Abstract
Artemisia princeps (AP) is a flowering perennial used as a traditional medicine and dietary supplement across East Asia. No study has yet assessed its effects on synaptic plasticity in hippocampus and much less in a model of ovarian hormone deficiency. We examined the influence of chronic oral AP ethanol extract treatment in ovariectomized rats on the induction of long-term depression in a representative synapse (CA3-CA1) of the hippocampus. Ovariectomized rats demonstrated lower trabecular mean bone mineral densities than sham, validating the establishment of pathology. Against this background of pathology, AP-treated ovariectomized rats exhibited attenuated long-term depression (LTD) in CA1 relative to water-treated controls as measured by increased field excitatory post-synaptic potentials (fEPSP) activation averages over the post-stimulation period. While pathological significance of long-term depression (LTD) in ovariectomized rats is conflicting, that AP treatment significantly affected its induction offers justification for further study of its influences on plasticity and its related disorders.
Keywords: Artemisia princeps, Oriental medicine, ovariectomy, long-term depression (LTD), hippocampal synaptic plasticity
INTRODUCTION
A large body of evidence suggests a relationship between ovarian hormones and a number of neuropsychological symptoms. A number of studies have linked hormones with the epidemiology of depressive syndromes, the base incidence of which is higher in females than males across the lifespan [1, 2, 3, 4, 5, 6, 7]. Depression and anxiety-like symptoms are expressed at greater rates in perimenopausal relative to post- [8] or premenopausal women [9, 10, 11], even in the absence of premenopausal depressive tendencies [12]. Additionally, estrogen receptor gene polymorphisms have been linked with not only depressive symptomology in postpartum subjects [13] but also Alzheimer's risk in elderly women and men [14]. Sudden loss of ovarian hormones inherent in oopherectomy may also be associated with higher risk of dementia in women [15]. Conversely, increased serum estradiol, specifically, has been associated with improved mood in both perimenopausal [16] and postmenopausal [17] women and in postpartum depression patients [18], and oral progesterone analogue contraceptives have been shown to improve depressive mood in reproductive-age women [19]. Moreover, levels of frontal cortical estradiol receptor α (ERα) correlated positively with cognitive function in both female and male Alzheimer's patients [20], and hormone replacement therapy has exhibited beneficial effects on both cognitive function and later dementia risk in postmenopausal women, though therapeutic effects are often contingent on the age of administration [21].
Despite the profusion of epidemiological evidence hinting interactions between ovarian hormones and the brain, the physiological effects of these chemicals on neural circuits, as well as the influences of these circuits on subsequent neural function and behaviors, have yet to be characterized in full detail. Perhaps the most widely studied of these brain regions is the hippocampus, in which estradiols or their receptors have been demonstrated to influence ACh release [22], monoamine levels [23], spine number and density of CA1 pyramidal neurons [24], and dentate gyrus granule cell proliferation [25] and survival [26]. As abnormalities in hippocampal plasticity have been associated with disorders in which estradiols have also been implicated, as in the reduced hippocampal neurogenesis seen in major depression patients [27] and reduced hippocampal and serum brain-derived neurotrophic factor in Alzheimer's patients [28], it is reasonable to hypothesize that understanding the activity of estradiols in this region may be key to unlocking their possible roles in these and possibly other pathologies.
One of the less well studied routes by which ovarian function may affect hippocampal synaptic plasticity is by influencing long-term depression (LTD), the process by which a neuron exhibits reduced excitability to stimulation at one or more synapses over a prolonged (>15-20 minutes) time frame [29]. Disruptions in LTD have been associated with abnormalities in working memory, episodic memory, reversal learning, and stress-induced inhibitions of memory retrieval in rodents; inversely, animal models of Fragile X syndrome as well as depressive and schizoprenic pathologies also exhibit disruptions in LTD [30]. The few studies on the effects of estradiols on hippocampal LTD in female mammals suggest a complex relationship dependent on age and time course of treatment [17]. LTD may be induced in rodent hippocampal slice cultures through a number of means, including low-frequency stimulation (LFS), stimulation synchronized with the negative phase of the theta rhythm [29], and application of N-methyl-D-aspartic acid (NMDA) [31]. Consistent with these results, paired-pulse LFS of acute hippocampal slices from ovariectomized female adult rats exhibited LTD in CA1 that was attenuated by in vitro application of estradiol benzoate [32]. In contrast, adult ovariectomized female rats reportedly demonstrated attenuated paired-pulse LFS-induced LTD relative to age-matched low-estrogen female controls in estrus either five days or five weeks following surgery, leading the authors to conclude that the chronic estrogen loss of ovariectomy leads to inhibitory effects on LTD relative to the potentiation seen in the short-term estrogen loss of estrus [33]. These results are consistent with another study suggesting that estradiol potentiates LTD by lowering the frequency threshold for its induction [34]. Taken together, these results support the idea that ovarian hormones exert multifaceted time-dependent influences on hippocampal LTD and that this relationship is disrupted by their loss.
In this study, we compared the effects of long-term treatment of AP ethanol extract with the those of commonly prescribed osteoporosis medication sodium alendronate (Fosamax) on LTD induced in CA1 following low-frequency stimulation to the CA2 stratum radiatum in hippocampal slice cultures of mature ovariectomized female rats. We hypothesized that the same single-pulse LFS-induced long-term depression found in the ovariectomized rats of Sharrow et al. [32]. would be replicable in our own rat models of ovarian hormone loss and that AP would also exhibit significant LTD-related biological activities in the estrogen-poor environment, justifying further study on the effects of its extracts and components on central nervous system function.
MATERIALS AND METHODS
Artemisia princeps Pampanini preparation
The stalk and leaves of Artemisia princeps Pampanini (AP) was purchased from a local supplier (MANI F&B, Incheon, South Korea). A dried voucher specimen was deposited in the Herbarium of the Graduate School of East-West Medical Science (KHUAP-01). The raw plant material was submerged in 80% ethanol in a 1:20 mass ratio in a flask heated to 60℃ and sonicated (3210; Branson, Wilmington, USA) at 60 kHz for 1 hour; precipitate was isolated by vacuum filtration. This process was repeated for two more rounds, after which the precipitate was suspended in distilled water at a 1:20 ratio in an Erlenmeyer flask heated to 60℃ and sonicated at 60kHz for 1 hour. The collected extract was concentrated by evaporator (N-1N Rotatory evaporator; EYELY Sunil, Seoul, South Korea), freeze dried (FD8512; Ilshin, Seoul, South Korea), and stored at 4℃.
High-pressure liquid chromatography analysis
High-pressure liquid chromatography (HPLC) analysis was carried out on a Waters system (Milford, MA, USA) consisting of a separation module (e2695) with integrated column heater, as well as an autosampler and photodiode array detector (2998). UV absorbance was monitored from 200 to 400 nm; peak areas were integrated at 370 nm. Injection volume was 10 µL. A 250×4.6 mm column (YMC-Triart C18; YMC Co. Ltd., Kyoto, Japan) with particle size 5 µm was installed in a column oven and maintained at 40℃. The mobile phase consisted of water containing 1% acetic acid (solvent A) and acetonitrile (solvent B). The gradient was 0.0 min, 20% B; 20.0 min, 40% B; 35.0 min, 45% B; flow rate was maintained at 1.0 mL/min. AP extract was dissolved in methanol at a concentration of 10 mg/mL prior to analysis.
Animal models and experimental diet
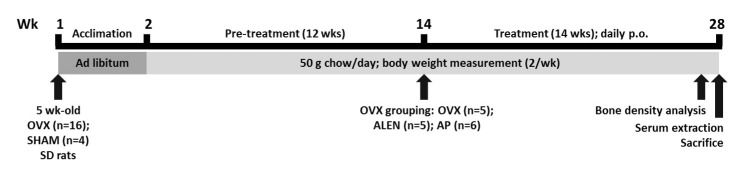
All experimental protocols were approved by the Animal Care and Use Review Committee (KHUASP (SU) 13-01) of Kyung Hee University. Sixteen ovariectomized and four sham female Sprague-Dawley rats of 5 weeks old were purchased from SLC (Shizuoka, Japan). All rats had undergone anesthesia (3% isoflurane dissolved in oxygen), abdominal incision, and suturing, while only the ovariectomized animals received tubal ligation and ovary excision. Rats were individually caged in a temperature- (22±2℃) and humidity- (55±5%) controlled environment with a 12-h light/dark cycle. They had ad libitum access to water and pellet chow for the duration of the experiment. The body mass of all animals was measured twice a week throughout the experiment. Starting at week 14, ovariectomized animals were randomized into three groups (OVX, ALEN, and AP) in addition to sham. SHAM (n=4) and OVX (n=5) groups received daily administrations of 0.1 mL/kg distilled water by oral gavage. ALEN (n=5) and AP (n=6) rats received daily gavage administrations of 10 mg/kg sodium alendronate (commonly used osteoporosis medication positive control) and 300 mg/kg AP extract, respectively. All treatments continued for 14 weeks. One rat in the ALEN group died during pre-imaging anesthesia at the end of the experiment; its histomorphometric data were thus incomplete and omitted from all final analyses. At the beginning of week 28, following 12 hours of overnight fasting, the rats were anesthetized with intraperitoneally administered zolazepan/tiletamine (Zoletil 50, Virbac) (0.1 mL/100 g) and xylazine (Rompun, Bayer) (0.03 mL/100 g) as well as inhalational ethyl ether (99.5%) and sacrificed by fast decapitation (Fig. 1).
Fig. 1. Study design. Ovariectomized (n=16) and sham (n=4) rats (SLC, Inc., Shizuoka, Japan) were fed pellet chow ab libitum for one week after arrival to habituate to laboratory conditions. Starting the second week, rats were fed 50 g of pellet chow per day until the end of the experiment, chow consumption was measured daily, and body weights of a sample of 2-3 rats per group was measured twice per week. At the fourteenth week, ovariectomized rats were randomly sorted into three groups of differing drug treatments: distilled water (OVX; n=5), 10 mg/kg/d alendronate sodium (ALEN, n=5), or 300 mg/kg/d Artemisia princeps Pampanini ethanol extract (AP, n=6). All drugs were administered daily by oral gavage at a volume of approximately 0.1 mL/kg until the end of the experiment. On the second to last day, rats were anesthetized, and cortical and trabecular bone volume fraction and mineral density of the left tibia was scanned. On the last day, rats were anesthetized; after serum extraction by cardiac puncture (results not shown), the rats were sacrificed by fast decapitation and whole brains extracted for electrophysiological analysis.
Morphological analysis
One day prior to sacrifice, the left tibia of all sham rats and three randomly selected animals per experimental group were scanned by micro-computed tomographic analysis (µCT). Rats were anesthetized with zolazepan/tiletamine (Zoletil 50, Virbac) (0.1 cc/100 g) and xylazine (Rompun, Bayer) (0.03 mL/100 g) administered intraperitoneally in saline at a volume of 0.1 mL/kg immediately before scanning. Cortical and trabecular microstructure were scanned at 50 kV, 200 µA, at a rotation step of 0.4°. NRecon cone-beam algorithm software (SkyScan 1076, Kontich, Belgium) was used for image preprocessing; processed data were imported into CTan software (SkyScan) for image generation and analysis. Bone volume (BV) as a fraction of total tissue volume and bone mineral density (BMD) of the cortical and trabecular bones were measured to confirm the pathological effect of ovariectomy on bone integrity. Additional measurements were taken for use in another experiment, as reported above.
Preparation of hippocampal slices
Following sacrifice, the whole brain of each rat was quickly extracted using a rongeur (Fine Science Tools Inc., CA, USA) and immediately put into ice-cold oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (114 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, pH 7.4). NaOH was used for pH adjustment. Whole-brain slices (300 µm) were cut with a vibratome (MA752, Campden Instruments). The slices were stabilized at room temperature for at least 1 h in the aCSF.
Preparation of acute hippocampal slices on the microelectrode array probes
A single stabilized hippocampal slice was carefully removed from a membrane insert with a needle and then placed on an 8x8 microelectrode array (MEA) of 10 µm-diameter electrodes spaced 100 µm apart (Multi Channel Systems, Reutlingen, Germany) precoated with 0.01% polyethylenimine and connected to a stimulator, amplifier, temperature control unit, and computer for data acquisition. The slice was stabilized in aCSF (in which 114 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, pH 7.4) for 1 hr at 33℃ with 95% O2 and 5% CO2 gas aeration. Extraneous aCSF was then removed using a pipette. The MEA containing the hippocampal slice was transferred to an MEA1060 amplifier interface. The solution in the array was grounded using an Ag/AgCl pellet. Data were sampled from every channel at 25 kHz speed and recorded using Recorder-Rack software (MEA systems, MCS software). The stimulating channel was disconnected from the sampling device during stimulation.
Induction of LTD for hippocampal slice electrophysiology
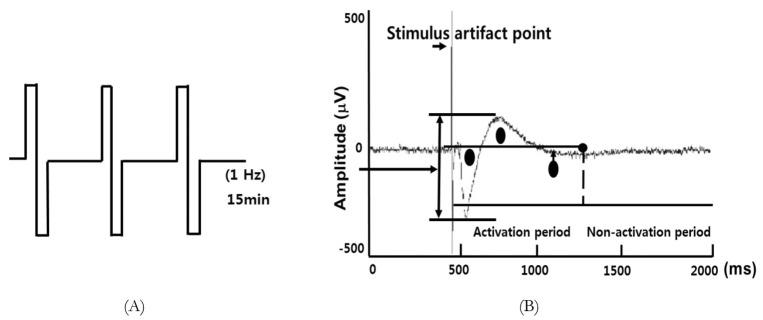
Single slices from multiple animals (n=3~4) were used for each experimental group. Bipolar electrical stimulation was applied to the CA2 stratum radiatum region to stimulate the Schaffer collateral (SC) and commissural pathways. The intensity of bipolar test pulse (or baseline) stimulation was set at 100 mA; this value was optimized to provide 40~65% of the maximum tissue response and delivered once every 60 seconds. Baseline responses were evoked for at least 30 min, of which the last 10 minutes were recorded, before the low-frequency conditioning stimulation (1Hz for 15 minutes; 900 total pulses; Fig. 2A) was applied to induce LTD. After the conditioning stimulation, field excitatory postsynaptic potentials (fEPSPs) were recorded every 60 sec for another 75 min from 59 microelectrodes spanning the hippocampus. During experiments, the slices were continuously perfused with fresh aCSF solution (bubbled with 95% O2, 5% CO2) at the rate of 3 mL/min.
Fig. 2. Long-term depression (LTD) induction and activity calculation in rat hippocampal slices. (A) Low-frequency stimulation (1 Hz, 15 min) was applied to induce LTD in hippocampal slices from sacrificed rats. (B) Activity was calculated as the area under the field potential trajectory during the activation period shown. Regions included in the calculations are represented by circles. Stimulus artifact points (indicated by the labeled arrow) were omitted from the calculations.
Electrophysiology data processing
MC_Rack (v.3.2.1.0, Multi Channel Systems) and a custom program (Dr. Tae-Sung Kim, department of medical-engineering Kyung-hee university) written in MatLab (v.7.0.1, The Mathworks inc.) was used to analyze the data. This program integrated the field potential trajectory, minus artifact (Fig. 2B).
Statistical analysis
Results are expressed as means±standard error (S.E.). Between-group differences were calculated for two groups by independent student's t-test given that all independent variable groups showed no difference from normality as indicated by a nonsignificant Shapiro-Wilk test result, as well as equality of variances as indicated by Levene's test. Differences among three or more groups were calculated with univariate analysis of variance or repeated measures univariate analysis of variance using the Huynh-Feldt correction for deviations from sphericity [35] followed by Scheffe's S post-hoc analysis given that all independent variable groups demonstrated normality as indicated by a nonsignificant Shapiro-Wilk test result and homogeneity as demonstrated by Levene's test; it was decided ahead of time that normal non-homogeneous samples would be compared with Tumhane's T2 post-hoc analysis. Non-parametric Mann-Whitney and Kruskal-Wallis tests were used to evaluate group differences for two or more, respectively, distributions violating the assumption of normalcy as demonstrated by a significant Shapiro-Wilk result. Alpha was set at p=0.05. Parametric analyses were performed in SPSS (Version 20.0, SPSS Inc, Chicago, USA). Non-parametric analyses were performed in SPSS (Version 20.0, SPSS Inc, Chicago, USA) and non-parametric post-hoc tests in R (R Project for Statistical Computing, v. 3.0.2).
RESULTS
High-pressure liquid chromatography results
HPLC analysis confirmed that the AP contained eupatilin (0.0463±0.006 µg/mg), the retention time of which is shown in Supplemental.
Effect of ovariectomy on body mass, food intake, and food efficiency ratio
While an equal variances independent samples t-test revealed no significant effect of ovariectomy (n=16) on pre-surgery body mass (152.75±0.94 g versus sham 152.75±1.44 g; t(20)=0.000, p=1.000), ovariectomized rats showed significantly higher body masses versus sham by Week 13 (354.74±7.10 versus 275.65±9.73 g; t(20)=5.217, p<001). Similarly, pretreatment weight gain from weeks 1 to 14 was significantly higher in all ovariectomized than sham rats (201.99±28.26 g versus sham 122.90±8.73 g; t(20)=5.286, p<0.01). Mann-Whitney tests revealed a continued significant effect of ovariectomy on body weight at Week 28 (151.85±6.85 g versus sham 92.48±5.98 g; two-tailed p<0.01) but no significant effect of ovariectomy on weight gain from Weeks 14 to 28 (11.28±6.18 g versus sham 21.31±3.58 g; p=0.134).
Mann-Whitney tests revealed significantly higher daily dietary intakes from Weeks 1-13 for as-yet untreated ovariectomized (n=6; 301.87±12.58 g/d) relative to sham (n=2; 230.52±5.43 g/d) rats (U=0.000, two-tailed p<0.05).
Effect of treatment on body mass, food intake, and food efficiency ratio
Sham and water-treated, ALEN -treated, and AP-treated ovariectomy groups showed no between-group differences in body mass before surgery (Levene's test F3,16=0.495 p=0.691; univariate analysis of variance F3,16=0.151, p=0.927), but univariate analysis showed a significant effect of treatment on body mass by Week 13 (Levene's test F3,16=0.344, p=0.794; F3,16=8.260, p<0.01). Post-hoc analysis with Scheffe's S procedure revealed significantly higher mean mass for ovariectomized controls versus sham (79.47±19.16 g, p<0.05), for ovariectomized ALEN treatment group versus sham (83.47±19.16, p<0.05), and for ovariectomized AP treatment group versus sham (75.12±18.43, p<0.01) but no between-group difference for the two ovariectomized treatment groups versus ovariectomized controls (Scheffe's S p>0.05 for ALEN - and AP-treated rats versus ovariectomized controls).
A similar pattern was seen after treatment at Week 28, as Kruskal-Wallis analysis showed a significant effect of treatment group on body weight (χ2(3)=9.183, p<0.05). Pairwise post-hoc analysis with a multiple comparisons Kruskal test with Bonferonni correction showed higher body weight than sham (286.93±9.21 g) for ovariectomized rats treated with water (375.28±9.44 g; mean rank difference 10.20, p<0.05), ALEN (371.44±12.15 g; mean rank difference 9.60, p<0.05), and AP (375.77±12.54 g; mean rank difference 10.17, p<0.05), but no evident differences among the three ovariectomized treatment groups. Kruskal-Wallis analysis on weight gain from weeks 14 to 28 did not show a significant effect of treatment group (p=0.243).
Univariate ANOVA did not show a significant effect of treatment group on dietary intakes from weeks 14 to 28 (F3,7=0.348, p=0.792).
The mean values of body mass before surgery and at the beginning of weeks 2, 13, and 28, as well as changes in body weight and average dietary intakes during the pretreatment period (weeks 2 to 13) and treatment period (14 to 28) are shown in Table 1.
Table 1. Effects of ovariectomy and treatment on body weight, weight gain, and dietary intake.
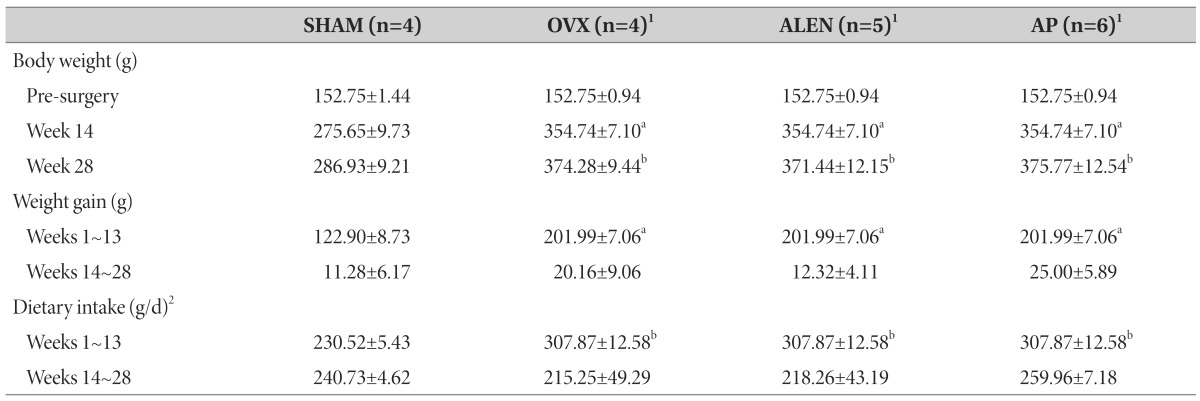
Sham-operated rats treated with distilled water (SHAM) and ovariectomized rats treated with distilled water (OVX), 10 mg/kg/d sodium alendronate (ALEN), or 300 mg/kg/d Artemisia princeps ethanol extract (AP) daily by oral gavage (0.1 mL/kg) for 15 weeks starting at Week 14. Data expressed as group mean±SEM. aEqual variances independent samples t-test versus sham p<0.05. bMann-Whitney mean rank test versus sham p<0.05. 1Pre-Week-13 figures reported as untreated ovariectomized rat group (n=16). 2Figures calculated from a subset of sham (n=2), ovariectomy controls (n=2), alendronate (n=2), and AP rats (n=2).
Effect of ovariectomy and treatment on trabecular and cortical bone histomorphometry
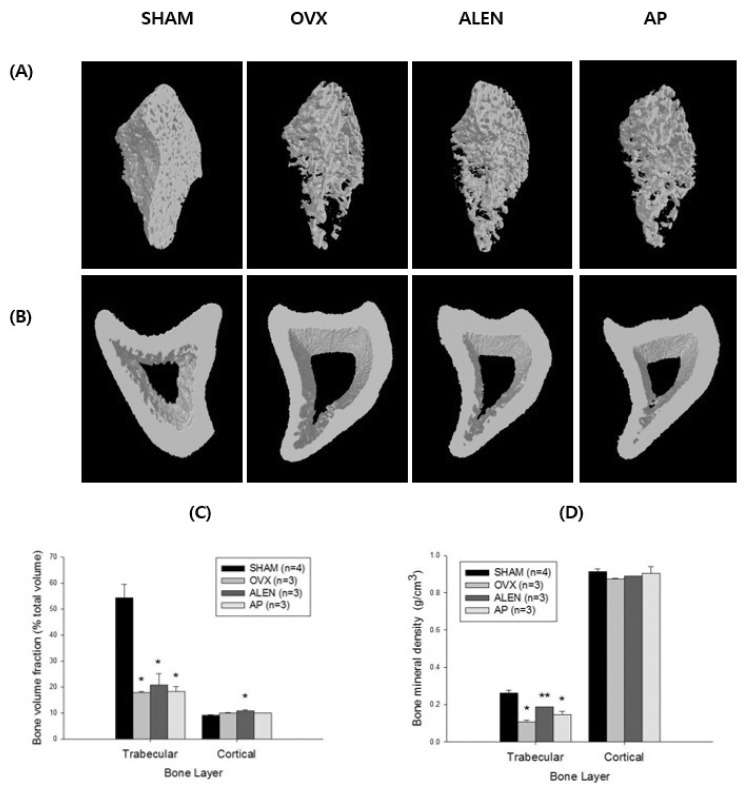
Univariate ANOVA on sham (n=4) and water-treated (n=3), ALEN-treated (n=3), and AP-treated (n=3) ovariectomy groups showed a significant effect of treatment on trabecular bone volume fraction (F3,9=22.186, p<0.001); Scheffe's S post-hoc analysis revealed significantly lower trabecular bone volume fraction than sham in ovariectomized rats treated with water (17.93±0.47% versus 54.40±5.26%; mean difference -36.47%, p<0.01), ALEN (20.81±4.41%; mean difference -33.59%, p<0.01), and AP (18.38±1.84%; mean difference -36.02%, p<0.01) but no differences among the three treatment groups. Kruskal-Wallis test showed a significant effect of treatment on trabecular bone mineral density (χ2(3)=10.840, p<0.05). Pairwise post-hoc analysis with Bonferroni correction for multiple comparisons [36] revealed significantly reduced trabecular bone mineral density in ovariectomized (0.11±0.0088 g/cm3; mean rank difference 9.0, p<0.001) and AP-treated groups (0.15±1.85 g/cm3; mean rank difference 7.0, p<0.001) relative to sham (0.26±0.028 g/cm3) but only a marginally significant difference between sham and the ALEN -treated ovariectomized rat values (0.19±0.0058 g/cm3; mean rank difference 3.5, p=0.06). In addition, ALEN -treated ovarictomized rats exhibited a significantly higher trabecular bone mineral density than water-treated ovariectomized controls (mean difference 5.5 g/cm3, p<0.01).
Univariate ANOVA also showed a significant effect of treatment on cortical bone volume (F3,9=5.659, p<0.05); Scheffe's S posthoc analysis revealed significantly higher cortical bone volume fraction in ovariectomized rats treated with ALEN than in sham (10.77±0.40% versus 9.11±0.32%; mean difference 1.66%, p<0.05) but no other between-group differences. Kruskal-Wallis test did not show a significant effect of treatment on cortical bone mean density (χ2(3)=4.193, p=0.241). See Fig. 3 for a summary of treatment effects on bone histomorphometric parameters.
Fig. 3. Bone histomorphological parameters at Week 28. Histomorphological parameters for left tibia of 33-week-old sham-operated rats treated with distilled water (SHAM, n=4) and ovariectomized rats treated with distilled water (OVX, n=3), 10 mg/kg/d sodium alendronate (ALEN, n=3), or 300 mg/kg/d Artemisia princeps Pampanini ethanol extract (AP, n=3) daily by oral gavage (0.1 mL/kg) for 15 weeks following a 13-week pretreatment period. (A) Trabecular bone scans at 28 weeks. Note how the bone, which appears much less dense in the OVX and AP than SHAM groups, appears to make a partial recovery in the ALEN group. (B) Cortical bone scans at 28 weeks. Note the absence of visible recovery in all ovariectomized groups. (C) Trabecular and cortical bone volume as a percent of total tissue volume by group. Univariate ANOVA showed a significant effect of treatment for trabecular bone volume (F=22.186, p<0.001) with lower values for all ovariectomized groups relative to sham controls. No differences between sham and ovariectomized controls were found for cortical bone volume, though the alendronate-treated group did show significantly higher values than sham (effect of treatment F3,9=5.659, p<0.05; alendronate versus sham mean difference 1.66%, p<0.05). (D) Trabecular and cortical bone mineral density by group. Like bone volume, trabecular bone density also decreased significantly in all ovariectomized groups relative to sham (Kruskal-Wallis χ2(3)=10.840, p<0.05), while no significant differences were seen in cortical bone density. Partial recovery in trabecular bone density relative to OVX controls was seen in the ALEN group. Values represent means±SEM. *Scheffe's S or Kruskal-Wallis pairwise post-hoc versus SHAM p<0.05. **Kruskal-Wallis pairwise post-hoc versus OVX p<0.05 and versus SHAM p<0.1.
Effect of treatment on pre-stimulation EPSP activation values
Univariate ANOVA did not show a significant effect of treatment on pre-stimulation EPSP total activation averages in acute hippocampal slices from ovariectomized rats treated with water (n=3), ALEN (n=4), or AP (n=3) (F2,7=2.464, p=0.155).
Effect of treatment on acute hippocampal slice LTD induction
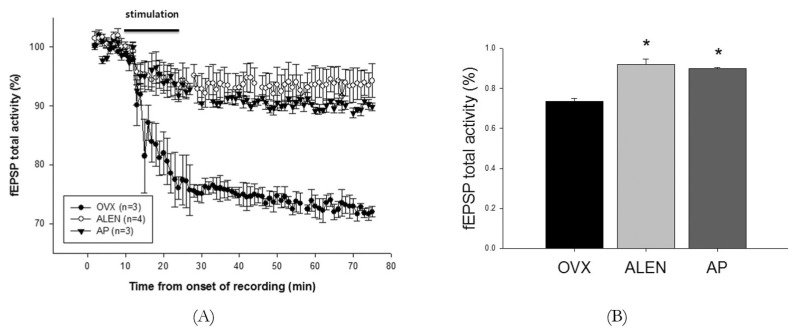
Total activation values from each of the 75 minutes of fEPSP recording from CA1 were averaged across CA1 channels (n=2-4 per slice) and normalized to percent of baseline, which was defined as the average of the first ten minutes of pre-stimulation recording. A repeated measures ANOVA with fixed factor treatment and within-subjects repeated measure time (before, during, and following stimulation) on individual subject averages showed a significant within-subject effect of time (F2,7=90.521, p<0.001), a significant between-subject effect of treatment (F2,7=24.929, p<0.01), and a significant within-subject interaction between time and treatment (F2,7=13.632, p<0.001) on total activation averages. Post-hoc analysis for treatment with Scheffe's S procedure revealed significantly higher values for both ALEN and AP treatment groups relative to water-treated ovariectomized controls (mean difference 10.07%, p<0.01; 8.78%, p<0.01, respectively) but no significant difference of AP from ALEN (mean difference -1.29%, p=0.704). Univariate ANOVA showed no effect of treatment on time-averaged fEPSP total activation percent before stimulation as they were, by definition, normalized to a mean of 100% for each group, but showed a significant effect both during (F2,7=11.241; p<0.01) and after (F2,7=26.797; p=0.001). Scheffe's S post-hoc analysis revealed significantly higher values for ALEN and AP treatment groups relative to water-treated ovariectomized controls during stimulation (94.98±2.19%, mean difference 11.88%, p<0.01; 93.10±0.31%, mean difference 10.00%, p<0.05, respectively; control 83.10±2.00%) and after stimulation (92.00±2.35%, mean difference 18.00%, p<0.01; 90.00±0.58%, mean difference 16.68%, p<0.01, respectively; control 73.67±1.20%) but no significant difference of AP relative to ALEN during either time frame (mean difference during stimulation -1.87%, p=0.780; mean difference after stimulation -2.00%, p=0.761). See Fig. 4 for activation trends across time for each group.
Fig. 4. Integrated activation-period microelectrode array field potential sums following low-frequency stimulation of hippocampal slice cultures. Sum of all integrated activation-period field potentials from three of 59 single microelectrode recordings of hippocampal slice cultures from 33-week-old ovariectomized rats treated with distilled water (OVX, n=3 slices), 10 mg/kg/d sodium alendronate (ALEN, n=3 slices), or 300 mg/kg/d Artemisia princeps ethanol extract (AP, n=3 slices) daily by oral gavage (0.1 mL/kg) for 15 weeks following a 13-week pretreatment period. Recordings followed 15 minutes of low-frequency stimulation (100 mA, 1Hz) to the CA2 stratum radiatum to stimulate LTD-inducing Schaffer collateral signaling. (A) Time course of activity totals. Starting at 10 minutes following stimulation, OVX rats exhibited significantly lower activation than that of ALEN or AP rats, a difference maintained until the end of the experiment. (B) Group averages of normalized total activity for 50 minutes following stimulation. ALEN and AP activation were both significantly higher than that of the OVX group (F2,7=26.797; p=0.001; Scheffe's post hoc S mean difference 11.88%, p<0.01; mean difference 10.00%, p<0.05, respectively) but did not differ significantly from each other. Values represent means±SEM. Stars represent significant between-group differences as calculated by Scheffe's S post hoc analysis with alpha set at p<0.05.
DISCUSSION
The present study assessed the possible effect of Artemisia princeps Pamp ethanol extract on long-term depression at the CA3-CA1 synapse in hippocampal slice cultures from rat models of ovarian hormone deficiency. As Artemisia princeps and its components have demonstrated a number of bioactivities, including insulin regulation [37,38, 39], antioxidant [40], proinflammatory [41] or anti-inflammatory [42, 43], and cytotoxic [44, 45] effects, it was hypothesized that it may also influence hippocampal LTD in a model of ovarian hormone deficiency.
Calculations of body weight and food intake confirmed the presence of physiological abnormalities in the ovariectomized relative to sham group. Though pre-surgery body weights by group showed no significant differences, body weights had increased significantly in ovariectomized relative to sham rats by Week 14, a difference that was maintained to the end of the experiment even though food intake from Weeks 14~28 showed no significant differences among the groups. It is reasonable to attribute this difference at least in part to higher food intakes by ovariectomized relative to sham rats prior to treatment in the first thirteen weeks of analysis. This result mirrors previous observations of ovariectomized rats, in which ovariectomy is associated with not only obesity but also hyperphagic behaviors relative to control [46], a difference that can disappear with time [47].
Similarly, cortical and trabecular bone volume and mean density in the left tibia showed abnormalities in ovariectomized relative to sham rats. Both trabecular bone volume and mean density were shown to have decreased relative to sham in all three ovariectomized groups twenty-eight weeks following ovariectomy, while cortical bone abnormalities were not yet present, a result that also recapitulates previous findings of trabecular bone volume fraction and/or mineral density reductions without statistically significant changes in mineral density or area in ovariectomized relative to sham rats less than eight months following surgery [48, 49, 50], thus validating the establishment of pathology in our ovariectomized rats. Notably, some reversal in trabecular bone loss was apparent in the group treated with alendronate for fifteen weeks prior to measurement, as trabecular bone mineral density was higher in this group than water-treated ovariectomized controls, as well as the AP treatment group. This is a result that would be expected in a model of osteoporosis given that alendronate is a popular therapeutic of this condition.
Against this background of pathology, we found that long-term treatment with Artemisia princeps Pamp extract attenuated long-term depression at the CA3/CA1 synapse relative to that exhibited in vehicle-treated ovariectomized rats. These findings may have implications for the current understanding of the mechanisms behind LTD generation in rodent models of chronic ovarian hormone deficiency and signal the need for further research on possible applications for AP in the management of affective and cognitive symptoms engendered by pathological changes in synaptic plasticity.
Determining the extent to which the LTD observed in the ovariectomized rats of this study represents a pathological departure from the norm is vital to understanding the nature of its alteration in our treated groups. While the induction of LTD in the CA1 pyramidal neurons by the LFP in acute hippocampal slice cultures from adult, but not aged, rats like the ones we used is reportedly unusual [51, 52], it is not unprecedented. The first report of homosynaptic LTD in CA1 induced in vitro was by LFS to adult male slices in 1992 [53], and more cases have been subsequently reported [29], with allegedly greater ease of production in Sprague-Dawley or Wistar than hooded rats [30]. Moreover, both stress [54] and exposure to novelty [55] has been shown to enhance LFS-induced LTD [30], and the bone-scanning protocol to which some rats were exposed one day before euthanasia, as well as the conditions to which all rats were exposed immediately prior to euthanasia, presumably encompassed both features, presenting these factors as a possible confounds among the three tested groups.
Of the few studies done to date inducing homosynaptic LTD in ovariectomized rats [32, 33, 34, 56], only one, to our knowledge, has directly compared ovariectomized rat measures with sham controls [33]. This study demonstrated LTD in CA1 from ovariectomized rats in response to paired-pulse stimulation to the Schaffer collaterals. LTD was, however, stronger rather than attenuated in both sham rats and ovariectomized rats treated with short-term injection of 17β estradiol, a result corroborated by previous research similarly showing enhanced LTD to paired-pulse stimulation in 17β estradiol-treated ovariectomized adult rats [34, 56]. This suggests that at least under some circumstances, LTD stimulation by LFS in adult rats, despite the difficulties inherent in its induction [51, 52], may be considered a feature of normal tissue rather than symptomatic behavior to be treated.
Two other experiments, however, show that the conditions under which LTD in adult ovariectomized rats may be considered normal features of brain tissue may be limited. One reported attenuated homosynaptic LTD in acute slices from 17β estradiol -treated ovariectomized rats relative to vehicle-treated controls [56] or in 17β estradiol-perfused acute hippocampal slices from ovariectomized rats relative to vehicle-perfused controls [32]. Even though these studies did not have sham controls, their results link the estrogen deficiency of ovariectomy with enhanced rather than attenuated LTD, suggesting that their stimulation protocols would potentially result in attenuated LTD in sham relative to ovariectomized rats as well. The differing results of the first study have been attributed to the use of its lower-frequency single-pulse stimulation than in experiments demonstrating estrogenic enhancement in LTD [56], while those of the second by the use of acute estradiol application rather than a longer-term in vivo injection schedule [33]. It is thus possible that our own protocol, which also differs from the conflicting studies in stimulation type (single versus paired-pulse), age of ovariectomy, and other contextual details, may exhibit similar patterns of attenuated rather than enhanced LTD in hormonally normal controls relative to ovariectomized rats, had the electrophysiology experiments included a sham group.
While the etiology and significance of the LTD exhibited by ovariectomized controls in our study are thus admittedly ambiguous, that it was clearly attenuated in rats treated long-term with 300 mg/kg/d Artemisia princeps Pamp ethanol extract suggests bioactivities of this substance on hippocampal plasticity, particularly in inhibiting the mechanisms by which hippocampal long-term depression are effected in an environment bereft of gonadally derived ovarian hormones. It is worthy of note that this effect was exerted in a model of ovarian hormone deficiency; whether it might also appear in sham or naïve controls is important to understanding its possible mechanisms.
The dearth of research on AP's molecular routes of action as well as the complexity of its multi-component makeup make it difficult to pinpoint the mechanisms by which it exhibited the modulatory effects we demonstrated here. Among the most well studied are its antioxidant [40], and anti-inflammatory [42, 43] properties, which present a viable route for LTD attenuation in environments marked not only by ovarian hormone deficiency, relevant here perhaps for its association with oxidative stress [57, 58] and inflammation [59] in rodents as well as a reduction in biomarkers for stress compensation in the primate midbrain [60], but any manipulation that increases cellular stress in the brain. Indeed, while research on the effects of oxidative stress on LTD is sparse, behavioral stress, as mentioned, has been shown to enhance LTD in CA1 [54]; in addition, inflammatory cytokines have also demonstrated supportive or enhancing effects on homosynaptic LTD in CA1. For example, treatment of acute hippocampal slices from adult rats with interferon alpha (IFNα) enhanced glutamate-induced reductions in EPSP amplitude [61]; similarly, application of interleukin-1beta (Il-1β) produced a depression that both blocked and was blocked by LFS, suggesting a shared mechanism of action [62]. Tumor necrosis factor alpha (TNFα) has also been suggested to be necessary for LFS-induced LTD in CA1, as its induction was shown to be impaired in TNF-receptor knockout mice [63]. It is thus possible that AP altered the plasticity patterns demonstrated in ovariectomized controls by reducing the levels of these and other inflammatory cytokines implicated in LTD, as it has been previously reported to do for Il-1β and TNFα in serum [42] and Il-4 and TNFα in epidermal tissue [43], thereby attenuating the influence of these factors on hippocampal plasticity. Eupatilin, one of the active ingredients confirmed by HPLC analysis to be present in the sample used in our study, has similarly demonstrated inhibitory effects on Il-1β, Il-6, and TNFα in lipopolysaccharide-stimulated macrophages [64] and Il-1β and TNFα in a mouse model of inflammatory edema [65] in addition to acutely enhancing CA1 neuron counts following ischemia-reperfusion injury in mice [66], suggesting a possible role of this compound in the attenuation of LTD by chronic AP treatment suggested by our findings.
Unfortunately, the current state of research on AP and its components can lead us only to such unsatisfyingly general explanations of its action as that attempted above, as to present little effort has been made to understand the molecular mechanisms of its putative bioactivities. On the other hand, an examination of alendronate, which has more well studied mechanisms of action and exerted an effect similar to that of AP in our analysis, might provide an alternative direction for an examination of the findings demonstrated by our study. Alendronate is a member of the bisphosphonate family, a group of structurally similar molecules whose most well known function is their therapeutic use in inhibiting excess bone resorption, a process thought to be effected through the chemicals' absorption into bone tissue and chelation with Ca2+ ion, thereby preventing mineral loss to serum [67]. Even though a large body of research has described the various physiological effects of alendronate and other bisphosphonates, we have not seen any describing the influences on hippocampal plasticity that we demonstrated.
Alendronate is thought to be able to pass the blood-brain barrier [68], suggesting that it is at least theoretically capable of exerting direct effects on central nervous system activity. Indeed, daily oral administration of 3 mg/kg alendronate for one week was shown to reduce acetylcholinesterase activity in the prefrontal cortex of rats [68]; the same dosing schedule for nine days also yielded significant reductions in brain cholesterol synthesis rates in hippocampus, frontal lobe, and spinal cord [69]. Acute and subchronic administrations of 10~80 mg/kg alendronate have also demonstrated antinociceptive effects in rat models of visceral (acetic acid) but not inflammatory (formalin) pain [70], though this finding is as parsimoniously explained by possible influences on peripheral tissues as that on the central nervous system. Given some evidence that at least one of the molecular targets of alendronate might be a subset of protein tyrosine phosphatases [71, 72], which as a group have been implicated in neural functions from the management of hippocampal neuron calcium stores [73] to behavioral habituation to new environments [74], its biological influences might be far more diverse than the inhibitory effects on bone resorption for which it is commonly exploited.
To our knowledge, neither AP nor alendronate has demonstrated direct effects on neuroplasticity in any experiments to date. As a result, our finding that long-term administration of both drugs appears to inhibit the induction of long-term depression at the CA3-CA1 synapse justifies the further exploration of their neurological bioactivities, either for their therapeutic benefits or for their possible adverse patient effects.
Long-term oral treatment with Artemisia princeps Pamp ethanol extract attenuates low-frequency-stimulation-induced homosynaptic long-term depression in CA1. These results were similar to those shown for alendronate and justify further focused research on the extent and mechanisms of the possible effects of both substances on hippocampal plasticity and the disorders with which it is associated, especially in the context of ovarian hormone deficiency and menopause.
ACKNOWLEDGEMENTS
This work was supported by a grant from Kyung Hee University (KHU-2012 0575). Additionally, we would like to thank to Wonwoo Lee, a Joongdong high school student, for his assistance of our experiment.
Supplementary Material
High-pressure liquid chromatography analysis of Artemisia princeps Pampanini extract. HPLC chromatogram of eupatilin. (A) Standard reference compound eupatilin at 1 mg/mL MeOH (B) AP at 10 mg/mL MeOH. UV absorbance was monitored from 200 to 400 nm, and the relevant peak (denoted by the triangle and diamond) was integrated at 370 nm. Injection volume was 10 µL; the column (YMC Co. Ltd., Japan) was maintained at 40℃. The mobile phase consisted of 1% acetic acid (solvent A) and acetonitrile (solvent B) in water. The gradient was 0.0 min, 20% B; 20.0 min, 40% B; 35.0 min, 45% B at a flow rate of 1.0 mL/min.
References
- 1.Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C. Gender differences in depression and anxiety: the role of age. Psychiatry Res. 2013;210:1301–1303. doi: 10.1016/j.psychres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Jane Costello E, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? J Child Psychol Psychiatry. 2006;47:1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- 3.Munhoz TN, Santos IS, Matijasevich A. Major depressive episode among Brazilian adults: a cross-sectional population-based study. J Affect Disord. 2013;150:401–407. doi: 10.1016/j.jad.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Xu Y, Nie H, Zhang Y, Wu Y. The prevalence of depressive symptoms among the older in China: a meta-analysis. Int J Geriatr Psychiatry. 2012;27:900–906. doi: 10.1002/gps.2821. [DOI] [PubMed] [Google Scholar]
- 6.Barcelos-Ferreira R, Izbicki R, Steffens DC, Bottino CM. Depressive morbidity and gender in community-dwelling Brazilian elderly: systematic review and meta-analysis. Int Psychogeriatr. 2010;22:712–726. doi: 10.1017/S1041610210000463. [DOI] [PubMed] [Google Scholar]
- 7.Luppa M, Sikorski C, Luck T, Ehreke L, Konnopka A, Wiese B, Weyerer S, König HH, Riedel-Heller SG. Age- and gender-specific prevalence of depression in latest-life--systematic review and meta-analysis. J Affect Disord. 2012;136:212–221. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Seritan AL, Iosif AM, Park JH, DeatherageHand D, Sweet RL, Gold EB. Self-reported anxiety, depressive, and vasomotor symptoms: a study of perimenopausal women presenting to a specialized midlife assessment center. Menopause. 2010;17:410–415. doi: 10.1097/gme.0b013e3181bf5a62. [DOI] [PubMed] [Google Scholar]
- 9.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers M, Randolph JF., Jr Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF, Jr, Matthews KA. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161:2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 13.Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression--a pilot study. Arch Womens Ment Health. 2013;16:499–509. doi: 10.1007/s00737-013-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis. 2012;10:175–178. doi: 10.1159/000334764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joffe H, Petrillo LF, Koukopoulos A, Viguera AC, Hirschberg A, Nonacs R, Somley B, Pasciullo E, White DP, Hall JE, Cohen LS. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96:E1044–E1054. doi: 10.1210/jc.2010-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colangelo LA, Craft LL, Ouyang P, Liu K, Schreiner PJ, Michos ED, Gapstur SM. Association of sex hormones and sex hormone-binding globulin with depressive symptoms in postmenopausal women: the Multiethnic Study of Atherosclerosis. Menopause. 2012;19:877–885. doi: 10.1097/gme.0b013e3182432de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiller CE, O'Hara MW, Rubinow DR, Johnson AK. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–144. doi: 10.1016/j.physbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber JC, Heskamp ML, Schramm GA. Effect of an oral contraceptive with chlormadinone acetate on depressive mood : analysis of data from four observational studies. Clin Drug Investig. 2008;28:783–791. doi: 10.2165/0044011-200828120-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JF, Bienias JL, Shah A, Meeke KA, Schneider JA, Soriano E, Bennett DA. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer's disease: relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008;5:45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 2011;191:148–158. doi: 10.1016/j.neuroscience.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroids maintain 24 h acetylcholine release in the hippocampus: organizational and activational effects in behaving rats. J Neurosci. 2009;29:3808–3815. doi: 10.1523/JNEUROSCI.5301-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubbers LS, Zafian PT, Gautreaux C, Gordon M, Alves SE, Correa L, Lorrain DS, Hickey GJ, Luine V. Estrogen receptor (ER) subtype agonists alter monoamine levels in the female rat brain. J Steroid Biochem Mol Biol. 2010;122:310–317. doi: 10.1016/j.jsbmb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 25.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 26.McClure RE, Barha CK, Galea LA. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2013;63:144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Wainwright SR, Galea LA. The neural plasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast. 2013;2013:805497. doi: 10.1155/2013/805497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 30.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 31.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharrow KM, Kumar A, Foster TC. Calcineurin as a potential contributor in estradiol regulation of hippocampal synaptic function. Neuroscience. 2002;113:89–97. doi: 10.1016/s0306-4522(02)00151-3. [DOI] [PubMed] [Google Scholar]
- 33.Day M, Good M. Ovariectomy-induced disruption of long-term synaptic depression in the hippocampal CA1 region in vivo is attenuated with chronic estrogen replacement. Neurobiol Learn Mem. 2005;83:13–21. doi: 10.1016/j.nlm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Desmond NL, Zhang DX, Levy WB. Estradiol enhances the induction of homosynaptic long-term depression in the CA1 region of the adult, ovariectomized rat. Neurobiol Learn Mem. 2000;73:180–187. doi: 10.1006/nlme.1999.3929. [DOI] [PubMed] [Google Scholar]
- 35.Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Behav Stat. 1976;1:69–82. [Google Scholar]
- 36.de Mendiburu F. Package 'agricolae'. Statistical procedures for agricultural research. [place unknown]: Comprehensive R Archive Network; 2014. [Google Scholar]
- 37.Yamamoto N, Kanemoto Y, Ueda M, Kawasaki K, Fukuda I, Ashida H. Anti-obesity and anti-diabetic effects of ethanol extract of Artemisia princeps in C57BL/6 mice fed a high-fat diet. Food Funct. 2011;2:45–52. doi: 10.1039/c0fo00129e. [DOI] [PubMed] [Google Scholar]
- 38.Jung UJ, Baek NI, Chung HG, Bang MH, Yoo JS, Jeong TS, Lee KT, Kang YJ, Lee MK, Kim HJ, Yeo JY, Choi MS. The anti-diabetic effects of ethanol extract from two variants of Artemisia princeps Pampanini in C57BL/KsJ-db/db mice. Food Chem Toxicol. 2007;45:2022–2029. doi: 10.1016/j.fct.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Kang YJ, Jung UJ, Lee MK, Kim HJ, Jeon SM, Park YB, Chung HG, Baek NI, Lee KT, Jeong TS, Choi MS. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic beta-cell function in type 2 diabetic mice. Diabetes Res Clin Pract. 2008;82:25–32. doi: 10.1016/j.diabres.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Kim MJ, Han JM, Jin YY, Baek NI, Bang MH, Chung HG, Choi MS, Lee KT, Sok DE, Jeong TS. In vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res. 2008;31:429–437. doi: 10.1007/s12272-001-1175-8. [DOI] [PubMed] [Google Scholar]
- 41.Kim TH, Lee SJ, Rim HK, Shin JS, Jung JY, Heo JS, Kim JB, Lee MS, Lee KT. In vitro and in vivo immunostimulatory effects of hot water extracts from the leaves of Artemisia princeps Pampanini cv. Sajabal. J Ethnopharmacol. 2013;149:254–262. doi: 10.1016/j.jep.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Han JM, Kim MJ, Baek SH, An S, Jin YY, Chung HG, Baek NI, Choi MS, Lee KT, Jeong TS. Antiatherosclerotic effects of Artemisia princeps Pampanini cv. Sajabal in LDL receptor deficient mice. J Agric Food Chem. 2009;57:1267–1274. doi: 10.1021/jf802639y. [DOI] [PubMed] [Google Scholar]
- 43.Ryu KR, Choi JY, Chung S, Kim DH. Anti-scratching behavioral effect of the essential oil and phytol isolated from Artemisia princeps Pamp. in mice. Planta Med. 2011;77:22–26. doi: 10.1055/s-0030-1250119. [DOI] [PubMed] [Google Scholar]
- 44.Ju HK, Lee HW, Chung KS, Choi JH, Cho JG, Baek NI, Chung HG, Lee KT. Standardized flavonoid-rich fraction of Artemisia princeps Pampanini cv. Sajabal induces apoptosis via mitochondrial pathway in human cervical cancer HeLa cells. J Ethnopharmacol. 2012;141:460–468. doi: 10.1016/j.jep.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Bang MH, Han MW, Song MC, Cho JG, Chung HG, Jeong TS, Lee KT, Choi MS, Kim SY, Baek NI. A cytotoxic and apoptosis-inducing sesquiterpenoid isolated from the aerial parts of Artemisia princeps PAMPANINI (Sajabalssuk) Chem Pharm Bull (Tokyo) 2008;56:1168–1172. doi: 10.1248/cpb.56.1168. [DOI] [PubMed] [Google Scholar]
- 46.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang JM, Sacco SM, Ward WE. Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr. 2008;138:2106–2110. doi: 10.3945/jn.108.093781. [DOI] [PubMed] [Google Scholar]
- 48.Yano T, Yamada M, Konda T, Shiozaki M, Inoue D. Risedronate improves bone architecture and strength faster than alendronate in ovariectomized rats on a low-calcium diet. J Bone Miner Metab. 2014;32:653–659. doi: 10.1007/s00774-013-0543-9. [DOI] [PubMed] [Google Scholar]
- 49.Yarrow JF, McCoy SC, Ferreira JA, Pingel JE, Conrad BP, Wronski TJ, Williams AA, Borst SE, Brown M. A rehabilitation exercise program induces severe bone mineral deficits in estrogen-deficient rats after extended disuse. Menopause. 2012;19:1267–1276. doi: 10.1097/gme.0b013e318255657f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otomo H, Sakai A, Ikeda S, Tanaka S, Ito M, Phipps RJ, Nakamura T. Regulation of mineral-to-matrix ratio of lumbar trabecular bone in ovariectomized rats treated with risedronate in combination with or without vitamin K2. J Bone Miner Metab. 2004;22:404–414. doi: 10.1007/s00774-004-0502-6. [DOI] [PubMed] [Google Scholar]
- 51.Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012;131:37–51. doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Foy MR. Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol Learn Mem. 2011;95:134–144. doi: 10.1016/j.nlm.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemp A, Manahan-Vaughan D. Hippocampal longterm depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamani MR, Desmond NL, Levy WB. Estradiol modulates long-term synaptic depression in female rat hippocampus. J Neurophysiol. 2000;84:1800–1808. doi: 10.1152/jn.2000.84.4.1800. [DOI] [PubMed] [Google Scholar]
- 57.Ceravolo GS, Filgueira FP, Costa TJ, Lobato NS, Chignalia AZ, Araujo PX, Tostes RC, Dantas AP, Fortes ZB, Carvalho MH. Conjugated equine estrogen treatment corrected the exacerbated aorta oxidative stress in ovariectomized spontaneously hypertensive rats. Steroids. 2013;78:341–346. doi: 10.1016/j.steroids.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues MF, Stotzer US, Domingos MM, Deminice R, Shiguemoto GE, Tomaz LM, Sousa NM, Ferreira FC, Leite RD, Selistre-de-Araújo HS, Jordão-Júnior AA, Baldissera V, Perez SE. Effects of ovariectomy and resistance training on oxidative stress markers in the rat liver. Clinics (Sao Paulo) 2013;68:1247–1254. doi: 10.6061/clinics/2013(09)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwasa T, Matsuzaki T, Kinouchi R, Gereltsetseg G, Murakami M, Munkhzaya M, Altankhuu T, Kuwahara A, Yasui T, Irahara M. Changes in central and peripheral inflammatory responses to lipopolysaccharide in ovariectomized female rats. Cytokine. 2014;65:65–73. doi: 10.1016/j.cyto.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Bethea CL, Reddy AP. The effect of long-term ovariectomy on midbrain stress systems in free ranging macaques. Brain Res. 2012;1488:24–37. doi: 10.1016/j.brainres.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendoza-Fernández V, Andrew RD, Barajas-López C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 2000;885:14–24. doi: 10.1016/s0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- 62.Ikegaya Y, Delcroix I, Iwakura Y, Matsuki N, Nishiyama N. Interleukin-1beta abrogates long-term depression of hippocampal CA1 synaptic transmission. Synapse. 2003;47:54–57. doi: 10.1002/syn.10154. [DOI] [PubMed] [Google Scholar]
- 63.Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 64.Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011;88:1121–1126. doi: 10.1016/j.lfs.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Cai M, Phan PT, Hong JG, Kim DH, Kim JM, Park SJ, Liu X, Han JE, Park H, Choi JW, Ryu JH. The neuroprotective effect of eupatilin against ischemia/reperfusion-induced delayed neuronal damage in mice. Eur J Pharmacol. 2012;689:104–110. doi: 10.1016/j.ejphar.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 67.Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 68.Cibicková L, Palicka V, Cibicek N, Cermáková E, Micuda S, Bartosová L, Jun D. Differential effects of statins and alendronate on cholinesterases in serum and brain of rats. Physiol Res. 2007;56:765–770. doi: 10.33549/physiolres.931121. [DOI] [PubMed] [Google Scholar]
- 69.Cibickova L, Hyspler R, Cibicek N, Cermakova E, Palicka V. Alendronate lowers cholesterol synthesis in the central nervous system of rats - a preliminary study. Physiol Res. 2009;58:455–458. doi: 10.33549/physiolres.931382. [DOI] [PubMed] [Google Scholar]
- 70.Goicoechea C, Porras E, Alfaro MJ, Martín MI. Alendronate induces antinociception in mice, not related with its effects in bone. Jpn J Pharmacol. 1999;79:433–437. doi: 10.1254/jjp.79.433. [DOI] [PubMed] [Google Scholar]
- 71.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 72.Morelli S, Bilbao PS, Katz S, Lezcano V, Roldán E, Boland R, Santillan G. Protein phosphatases: possible bisphosphonate binding sites mediating stimulation of osteoblast proliferation. Arch Biochem Biophys. 2011;507:248–253. doi: 10.1016/j.abb.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Koss DJ, Riedel G, Bence K, Platt B. Store-operated Ca2+ entry in hippocampal neurons: regulation by protein tyrosine phosphatase PTP1B. Cell Calcium. 2013;53:125–138. doi: 10.1016/j.ceca.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Erkens M, Bakker B, van Duijn LM, Hendriks WJ, Van der Zee CE. Protein tyrosine phosphatase receptor type R deficient mice exhibit increased exploration in a new environment and impaired novel object recognition memory. Behav Brain Res. 2014;265:111–120. doi: 10.1016/j.bbr.2014.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-pressure liquid chromatography analysis of Artemisia princeps Pampanini extract. HPLC chromatogram of eupatilin. (A) Standard reference compound eupatilin at 1 mg/mL MeOH (B) AP at 10 mg/mL MeOH. UV absorbance was monitored from 200 to 400 nm, and the relevant peak (denoted by the triangle and diamond) was integrated at 370 nm. Injection volume was 10 µL; the column (YMC Co. Ltd., Japan) was maintained at 40℃. The mobile phase consisted of 1% acetic acid (solvent A) and acetonitrile (solvent B) in water. The gradient was 0.0 min, 20% B; 20.0 min, 40% B; 35.0 min, 45% B at a flow rate of 1.0 mL/min.