Abstract
Nervous system development relies on the generation of neurons, their differentiation and establishment of synaptic connections. These events exhibit remarkable plasticity and are regulated by many developmental cues. Here we review the mechanisms of three classes of these cues: morphogenetic proteins, electrical activity and the environment. We focus on second messenger dynamics and their role as integrators of the action of diverse cues, enabling plasticity in the process of neural development.
Introduction
Nervous system development proceeds through the participation of many developmental cues. Some are intrinsic to the organism, such as morphogenetic proteins, while others are environmental, like changes in temperature, availability of water, nutrients and illumination. From the first steps of neural induction and folding of the neural plate through the establishment of functional circuits, the diversity of cellular events that need to take place in a spatiotemporally coordinated manner is remarkable. Neurogenesis, cell migration, neuronal differentiation, axonal routing, synaptogenesis and synaptic remodeling are all necessary to provide shape and function to the emerging brain and spinal cord. Each of these processes in turn comprises many subcellular and molecular events that are tightly orchestrated. For instance, neuronal differentiation involves the successful acquisition of many traits; these include the establishment of a specific neuronal morphology, consisting in dendrite arborization and axon outgrowth; adopting a characteristic electrophysiological phenotype through the expression of necessary ion channels; acquiring specific neurotransmitter phenotype, which will define the neuron as excitatory or inhibitory; and expressing a precise set of neurotransmitter receptors, which will enable the cell to respond to certain synaptic inputs.
The mainstream view in developmental neurobiology is that nervous system development follows a predetermined path, dictated by the genetically encoded specification of the neural progenitor for each neuronal subtype. However, many studies have challenged this view by discovering developmental plasticity in the acquisition of different neuronal phenotypes. These findings support a dynamic model for neuronal generation and specification, governed by multiple cues and by interactions between concurrent signaling pathways. Here we review studies that have contributed to our current understanding of the signaling mechanisms by which morphogenetic proteins, electrical activity and the environment interplay with second messenger dynamics to mediate specialization of neural cells.
Morphogenetic proteins
Morphogens direct formation of tissues and organs during development. They promote concerted growth to enable tissues to adopt appropriate scaled shapes in diverse species. In the nervous system, morphogenetic proteins are mostly known for affecting neural stem cells and progenitors. They induce not only cell proliferation but also expression of target genes that will direct these cells towards increasingly specific progenitor subclasses. A spatiotemporal decoding of morphogenetic protein gradients is achieved by a network of transcription factor expression, which constitutes the canonical outcome of morphogenetic protein action. Whether there is any room in this tight genetic program for interacting mechanisms of signaling like second messenger dynamics has not been the main focus of research in the field. In addition, the presence of morphogenetic proteins persists through adulthood, posing the question of whether these proteins are repurposed in later developmental processes to provide potential opportunity for plasticity in the maturing nervous system.
Neural stem cell specification and neural progenitor specialization
Before spinal neurons are born, neural progenitors undergo a process of specialization through which they become progressively restricted and specified to particular phenotypes depending on their relative localization along the presumptive spinal cord dorsoventral axis (Jessell, 2000; Briscoe and Therond, 2013). This is orchestrated by dorsoventral morphogenetic protein gradients that are established before and during neural tube closure. Chiefly, bone morphogenetic proteins (BMPs) and Sonic hedgehog (Shh) are present in opposing dorsoventral gradients and accordingly, regulate specification of dorsal and ventral progenitors, respectively. The transcription factor network that operates and defines domains of neural progenitors has been extensively studied and reviewed elsewhere (Briscoe and Therond, 2013). Recently, an elegant study in zebrafish embryos revealed that neural progenitors perceive a rather noisy morphogen signaling due to cell movement during neural tube patterning, which results in intermingled distributions of specified neural progenitors. A second cell adhesion-dependent and morphogen-independent sorting process takes place to generate sharply defined domains of specialized neural progenitors (Xiong et al., 2013).
Less explored has been the participation of second messenger signaling during this early phase of neural development. Prominently, cyclic adenosine monophosphate (cAMP) inhibits the canonical Shh pathway in spinal neural progenitors by a protein kinase A (PKA)-dependent mechanism. Expression of a dominant-negative form of PKA in the dorsal neural tube results in ectopic differentiation of ventral cells normally induced by Shh (Epstein et al., 1996; Hammerschmidt et al., 1996), while increased cAMP levels inhibit Shh-induced responses in neural plate explants (Ericson et al., 1996). In contrast, cyclic guanosine monophosphate (cGMP) enhances Shh-induced neural plate patterning (Robertson et al., 2001; Yamamoto and Suzuki, 2005). More recently, a conserved G-protein-coupled receptor has been identified at the primary cilium, which increases cAMP levels inhibiting canonical Shh signaling and Shh-dependent spinal neural progenitor specification (Mukhopadhyay et al., 2013). Understanding second messenger signaling involved in morphogen pathways is crucial for identifying the mechanisms underlying abnormal neural development and tumor formation, since aberrant morphogenetic protein signaling has been associated with both types of pathologies. Indeed, the atypical protein kinase Cι/λ (PKCι/λ) enhances hedgehog signaling and supports hedgehog-dependent tumor progression (Atwood et al., 2013). The atypical PKC is also recruited by BMP along with the canonical transcription factors Smad1/5/8 to regulate midline hinge point formation necessary for neural plate bending and neural tube closure (Eom et al., 2011). Strikingly, these transcription factors localize to tight junctions and participate in cytoskeletal dynamics necessary for apical constriction and interkinetic nuclear migration required for apicobasal polarity of midline hinge point cells (Eom et al., 2011). Altogether these studies suggest that second messenger signaling dynamically regulates even the earliest steps of nervous system development.
Further specification of spinal neural progenitors is also likely regulated by second messenger dynamics. The transition from phosphorylated to dephosphorylated forms of the transcription factor Olig2, presumably driven by PKA, respectively promotes specification of spinal cell phenotypes as different as motor neurons and oligodendrocytes (Li et al., 2011). Kinase activity is a pivotal link between morphogenetic protein action and second messenger dynamics. Indeed, extracellular signal regulated kinase (Erk)-mediated phosphorylation of Smad1/5/8 linker domain impedes their nuclear translocation and targets these BMP signaling effectors for degradation, preventing dorsal spinal neuron specification (Sapkota et al., 2007). Considering the wide range of second messengers that can directly or indirectly affect mitogen-activated protein kinase (MAPK) activity, the utilization of second messenger signaling and dynamics by BMPs is highly probable.
Axon guidance
The dogma of morphogenetic proteins acting exclusively on progenitor cells has been challenged by studies demonstrating a role for Wnts, BMPs and Shh in axon guidance (Charron et al., 2003; Kalil et al., 2011; Yam and Charron, 2013). In the developing spinal cord, commissural interneuron axons travel ventrally from their dorsally located cell body to then cross the midline guided by gradients of BMPs, Shh and Wnts. Second messengers and signaling involve Rho GTPases that activate the PI3K-Akt axis by BMP receptor type II activation, which also activates LIM kinase and induces Ca2+ influx through transient receptor potential channel TRPC1, activating calcineurin that induces attractive growth cone responses to a BMP gradient (Wen et al., 2007; Perron and Dodd, 2011). Phosphoinositides also participate in Wnt-mediated commissural axon routing presumably by activating atypical PKC (Di Marcotullio et al., 2006). Shh-mediated commissural interneuron axonal pathfinding is regulated by cAMP growth cone dynamics, which are important for modulating semaphorin-induced growth cone repulsion (Hutchins et al., 2012). Axonal pathfinding of retinal ganglion cells is also paradigmatically regulated by morphogens. A number of the participating second messengers are shared with the mechanisms involved in commissural interneuron axon guidance. Shh induces rapid increases in growth cone Ca2+ levels, which activate PKCα and subsequently integrin-linked kinase, which mediates retinal ganglion cell axon repulsion (Guo et al., 2012). Ca2+ is another shared second messenger for morphogen-regulated axon guidance in cortical neurons. Wnt5a mediates corpus callosum axon growth and guidance by a Ca2+-mediated mechanism that involves TRP channels and inositol triphosphate (IP3) receptors (Hutchins et al., 2011).
Synapse formation and synaptic plasticity
Further evidence of morphogen signaling acting in developing differentiated neurons has come from studies on the role of these proteins during synaptogenesis and synapse remodeling (Aberle et al., 2002; Marques et al., 2002; Budnik and Salinas, 2011; Harwell et al., 2012; Mitchell et al., 2012; Xiao et al., 2013). Wnt5a and Wnt7a stimulate formation and function of excitatory synapses in developing hippocampal neurons through localized dendritic Ca2+ dynamics and Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated mechanisms (Varela-Nallar et al., 2010; Ciani et al., 2011). In Drosophila neuromuscular junction, retrograde BMP signaling regulates synaptic homeostasis by modulating Ca2+ and CaMKII activity in presynaptic neurons, which in turn regulate neurotransmitter release (Haghighi et al., 2003). The second messenger signaling involved in Shh-driven synapse formation in hippocampal (Mitchell et al., 2012) and corticofugal projection neurons (Harwell et al., 2012) remain to be determined, although these studies suggest that transcription factor Gli-dependent canonical pathway may not participate in these instances of Shh signaling.
Neural activity
During spinal cord and brain development electrical activity spontaneously manifests in embryonic neurons (Spitzer, 2006; Rosenberg and Spitzer, 2011). This activity is mostly calcium-mediated and becomes apparent either prior to or during synapse formation. In the embryonic spinal cord, calcium-dependent electrical activity has been identified in mouse (Hanson and Landmesser, 2003), rat (Ren and Greer, 2003), chick (Sernagor et al., 1995; Chub and O'Donovan, 1998; O'Donovan et al., 1998), Xenopus laevis (Gu et al., 1994; Gu and Spitzer, 1995; Borodinsky et al., 2004), Xenopus tropicalis (Marek et al., 2010) and zebrafish (Warp et al., 2012; Plazas et al., 2013), arguing for the universal character of this developmental feature. Many studies have investigated the role of this early electrical activity in different aspects of nervous system development. The findings argue for a broad role of electrical activity in neural development. Instead of electrical activity being confined to refinement of pre-patterned circuits, it participates in events spanning the whole spectrum of nervous system development, from neural cell proliferation and neurogenesis to axonal pathfinding and synapse formation.
Neurogenesis
Neurotransmitter signaling components are present in the developing nervous system long before synapses are formed. Gamma-aminobutyric acid (GABA), glutamate, dopamine and noradrenaline are expressed in the presumptive embryonic spinal cord, the medial-posterior neural plate, of Xenopus laevis embryos (Rowe et al., 1993; Root et al., 2008). Similarly, in chick and mouse embryos, serotonin is released from the notochord during neural tube formation (Wallace, 1982; Lauder et al., 1988). The presence of neurotransmitters and neurotransmitter receptors in presynaptogenic stages of the embryonic spinal cord suggests that they may participate in developmental processes by non-synaptic mechanisms. Indeed, knockdown of the glycine receptor subunit α2 in zebrafish embryos induces a decrease in the number of spinal interneurons and an increase in mitotic progenitor cells (McDearmid et al., 2006), suggesting a role for glycine signaling in interneuron neurogenesis and differentiation. Moreover, reversing the chloride gradient characteristic of early developmental stages to the adult form by overexpressing the K+/Cl− cotransporter 2 (KCC2) in zebrafish embryos, decreases neurogenesis in the spinal cord and leads to fewer motor neurons and interneurons (Reynolds et al., 2008). In contrast, spontaneous glycine-induced Ca2+ transients in spinal cord progenitors promote the interneuron neurogenic program (Brustein et al., 2013). Additionally, in zebrafish embryos dopamine from descending brain axons induces the generation of motor neurons at the expense of a decrease in V2 interneurons (Reimer et al., 2013).
Recruitment of second messenger dynamics by electrical activity and neurotransmitter signaling is diverse but Ca2+ dynamics is a clear point of convergence. Ca2+ transients are present in precursor cells of the neocortical ventricular zone of rat embryos (Owens and Kriegstein, 1998), and in particular, Ca2+ waves propagated across radial glial cells regulate proliferation in the developing neocortex (Weissman et al., 2004). Neonatal inhibition of GABAA receptor-induced Ca2+ transients in neural precursor cells of the subventricular zone diminishes their proliferation and subsequently decreases newborn neuron density (Young et al., 2012). Also in vitro, proliferation of rat cerebellar granule cell precursors is modulated by Ca2+ dynamics (Borodinsky and Fiszman, 1998) triggered by depolarizing GABA signaling (Fiszman et al., 1999). Moreover, an in vitro screening of isoxazole small molecules that trigger robust neuronal differentiation of adult neural stem cells indicates that these drugs elicit glutamate- and Ca2+-mediated signaling that recruits MEF2 by derepressing the inhibitory action exerted by histone deacetylase 5 (Schneider et al., 2008). Interestingly, membrane depolarization regulates splicing of the neural cell adhesion molecule (NCAM) by causing H3K9 hyper-acetylation in a specific internal region of the ncam gene (Schor et al., 2009) and localization of different NCAM splice variants is associated with different states of neuronal differentiation and function (Pollerberg et al., 1985; Polo-Parada et al., 2004). Another transcription factor responding to changes in electrical activity is the Nuclear Factor of Activated T-Cells (NFATC), which is activated under depolarizing resting membrane potential characteristic of immature cerebellar granule neurons, by its Ca2+-calcineurin dependent-dephosphorylation and nuclear translocation, which in turn prevents Nuclear Factor I (NFI) repressor activity of late gene transcription. In contrast, when cerebellar granule cells mature and acquire hyperpolarized resting membrane potential, NFATC is phosphorylated releasing NFI to promote late gene transcription (Ding et al., 2013).
Neuronal differentiation
Becoming a differentiated neuron involves acquiring several features that altogether define the particular neuronal identity. For instance, a vertebrate spinal motor neuron can be defined by the ventrolateral localization of its cell body, innervation of muscle, the cholinergic phenotype and a specific connectivity profile with other spinal neurons. Are these features acquired all at once? Is the spinal neuron a blank canvas in which different phenotypic characteristics manifest de novo? Or rather, is the process of neuronal differentiation iterative and gradually achieved from some common ground immature neuronal phenotype? Is neural progenitor specification determinant for the particular spinal neuron phenotype that originates from it? Most of these questions remain to be fully answered but the fact that neural activity participates in the process of neuronal differentiation argues for a dynamic event.
Morphological differentiation
Acquisition of a specific connectivity profile starts with outgrowth of dendrites and axon and turning of growth cones towards appropriate targets. All these important events are activity dependent. Dendritogenesis is regulated by GABA-mediated depolarization of newborn neurons originated from the mouse subventricular zone (Young et al., 2012). Expression and activity-dependent NeuroD phosphorylation is necessary for the elaboration of dendrites in cerebellar granule cells in vitro and in vivo through a CaMKII-mediated mechanism (Gaudilliere et al., 2004). Similarly, in the adult hippocampus, neural activity from the mature network elicits depolarizing GABAergic inputs on neural progenitor cells that induce an increase in [Ca2+]i and NeuroD transcription, resulting in the promotion of neuronal differentiation (Tozuka et al., 2005). Other key transcriptional mechanisms have been identified that link neuronal activity to acquisition of neuronal morphology: neuronal activity promotes phosphorylation of Methyl-CpG-binding protein 2 (Mecp2) transcription factor through a Ca2+-dependent mechanism, which regulates dendritic patterning, spine morphogenesis and brain-derived neurotrophic factor (BDNF) transcription (Zhou et al., 2006).
In addition, neurotransmitter signaling regulates axonal pathfinding in a second messenger-dependent manner. In spinal neurons, neurotransmitters like acetylcholine induce growth cone turning responses dependent on intracellular cAMP levels (Song et al., 1997). Stereotypical responses of growth cones to different guidance cues are altered upon brief periods of electrical stimulation of Xenopus spinal neurons grown in vitro by Ca2+ influx- and cAMP-dependent mechanisms (Ming et al., 2001). In vivo studies showed that knockdown of voltage-gated Na+ channel Nav1.6 perturbs axonal pathfinding of some subtypes of zebrafish developing motor neurons (Pineda et al., 2006). Moreover, these motor neurons exhibit synchronized Ca2+ spike activity that is important for appropriate axonal pathfinding through an activity-based competition mechanism and PlexinA3-mediated axon guidance (Plazas et al., 2013). This dependence of motor neuron axonal routing on spontaneous electrical activity is also apparent in the chick spinal cord (Hanson and Landmesser, 2004; Kastanenka and Landmesser, 2013). Activity likely operates by regulating neural cell adhesion molecule patterns of expression in growing axons (Hanson and Landmesser, 2004).
Neurophysiological differentiation
Neurotransmitter phenotype
Depolarization induces differentiation of dopaminergic neurons of the sensory ganglia through an L-type voltage-gated Ca2+ channel dependent mechanism (Brosenitsch et al., 1998). The model suggests that in the carotid body only a subpopulation of petrosal ganglion neurons receive sufficient depolarizing inputs during development, which enables them to sustain a stable dopaminergic phenotype. In contrast, cells that are not differentiated to receive these synaptic inputs until after birth loose the dopaminergic phenotype (Brosenitsch et al., 1998). Evidence of electrical activity and Ca2+ signaling regulating neurotransmitter specification has also been demonstrated in Xenopus laevis spinal cord and brain. Spontaneous Ca2+ spike frequency regulates the number of GABAergic neurons in cultured immature spinal neurons (Gu and Spitzer, 1995) and accelerates GABAergic differentiation of primary cultures from embryonic striatal cells (Ciccolini et al., 2003). Moreover, in vivo perturbations of Ca2+ spike activity induces homeostatic changes in the number of excitatory and inhibitory spinal neurons; when activity is enhanced more GABAergic and glycinergic neurons are specified and when activity is suppressed more glutamatergic and cholinergic neurons are present in the developing spinal cord (Borodinsky et al., 2004). Activity-mediated changes in neurotransmitter phenotype expression vary among different subclasses of neurons. For instance, activity-induced dopaminergic phenotype specification is particularly favored in a subclass of GABAergic neurons of the ventral suprachiasmatic nucleus and spinal cord (Velazquez-Ulloa et al., 2011). Activity-dependent choice between glutamatergic and GABAergic phenotypes is also apparent in Xenopus tropicalis spinal cord and it operates by controlling transcription of homeobox protein Tlx3, which favors glutamatergic over GABAergic specification (Marek et al., 2010). Ca2+ spikes are responsible for phosphorylating c-Jun, which binds to the tlx3 promoter region and represses its expression (Marek et al., 2010). Similarly, developing rat hippocampal granule cells exhibit a glutamatergic/GABAergic dual phenotype (Walker et al., 2001; Gutierrez et al., 2003; Kasyanov et al., 2004) that matures towards the glutamatergic-only phenotype as development progresses (Gutierrez et al., 2003). Interestingly, epileptic seizures or BDNF-treatment brings back the dual phenotype (Gomez-Lira et al., 2005) suggesting an electrical activity-dependent control of neurotransmitter phenotype specification in the mammalian hippocampus. Activity-dependent regulation of the transcription factor Lmx1b expression in the developing hindbrain of frog larvae changes the number of serotonergic neurons, which changes swimming behavior of the animal (Demarque and Spitzer, 2010).
Electrical properties
Acquisition of appropriate electrical features in developing neurons is critical to the functional outcome of the neuron as well as to the circuit in which the cell participates. Blocking synaptic activity in developing Drosophila embryos alters the electrical properties of embryonic motor neurons. Increases in Na+ and K+ currents occur, leading to an overall increase in intrinsic excitability (Baines et al., 2001). Importantly, these changes are reversible and restoring synaptic activity rescues the electrical phenotype (Baines et al., 2001), suggesting dynamic mechanisms regulating electrical differentiation of developing neurons.
In the embryonic rat spinal cord, the changes in motor neuron electrical properties that accompany its differentiation depend on Ca2+-dependent electrical activity (Xie and Ziskind-Conhaim, 1995). Both the level of expression and kinetics of the A-type K+ channel in embryonic lumbar motor neurons of the chick are also dependent on spontaneous spinal neuron electrical activity (Casavant et al., 2004).
Environmental cues
Robustness of nervous system development relies on adapting to environmental alterations that may occur. Participation of second messenger dynamics in neuronal development supports this concept. Indeed, axonal arborization is enhanced in Mushroom Body Cells of Drosophila embryos grown at high temperatures. This effect is mediated by an increase in Ca2+ current and a decrease in K+ current, which result in an increase in spontaneous Ca2+ transients. In turn, the increase in neurite outgrowth is dependent on cAMP dynamics (Peng et al., 2007). Changes in ionic conductances triggered by environmental stimuli are also apparent in the developing optic tectum of Xenopus tadpoles. Enhanced visual stimulation leads to a decrease in Ca2+-permeable AMPAR-mediated synaptic drive provoking a decrease in action potential firing in tectal neurons and a compensatory increase in voltage-gated Na+ current which brings cells back to normal spiking levels (Aizenman et al., 2003). This Ca2+-mediated homeostatic mechanism is constrained within a developmental window and since this process improves stimulus detection in the background of enhanced visual stimulation, it may facilitate developmental plasticity and adaptability (Aizenman et al., 2003).
Importantly, environmental stimuli change Ca2+-mediated activity which alters specification of neurotransmitter phenotype. Altering light exposure that changes the sensory input to the circuit controlling adaptation of skin pigmentation to background, changes the number of neurons expressing dopamine in Xenopus laevis tadpoles, which results in changes in camouflage coloration in response to illumination (Dulcis and Spitzer, 2008). The stimulus-induced changes in neurotransmitter phenotype specification are not restricted to the developing nervous system or to the frog; interneurons of the adult rat hypothalamus switch between somatostatin and dopaminergic phenotypes in response to changes in duration of daily photoperiod, which has concomitant behavioral consequences (Dulcis et al., 2013).
Second messenger signaling as key integrator of multiple developmental cues
Review of these three pillars of influential factors in neural development, morphogens, neural activity and environment, which are far from being a complete list, prompts the question of how signaling and the action of different developmental cues are integrated in a single developing neuron.
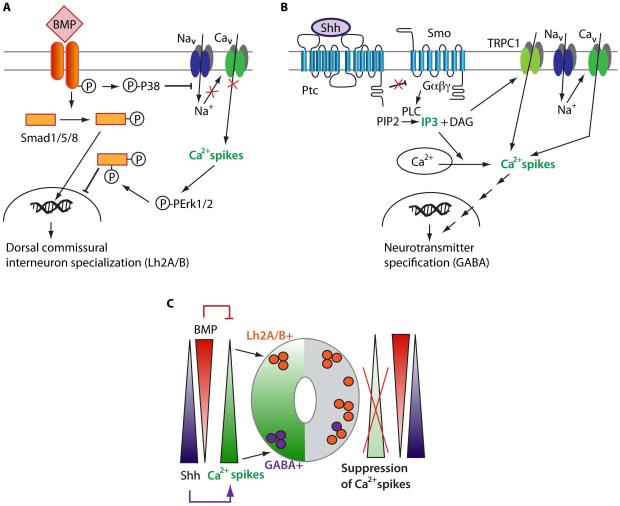
Our previous work showed that in immature spinal neurons, Shh (Belgacem and Borodinsky, 2011) and BMPs (Swapna and Borodinsky, 2012) acutely regulate Ca2+ spike activity (Figure 1). Shh enhances Ca2+ spike activity through a Smoothened-mediated mechanism that involves TRPC1 and IP3 transients at the neuronal primary cilium (Belgacem and Borodinsky, 2011). In contrast, BMP4/7 inhibits Ca2+ spike activity through a p38-dependent mechanism by a potential phosphorylation-mediated inhibition of voltage-gated Na+ channels (Swapna and Borodinsky, 2012). This modulation of Ca2+ spike activity by morphogenetic proteins has consequences in the process of spinal neuron differentiation. Ectopic Shh enhances the number of GABAergic spinal neurons by an electrical activity-dependent mechanism (Belgacem and Borodinsky, 2011). On the other hand, ectopic BMP increases the number of Lh2A/B-expressing commissural interneurons and expands their localization to ventral regions by inhibiting Ca2+ spike activity (Swapna and Borodinsky, 2012), suggesting that this spontaneous neural activity is necessary for the domain-restricted differentiation of spinal neurons (Figure 1). These studies reveal that the interaction between morphogenetic proteins and electrical activity is bidirectional: morphogens modify electrical activity, which in turn changes specialization of neurons driven by morphogens. Moreover, it has been recently shown that Ca2+ spike activity-dependent specification in developing spinal neurons is non-cell autonomous and instead is mediated by activity-dependent release of BDNF which in turn regulates expression of the glutamatergic/GABAergic transcription factor selector Tlx3 through a c-Jun N-terminal kinase-dependent mechanism (Guemez-Gamboa et al., 2014) demonstrating further the crosstalk among developmental cues during neuronal differentiation. The other important concept that derives from these studies is that neuronal specification is not sealed with the specialization of neural progenitors but instead, electrical activity and morphogenetic proteins interplay in maturing neurons to further modulate neuronal differentiation. The interaction of morphogens with novel signaling pathways emerging as neurons progress in their differentiation suggests that morphogen signaling dynamically changes during nervous system development. In turn, morphogenetic proteins may participate in neural function beyond morphogenesis. Indeed, Shh, BMPs and Wnts play important roles in synaptogenesis and synaptic plasticity, as described in previous sections. Moreover, even in the adult nervous system, Wnt action contributes to neurotransmitter receptor localization in postsynaptic cells and mediates activity-dependent synaptic plasticity in C. elegans (Jensen et al., 2012).
Figure 1. Interplay between Ca2+ spike activity and morphogenetic proteins in the developing spinal cord.
A. BMP signaling recruits p38 MAPK that phosphorylates and inhibits Nav1.6, thus decreasing Ca2+ spikes in embryonic spinal neurons. Low levels of Ca2+ spike activity are necessary for the specification of dorsal commissural spinal neurons through a Smad1/5/8-dependent mechanism. Based on the study by Swapna and Borodinsky, 2012. B. Shh activates Smo, which recruits PLC leading to IP3 transients that correlate with TRPC1 and Cav-mediated Ca2+ spikes that regulate neurotransmitter specification in developing spinal neurons. Based on the study by Belgacem and Borodinsky, 2011. C. The opposing dorsoventral gradients of BMP and Shh generate a gradient of Ca2+ spike activity that is important for spinal neuron differentiation. Ptc: Patched1; Smo: Smoothened; PLC: phospholipase C.
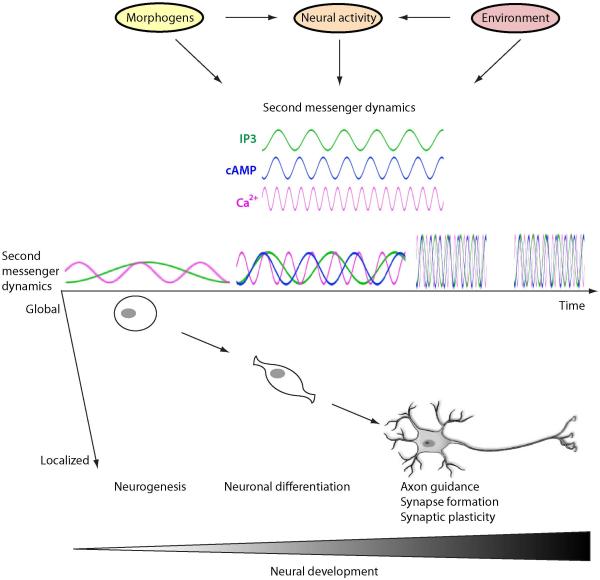
Clearly, Ca2+ signaling serves not only as a hub where diverse signaling pathways converge but also as an integrator of the information carried by different stimuli and first messengers (Figure 2). Depending on Ca2+ transient frequency and its global or subcellular localization, this signaling has a wide-ranging impact on different aspects of neuronal development, from neuromorphogenesis and neurite outgrowth (Gu and Spitzer, 1995; Gomez and Spitzer, 1999; Gomez et al., 2001; Ciccolini et al., 2003) to neurotransmitter specification (Gu and Spitzer, 1995; Ciccolini et al., 2003; Borodinsky et al., 2004). The intercalation of second messenger signaling in morphogenetic protein pathways may also contribute to the temporal pattern of transcription factor expression that is critical for neural progenitor cell proliferation/specialization of the perinatal mouse ventral telencephalon (Imayoshi et al., 2013) and for embryonic mouse ventral spinal progenitor specification (Dessaud et al., 2007; Balaskas et al., 2012).
Figure 2. Integration of diverse developmental cues by second messenger signaling.
Morphogenetic proteins, neural activity and environmental factors all converge in characteristic second messenger dynamics that vary from global to localized as neural cells differentiate and mature. The kinetics of second messenger signaling are developmentally regulated due to temporal changes in developmental cues and in expression and activation profiles of ion channels and signaling molecules.
The profile of second messenger dynamics changes as cells progress in their intrinsic differentiation, but also varies in response to cues in the changing internal and external environment. In turn, this provides plasticity to the process of neuronal differentiation and great adaptability to the developing nervous system.
Acknowledgements
Research in the Borodinsky lab has been supported by the Basil O’Connor Starter Scholar Research Award Grant 5-FY09-131 from the March of Dimes Foundation, Klingenstein Foundation Award in Neuroscience, NSF 1120796, NIH-NINDS R01NS073055 and SHC 86500-NCA and 85220-NCA grants to L.N.B., Shriners Postdoctoral Fellowships to Y.H.B., I.S, E.B.S. and A.M.H. and CIRM Predoctoral fellowship to M.K.T.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494:484–488. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, Briscoe J, Ribes V. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148:273–284. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci U S A. 2011;108:4482–4487. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Fiszman ML. Extracellular potassium concentration regulates proliferation of immature cerebellar granule cells. Brain Res Dev Brain Res. 1998;107:43–48. doi: 10.1016/s0165-3806(97)00217-4. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature reviews Molecular cell biology. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Brosenitsch TA, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Cote S, Ghislain J, Drapeau P. Spontaneous glycine-induced calcium transients in spinal cord progenitors promote neurogenesis. Developmental neurobiology. 2013;73:168–175. doi: 10.1002/dneu.22050. [DOI] [PubMed] [Google Scholar]
- Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casavant RH, Colbert CM, Dryer SE. A-current expression is regulated by activity but not by target tissues in developing lumbar motoneurons of the chick embryo. Journal of neurophysiology. 2004;92:2644–2651. doi: 10.1152/jn.00307.2004. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chub N, O'Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)(+)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 2011;108:10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–111. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- Ding B, Wang W, Selvakumar T, Xi HS, Zhu H, Chow CW, Horton JD, Gronostajski RM, Kilpatrick DL. Temporal regulation of nuclear factor one occupancy by calcineurin/NFAT governs a voltage-sensitive developmental switch in late maturing neurons. J Neurosci. 2013;33:2860–2872. doi: 10.1523/JNEUROSCI.3533-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138:3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res. 1999;115:1–8. doi: 10.1016/s0165-3806(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Xu L, Meng D, Spitzer NC. Non-cell-autonomous mechanism of activity-dependent neurotransmitter switching. Neuron. 2014;82:1004–1016. doi: 10.1016/j.neuron.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Standley C, Bellve K, Fogarty K, Bao ZZ. Protein kinase Calpha and integrin-linked kinase mediate the negative axon guidance effects of Sonic hedgehog. Mol Cell Neurosci. 2012;50:82–92. doi: 10.1016/j.mcn.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the "glutamatergic" granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73:1116–1126. doi: 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BI, Li L, Kalil K. Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol. 2011;71:269–283. doi: 10.1002/dneu.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BI, Li L, Kalil K. Wnt-induced calcium signaling mediates axon growth and guidance in the developing corpus callosum. Science signaling. 2012;5:pt1. doi: 10.1126/scisignal.2002523. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara T, Ishidate F, Kageyama R. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342:1203–1208. doi: 10.1126/science.1242366. [DOI] [PubMed] [Google Scholar]
- Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, Maxfield D, Francis MM, Madsen DM, Maricq AV. Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell. 2012;149:173–187. doi: 10.1016/j.cell.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kalil K, Li L, Hutchins BI. Signaling mechanisms in cortical axon growth, guidance, and branching. Frontiers in neuroanatomy. 2011;5:62. doi: 10.3389/fnana.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastanenka KV, Landmesser LT. Optogenetic-mediated increases in in vivo spontaneous activity disrupt pool-specific but not dorsal-ventral motoneuron pathfinding. Proc Natl Acad Sci U S A. 2013;110:17528–17533. doi: 10.1073/pnas.1316457110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Tamir H, Sadler TW. Serotonin and morphogenesis. I. Sites of serotonin uptake and -binding protein immunoreactivity in the midgestation mouse embryo. Development. 1988;102:709–720. doi: 10.1242/dev.102.4.709. [DOI] [PubMed] [Google Scholar]
- Li H, de Faria JP, Andrew P, Nitarska J, Richardson WD. Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron. 2011;69:918–929. doi: 10.1016/j.neuron.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KW, Kurtz LM, Spitzer NC. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat Neurosci. 2010;13:944–950. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Liao M, Drapeau P. Glycine receptors regulate interneuron differentiation during spinal network development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9679–9684. doi: 10.1073/pnas.0504871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Petralia RS, Currier DG, Wang YX, Kim A, Mattson MP, Yao PJ. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. J Cell Sci. 2012;125:4207–4213. doi: 10.1242/jcs.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. Journal of neurobiology. 1998;37:131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Berke BA, Zhu Y, Lee WH, Chen W, Wu CF. Temperature-dependent developmental plasticity of Drosophila neurons: cell-autonomous roles of membrane excitability, Ca2+ influx, and cAMP signaling. J Neurosci. 2007;27:12611–12622. doi: 10.1523/JNEUROSCI.2179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron JC, Dodd J. Inductive specification and axonal orientation of spinal neurons mediated by divergent bone morphogenetic protein signaling pathways. Neural Dev. 2011;6:36. doi: 10.1186/1749-8104-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda RH, Svoboda KR, Wright MA, Taylor AD, Novak AE, Gamse JT, Eisen JS, Ribera AB. Knockdown of Nav1.6a Na+ channels affects zebrafish motoneuron development. Development. 2006;133:3827–3836. doi: 10.1242/dev.02559. [DOI] [PubMed] [Google Scholar]
- Plazas PV, Nicol X, Spitzer NC. Activity-dependent competition regulates motor neuron axon pathfinding via PlexinA3. Proc Natl Acad Sci U S A. 2013;110:1524–1529. doi: 10.1073/pnas.1213048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollerberg EG, Sadoul R, Goridis C, Schachner M. Selective expression of the 180-kD component of the neural cell adhesion molecule N-CAM during development. J Cell Biol. 1985;101:1921–1929. doi: 10.1083/jcb.101.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Bose CM, Plattner F, Landmesser LT. Distinct roles of different neural cell adhesion molecule (NCAM) isoforms in synaptic maturation revealed by analysis of NCAM 180 kDa isoform-deficient mice. J Neurosci. 2004;24:1852–1864. doi: 10.1523/JNEUROSCI.4406-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Norris A, Ohnmacht J, Patani R, Zhong Z, Dias TB, Kuscha V, Scott AL, Chen YC, Rozov S, Frazer SL, Wyatt C, Higashijima S, Patton EE, Panula P, Chandran S, Becker T, Becker CG. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Developmental cell. 2013;25:478–491. doi: 10.1016/j.devcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. Journal of neurophysiology. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Brustein E, Liao M, Mercado A, Babilonia E, Mount DB, Drapeau P. Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1588–1597. doi: 10.1523/JNEUROSCI.3791-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CP, Gibbs SM, Roelink H. cGMP enhances the sonic hedgehog response in neural plate cells. Dev Biol. 2001;238:157–167. doi: 10.1006/dbio.2001.0392. [DOI] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SJ, Messenger NJ, Warner AE. The role of noradrenaline in the differentiation of amphibian embryonic neurons. Development. 1993;119:1343–1357. doi: 10.1242/dev.119.4.1343. [DOI] [PubMed] [Google Scholar]
- Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Schneider JW, Gao Z, Li S, Farooqi M, Tang TS, Bezprozvanny I, Frantz DE, Hsieh J. Small-molecule activation of neuronal cell fate. Nat Chem Biol. 2008;4:408–410. doi: 10.1038/nchembio.95. [DOI] [PubMed] [Google Scholar]
- Schor IE, Rascovan N, Pelisch F, Allo M, Kornblihtt AR. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc Natl Acad Sci U S A. 2009;106:4325–4330. doi: 10.1073/pnas.0810666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Chub N, Ritter A, O'Donovan MJ. Pharmacological characterization of the rhythmic synaptic drive onto lumbosacral motoneurons in the chick embryo spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:7452–7464. doi: 10.1523/JNEUROSCI.15-11-07452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Swapna I, Borodinsky LN. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proc Natl Acad Sci U S A. 2012;109:16336–16341. doi: 10.1073/pnas.1202818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Ulloa NA, Spitzer NC, Dulcis D. Contexts for dopamine specification by calcium spike activity in the CNS. J Neurosci. 2011;31:78–88. doi: 10.1523/JNEUROSCI.3542-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Wallace JA. Monoamines in the early chick embryo: demonstration of serotonin synthesis and the regional distribution of serotonin-concentrating cells during morphogenesis. The American journal of anatomy. 1982;165:261–276. doi: 10.1002/aja.1001650304. [DOI] [PubMed] [Google Scholar]
- Warp E, Agarwal G, Wyart C, Friedmann D, Oldfield CS, Conner A, Del Bene F, Arrenberg AB, Baier H, Isacoff EY. Emergence of patterned activity in the developing zebrafish spinal cord. Current biology : CB. 2012;22:93–102. doi: 10.1016/j.cub.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, Schneggenburger R. BMP signaling specifies the development of a large and fast CNS synapse. Nat Neurosci. 2013;16:856–864. doi: 10.1038/nn.3414. [DOI] [PubMed] [Google Scholar]
- Xie H, Ziskind-Conhaim L. Blocking Ca(2+)-dependent synaptic release delays motoneuron differentiation in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5900–5911. doi: 10.1523/JNEUROSCI.15-09-05900.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F, Tentner AR, Huang P, Gelas A, Mosaliganti KR, Souhait L, Rannou N, Swinburne IA, Obholzer ND, Cowgill PD, Schier AF, Megason SG. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell. 2013;153:550–561. doi: 10.1016/j.cell.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki N. Expression and function of cGMP-dependent protein kinase type I during medaka fish embryogenesis. J Biol Chem. 2005;280:16979–16986. doi: 10.1074/jbc.M412433200. [DOI] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Wu S, Ikeda-Matsuo Y, Kubera C, Bordey A. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 2012;32:13630–13638. doi: 10.1523/JNEUROSCI.2864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]