Abstract
The dopaminergic system is involved in the regulation of food intake, which is crucial for the maintenance of body weight. We examined the relationship between striatal dopamine (DA) D2/3 receptor availability and body mass index (BMI) in 25 non-obese healthy male subjects using [11C]raclopride and positron emission tomography. None of [11C]raclopride binding potential (BP) values (measures of DA D2/3 receptor availability) in striatal subregions (dorsal caudate, dorsal putamen, and ventral striatum) in the left and right hemispheres was significantly correlated with BMI. However, there was a positive correlation between the right-left asymmetry index of [11C]raclopride BP in the dorsal putamen and BMI (r=0.43, p<0.05), suggesting that greater BMI is linked with higher receptor availability in the right dorsal putamen relative to the left in non-obese individuals. The present results, combined with previous findings, may also suggest neurochemical mechanisms underlying the regulation of food intake in non-obese individuals.
Keywords: Dopamine, striatum, body mass index, asymmetry
INTRODUCTION
Food intake is firmly related with individual body type (i.e., lean vs. obese) and is supposed to be regulated by feeling of hunger to maintain the natural state of homeostasis. The hypothalamus has been thought as a core brain structure for controlling the food consumption [1]. However, when sufficient food is available, eating behavior is mainly provoked by the reward value of food such as taste or quality [2], and abnormal eating behavior seems to be more related with the common reward pathway which is modulated by dopamine (DA) [3].
Gaining weight is one of the consequences of deficit in dopaminergic modulation, as evidenced by an association of depressive symptoms and body mass index (BMI) [4] and increase in body weight after deep brain stimulation [5] and dopaminergic medications [6] in patients with Parkinson's disease. Decreased striatal DA D2/3 receptor availability has been shown in obese subjects, which was correlated inversely with BMI [7]. These data suggest involvement of dopaminergic deficit in pathological eating behavior and obesity.
Anatomical, functional, and metabolic asymmetries between the hemispheres in the healthy brain have been widely accepted [8,9]. More recently, there have been growing interests in the neurochemical asymmetry and its associations with neuropsychiatric conditions such as stress [10] and cognitive decline [11] have been reported. Although some studies suggested a link between dopaminergic function and BMI in pathological eating behavior and obesity [12,13], how the dopaminergic system is related with the individual difference of BMI in non-obese subjects is largely unknown. Moreover, few study sought to test a possible association between dopaminergic asymmetry and BMI.
This study aimed at determining the relationship of DA D2/3 receptor availability in striatal subregions and its asymmetry with BMI in non-obese subjects using [11C]raclopride, a DA D2/3 receptor radioligand, and positron emission tomography (PET).
MATERIALS AND METHODS
Subjects
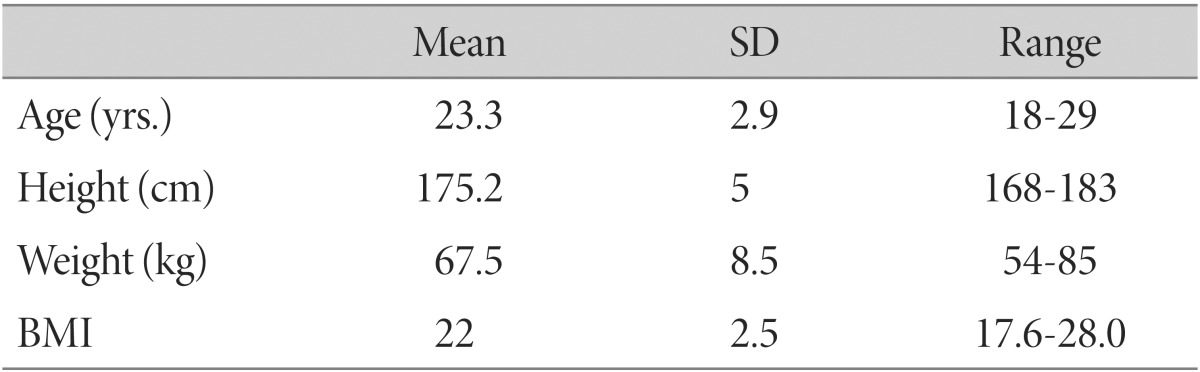
Non-obese healthy males were recruited by advertisement. We excluded individuals with a history of neurological or psychiatric disorders such as epilepsy, head injury, and depression. BMI, calculated as weight (kg)/height2 (m2), was acquired during the recruitment procedures, and obese individuals, defined as BMI>30 kg/m2, were excluded. Twenty-five non-obese healthy male subjects (mean (±SD) age 23.3±2.9 y [18-29 y]; mean BMI 22.0±2.5 [17.6-28.0]; mean body weight 67.5±8.5 kg [54.0-85.0 kg]) participated in the study after having given written informed consent (Table 1). All subjects were right-handed. Five subjects were smokers, who were asked not to change their smoking habits prior to the scan.
Table 1. Subject demographics.
PET scan
PET scans were acquired using a Siemens ECAT EXACT 47 PET scanner (CTI/Siemens, Knoxville, TN, USA) in 15 subjects or a GE Advance PET scanner (GE Medical Systems, Waukesha, WI, USA) in 10 subjects. Image acquisition protocols were the same for the two scanners and images were reconstructed using parameters recommended by the manufacturer of each scanner. We analyzed the images of all subjects as a one pool. After a 10-min transmission scan, [11C]raclopride was delivered in a 48-ml syringe (average activity 29.3±16.8 mCi) and administered by a computer-operated pump with a fixed time schedule: at time 0, a bolus dose of 21 ml was given over 1 min and then the rate of infusion was decreased to 0.20 ml/min and was maintained for the remaining time. The bolus to infusion rate ratio (Kbol) was 105 min. This protocol was selected based on the optimization procedure developed by Watanabe and coworkers, which was known to be optimal in establishing equilibrium state in approximately 30 min after initiation of the radioligand injection [14].
Emission data were collected in the three-dimensional mode for 120 min as 30 successive image frames of increasing duration (3×20 s, 2×1 min, 2×2 min, 1×3 min, and 22×5 min) were recorded. PET images obtained using the Siemens ECAT EXACT 47 PET scanner were reconstructed using a Shepp-Logan filter (cut-off frequency=0.35 mm) and displayed in a 128×128 matrix (pixel size=2.1×2.1 mm with a slice thickness of 3.4 mm). Images from the GE Advance PET scanner were reconstructed in a 128×128 matrix (pixel size=1.95×1.95 mm with a slice thickness of 4.25 mm) using a Hanning filter (cut-off frequency=4.5 mm).
Image analysis
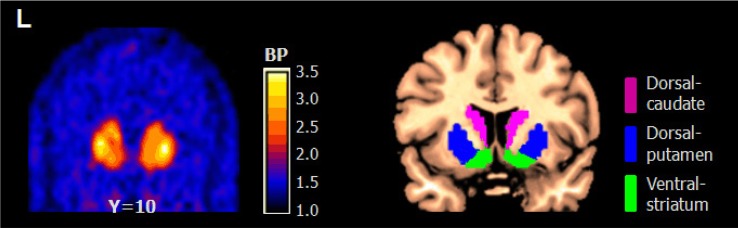
The resting state DA D2/3 receptor availability was assessed using PET images of 30-50 min after [11C]raclopride injection, during which the binding of the radioligand attained equilibrium. Four PET frames during this period were realigned and summed for coregistration with individual MR images and transformation into standardized stereotaxic space by means of automated feature-matching to the MNI template. [11C]Raclopride binding potential (BP) as a measure of DA D2/3 receptor availability was calculated in a voxel-wise manner to generate parametric BP images, using the cerebellum as the reference region, as (Cvoxel-Ccb)/Ccb [15], where Cvoxel is the activity in each voxel and Ccb is the mean activity in the cerebellum. Regions of interests (ROIs) were drawn manually on coronal slices of high-resolution brain MR image (Colin brain) on the left and right striatal subregions (dorsal putamen, dorsal caudate, and ventral striatum). The boundaries of ROIs were delineated according to a previously developed method [16]. Using these ROIs, BP values in striatal subregions were extracted from individual BP images (Fig. 1). Also, the asymmetry index of BP (AIBP) was calculated as (right-left)/(right+left) for each striatal subregion, so that a positive value indicates higher AIBP in the right side relative to the left. The relationships of [11C] raclopride BP and AIBP with BMI were tested using the two-tailed Pearson correlation with SPSS 16.0 (Chicago, Illinois).
Fig. 1. Example of parametric [11C] raclopride BP image in one subject (Left; transformed in to MNI standard space) and map of predefined ROI for striatum (Right).
RESULTS
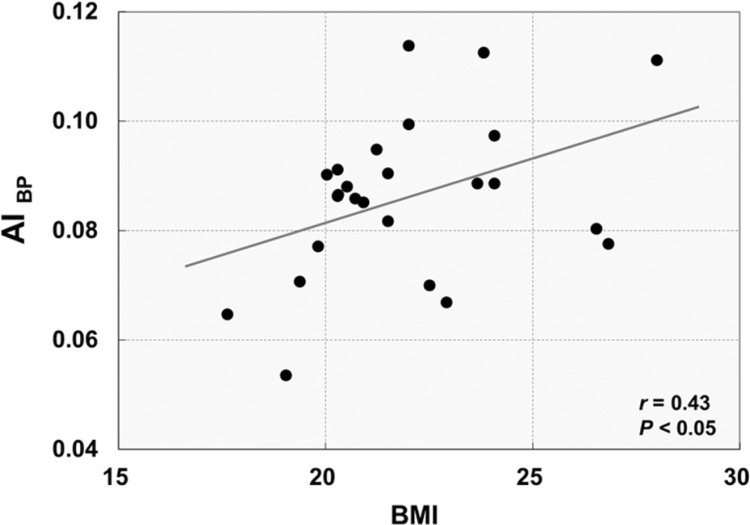
[11C]Raclopride BP in either of the six striatal subregions had no significant correlation with BMI (r=-0.25, p=0.23 in the left dorsal putamen; r=-0.14, p=0.52 in the right dorsal putamen; r=-0.22, p=0.30 in the left dorsal caudate; r=-0.18, p=0.40 in the right dorsal caudate; r=-0.18, p=0.40 in the left ventral striatum; r=-0.19, p=0.36 in the right ventral striatum). However, there was a significant positive correlation between the AIBP in the dorsal putamen and BMI (r=0.43, p<0.05) (Fig. 2), suggesting that greater BMI is associated with higher D2/3 receptor availability in the right dorsal putamen relative to the left. The AIBP in either dorsal caudate and ventral striatum had no significant correlation with BMI (r=0.01, p=0.98 in the dorsal caudate; r=-0.13, p=0.53 in the ventral striatum).
Fig. 2. Relationship between the AIBP and BMI in the dorsal putamen. The asymmetry index of BP (AIBP) was calculated as (right-left)/(right+left), so that a positive value indicates higher AIBP in the right side relative to the left (r=0.43, p<0.05; two-tailed Pearson correlation).
DISCUSSION
In the present study, we examined the relationship of DA D2/3 receptor availability in striatal subregions and its asymmetry with BMI in non-obese healthy male subjects using [11C]raclopride PET. There was no direct relationship between striatal D2/3 receptor availability and BMI in our non-obese subjects. This is consistent with the report by Wang et al. [7] using [11C]raclopride PET. Although they found an inverse correlation between striatal D2 receptor availability and BMI in obese individuals, no such a correlation was observed in non-obese controls. However, we found an association of BMI with the right-left asymmetry in D2/3 receptor availability in the dorsal putamen in non-obese subjects.
As a part of the habit learning and reward system, striatum is a core structure of the dopaminergic neuronal circuitry that mediates the reinforcing effect of food and other rewards, including drugs abused by humans. Functional differences between dorsal and ventral striatum in the food motivation were reported. The action of dorsal striatum was more essential for the feeding behavior itself and its pleasantness [13], while ventral striatum was more sensitive for the food cues and expectation level of given food stimulation [17]. Also, studies in mice [12] as well as humans [18] suggested differential roles of DA in dorsal and ventral striatum in regulating food intake. The notion was that DA in dorsal striatum is implicated in maintaining caloric requirements for survival, while DA in ventral striatum is involved in the rewarding properties of food. This may be linked, directly or indirectly, with the association between BMI and the asymmetry in D2/3 receptor availability in the dorsal putamen in our non-obese subjects, in that food intake in normal weight individuals is likely controlled by caloric requirements, not by the reinforcing property of food.
Much evidence suggests that the human brain is anatomically and functionally lateralized. While asymmetries in DA and other neurotransmitters have been reported in postmortem human brain [19], molecular and functional imaging techniques revealed evidence for neurochemical asymmetries in living human brain, providing more opportunities to directly examine the relationship between the brain laterality and human behavior and function. PET and SPECT (single-photon emission computed tomography) studies in healthy subjects have shown hemispheric asymmetries in dopaminergic markers in the striatum, including DA D2/3 receptor availability [20], DA transporter density [21], and DA synthesis capacity [22]. Although these studies reported a population bias towards higher values of radioligand binding in the right as compared to left striatum based on group averages, there were considerable individual differences, not only in the magnitude, but also in the direction of asymmetry. In animals, individual differences in dopaminergic asymmetry have been shown to covary with, or predict, individual differences in spatial behavior and stress reactivity, as well as susceptibility to stress pathology and drug sensitivity [23]. In humans, associations between cognitive functions and the pattern of asymmetry in DA D2/3 receptor availability have been reported [24]. Our findings reveal a significant association between BMI and the direction and magnitude of asymmetry in striatal D2/3 receptor availability in non-obese subjects.
In our non-obese subjects, greater BMI was linked with higher D2/3 receptor availability in the right dorsal putamen relative to the left. This is in contrast to a previous study showing that greater positive incentive motivation was associated with higher D2/3 receptor availability in the left relative to the right putamen [24]. The opposite direction of asymmetry may hint at different neurochemical mechanisms underlying the regulation of food intake between obese and non-obese individuals.
Our study has several limitations. First, three of our subjects had BMI higher than 25 their BMIs can be classified into overweight (23.0-24.9) or obesity (≥25.0) groups according to Asian criteria. However, our subject group is composed of fit healthy young adults and taking into account that BMI is related not only to fat free mass but to a lesser extent, also to body build, we classified those subjects as non-obese overweight subjects following the opinion of WHO Expert Consultation [25] that suggested retaining the current international classifications for obesity (≥30.0). To exclude the possible effect of including borderline over weight subjects in our current study, we re-tested our statistical analysis with 22 subjects after excluding those three subjects. The results exhibited a higher correlation than the analysis done with 25 subjects and showed also an increased significance level (r=0.55, p=0.008). Second, since [11C]raclopride binding is sensitive to competition with endogenous DA, it is difficult to determine whether the asymmetry of DA D2/3 receptor availability represents that of receptor density or that of levels of endogenous DA. DA D2/3 binding as measured by [11C]raclopride is heterogeneous within the striatal regions with higher binding in the dorsal striatum than in the ventral striatum [26]. Thus, [11C]raclopride PET might not have a sensitivity good enough for detecting subtle interindividual and interregional differences in D2/3 receptor availability in the ventral striatum. Further studies are needed to explore the dopaminergic system in limbic striatal and extrastriatal regions using radioligands that have higher affinity and selectivity for DA D3 receptors. Finally, relatively small sample which consisted only of males, limiting the generalizability of our findings.
In conclusion, the present results suggest an association between BMI and the pattern of asymmetry in DA D2/3 receptor availability in the dorsal putamen in non-obese individuals, such that greater BMI is associated with higher receptor availability in the right dorsal putamen relative to the left. Indeed, the information that is related with neurochemical lateralization of DA not only gives a hint in predicting the clinical course of obesity onset or development of food intake related disease such as anorexia nervosa and bulimia nervosa more importantly, it would work as a biomarker to predict treatment prognosis in those disease. Our results, combined with previous findings, may also suggest neurochemical mechanisms underlying the regulation of food intake in non-obese individuals. These may have important implications for understanding and predicting individual differences in responding to food related rewards and the development of "obesity" from "non-obese state."
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF-2009-0078370, NRF-2006-2005087) funded by the Ministry of Science, ICT and Future Planning of Republic of Korea and a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI09C1444/HI14C1072). This study was also supported by a grant from the Seoul National University Bundang Hospital Research Fund (02-2012-047).
Footnotes
We state that there is no conflict of interest for this article.
References
- 1.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffery RW, Linde JA, Simon GE, Ludman EJ, Rohde P, Ichikawa LE, Finch EA. Reported food choices in older women in relation to body mass index and depressive symptoms. Appetite. 2009;52:238–240. doi: 10.1016/j.appet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barichella M, Marczewska AM, Mariani C, Landi A, Vairo A, Pezzoli G. Body weight gain rate in patients with Parkinson's disease and deep brain stimulation. Mov Disord. 2003;18:1337–1340. doi: 10.1002/mds.10543. [DOI] [PubMed] [Google Scholar]
- 6.Kumru H, Santamaria J, Valldeoriola F, Marti MJ, Tolosa E. Increase in body weight after pramipexole treatment in Parkinson's disease. Mov Disord. 2006;21:1972–1974. doi: 10.1002/mds.21086. [DOI] [PubMed] [Google Scholar]
- 7.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Dupont P, Baete K, Van Paesschen W, Van Laere K, Nuyts J. Detection of inter-hemispheric metabolic asymmetries in FDG-PET images using prior anatomical information. Neuroimage. 2009;44:35–42. doi: 10.1016/j.neuroimage.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Pujol J, López-Sala A, Deus J, Cardoner N, Sebastián-Gallés N, Conesa G, Capdevila A. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage. 2002;17:670–679. [PubMed] [Google Scholar]
- 10.Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- 11.Vernaleken I, Weibrich C, Siessmeier T, Buchholz HG, Rösch F, Heinz A, Cumming P, Stoeter P, Bartenstein P, Gründer G. Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage. 2007;34:870–878. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 13.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 14.Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–530. [PubMed] [Google Scholar]
- 15.Ito H, Hietala J, Blomqvist G, Halldin C, Farde L. Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C] raclopride binding. J Cereb Blood Flow Metab. 1998;18:941–950. doi: 10.1097/00004647-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 19.Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- 20.Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Müller-Gärtner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998;19:781–787. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 22.Hietala J, Syvälahti E, Vilkman H, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Eronen E, Ruotsalainen U, Salokangas RK. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 23.Carlson JN, Glick SD. Cerebral lateralization as a source of interindividual differences in behavior. Experientia. 1989;45:788–798. doi: 10.1007/BF01954054. [DOI] [PubMed] [Google Scholar]
- 24.Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 26.Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]