Abstract
Background
The carpal tunnel is a fibro-osseous structure containing the median nerve and flexor tendons. Its cross-sectional area has been shown to increase during compressive force application to the carpal bones in modeling and in vitro studies. The purpose of this study was to investigate the morphological and positional changes of the carpal arch and median nerve while in vivo compressive force was applied in the radioulnar direction across the wrist.
Methods
Ultrasound images of the carpal tunnel and its contents were captured for 11 healthy, female volunteers at the distal tunnel level prior to force application and during force application of 10 and 20 N.
Findings
With applied force, the carpal arch width significantly decreased, while the carpal arch height and area significantly increased (P < 0.001). The median nerve shape became more rounded as the compressive force magnitude increased, reflected by decreases in the nerve’s flattening ratio and increases in its circularity (P < 0.001). The applied force also resulted in nerve displacement in the radial-volar direction.
Interpretation
This study demonstrates that noninvasively applying radioulnar compressive force across the wrist may potentially provide relief of median nerve compression to patients suffering from carpal tunnel syndrome.
Keywords: carpal tunnel syndrome, compression, force, median nerve, wrist
1 Introduction
The carpal tunnel is a fibro-osseous structure within the wrist bounded by the inter-connected carpal bones and the transverse carpal ligament (TCL). The tunnel serves as a passageway for the digital flexor tendons and the median nerve. The delicate nature of the median nerve lends itself susceptible to compression within the carpal tunnel; prolonged compression of this nerve may lead to the median nerve neuropathy known as carpal tunnel syndrome.
When subjected to stress, the median nerve may displace or undergo morphological changes to minimize its insult within the carpal tunnel [1]. Previously, the deformation and displacement of the median nerve has been investigated in response to wrist and finger motion in patients with carpal tunnel syndrome [2–5] as well as in asymptomatic volunteers [1, 6, 7]. These aforementioned studies have shown that the median nerve displaces in three dimensions and its shape often changes to better accommodate the space that is unoccupied by the other tunnel contents.
The amount of space available for the median nerve, and the additional carpal tunnel contents, is furnished by the boundaries of the carpal tunnel. The morphology of the carpal tunnel boundary has been shown to change with varying wrist postures[8] and after the surgical intervention of carpal tunnel release [9–11]. These structural changes are likely related to the soft tissue components of the carpal tunnel boundary which provides the carpal tunnel with some degree of compliance [8, 12–15]. Recent studies support that the cross-sectional area of the carpal tunnel can be increased by narrowing the carpal arch width (CAW), i.e., distance between the trapezium and hook of hamate [13, 14, 16, 17]. Geometric modeling [16] and in vitro [13, 16] studies have shown that CAW narrowing is associated with palmar bowing of the TCL which increases the height and cross-sectional area of the carpal arch. These changes may provide additional space within the carpal tunnel for its contents; however, such previous studies have not investigated the impact of CAW narrowing on the tunnel contents. Additionally, the in vitro strategy of force application to achieve CAW narrowing involved directly applying invasive force to the carpal bones or transverse carpal ligament [13, 15]. Non-invasive, in vivo narrowing of the CAW by applying external transverse force across the wrist has not been explored.
Therefore, the purpose of this study was to investigate the in vivo morphological and positional changes of the carpal arch and the median nerve during transverse, compressive force application across the wrist. It was hypothesized that force applied across the wrist would result in a decrease of the CAW, accompanied by an increase in the carpal arch height and area. It was also hypothesized that the median nerve shape would become rounder and the nerve would displace in the volar direction due to the additional space created within the carpal arch.
2 Methods
2.1 Human subjects
Twelve healthy, right-handed female volunteers were enrolled in this study; however, one participant was excluded because not all of this study’s anatomical landmarks of interest could be identified within the same ultrasound imaging plane (n=11, 24.8 (SD 5.5) years old). The participants had no history of injury, surgical intervention, or musculoskeletal/neuromuscular disorders affecting the right hand or wrist. The study was approved by the institutional review board and written informed consent was obtained from each volunteer prior to study participation.
2.2 Compression system
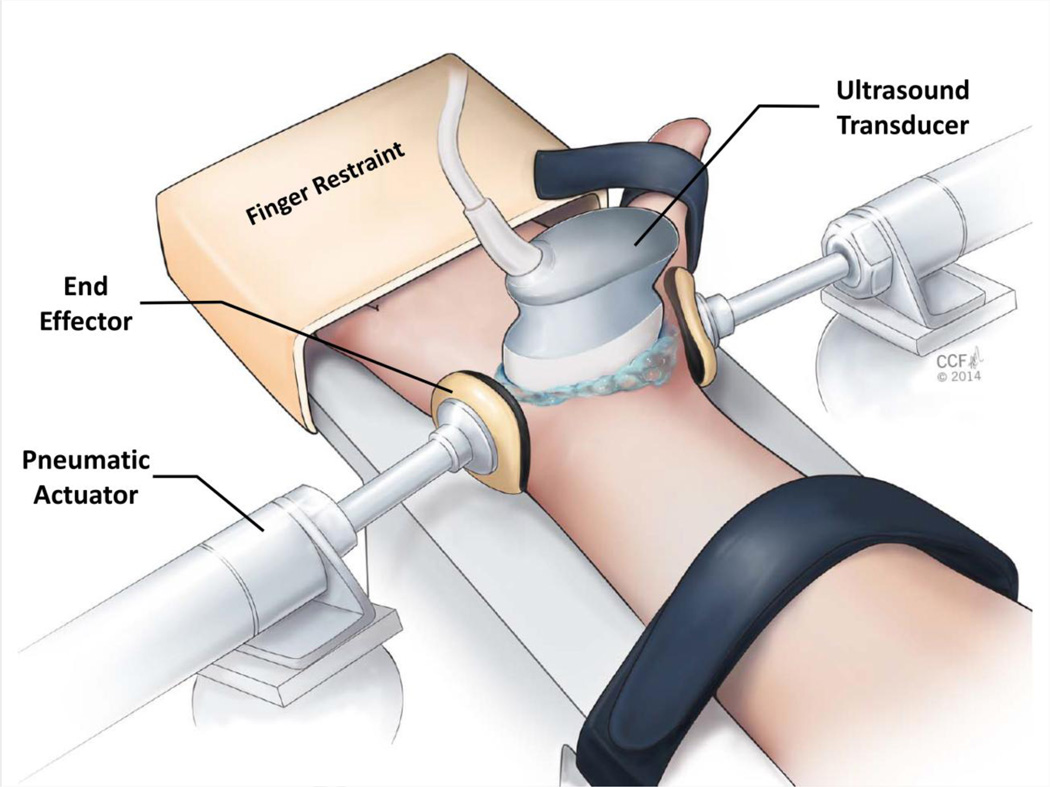
A custom system was developed to non-invasively apply transverse compression across the wrist at the distal level of the carpal tunnel (Figure 1). The system included 1) a height adjustable support for the hand, wrist, and forearm, 2) two six degrees-of-freedom alignment mounts, 3) two pneumatic actuators (Bimba Manufacturing, Monee, IL, USA), 4) two end effectors, 5) an air pressure regulator (VBM Medical, Noblesville, IN, USA), 6) plastic tubing, and 7) a digital pressure gage. Each actuator was rigidly attached to an alignment mount and an end effector was securely fastened to each actuator’s extension rod. The end effectors consisted of concave pieces of thermoplastic that were molded to comfortably fit the curvature of the hand/wrist. Before molding each thermoplastic piece, it had a cross-sectional area of 9.6 cm2 and was 0.3 cm thick. For added comfort, a thin piece of foam (0.3 cm thick) was added to the surface of the end effectors that made contact with the wrist. The plastic tubing was used to connect the pressure regulator, digital pressure gage, and actuators. The pressure regulator generated and controlled the desired force magnitude according to a calibration performed that related the regulator pressure to the amount of force applied by the actuators.
Figure 1.
Experimental set-up for in vivo wrist compression and ultrasound imaging.
2.3 Experimental procedures
Each participant lay supine on a testing bed. Their right arm was abducted 30° and placed on the height adjustable support of the compression system so that their palm faced up. Their four fingers were stabilized in the extended position and secured using a restraint; their thumb was naturally abducted and held with a Velcro® strap. Additionally, the forearm was stabilized using a Velcro® strap (Figure 1). To guide the alignment of the end effectors, an ultrasound system (Acuson S2000, Siemens Medical Solutions USA, Mountain View, CA, USA) with an 18L6 HD linear array transducer (Siemens Medical Solutions USA, Mountain View, CA, USA) was used to identify the axial imaging plane that clearly contained the hook of hamate and ridge of the trapezium, corresponding to the distal level of the carpal tunnel. The position of the ultrasound transducer was outlined for each subject using a skin marking pen. Then, the actuators were adjusted so that the line of action of the end effectors coincided with the determined imaging plane, and the center of the end effectors were positioned at the mid-point between the volar and dorsal surface of the wrist.
Transverse, compressive forces of 10 N and 20 N were applied to the wrist at the distal level of the carpal tunnel for each study volunteer. The air pressure regulator supplied a single air pressure through bifurcated tubes to simulateously actuate the two end effectors, applying the specified force magnitude to both the radial and ulnar aspects of the wrist. Each force level was applied four times, for a total of eight trials in a randomized order. At the beginning of each trial, the ultrasound transducer was oriented perpendicularly to the palm of the participant with an axial imaging plane that provided visualization of the hook of hamate, ridge of trapezium, median nerve, and the thenar muscles’ ulnar point (TUP) [18]. Then, three ultrasound images were captured without force application (unloaded, 0 N condition). Next, the pressure regulator generated and maintained the desired, predetermined force output for 3 minutes. After 3 minutes of continuous force application, three additional ultrasound images were obtained of the carpal tunnel following the above described procedure. After collecting the loaded ultrasound images, the compressive force was released for that trial. Between consecutive trials, participants removed their arm from the compression system and were given a 5-minute resting period. Throughout imaging, the ultrasound system was operated in two-dimensional B-mode, with tissue harmonic imaging at an imaging frequency of 8 MHz and an image depth of 2.5 cm.
2.4 Data analyses
One trial for each of the 10 N and 20 N force conditions was used for analyses for each participant; the remaining trials were used as backup. For each trial, the three unloaded images and the three loaded images were examined by a single investigator (TLM) who has received musculoskeletal ultrasound training. The examiner was blinded to the magnitude of the compressive force for each image. The ImageJ (US National Institutes of Health, Bethesda, MD, USA) point tool was used to determine the coordinates of the most volar point of the hook of hamate and the ridge of trapezium on each image. Similarly, the TUP was identified and its coordinates were recorded. The multipoint selection tool in ImageJ was used to trace the median nerve within its hyperechoic border. The nerve tracing provided quantification of the median nerve’s shape descriptors, including the nerve perimeter, area, flattening ratio ( of fit ellipse), and circularity (). The flattening ratio and circularity of the median nerve provided an indication of the nerve’s roundness. For both parameters, a value of 1.0 indicates a perfect circle. A flattening ratio greater than 1.0 or a circularity of less than 1.0 reflects a more elliptical cross-sectional shape. Additionally, the coordinates of the median nerve centroid were found.
All of the coordinates obtained from ImageJ were transformed to an anatomical coordinate system using a custom MATLAB (MathWorks, Natick, MA, USA) program. The trapezium served as the origin of the anatomical coordinate system. The x-axis was defined as the line that passed through the points representing the trapezium and the hook of hamate pointing in the ulnar direction. The y-axis was perpendicular to the x-axis and pointed in the volar direction. Additionally, the program calculated the CAW, carpal arch height (CAH), and carpal arch area (CAA). The CAW was defined as the distance between the hook of hamate and the trapezium. The CAH was the perpendicular distance from the TUP to a line connecting the hook of hamate and the trapezium. The CAA was calculated as ½*CAW*CAH with the approximation of area by a triangle [19].
2.5 Statistical analysis
For each participant, data was averaged across the representative images for each specific force magnitude and statistical analysis was completed using SigmaStat 3.4 (Systat Software Inc, San Jose, CA, USA). One-way repeated measures ANOVAs were used to investigate how the magnitude of compressive force (0, 10, and 20 N) affected each dependent variable of the carpal arch (CAW, CAH, CAA) and the nerve (perimeter, area, flattening ratio, circularity, and centroid location). Post-hoc Tukey’s tests were used for all pairwise comparisons. A significance level of α = 0.05 was used for statistical comparisons.
3 Results
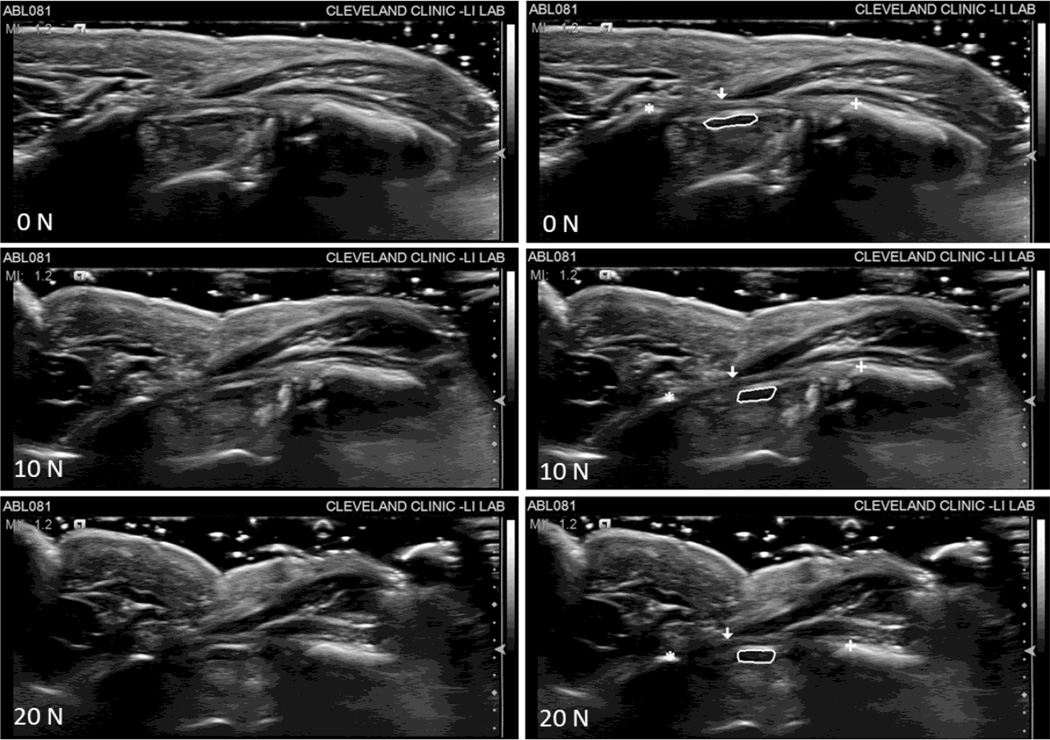
Ultrasound images captured the effects that compressive force application across the wrist has on the carpal arch and the median nerve (Figure 2). In general as the magnitude of compressive force increased, the CAW decreased resulting in corresponding increases in the CAH and CAA. Additionally, the median nerve shape became rounder and the nerve displaced in the radial-volar direction during the application of compressive force (Figure 3).
Figure 2.
Ultrasound images at each force level (0, 10, and 20 N) for a representative subject. The images on the left are unlabeled and the images on the right reflect the selections made in ImageJ for each landmark of interest, specifically the hook of hamate ( ), ridge of the trapezium (
), ridge of the trapezium ( ), thenar muscles ulnar point (
), thenar muscles ulnar point ( ), and the median nerve (solid line).
), and the median nerve (solid line).
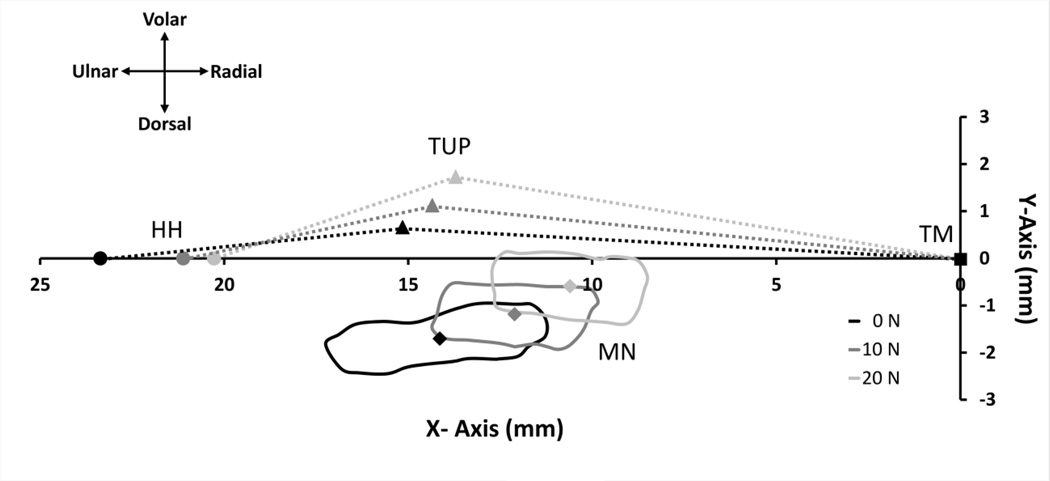
Figure 3.
The displacement of the hook of hamate (HH,  ), thenar muscles ulnar point (TUP,
), thenar muscles ulnar point (TUP,  ), and median nerve (MN, solid line) with centroid (
), and median nerve (MN, solid line) with centroid ( ) for a representative subject at 0, 10, and 20 N of wrist compression relative to the anatomically defined coordinate system with its origin at the trapezium (TM,
) for a representative subject at 0, 10, and 20 N of wrist compression relative to the anatomically defined coordinate system with its origin at the trapezium (TM,  ). The area beneath the dotted lines bounded by the X-Axis represents the carpal arch area.
). The area beneath the dotted lines bounded by the X-Axis represents the carpal arch area.
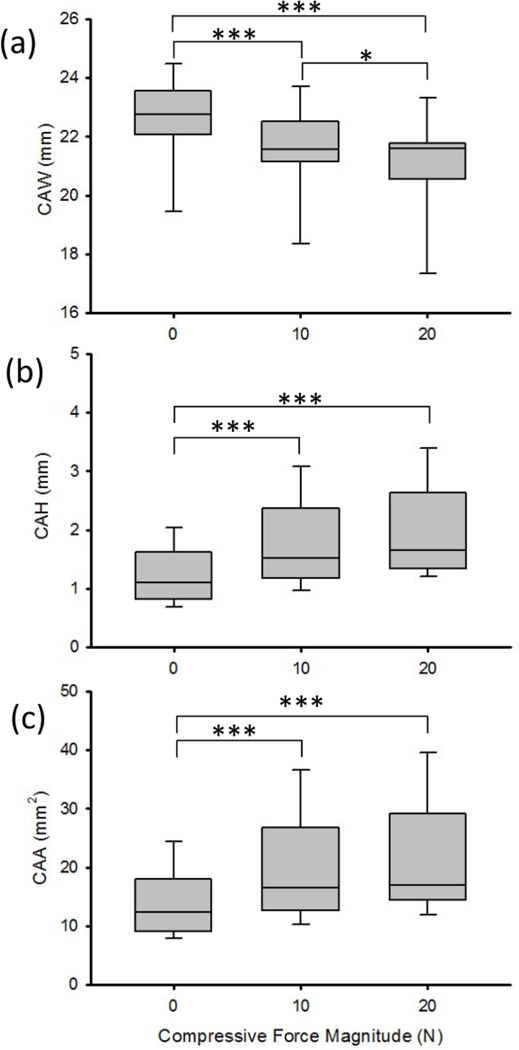
The CAW, CAH, and CAA were significantly affected by the factor of force (P < 0.001, Figure 4). The unloaded (0 N) CAW was 22.55 (SD 1.47) mm and it decreased by 0.98 (SD 0.40) mm and 1.43 (SD 0.61) mm during compressive force application of 10 N (P < 0.001) and 20 N (P < 0.001), respectively (Figure 4a). These decreases in the CAW were accompanied by increases in the CAH and CAA (P < 0.001). Specifically, the CAH significantly increased by 0.57 (SD 0.37) mm during 10 N (P < 0.001) and 0.79 (SD 0.51) mm during 20 N (P < 0.001) of applied force, relative to an initial height of 1.22 (SD 0.48) mm for 0 N (Figure4b). The CAA at 0 N was 13.90 (SD 5.93) mm2 and it significantly increased to 19.55 (SD 8.87) mm2 during 10 N (P < 0.01) and to 21.19 (SD 9.24) mm2 during 20 N (P < 0.001) of applied compressive force (Figure 4c). However, there were no significant differences in the carpal arch height or area for the pairwise comparison between the 10 N and 20 N force levels.
Figure 4.
The (a) carpal arch width (CAW), (b) height (CAH), and (c) area (CAA) at the different magnitudes of compressive force applied across the wrist. * P < 0.05, *** P < 0.001
Compressive force application also impacted the median nerve’s flattening ratio (P < 0.001), circularity (P < 0.001), and perimeter (P = 0.007), but its area was not significantly affected (P = 0.15, Table 1). The median nerve increased in roundness as shown by its decreasing flattening ratio, specifically 3.71 (SD 0.72), 3.06 (SD 0.61), and 2.75 (SD 0.61) at 0, 10, and 20 N, respectively. Post-hoc analysis of the flattening ratio revealed significant differences among each pairwise comparison (Table 1). Similar to the flattening ratio, the median nerve circularity also reflected that the nerve became more rounded with increased applied force. The initial nerve circularity was 0.56 (SD 0.09) at 0 N and force application of 10 N and 20 N increased the circularity to 0.64 (SD 0.08, P <0.001) and 0.68 (SD 0.09, P <0.001), respectively. All pairwise comparisons of the median nerve circularity at varying force levels were significant (Table 1). With increasing compressive force magnitude, the perimeter of the median nerve decreased from 12.57 (SD 1.60) mm at 0 N to 11.59 (SD 1.28) mm at 20 N (P <0.01).
Table 1.
Median nerve shape descriptors [mean (standard deviation)]
| Force (N) |
Area (mm2) |
Perimeter (mm) |
Flattening Ratio |
Circularity |
|---|---|---|---|---|
| 0 | 6.93 (1.31) | 12.57 (1.60) | 3.71 (0.72) | 0.56 (0.09) |
| 10 | 7.54 (1.58) | 12.15 (1.25) | 3.06 (0.61)*** | 0.64 (0.08)*** |
| 20 | 7.27 (1.36) | 11.59 (1.28)** | 2.75 (0.61)***† | 0.68 (0.09)***† |
Note: ** and *** denote a significant difference in comparison to the 0 N force level of P < 0.01 and P < 0.001, respectively;
denotes a significant difference between the 10 N and 20 N force levels with P < 0.05
In addition to shape changes of the nerve, its location also changed moving in the radial-volar direction during force application (P < 0.001) (Figure 3). The location of the nerve’s centroid at 0 N was 10.50 (SD 1.83) mm ulnar to the trapezium and 1.44 (SD 0.64) mm dorsal to the CAW line (x-axis). The application of 10 N of force across the wrist significantly displaced the nerve’s centroid 0.82 (SD 0.65) mm in the radial direction and 0.43 (SD 0.32) mm in the volar direction (P < 0.01). When 20 N of force was applied, the nerve had a relative centroid displacement of 1.09 (SD 0.75) mm and 0.67 (SD 0.38) mm in the radial and volar directions, respectively, compared to its location at 0 N (P < 0.001). The pairwise comparison between the nerve’s centroid location at 10 N and 20 N was not significantly different (P > 0.05). Relative to its 0 N centroid position, the Euclidean displacement of the nerve’s centroid was 1.02 (SD 0.56) mm for the 10 N force condition and 1.42 (SD 0.53) mm for the 20 N force condition.
4 Discussion
In this study, in vivo transverse compressive force was externally applied across the wrist to investigate the effects of force application on the carpal arch and the median nerve. The corresponding changes in the morphology and position of the carpal arch and nerve were captured using ultrasonography. It was shown that compressive force applied transversely across the wrist resulted in morphological changes to the carpal arch including decreases in the CAW and increases in the CAH and CAA. Additionally, the applied force caused changes in the median nerve shape and its relative position within the carpal tunnel.
Narrowing the CAW and its effects on the carpal tunnel have been previously demonstrated in modeling [16] and in vitro studies [13, 16]. The current study is the first in vivo study to apply transverse forces across the wrist for the purpose of narrowing the CAW. In this study, a compressive force of 10 N resulted in 0.98 mm of CAW decrease, 0.57 mm in CAH increase, and 5.65 mm2 in CAA increase. These results are similar to those of an in vitro study where point force was directly applied to the carpal bones and 1 mm of CAW narrowing resulted in 0.4 mm of CAH increase and 4.0 mm2 of CAA increase [13]. The current study also agrees with an in vivo study that found a CAW narrowing of 0.8 mm occurs during maximum thumb and index finger pinching, increasing the CAH and CAA by 0.5 mm and 5.1 mm2, respectively [19]. The morphological changes of the carpal arch induced by force application across the wrist in the current study further supports the literature where the carpal tunnel is seen to be a compliant structure [8, 12–15]. Altering the morphology of the carpal tunnel by decreasing the CAW to increase the carpal arch height and area provides additional space for the carpal tunnel contents and may lead to the reduction of pressure within the carpal tunnel.
The contents of the carpal tunnel have been shown to change shape and relative location according to the amount of available space within the tunnel [4, 11, 20]. The shape of the median nerve within the carpal tunnel has been investigated during wrist and finger motion [1, 6, 7] and the nerve has been reported to be more flattened in patients with carpal tunnel syndrome [11, 20–22]. The median nerve’s shape is often quantified by its flattening ratio and this descriptor has been suggested for use as a diagnostic criteria for carpal tunnel syndrome [23]. After carpal tunnel release surgery, the flattening ratio of the median nerve has been found to decrease [21, 22, 24] as the elevated pressure within the carpal tunnel is reduced and the amount of available space for the carpal tunnel contents is increased [25]. In this study, the flattening ratio of the median nerve decreased with increasing transverse force application across the wrist. Similarly, the circularity of the median nerve increased with force application indicating a more rounded cross-sectional shape. These complementary parameters support the postulation that narrowing the CAW by applying transverse compressive force across the wrist may reduce pressure on the median nerve as indicated by a less flattened nerve shape resulting from decreased intracarpal tunnel pressure and increased area available to the carpal tunnel contents.
In addition to the change in median nerve shape, the nerve also displaced radially and volarly under the applied wrist compression force. Previous studies have shown that in healthy subjects the median nerve tends to displace towards open areas within the carpal tunnel to escape compression [1]. In this study, the movement of the nerve in the radial and volar direction may be a result of the increase in CAH which created more space within the carpal arch formed beneath the TCL. It is likely that the additional space created within the carpal arch was in the radial-volar region since that was the general direction of displacement of the thenar muscles attachment site (TUP) during force application [19]. A volar displacement of the median nerve has also been shown to occur after carpal tunnel release surgery in patients with carpal tunnel syndrome [11, 20].
There are several limitations to consider in this study. Firstly, a limited number of force magnitudes were investigated based off of pilot experimentation with the consideration of achieving narrowing of the CAW while maintaining the comfort of the research participants. The forces were applied across the wrist at the distal level of the carpal tunnel following a holistic approach which was necessary given the nature of this in vivo experiment. The line of action for force application was determined using a combination of surface landmarks and the determined ultrasound imaging plane. Force dissipation to the surrounding soft tissues of the wrist was not accounted for; however, all subjects had a similar BMI (20.1, SD 2.1 kg/m2), and therefore it is likely that the amount of force dispersed was similar across subjects. Secondly, the morphological and positional changes of the carpal arch and median nerve were examined in a two-dimensional ultrasound image obtained at the distal level of the carpal tunnel. Additional changes to the carpal tunnel and median nerve at other levels of the carpal tunnel were not investigated in this study and warrant future examination. Thirdly, the temporal effects of force application on the carpal arch and median nerve changes were not explored in the current study. This study was an initial in vivo investigation to reveal carpal arch and median nerve morphological and positional changes. Fourthly, the CAA was approximated by a triangle formed by the hook of hamate, the TUP, and the trapezium. This approximation was previously used [19] and shows the relative change of CAA increase with relation to CAW narrowing, although it may slightly underestimate the arch area. Finally, only female participants were recruited in this study to focus on the force effects without the potential confounding factor of gender differences. The results of the current study may likely be applicable to male subjects, although this warrants further investigation.
In conclusion, this study demonstrated that in vivo narrowing of the CAW is physiologically achievable by applying compressive force across the wrist. It was found that this applied force impacted not only the CAW, CAH, and CAA, but also the median nerve. The median nerve shape became rounder and the nerve displaced in a radial-volar direction. Manipulation of the carpal tunnel to increase its cross-sectional area may decrease intracarpal tunnel pressure and provide relief of median nerve compression. This strategy of carpal arch with narrowing may be clinically useful to alleviate median nerve compression in patients with carpal tunnel syndrome.
Highlights.
Carpal arch and median nerve changes were examined during radioulnar wrist compression.
Wrist compression decreased the carpal arch width and increased the carpal arch area.
The median nerve displaced volarly and became more circular under wrist compression.
Radioulnar wrist compression augments space available in the carpal arch, potentially alleviating compression of the median nerve.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR062753. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Conflicts of interest: none.
Contributor Information
Tamara L. Marquardt, Email: marquat@ccf.org.
Joseph N. Gabra, Email: gabraj@ccf.org.
References
- 1.Wang Y, Zhao C, Passe SM, Filius A, Thoreson AR, An KN, Amadio PC. Transverse ultrasound assessment of median nerve deformation and displacement in the human carpal tunnel during wrist movements. Ultrasound Med Biol. 2014;40:53–61. doi: 10.1016/j.ultrasmedbio.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Doesburg MH, Henderson J, Yoshii Y, Mink van der Molen AB, Cha SS, An KN, Amadio PC. Median nerve deformation in differential finger motions: ultrasonographic comparison of carpal tunnel syndrome patients and healthy controls. J Orthop Res. 2012;30:643–648. doi: 10.1002/jor.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Doesburg MH, Henderson J, Mink van der Molen AB, An KN, Amadio PC. Transverse plane tendon and median nerve motion in the carpal tunnel: ultrasound comparison of carpal tunnel syndrome patients and healthy volunteers. PLoS One. 2012;7:e37081. doi: 10.1371/journal.pone.0037081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Filius A, Zhao C, Passe SM, Thoreson AR, An KN, Amadio PC. Altered median nerve deformation and transverse displacement during wrist movement in patients with carpal tunnel syndrome. Acad Radiol. 2014;21:472–480. doi: 10.1016/j.acra.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshii Y, Ishii T, Tung WL, Sakai S, Amadio PC. Median nerve deformation and displacement in the carpal tunnel during finger motion. J Orthop Res. 2013;31:1876–1880. doi: 10.1002/jor.22462. [DOI] [PubMed] [Google Scholar]
- 6.Yoshii Y, Villarraga HR, Henderson J, Zhao C, An KN, Amadio PC. Ultrasound assessment of the displacement and deformation of the median nerve in the human carpal tunnel with active finger motion. J Bone Joint Surg Am. 2009;91:2922–2930. doi: 10.2106/JBJS.H.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Doesburg MH, Yoshii Y, Villarraga HR, Henderson J, Cha SS, An KN, Amadio PC. Median nerve deformation and displacement in the carpal tunnel during index finger and thumb motion. J Orthop Res. 2010;28:1387–1390. doi: 10.1002/jor.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Elias M, Sanchez-Freijo JM, Salo JM, Lluch AL. Dynamic changes of the transverse carpal arch during flexion-extension of the wrist: effects of sectioning the transverse carpal ligament. J Hand Surg Am. 1992;17:1017–1019. doi: 10.1016/s0363-5023(09)91049-8. [DOI] [PubMed] [Google Scholar]
- 9.Viegas SF, Pollard A, Kaminksi K. Carpal arch alteration and related clinical status after endoscopic carpal tunnel release. J Hand Surg Am. 1992;17:1012–1016. doi: 10.1016/s0363-5023(09)91048-6. [DOI] [PubMed] [Google Scholar]
- 10.Gartsman GM, Kovach JC, Crouch CC, Noble PC, Bennett JB. Carpal arch alteration after carpal tunnel release. J Hand Surg Am. 1986;11:372–374. doi: 10.1016/s0363-5023(86)80144-7. [DOI] [PubMed] [Google Scholar]
- 11.Richman JA, Gelberman RH, Rydevik BL, Hajek PC, Braun RM, Gylys-Morin VM, Berthoty D. Carpal tunnel syndrome: morphologic changes after release of the transverse carpal ligament. J Hand Surg Am. 1989;14:852–857. doi: 10.1016/s0363-5023(89)80089-9. [DOI] [PubMed] [Google Scholar]
- 12.Tung WL, Zhao C, Yoshii Y, Su FC, An KN, Amadio PC. Comparative study of carpal tunnel compliance in the human, dog, rabbit, and rat. J Orthop Res. 2010;28:652–656. doi: 10.1002/jor.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZM, Gabra JN, Marquardt TL, Kim DH. Narrowing carpal arch width to increase cross-sectional area of carpal tunnel--a cadaveric study. Clin Biomech (Bristol, Avon) 2013;28:402–407. doi: 10.1016/j.clinbiomech.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li ZM, Masters TL, Mondello TA. Area and shape changes of the carpal tunnel in response to tunnel pressure. J Orthop Res. 2011;29:1951–1956. doi: 10.1002/jor.21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiu KH, Kim JH, Li ZM. Biomechanics of the transverse carpal arch under carpal bone loading. Clin Biomech (Bristol, Avon) 2010;25:776–780. doi: 10.1016/j.clinbiomech.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131:081011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Marquardt TL, Gabra JN, Shen ZL, Evans PJ, Seitz WH, Li ZM. Pressure-morphology relationship of a released carpal tunnel. J Orthop Res. 2013;31:616–620. doi: 10.1002/jor.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen ZL, Li ZM. Ultrasound assessment of transverse carpal ligament thickness: a validity and reliability study. Ultrasound Med Biol. 2012;38:982–988. doi: 10.1016/j.ultrasmedbio.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen ZL, Li ZM. Biomechanical interaction between the transverse carpal ligament and the thenar muscles. J Appl Physiol (1985) 2013;114:225–229. doi: 10.1152/japplphysiol.01273.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ablove RH, Peimer CA, Diao E, Oliverio R, Kuhn JP. Morphologic changes following endoscopic and two-portal subcutaneous carpal tunnel release. J Hand Surg Am. 1994;19:821–826. doi: 10.1016/0363-5023(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 21.El-Karabaty H, Hetzel A, Galla TJ, Horch RE, Lucking CH, Glocker FX. The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol. 2005;45:223–227. [PubMed] [Google Scholar]
- 22.Horch RE, Allmann KH, Laubenberger J, Langer M, Stark GB. Median nerve compression can be detected by magnetic resonance imaging of the carpal tunnel. Neurosurgery. 1997;41:76–82. doi: 10.1097/00006123-199707000-00016. discussion 82–73. [DOI] [PubMed] [Google Scholar]
- 23.Buchberger W, Judmaier W, Birbamer G, Lener M, Schmidauer C. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793–798. doi: 10.2214/ajr.159.4.1529845. [DOI] [PubMed] [Google Scholar]
- 24.Nadar MS, Dashti MH, Cherian J. Finger position alters the median nerve properties within the carpal tunnel: a pre-post MRI comparison study. PLoS One. 2013;8:e79273. doi: 10.1371/journal.pone.0079273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks JJ, Schiller JR, Allen SD, Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon) 2003;18:685–693. doi: 10.1016/s0268-0033(03)00052-4. [DOI] [PubMed] [Google Scholar]