Abstract
Recently, we reported that the A3 adenosine receptor (A3AR) agonist LJ529 (2-chloro-N6-(3-iodobnzyl)-5'-N-methylcarbamoyl-4'-thioadenosine) reduces cerebral ischemic injury via inhibition of recruitment of peripheral inflammatory cells into ischemic brain lesion. A3AR agonists, however, are known to possess anti-platelet activity, which may deter the combination therapy with tissue plasminogen activator for the therapy of cerebral ischemic stroke. Thus, the present study investigates the neuroprotective/anti-ischemic effect of a synthetic seco-nucleoside, LMT497 ((S)-2-((R)-1-(2-chloro-6-(3-iodobenzylamino)-9H-purin-9-yl)-2-hydroxyethoxy)-3-hydroxy-N-methylpropanamide) with little anti-platelet activity. LMT497 neither showed A3AR binding activity nor anti-platelet activity. In our present study LMT497 significantly attenuated the injury/death of cortical neurons exposed to oxygen-glucose deprivation (OGD) followed by re-oxygenation (R). LMT497 significantly reduced the ascending cellular level of reactive oxygen species under ischemic conditions by increasing the superoxide dismutase (SOD) levels. LMT497 also inhibited the migration of microglia which mediates inflammatory responses in ischemia. In rats subjected to middle cerebral artery occlusion (MCAO, 1.5 h) followed by reperfusion, LMT497 largely reduced brain infarction volume, and edema, and improved neurological score. Therapeutic efficacy of LMT497 was obtained by twice treatments even at 10 h and 18 h after the onset of ischemia. Collectively, LMT497 could be a therapeutic drug candidate with a wide therapeutic time window for the treatment of cerebral ischemic stroke.
Keywords: cerebral ischemia, MCAO, oxidative stress, inflammation, LMT497, Cl-IB-MECA
INTRODUCTION
Through excitotoxic, oxidative and inflammatory intertwining pathways, ischemic stroke causes irreparable cerebral injury which leads to disability and death worldwide. Although acute ischemic stroke causes 10-12% of all deaths in western countries, advances in therapeutics and treatments are still in its infancy [1]. Currently, tissue plasminogen activator (t-PA), the only FDA-approved drug for this intractable disease, is limited due to its short therapeutic time window. Moreover, due to the high risk of hemorrhage and edema after 3 h from the onset of ischemia, only 10% of patients are eligible for this treatment [2, 3]. Therefore, there is an urgency to find new treatments and therapeutics against ischemic stroke [4].
Several studies have shown the neuroprotective effects of A3 adenosine receptors (A3AR) against ischemic stroke. From knockout mouse models to agonist administration experiments on ischemic models, A3AR has much evidence to support its anti-ischemic properties. Recently, we have reported that an A3AR agonist LJ529 (2-chloro-N6-(3-iodobnzyl)-5'-N-methylcarbamoyl-4'-thioadenosine) possessed anti-ischemic properties through the inhibition of inflammatory cell migration into the ischemic brain region. However, A3AR agonists such as LJ529 possess antiplatelet activity, which increases the risk of hemorrhagic ruptures among ischemic stroke patients [5]. When combining antiplatelet treatments with recanalization therapy (e.g. t-PA) the risk of hemorrhagic bleeding hinders the progression of many clinical trials. Therefore it is ideal to focus on a treatment with the neuroprotection of the A3AR agonists without the negative side effects caused by the anti-platelet properties.
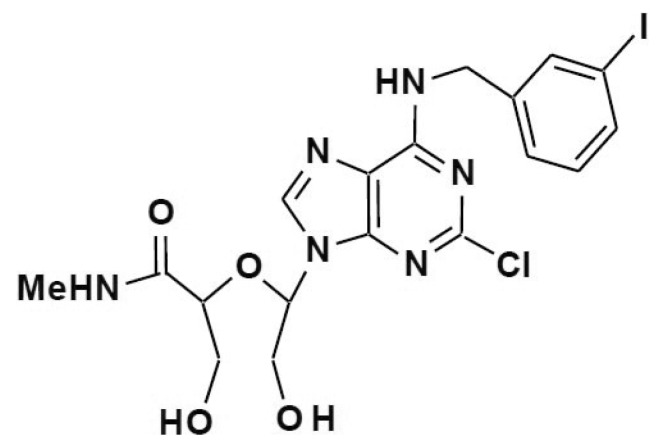
In the present study, we examined the anti-ischemic mechanism of LMT497, ((S)-2-((R)-1-(2-chloro-6-(3-iodobenzylamino)-9H-purin-9-yl)-2-hydroxyethoxy)-3-hydroxy-N-methylpropanamide), a synthetic seco-nucleoside of a well-known A3AR agonist, Cl-IB-Meca (Fig. 1). Unlike Cl-IB-Meca however, LMT497 has no A3AR binding activity and has reduced anti-platelet activity compared to our previously tested compound, LJ529.
Fig. 1. Structure of LMT497.
Our results demonstrated that LMT497 reduced intracellular overproduction of reactive oxygen species (ROS) and mitigated inflammatory responses by inhibiting microglial migration in vitro. LMT497 markedly reduced cerebral injury in rats subjected to middle cerebral artery occlusion (MCAO 1.5 h) followed by reperfusion. More importantly, LMT497 was significantly effective when administrated 10 h after the onset of ischemic stroke. Therefore, with this extended therapeutic time window, along with its multi-facetted neuroprotective effects, LMT497 is a viable candidate against ischemic stroke several hours after the onset of injury.
MATERIALS AND METHODS
Materials
5-(and-6)-chloromethyl-2', 7'-dichlorodihydrofluorescein diacetate and probenecid were obtained from Life Technologies Corporation (Carlsbad, CA). Dulbecco's modified Eagle medium (DMEM), minimum essential media (MEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA). Laminin and Monocyte Chemoattractant Protein-1 (MCP-1) were purchased from BD biosciences (San Jose, CA). EBSS and HEPES buffer solution were obtained from Welgene (Daegu, South Korea). 2, 2'-azobis-(2-methylpropionamide)-dihydrochloride (AAPH), 1, 1-diphenyl-2-picrylhydrazyl (DPPH), Poly-D-Lysine (PDL), Harris hemotatoxylin, and all other drugs were purchased from Sigma-Aldrich co (St. Louis, MO). LMT497 (Seco-Cl-IB-MECA) was synthesized by Dr. Yongseok Choi at the Department of Biotechnology, School of Life Sciences and Biotechnology, Korea University (For details of chemical synthesis, see the supplementary Data).
Animal
Male Sprague-Dawley rats (260 to 300 g; Charles River Laboratories, Seoul, South Korea) were acclimated to their environments. All animal experiments were performed in accordance with the NIH guideline and approved by the Institutional Animal Care & Use Committee. The animal IRB number used for in vitro was KUIACUC-2014-29 and the IRB number used for in vivo was KUIACUC-2014-14.
Focal cerebral ischemia and drug treatment
Rats were initially anesthetized with 3% isoflurane in a 70% N2O and 30% O2 mixture via facemask and maintained with 2% isoflurane. Focal cerebral ischemia was induced by endovascular middle cerebral artery occlusion (MCAO) on the right side of the brain as previously described [6]. Briefly, a 4-0 monofilament nylon suture (Ethicon Johnson & Johnson, Brussels, Belgium) with a heat-blunted end was inserted into the right external carotid artery and advanced into the internal carotid artery. After 90 minutes of MCAO, rats were reperfused by removing the suture from the vessel. During the surgery, body temperature was maintained at 37℃ with a homeothermic blanket (Panlab, Barcelona, Spain) and checked with a rectal temperature probe. LMT497 (2 mg/kg) was initially dissolved in dimethyl sulfoxide 5%, Cremophor EL 10% (poluoxyl-35 hydrogenated castor oil; Merck KGaA, Darmstadt, Germany), and saline 85% respectively. Drugs were injected intravenously twice per experiment at 3 h/8 h or 10 h/18 h after the onset of MCAO.
Measurement of infarct volume
After 24 h of MCAO, rats were anesthetized with chloral hydrate. Brains were extracted from rats and cut into 2 mm coronal sections and stained with 2% triphenyltetrazoliumchloride (TTC; Alfa Aesar A Johnson Matthey Company, Ward Hill, USA) at 37℃ for 10 minutes. The total infarction volume was calculated by integrating 6 sections, manually measuring the infarct area and compensating for brain edema as described previously [7, 8]. The percentage of edema was calculated by measuring the difference in volume between ipsilateral and contralateral hemispheres [% Edema = (Vi - Vc)/Vc; Vi, ipsilateral volume; Vc, contralateral volume].
Neurological score
Neurological scores were evaluated after 24 h of initial MCAO, as described previously [9]. Scores are divided as follows: 0, no observable deficit of motor function; 1, contralateral forelimb flexion when rat was lifted by tail; 2, decreased resistance to lateral push (and forelimb flexion) without circling; 3, same behavior as grade 2, with circling; 4, no spontaneous motor activity.
Mixed cortical neuronal/glial co-cultures
Primary mixed cortical neuronal/glial co-cultures were prepared from embryonic (E16-E17 day-old) Sprague-Dawley rats. Meninges-free cortices were dissociated by triturating with Pasteur pipette. Dissociated cerebrocortical cells (1.35 × 103cells/mm) were added to pre-coated culture plates with poly-D-lysine (10 µg/mL) and laminin (4 µg/mL). Cells were then maintained in DMEM containing 10% fetal bovine serum and B27-supplement in a 37℃ in a humidified incubator containing 95% air and 5% CO2. Experiments were performed 12-14 days after initial plating of cultures. Cultures consisted of 50-60% neurons and 40-50% glial cells (> 95% astrocytes), assessed by immunostaining with neuronal marker NeuN and astroglial marker GFAP.
Microglial cell culture
Pure microglial cells were prepared from primary mixed glial cell cultures. Cerebral cortices from neonatal Sprague-Dawley rats (1 to 2 days old) were triturated to single cells. They were then plated onto poly-D-lysine (1 µg/mL) coated 75-cm2 T-flasks and maintained in modified Eagle's medium (MEM) containing 10% fetal bovine serum. Seven to nine days after plating, microglia was detached from the flasks by mild shaking (37℃, 2 min at 200 r.p.m.) and was set for experimental use.
Oxygen-Glucose Deprivation (OGD) and Reoxygenation
For an in vitro model of hypoxic/ischemic insult, cells were placed in an anaerobic chamber (partial pressure of oxygen <2mm Hg; 95% N2, 5% CO2), at 37℃ for 1 h, while the culture medium was replaced with a glucose-free DMEM. OGD was terminated by replacing culture medium with DMEM supplemented with 25 mM glucose, and by returning cells to normoxic conditions. Cells were treated with LMT497 (10 µM), during the entire period of OGD/R.
Assessment of cell injury or death
Cell injury or death was assessed by morphological examination with a phase-contrast microscope (Leica, Solms, Germany), and by measuring the amount of LDH released into the culture medium using a diagnostic kit (Sigma-Aldrich, Co., St. Louis, MO). The degree of cell injury was expressed as a percentage of total LDH release, which was defined as the amount of LDH released from the cells after repeated freeze/thaw cycles. Dose-response experiments of LMT497 in the OGD/R model were performed between the ranges of 0.1 and 100 µM.
Measurement of intracellular oxidative stress: CM-H2DCF-DA assay
The intracellular reactive oxygen species (ROS) level was measured with CM-H2DCF-DA, which diffuses through cell membranes and hydrolyzed by intracellular esterase to its nonfluorescent form, CM-DCF-H. CM-DCF-H then reacts with free radicals to form highly fluorescent CM-DCF. After 3 h reoxygenation, cells were loaded with 1 µM CM-H2DCF-DA in EBSS containing 2.5 mM probenecid for 10 min. After removing the loading medium, the DCF fluorescence in three non-overlapping optical regions (825 × 625 µm2) per sample was measured at Ex488 nm/Em525 nm with a fluorescence microscope (Leica, Solms, Germany) equipped with a digital camera. The fluorescence intensity was quantified using an image analyzer (TOMORO ScopeEYE 3.5) by a treatment-blinded examiner.
Measurement of ROS scavenging activity: ORAC assay
For oxygen radical absorbance-capacity (ORAC) assays, different concentrations of LMT497 or Trolox were allowed to react with peroxyl radicals generated from 2,2'-azobis-(2-methylpropionamide)-dihydrochloride (AAPH; 60 mM), and ROS scavenging activity was determined by monitoring the loss of fluorescein (50 nM) fluorescence caused by peroxyl radicals. Fluorescence decay was measured every 5 min for 3 h at 37℃ at (Ex485nm/ Em530nm), respectively, using a fluorescence microplate reader. Peroxyl radical scavenging capacity was quantified by calculating the area under the curve (AUC) on the basis of kinetic curves using the following equation: AUC = (0.5 + f1/f0 + f2/f0 + f3/f0 + … + fn-2/f0 + fn-1/f0 + fn/f0) × 5, where fi is the fluorescence reading at time i (minutes). The net AUC = AUCsample - AUCblank. The concentration of LMT497 producing the same net AUC, when compared to 50 mM Trolox, was presented as its Trolox Equivalents (TE).
Measurement of ROS scavenging activity: DPPH assay
For the DPPH reduction assay, LMT497 or Vitamin C (0.1, 1, 10, 25, 50, 100 µM) were mixed with 23.6 µg/mL of DPPH, an organic nitrogen radical generator. After 30 min of incubation at 37℃, the absorbance was measured at 517 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The scavenging activity of free radicals was expressed as the percentage of maximum inhibition [(Absmaximum - Abssample)/(Absmaximum - Absminimum) × 100], obtained from a standard curve generated using vitamin C.
Measurement of Superoxide Dismutase (SOD) and catalase activity
For the measurement of SOD activity, cells were suspended in cold HEPES buffer (20 mM HEPES pH 7.2, 1 mM EGTA, 210 mM mannitol, 70 mM sucrose), and the resulting cell lysates were centrifuged at 1500' g for 5 min at 4℃. SOD activity was measured using a SOD assay kit (Cayman Chemical Company, Ann Arbor, MI). In this assay, tetrazolium salt reacts with superoxide anion to produce formazan, which is detected at 450 nm with an ELISA microplate reader (SpectraMax190; Molecular Devices, Sunnyvale, CA).
For the measurement of catalase activity, cells were homogenized with cold phosphate buffer (50 mM potassium phosphate, pH 7.0, containing 1 mM EDTA), and the resulting cell lysates were centrifuged at 10,000'g for 15 min at 4℃. Catalase activity was determined in supernatants by the reaction of catalase with methanol in the presence of an optimal concentration of H2O2. Formaldehyde production was determined using a catalase assay kit (Cayman Chemical Company) and was quantified by measuring absorbance at 540 nm using an ELISA microplate reader (SpectraMax190, Molecular Devices).
Measurement of intracellular superoxide levels
The intracellular levels of superoxide were determined by measuring the red fluorescence of hydroethidium (HE) produced by oxidation of dihydroethidium (DHE) [10].Cells were loaded with 5 µM DHE for 20 min before the termination of OGD/R (1 h/3 h), after which the medium was replaced with fresh medium. HE fluorescence in three non-overlapping optical sections was examined using a fluorescence microscope (DM IL HC Fluo; Leica Microsystems GmbH, Wetzlar, Germany) equipped with a digital camera (DFC420C, Leica Microsystems GmbH, Wetzlar, Germany). The intensity of the fluorescence was quantified using an image analyzer (TOMORO ScopeEye 3.5; Saramsoft Co.).
Chemotaxis
Monocyte Chemoattractant Protein 1(MCP-1) was placed in the bottom chamber of a chemotaxis chamber (Neuro Probe, Cabin John, MD) and microglia (5 × 104 cells/mL in serum-free MEM) in the upper chamber was allowed to migrate to the bottom section for 2 hours, through membrane pores (8 mm2 filter area; Neuro Probe, Gaithersburg, MD). Migrated cells on the bottom-side filter were stained for nuclei with Harris's hematoxylin and then counted.
Statistical analysis
Data were expressed as mean±standard error of the mean (SEM) and analyzed for statistical significance by employing an analysis of variance (ANOVA) and followed by the post hoc Tukey's test for multiple comparisons. Otherwise, the data were expressed as median and interquartile range (IQR: Q1-Q3) and analyzed by Kruskal-Wallis test followed by Mann-Whitney U test. p values < 0.05 were considered significant after Bonferroni correction.
RESULTS
LMT497 inhibits OGD/R-induced cortical cell injury
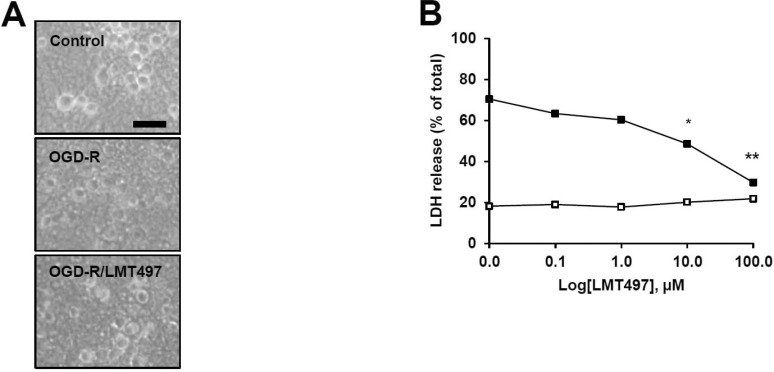
To investigate the neuroprotective effects of LMT497 during ischemic conditions in vitro, cortical cells were placed in an anaerobic chamber for 1 h and were re-oxygenated for 8 h in normoxic conditions. LMT497 (10 µM) reduced the bursting and swelling of the neuronal cell bodies induced by OGD/R in microscope phase-contrast observation (Fig. 2A). Using the LDH assay to evaluate the viability of neuronal cells, LMT497 dose-dependently attenuated OGD/R-evoked LDH release significantly compared to the untreated OGD/R group (Fig. 2B).
Fig. 2. Neuroprotective effect of LMT497 in vitro. Cortical neurons were treated with LMT497 [LMT; at 10 µM and B indicated concentrations] and exposed to oxygen-glucose deprivation (OGD, 1 h) and subsequent re-oxygenation (8 h) (OGD/R). (A) Neuronal cell injury or death was assessed by morphological observation using representative phase-contrast images. Scale bar=50 µm. (B) Cell death was quantified as the % of total LDH release. n=6; *p<0.05, **p<0.001, significantly different from the control group.
LMT497 reduces OGD/R-induced intracellular oxidative stress but does not directly scavenge free radicals
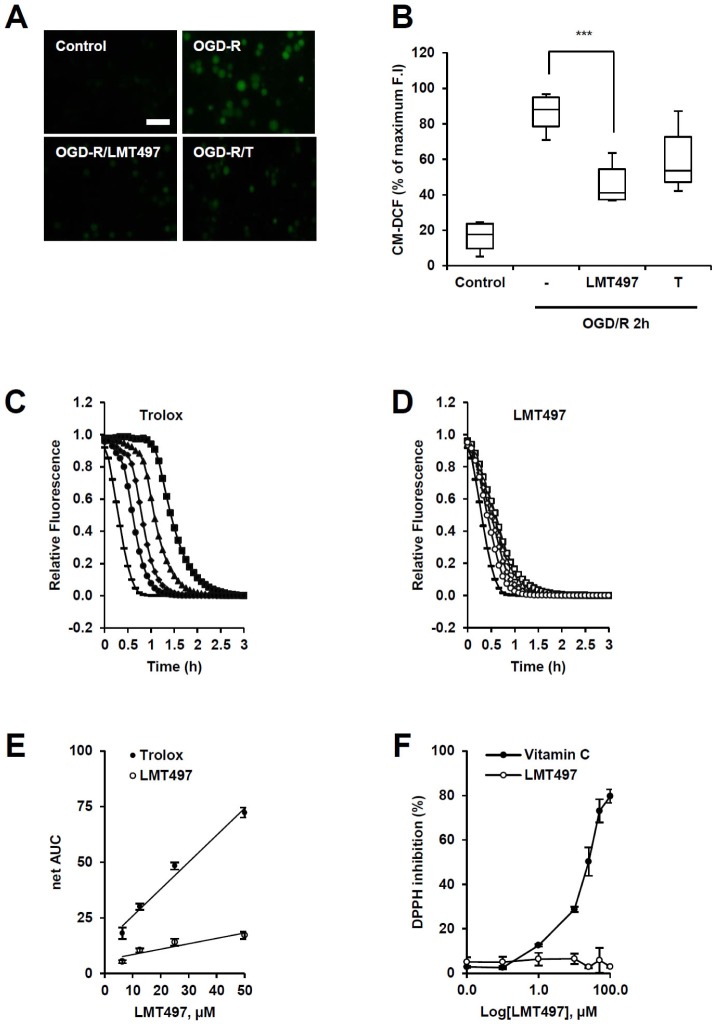
It is known that oxidative stress occurs in cerebral ischemic stroke due to the imbalance of NO and ROS [11]. Therefore, to assess the anti-oxidative effect of LMT497 on OGD/R-induced ROS generation in cortical neurons, the DCF-DA assay was performed. LMT497-treated cells showed significantly decreased fluorescence in ischemic conditions, compared to the non-treated control (Fig. 3A, B). To further examine the ROS scavenging activities of LMT497 in a cell-free system, ORAC (hydrogen atom transfer) and DPPH (electron transfer) assays were done [12]. In the ORAC assay, LMT497 did not significantly slow the rapid AAPH-induced decay in fluorescein fluorescence (Fig. 3C, D). LMT497 did not react significantly with DPPH radicals (Fig. 3F).
Fig. 3. Anti-oxidative effect of LMT497. (A, B) LMT497 reduced OGD/R-evoked oxidative stress in a cell culture system seen through DCF-DA assay. Cortical neurons were treated with LMT497 (10 µM) or Trolox (T; 10 µM) directly before the initiation of OGD. Cells were loaded with CM-H2DCF-DA for 10 minutes after reoxygenation, and intracellular ROS production was measured at 3 h after initiation of reoxygenation by an increase of fluorescence intensity (F.I). (A) Representative fluorescence images. Scale bar 50 µm. (B) Quantification of CM-DCF fluorescence. Horizontal bar, median; vertical box, interquartile ranges (Q1-Q3); and whiskers, minimum/maximum. n=6. ***p<0.001 (C-F) ROS scavenging activity of LMT497 in a cell free system. (C-E) The ORAC assay. (C, D) Representative time-dependent decay graphs of relative F.I in the presence of Trolox or LMT497 at different concentrations (-=untreated; •,○=6.25 µM; ▴,▵=12.5 µM; ✦,◊=25 µM; ▪,□=50 µM). (E) Best-fit lines between the net AUC (=AUCsample-AUCblank) and different concentrations of LMT497 of Trolox. n=4. (F) The DPPH assay. The dose-response curves of DPPH inhibition in the presence of LMT497 in comparison to vitamin C. n=4.
LMT497 increases SOD activity and decreases OGD/R-induced superoxide level
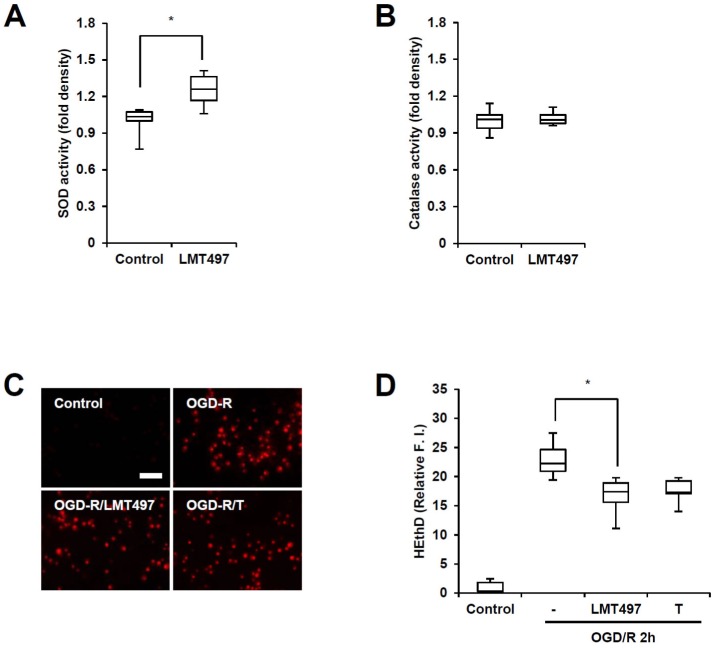
In regards to anti-oxidative defense mechanisms, enzymes such as SOD and catalase have the abilities to alleviate oxidative damage caused by ischemic stroke [13]. To further investigate the anti-oxidative capabilities of LMT497, the levels of SOD and catalase were tested as representative anti-oxidant enzymes. LMT497 significantly increased SOD activity in cortical cells in non-ischemic conditions, but failed to show significance when tested for catalase activity (Fig. 4A, B). Since SOD catalyzes the dismutation of superoxide to hydrogen peroxide and oxygen [13], it was necessary to examine whether LMT497 could decrease the level of superoxide under ischemic conditions. Similar to Trolox, LMT497 significantly decreased OGD/R-induced HE fluorescence, which indicates the decline of superoxide levels. (Fig. 4C, D)
Fig. 4. Effects of LMT497 on SOD/catalase activity and ODG/R-induced superoxide generation. SOD (A) and catalase (B) assays were performed 4 h after incubation with vehicle or LMT497 10 µm. Data are presented as medians±interquartile ranges. n=6; *p<0.05 compared with indicated group (C, D) OGD/R-induced intracellular superoxide levels in cortical cells. Cortical cells were treated with LMT497 (10 µm) Or Trolox (T; 10 µm) before OGD. (C) Representative images. Scale bar=50 µm. (D) Quantification of HE fluorescence. n=6; **p<0.01 significantly different from the control group.
LMT497 inhibits MCP-1-induced microglial migration
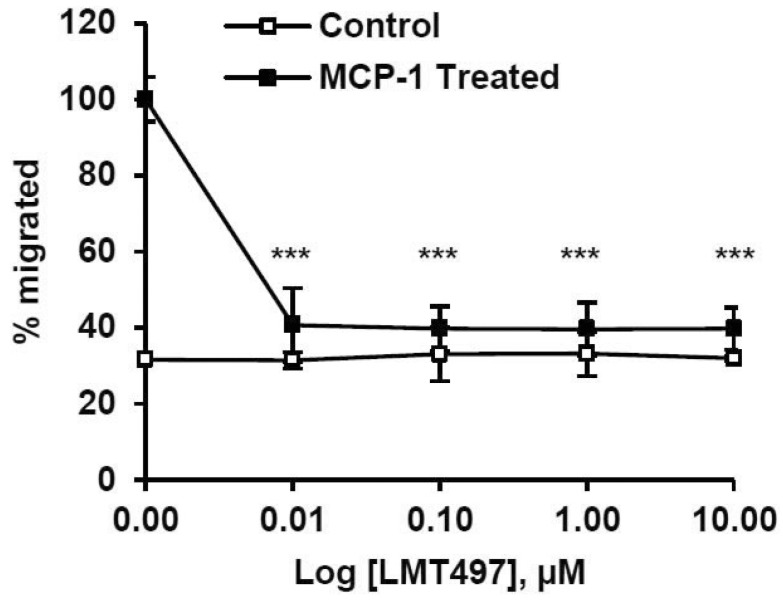
The inflammatory process plays a vital role during the ischemic cascade. The resident immune cells, primarily microglia, are activated once ischemic stroke disables the endogenous inhibitory signals and triggers. Microglia then migrates towards the damaged penumbra area to release anti- and pro-inflammatory cells [14]. To examine whether LMT497 has anti-inflammatory properties, a microglial migration chemotaxis experiment was done. LMT497 significantly inhibited MCP-1 (100 ng/mL)-induced migration of microglia compared to the untreated control (Fig. 5).
Fig. 5. Inhibition of MCP-1 induced microglial chemotaxis by LMT497. MCP-1 evoked migration of microglia was quantified using a chemotaxis chamber. n=6; ***p<0.001 significantly different from the MCP-1 treated microglia in the absence of LMT497.
LMT497 possesses neuroprotective qualities in an MCAO rat model
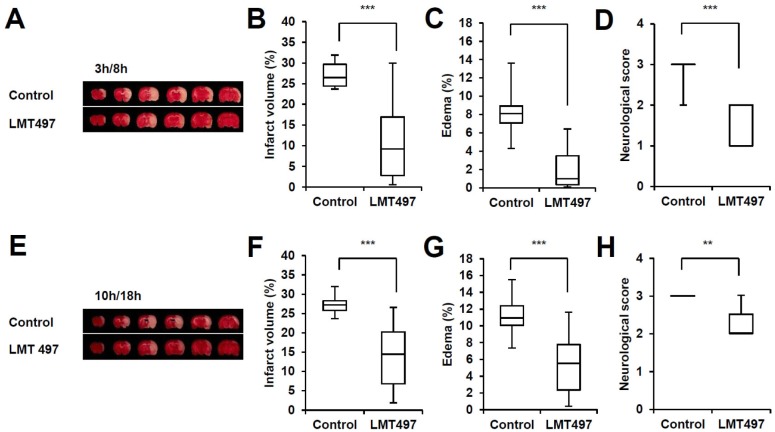
In randomized experiments in rats, post-ischemic treatment of LMT497 (2 mg/kg, i.v., twice) significantly reduced ischemic infarction volume, edema and neurological score in two different time windows (3 h/8 h; 10 h/18 h) (Fig. 6). Results obtained from the initial i.v. administration (3 h/8 h) showed that LMT497 significantly diminished infarct volume (58.6%), edema (75.2%), and neurological score compared to the control group (Fig. 6A-D). To further expand the therapeutic time window, we administered LMT497 at 10 h/18 h post-ischemia. At this time point, LMT497 showed dimidiate infarct volume, edema, and neurological score compared to the control group (Fig. 6E-H).
Fig. 6. Protective effect of LMT497 in vivo ischemic model. All rats were treated MCAO for 90 min and sacrificed 24 hours after occlusion and brain coronal sections were stained with 2% triphenyltetrazolium chloride. (A-H): Intravenous injection of LMT497 (2 mg/kg) twice at 3 h/8 h (A-D) or 10 h/18 h (E-H). (A, E): TTC stained image. (B, F): quantification of infarct volume. (C, G): percentage of edema, (D, H): neurological score. Horizontal bar, median; vertical box, interquartile ranges (Q1 to Q3, minimum/maximum). n=7-10; **p<0.01, ***p<0.001, significantly different from the control group.
DISCUSSION
The recent recognition of cerebral ischemic stroke as a disease with multiple pathophysiological cascades has had a positive impact on the development of treatments and therapeutics [15, 16]. Currently the A3AR has been highly focused against inflammatory damage and moreover, has shown promise to be an effective anti-ischemic target [17, 18]
However A3AR agonists such as Cl-IB-Meca and LJ529 have also shown anti-platelet effects which increase the risk of hemorrhagic stroke. Thrombolytic agents such as t-PA are vital in breaking down blood clots during acute ischemic stroke, but are limited in use due to the increased threat of hemorrhagic bleeding [19, 20, 21]. It is particularly risky to combine antiplatelet drugs with thrombolytic therapy which further hinders the advancement of many clinical trials of neuroprotectants with antiplatelet properties [22, 23]. For this reason, we synthesized a derivative from Cl-IB-MECA without a C-C bond in a ribose ring to lessen the anti-platelet effect (data not shown) and retain the original anti-ischemic capabilities, without A3AR activity. We evaluated the potential neuroprotective action of a novel synthetic molecule LMT497 against ischemic conditions in vitro and in vivo models.
In the cerebral ischemic cascade, excitotoxicity, oxidative stress and neuroinflammation play important roles in neuronal cell injury and death [1, 24]. During the initial stages of ischemic stroke, immoderate extracellular glutamate release induces intracellular calcium dysregulation through NMDA receptors [1]. Accordant with A3AR agonist LJ529 results, LMT497 did not show significant inhibition against NMDA-induced neuronal cell death (data not shown), which indicates that the cytoprotection by LMT497 may not reflect anti-excitotoxic activity.
In addition to excitotoxicity, oxidative stress has shown substantial effect in stroke pathogenesis through ROS-induced damage [11]. During OGD/R conditions, LMT497 significantly inhibited the overall intracellular ROS production.
Although LMT497 did not scavenge ROS directly, LMT497 reduced ROS levels indirectly through the up-regulation of oxidative enzymes. Oxidative enzymes such as superoxide dismutase and catalase have pivotal roles in the defense against oxidative stress induced by ischemic stroke, which is important for the balance of intracellular ROS for cell survival [25, 26, 27]. In LMT497-treated groups, the level of SOD was significantly up-regulated. Similarly with A3AR agonists reported in a previous paper [28], the intracellular generation of superoxide anions induced by OGD/R was decreased by the administration of LMT497. These data indicate that the increase of SOD activity caused by LMT497 plays a crucial role in the decrease of overall ROS generation evoked by ischemic damage.
Under OGD conditions, the major cytotoxic mechanisms for neuronal cell death include mitochondrial malfunction, excitotoxicity and radicals formation. Therefore, the neuroprotective activity observed in Fig. 2 could be due to the up-regulation of antioxidant enzyme Mn-SOD. In contrast, the ischemic brain damage observed in Fig. 6 must be caused by various cytotoxic inflammatory responses in addition to the afore-mentioned cytotoxic mechanisms. We previously reported the importance of delayed inflammatory responses in the expansion of irreversible brain damage after the onset of ischemia [18]. As the ischemic cascade progresses, the migration of microglia, a small resident macrophage in the brain, towards the damaged area activates several pro-inflammatory cytokines into the cerebral nervous system. This inflammatory damage further down the ischemic cascade slowly renders the salvageable penumbra region of injury into the core region. [14, 29]. In this present study, LMT497 inhibited microglial migration induced by MCP-1, which suggests that LMT497 indirectly inhibits pro-inflammatory cytokines activated by microglia.
Although LMT497 does not possess A3AR binding affinity, it possesses anti-inflammatory effects, similar to several A3AR agonists. Therefore by inhibiting the migration of microglia and increasing SOD levels, we can assert that LMT497 possesses neuroprotective properties against ischemic damage through multiple therapeutic properties. Thus, we performed in vivo experiments to investigate whether LMT497 has anti-ischemic effects against animal models.
Consistent with LJ529 data, LMT497 post-ischemic treatment (3 h/8 h) after MCAO, markedly improved ischemic conditions. Notably, LMT497 treatment even at an extended time point (10 h/18 h) significantly protected the brain against cerebral injury, showing an inhibition in the infarct volume, edema, and an improved neurological score compared to controls. The protection shown by LMT497 during the 10 h/18 h time point of administration well exceeds many of the current time windows shown by other developing neuroprotectants against ischemia [2, 4]. Recent clinical evidence has shown that brain tissue is potentially salvageable even 8 h after symptom onset [30]. Moreover, a majority of stroke patients are unable to receive certain treatment either due to the uncertain time of the ischemic onset or ischemic onset has already passed 3 h after the onset [31]. Although stroke is a time-dependent disease, LMT497 has the capability to prevent damage even several hours after the onset.
Conclusively, drug cocktails for combination therapy or one drug targeting multiple pathways of anti-ischemic activity have recently been reported to be advantageous for the treatment of ischemic stroke [16]. Our results suggest that LMT497 is a multi-facetted neuroprotectant with both essential anti-oxidative and anti-inflammatory properties which aid in the amelioration of ischemic neuronal damage. Our in vivo model further confirmed the anti-ischemic properties of LMT497 with an extended therapeutic window. As a novel anti-ischemic compound, with an elongated time point of treatment, our study suggests LMT497 is a viable candidate against ischemic stroke. Nevertheless, to fully understand the mechanism of action of LMT497, further studies should be extended in vitro and in vitro as well as to elucidate the exact binding site.
ACKNOWLEDGMENTS
This work was supported by grants from the Korea Health Technology R&D Project (No. HI12C1353 to W.-K. Kim) funded by the Ministry of Health & Welfare and the Bio & Medical Technology Development Program (2011-0019440) through the National Research Foundation of Korea funded by the Ministry of Science and Technology.
SUPPLEMENTARY DATA
References
- 1.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Chapman SN, Mehndiratta P, Johansen MC, McMurry TL, Johnston KC, Southerland AM. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75–87. doi: 10.2147/VHRM.S39213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallevi H, Grotta JC. Antiplatelet therapy and the risk of intracranial hemorrhage after intravenous tissue plasminogen activator therapy for acute ischemic stroke. Arch Neurol. 2008;65:575–576. doi: 10.1001/archneur.65.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Shuaib A, Li Q. Quantification of infarct size on focal cerebral ischemia model of rats using a simple and economical method. J Neurosci Methods. 1998;84:9–16. doi: 10.1016/s0165-0270(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 8.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 9.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 10.Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 13.Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 14.Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5:73–90. [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. 2009;10:644–654. [PubMed] [Google Scholar]
- 17.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 18.Choi IY, Lee JC, Ju C, Hwang S, Cho GS, Lee HW, Choi WJ, Jeong LS, Kim WK. A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol. 2011;179:2042–2052. doi: 10.1016/j.ajpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuadrado E, Ortega L, Hernández-Guillamon M, Penalba A, Fernández-Cadenas I, Rosell A, Montaner J. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi K, Kawahara K, Miyagi N, Uchikado H, Kuramoto T, Morimoto Y, Tancharoen S, Miura N, Takenouchi K, Oyama Y, Shrestha B, Matsuda F, Yoshida Y, Arimura S, Mera K, Tada K, Yoshinaga N, Maenosono R, Ohno Y, Hashiguchi T, Maruyama I, Shigemori M. Edaravone: a new therapeutic approach for the treatment of acute stroke. Med Hypotheses. 2010;75:583–585. doi: 10.1016/j.mehy.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Medcalf RL. Plasminogen activation-based thrombolysis for ischaemic stroke: the diversity of targets may demand new approaches. Curr Drug Targets. 2011;12:1772–1781. doi: 10.2174/138945011797635885. [DOI] [PubMed] [Google Scholar]
- 22.Clark WM, Madden KP, Lyden PD, Zivin JA. Cerebral hemorrhagic risk of aspirin or heparin therapy with thrombolytic treatment in rabbits. Stroke. 1991;22:872–876. doi: 10.1161/01.str.22.7.872. [DOI] [PubMed] [Google Scholar]
- 23.Bednar MM, Quilley J, Russell SR, Fuller SP, Booth C, Howard D, Gross CE. The effect of oral antiplatelet agents on tissue plasminogen activator-mediated thrombolysis in a rabbit model of thromboembolic stroke. Neurosurgery. 1996;39:352–359. doi: 10.1097/00006123-199608000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 25.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu W, Zhao H, Yenari MA, Sapolsky RM, Steinberg GK. Catalase over-expression protects striatal neurons from transient focal cerebral ischemia. Neuroreport. 2004;15:413–416. doi: 10.1097/00001756-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Sheng H, Bart RD, Oury TD, Pearlstein RD, Crapo JD, Warner DS. Mice overexpressing extracellular superoxide dismutase have increased resistance to focal cerebral ischemia. Neuroscience. 1999;88:185–191. doi: 10.1016/s0306-4522(98)00208-5. [DOI] [PubMed] [Google Scholar]
- 28.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrop hils. Mol Pharmacol. 2008;74:685–696. doi: 10.1124/mol.108.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 30.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke. 1995;26:2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- 31.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.