaud G, Knebelmann B, Harris PC, et al. Therapeutic mTOR inhibition in autosomal dominant polycystic kidney disease: What is the appropriate serum level? Am J Transplant. 2010;10:1701–1706. doi: 10.1111/j.1600-6143.2010.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perico N, Antiga L, Caroli A, et al. Sirolimus therapy to half the progression of ADPKD. J Am Soc Nephrol. 2010;21:1031–1040. doi: 10.1681/ASN.2009121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stallone G, Infante B, Grandaliano G, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:3560–3567. doi: 10.1093/ndt/gfs264. [DOI] [PubMed] [Google Scholar]
- 100.Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 101.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 102.Braun WE, Schold JD, Stephany BR, et al. Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:881–888. doi: 10.2215/CJN.02650313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sweeney WE, Jr., von Vigier RO, Frost P, et al. Src inhibition ameliorates polycystic kidney disease. Journal of the American Society of Nephrology. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elliott J, Zheleznova NN, Wilson PD. c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation. American journal of physiology Cell physiology. 2011;301:C522–529. doi: 10.1152/ajpcell.00163.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cadnapaphornchai M, George D, Wang W, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in Pediatric Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.08350813. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]