Abstract
Background
Toxoplasma gondii is one of the most widely prevalent cyst forming Apicomplexan parasites with significant impact on animal production particularly in sheep, goats and pigs. The objectives of this cross-sectional study were to estimate the seroprevalence and to assess risk factors of Toxoplasma gondii infection in pigs. A systematic random sampling technique was used to collect 402 blood samples from pigs in Central Ethiopia. Direct Agglutination Test (DAT) was used to test sera. A questionnaire survey was made to assess potential risk factors and knowledge of farm attendants about toxoplasmosis.
Results
An overall seroprevalence of 32.1% [95% confidence interval (CI): 27.6%-36.9%] was found. Multivariable logistic regression analysis showed that extensively managed pigs (39.7%) are nearly twice (adjusted odds ratio [aOR]:=1.91, 95% CI: 1.01, 3.63) at higher risk of acquiring toxoplasmosis than intensively managed pigs (30.5%). Pigs supplied with feed containing animal byproducts had nearly four times (OR = 3.84, 95% CI: 2.01, 7.36) higher risk of acquiring T. gondii infection. Most of the farm attendants had little knowledge of health risks due to cats, neither to human nor to animals. Absence of rodent control, high neonatal mortality and history of abortion were found among herds of the studied pig farms.
Conclusions
T. gondii infections in pigs are wide spread. Extensive management systems and pig feed types containing animal byproducts are independent predictors of T. gondii seropositivity. The high seroprevalence suggests that pigs might serve as an important source of T. gondii infection for people. This is the first report of seroepidemiology of T. gondii infection in pigs in Ethiopia. Further studies are warranted for designing appropriate prevention and control strategies.
Keywords: Toxoplasma gondii, Seroprevalence, Risk factors, Pig, Central Ethiopia
Background
Pigs are important for nutrition and economic growth in many countries of the world [1]. In Ethiopia, the pig industry is still in its infancy and the population was estimated to be 29,000 [2]. In the past years adequate emphasis was not given for pig production in Ethiopia. Unlike other livestock distribution, swine farms are found predominantly in the central part of the country, particularly, in Addis Ababa and its surroundings.
Toxoplasma gondii is one of the most widely prevalent cyst forming Apicomplexan parasites. Cats and other felids play a crucial role in the epidemiology of toxoplasmosis as the definitive hosts, through shedding of millions of oocysts when infected [1,3]. Human beings and other warm-blooded animals, which serve as intermediate hosts, are infected primarily by ingesting food or water contaminated with sporulated oocysts or by ingesting meat that contain tissue cysts of T. gondii [3,4]. The parasite has a significant impact on animal production particularly in sheep, goats and pigs. Toxoplasma gondii causes mortality in pigs, especially neonatal pigs [1]. Most pigs acquire T. gondii infection postnatally by ingestion of oocysts from a contaminated environment or ingestion of infected tissues of animals [1,3]. Some pigs become infected prenatally by transplacental transmission of the parasite [1]. One-third of the human population is infected by this parasite worldwide. The routes of transmission differ in human populations depending on social culture, eating habits, religion, and/or environmental factors [5].
In Ethiopia Christian Orthodox and Muslim religion followers, which account for 43.5% and 33.9% of the total population, respectively, do not consume pork [6]. Although studies on Toxoplasma gondii seroprevalence and associated risk factors have been documented in different species of livestock in Ethiopia, [7-17] there are no studies conducted on seroepidemiology of T. gondii infection in pigs and the role of pigs in the transmission of toxoplasmosis to humans remains unknown in Ethiopia. Therefore, the objectives of this study were to estimate the seroprevalence and to assess the potential risk factors of acquiring T. gondii infection in pigs in selected areas of Central Ethiopia.
Methods
Description of the study areas
The study was carried out in four purposively selected areas located in Central Ethiopia, namely Akaki Kaliti sub-city, Kolfe Keraniho sub-city (Addis Ababa), Bishoftu and Ambo (Figure 1). The selection of the study areas was on the basis of availability of pig farms and accessibility.
Figure 1.
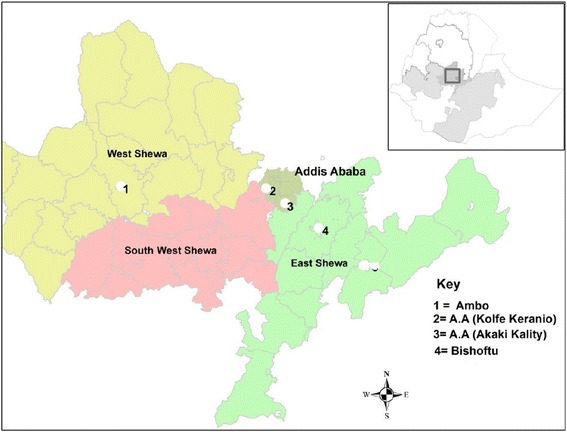
Study areas.
Addis Ababa is the capital city of Ethiopia located at longitude 38° 44′- 24″ E and latitude 9°1′ 48″ N. The altitude is from 2,200 to 3300 meters above sea level (masl). The annual rainfall is 800–1100 mm and the mean annual maximum and minimum temperature is about 21°C and 27°C, respectively [18].
Bishoftu is found in Adea district of East Shewa Zone of Oromia Regional State at a distance of 45 kms from Addis Ababa. Bishoftu town is situated at a longitude of 38°59′ E to 38.983°E and latitude of 08° 45′N to 8.750°N at an elevation of 1920 masl. The average rainfall is about 839 mm, while the mean minimum and maximum temperatures recorded for 27 years ranged from 7.9°C to 28°C with an overall average of 18.5°C [19].
Ambo district is found in West Shewa Zone of Oromia Regional State. The town is located at 8° 59′ 0″ N, 37° 51′ 0″ E at an elevation of 2101 masl. The mean annual temperature and rainfall of the town over 30 years (1981–2010) is about 18.64°C and 968.7 mm, respectively [20].
Study populations
The study was conducted in pigs originating from the four purposively selected areas in Central Ethiopia. The majorities of the pig populations studied were from the Bishoftu area, where many large and small scale commercial pig farms are concentrated. In the Bishoftu area, pigs are mainly kept under an intensive production system. In the Addis Ababa area, both intensive and extensive pig management systems are common, while in the Ambo area pigs are kept under an extensive management system. All pigs of more than three months of age belonging to both sexes (male and female) were considered for the purpose of this study. Age was determined using tooth eruption patterns [21].
Study designs and sample size
A cross-sectional study design was carried out from August, 2013 to May, 2014 to collect blood samples from pigs using a systematic random sampling technique. In the absence of previous studies on T. gondii infection in pigs in Ethiopia the required sample size was calculated by considering 50% expected prevalence (P) and 95% confidence interval (Z = 1.96) with a 5% desired absolute precision (d) using the formula N = (Z)2P(1-P)/d2 [22]. The calculated sample size (N) was 384; however, 402 pigs were sampled for the study. The total sample size was allocated to study site as follows. Bishoftu [246 animals: 139 young, 107 adult from six intensive farms], Ambo [57 animals: 13 young, 44 adult from two extensive farms], Akaki Kality [24 animals: 10 young and 14 adult from 4 extensive farms] and Kolfe Keraniho [75 animals: 8 young, 67 adult from one intensive farm].
Sample collection and transportation
Blood samples were aseptically collected from ear vein using sterile vacuum tubes without anti-coagulant, labeled and were immediately transported in an ice box with ice packs to the Ethio-Belgium Laboratory of the College of Veterinary Medicine and Agriculture of Addis Ababa University, Bishoftu for serological testing. Blood samples were centrifuged at 3200 RPM for 10 minutes; the separated sera were transferred to eppendorff tubes and kept at −20°C until serologically assayed.
Laboratory investigation
Direct Agglutination Test (DAT)
Toxoplasma gondii-specific IgG antibodies were detected by a direct agglutination test (DAT) (Toxo screen DA, biomerieux®, France) following the procedure described by the manufacturer of the kit. Sera were assayed at a screening dilution of 1/40 and 1/4000 in order to avoid the false negative results that might occur at low dilutions when using sera with high antibody titers. A titer of 1: 40 or 1: 4000 or both was considered indicative of T. gondii exposure. Sedimentation of antigen at the bottom of the well and clear agglutination above half of the well at either dilutions were recorded as negative and positive results respectively. Positive and negative controls were included in each test.
Questionnaire survey
A structured questionnaire was administered for each farm owner regarding herd size, age, sex (female/male), management system (intensive/extensive), feed type (with/without animal products), presence of domestic cats (yes/no), housing of domestic cats (totally outdoor/mixed [indoor and outdoor]), presence of rodents on the farm (yes/no), access for pigs to dead animals (yes/no), water source (pipe/mixed [pipe, river and pond]), presence of dogs (yes/no) and history of reproductive failure (abortion, still birth, neonatal mortality, mummification) were recorded during sample collection to assess potential risk factors for T. gondii infection in pigs. Furthermore, two people were interviewed from each of the 13 farms to assess farm attendants’ or owners’ knowledge about zoonotic importance of toxoplasmosis.
Data analysis
Data generated from the questionnaire survey and laboratory investigations were recorded and coded using Microsoft Excel spreadsheet (Microsoft Corporation) and analyzed using the STATA version 11.0 for Windows (Stata Corp. College Station, TX, USA). The seroprevalence was calculated as the number of seropositive pigs divided by the total number of pigs samples tested. Confidence limits for the proportions were established by exact binomial test with 95% confidence intervals (CI). All the variables assessed were categorical and variables with more than two categories were transformed into indicator (dummy) variables. To identify predictors of seropositivity, first the association of the potential risk factors (origin, age, sex, management system, presence of domestic cat, presence of dog, way of life of domestic cat, pig feed type, access to dead animal and water source) were analyzed by univariable logistic regression. Then, all non-collinear variables with P-value ≤ 0.25 in univariable logistic regression analysis were included in the final multivariable logistic regression model to construct the likely model (P < 0 .05). The model was reduced by backwards elimination of non-significant variables (P > 0.05) based on a likelihood ratio test to define the model that would best fit the data. In line with this, (with the exception of the management system, type of pig feed, presence of dog and age) all variables were dropped due to collinearity. Variables with small and incomparable frequencies in the contingency table were not considered. A significance level of α = 0.05 was used.
Ethics considerations
The animal part of the research project was reviewed and approved by the ethical committee for animal experimentation of the College of Veterinary Medicine and Agriculture, Addis Ababa University, Ethiopia. All animals were handled strictly in accordance with good animal practice. Verbal consent of owner’s permission to obtain samples from pigs and to participate in the questionnaire and interviews part of the study was approved by College of Health Sciences, Addis Ababa University (No. DRERC 001/11/MLS).
Results
Questionnaire
Among 26 farm attendants interviewed during the study 14 (53.8%) have completed university education, whereas only 2 (7.7%) were illiterate. Sixteen (61.5%) and 18 (69.2%) of the study participants do not have adequate knowledge about the role of cats in transmitting zoonotic diseases to humans and animals respectively (Table 1).
Table 1.
Educational status and knowledge about health risks of cats among farm attendants (n = 26)
| Variable | Category | Frequency (%) |
|---|---|---|
| Level of education | Illiterate | 2 (7.7) |
| Primary School | 4 (15.4) | |
| Secondary school | 6 (23.1) | |
| University | 14 (53.8) | |
| Knowledge of health risk to humans due to cats | Yes | 10 (38.5) |
| No | 16 (61.5) | |
| Knowledge of health risk to animals due to cats | Yes | 8 (30.8) |
| No | 18 (69.2) |
Out of 13 pig farms considered for this study, 12 (92.3%), 10 (76.9%) and 9 (69.2%) farms reported the presence of neonatal mortality, weak birth and history of abortion in their farms, respectively. Nine farms (69.2%) did not practice rodent control in their farms (Table 2).
Table 2.
Farm characteristics and reproductive problems in pig farms in study area (n = 13)
| Frequency (%) | ||
|---|---|---|
| Variable | Yes | No |
| History of abortion (s) | 9 (69.2) | 4 (30.8) |
| Presence of stillbirth | 8 (61.5) | 5 (38.5) |
| Presence of neonatal mortality | 12 (92.3) | 1 (7.7) |
| Presence fetal mummification | 9 (69.2) | 4 (30.8) |
| Presence of weak births | 10 (76.9) | 3 (23.1) |
| Contamination of stored animal feeds by cat faeces | 12 (92.3) | 1 (7.7) |
| Presence of feral cats | 13 (100) | 0 (0) |
| Presence of separate house for cats | 0 (0) | 13 (100) |
| Pig mortality in farms | 10 (76.9) | 3 (23.1) |
| Presence of rodent control | 4 (30.8) | 9 (69.2) |
Seroprevalence
The seroprevalence of IgG antibodies to T. gondii in pigs was 32.1% (129/402) (95% CI: 27.6%-36.9%). The highest animal level seroprevalence was observed in Kolfe Keraniho Sub-city (57.3%) followed by Akaki Kaliti Sub-city (45.8%), Ambo (33.3%) and Bishoftu (22.8%). These results are significantly different between study areas (P < 0.001). The seroprevalence of T. gondii infection was higher in extensively managed pigs (39.7%) than those pigs under intensive management (30.5%) (Table 3).
Table 3.
Results of univariable analysis of potential risk factors associated with Toxoplasma gondii seropositivity
| Variable category | Number tested | Test Pos. (%) | χ2 | P value | Univariable OR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Origin | 33.9 | <0.001 | |||||
| Bishoftu | 246 | 56 (22.8) | 1.0 | ||||
| Ambo | 57 | 19 (33.3) | 1.7 (0.91-3.17) | 0.098 | |||
| Akaki Kaliti | 24 | 11 (45.8) | 2.87 (1.22-6.76) | 0.016 | |||
| Kolfe Keraniho | 75 | 43 (57.3) | 4.56 (2.64-7.87) | <0.001 | |||
| Sex | 0.43 | 0.511 | |||||
| Female | 240 | 74 (30.8) | 1.0 | ||||
| Male | 162 | 55 (34.0) | 1.15 (0.75-1.76) | 0.511 | |||
| Age | 2.27 | 0.039 | |||||
| <12 months | 170 | 45 (26.5) | 1.0 | ||||
| ≥12 months | 232 | 84 (36.2) | 1.58 (1.02-2.43) | 0.039 | |||
| Management | 2.18 | 0.140 | |||||
| Intensive | 334 | 102 (30.5 | 1.0 | ||||
| Extensive | 68 | 27 (39.7) | 1.5 (0.87-2.57) | 0.142 | |||
| Presence of cats | 0.03 | 0.864 | |||||
| No | 61 | 19 (31.2) | 1.0 | ||||
| Yes | 341 | 110 (32.3) | 1.05 (0.58-1.89) | 0.864 | |||
| Presence of dogs | 16.2 | <0.001 | |||||
| No | 157 | 32 (20.4) | 1.0 | ||||
| Yes | 245 | 97 (39.6) | 2.56 (1.61-4.08) | <0.001 | |||
| Housing of domestic cats | 0.26 | 0.607 | |||||
| Totally outdoor | 138 | 42 (30.4) | 1.0 | ||||
| Mixed* | 264 | 87 (33) | 1.12 (0.72-1.75) | 0.607 | |||
| Pig feed type | 26.96 | <0.001 | |||||
| Without animal product | 327 | 86 (26.3) | 1.0 | ||||
| With animal product | 75 | 43 (57.3) | 3.77 (0.24-6.33) | <0.001 | |||
| Access to cadaver | 10.22 | 0.001 | |||||
| No | 230 | 59 (25.7) | 1.0 | ||||
| Yes | 172 | 70 (40.7) | 1.99 (1.30-3.04) | 0.001 | |||
| Water source | 0.28 | 0.599 | |||||
| Pipe | 333 | 105 (31.5) | 1.0 | ||||
| Mixed** | 69 | 24 (34.8) | 1.16 (0.67-2.0) | 0.599 | |||
| Rodent presence | 1.35 | 0.245 | |||||
| No | 12 | 2 (16.7) | 1.0 | ||||
| Yes | 390 | 127 (32.6) | 2.41 (0.52-1.18) | 0.260 | |||
* = indoor and outdoor, ** = pipe, river and pond.
Risk factors
For the univariable analysis the variable “presence of contamination of pig feed with cat faeces” was not considered in logistic regression because of smaller frequency. History of abortion, presence of still birth, neonatal mortality, fetal mummification, weak births and pig mortality were all excluded from the analysis because of lack of information on individual animals. The potential risk factors assessed for seropositivity of pigs are shown in Table 3.
Pigs reared on farms having access to dogs (OR = 2.56, 95% CI: 1.61, 4.08) had significantly higher seroprevalence than pigs reared on farms where dogs were not present. Similarly, origin, age, type of pig feed and access of pigs to cadavers were significantly associated with T. gondii seropositivity. The presence of rodents on farms, gender, source of water and management systems for pigs did not significantly influence the seropositivity of animals (P > 0.05) (Table 3).
The data obtained from the questionnaire surveys showed that all pigs in the study area had access to feral cats. Moreover, separate cat housing systems were not practiced for domestic cats. Domestic, as well as feral cats, have free access to pig farms. These variables were not subjected to statistical analysis as they were common to all farms (Table 3).
For multivariate logistic regression non-collinear variables with P-value <0.25 in univariable logistic regression were considered. Selection of one of the collinear variables was done on the basis of biological grounds to reason out scientifically the hypothesized risk factors in a better way. Sex, presence of domestic cats, housing of domestic cats, source of water and presence of rodents were variables excluded from the final model due to univariable P- value > 0.25. Origin and access for pigs to cadavers were removed due to collinearity. Finally, the management system, age category, type of pig feed and presence of dogs were entered into multivariable logistic regression models and the results are depicted in Table 4.
Table 4.
Results of multivariable logistic regression analysis of predictors of T. gondii infection in pig of study area
| Variable category | Adjusted OR (95% CI) | P -value |
|---|---|---|
| Management | 1.91 (1.01-3.63) | 0.047 |
| Type of pig feed | 3.84 (2.01-7.36) | <0.001 |
| Presence of dog | 1.48 (0.84-2.61) | 0.172 |
| Age | 1.12 (0.68-1.84) | 0.665 |
In the multivariate logistic regression analysis, management system and type of feed were the only risk factors significantly associated (P < 0.05) with pig seroprevalence (Table 4). The risk of acquiring toxoplasmosis in pigs raised on extensively managed farms was 1.91 times higher than pigs raised under intensive management. Type of feed was also a risk factor associated with toxoplasmosis, in that pigs fed on additional animal byproducts (slaughterhouse leftovers, dairy products (whey)) as feed supplement had a higher chance of acquiring T. gondii infection than pigs that were not supplemented with such animal products.
Discussion
Questionnaire
The survey results indicate that most of the respondents have little knowledge and awareness regarding the role of cats in the transmission of diseases either to humans (61.5%) or animals (69.2%), respectively. Therefore, this may contribute towards the transmission of various zoonotic diseases including toxoplasmosis. Several studies have indicated that contact with cats’ faeces is a major risk factor for pregnant women in acquiring T. gondii infection [23,24]. Furthermore, animals and humans can be infected by ingesting soil, water, or plant materials contaminated with T. gondii oocysts shed by infected cats [25]. In order to understand the transmission dynamics of zoonotic parasitic infections to humans, it is essential to have knowledge on the life cycle and prevalence of infections in both domestic and wild animals [26]. It is believed that people with a background of formal education are much more dedicated to health care than those without formal education and in most of the cases; they can have a tendency to gather information about health risk factors and its prevention [24].
The results of the questionnaire survey revealed widespread presence of neonatal mortality, history of abortion and presence of weak births among pig herds of the study areas. Previous studies showed that T. gondii infections result in tremendous problems for livestock husbandry and cause huge economic losses due to reproductive wastage [27,28]. Among domestic animals, abortion due to T. gondii in goats and sheep and high mortality rates in swine populations are widely noted [1,29]. Mortality due to T. gondii infection is more common in young pigs than in adult pigs [30]. Pigs infected transplacentally with T. gondii may be borne premature, dead, or weak, or may die soon after birth [31]. Recent reports from Korea have also demonstrated higher abortion rates (up to 44%) and unusually high sow mortality rates (up to 19%), that were primarily associated with toxoplasmosis [32]. The causes of reproductive failure in pigs have never been investigated in Ethiopia, however non-infectious (heat stress, hormonal disturbance, toxic chemicals, mineral and vitamin deficiencies) and infectious (porcine reproductive and respiratory syndrome virus, porcine parvovirus, pseudorabies virus, Japanese B encephalitis virus, classical swine fever virus, Leptospira spp, and Brucella suis) causes have been reported elsewhere [33].
Even though, the role of rodents as a reservoir of T. gondii has not yet been studied in domestic animals, including pigs in Ethiopia, the absence of rodent control on pig farms in this study might be an important factor for the transmission of pig toxoplasmosis. Kijlstra et al. [34] suggested that rodents can act as a reservoir for transmission of T. gondii and inadequate rodent control is considered to play a key role in T. gondii infection of pigs.
Seroprevalence
This is the first comprehensive survey of T. gondii infection in pigs in Ethiopia. The overall prevalence of the infection was found to be 32.1%. The result of the present study was much higher than the reports from Brazil (12.5%) using indirect fluorescent antibody tests (IFAT) [35], Central Italy (16.14%) using IFAT [36], Southern Italy (16.3%) [37] using Enzyme-Linked Immunosorbent Assay (ELISA), Serbia (9.2%) [38] and Portugal (15.6%) [39] using modified agglutination test (MAT). In contrast, the present result was lower than the report from Egypt (56.6%) using MAT [40]. However, this result is comparable with the reports from Northwestern Taiwan (28.8%) [41], South-west China (30.6%) [42], Peru (32.3%) [43], the Netherlands (31%) [44] and Switzerland (32%) [45].
The difference in seroprevalence between the present study and the aforementioned reports might be associated with the type of serological technique employed [46], cut-off values used, sample sizes and sampling techniques [47], type of rearing (free range vs. confinement) [1,3] climatic variations, density of feline and rodent control on farms [48,49]. The high seroprevalence of T. gondii in the present study might be associated with farm management systems and access of free roaming cats on pig farms. According to Arko-Mensah et al. [50] the risk factors influencing the prevalence of antibodies against T. gondii in Ghana was the age of the animals, breed, environmental conditions, and management practices.
The highest seroprevalence of T. gondii infection was found in pigs from the Kolfe Keraniho sub-city of Addis Ababa (57.3%) while the lowest seroprevalence was from the Bishoftu area (22.8%). The likely reasons for differences in seroprevalence among study areas might be associated with the difference in environmental temperature and management of pigs. Kolfe Keraniho is characterized by a warm and moist agro-climate compared to the mid highlands of Ambo and the relatively warmer and drier climatic condition in the midland of the Bishoftu area. The influence of the environment on the epidemiology of toxoplasmosis has been well documented [4]. It has been suggested that a warm and moist climate is more frequently associated with a high prevalence of T. gondii infection than hot dry ones [3]. This is due to the fact that the oocysts of T. gondii survive and sporulate easier in a moist and humid environment [37] whereas a dry climate has a deleterious effect on the persistence and dissemination of T. gondii oocysts, which in turn is likely to decrease the chance of oocyst survival, generally resulting in a low prevalence of toxoplasmosis [3].
Risk factors
Even though the seroprevalence of toxoplasmosis was not significantly associated with the presence of cats in the pig units/ farms, all pig farms in the study areas were accessed for either domestic or feral cats. Domestic cats have no separate housing system and have frequent outdoor access. Feed storage areas are easily accessed by feral and domestic cats leading to the possible contamination of pig feed and the environment with T. gondii oocysts shed in cats’ faeces. This finding corroborates the report of Fajardo et al. [51] suggesting that T. gondii infected cats spending time looking for rats in feed storage locations can defecate on the feed leading to its contamination with oocysts. Presence of cats is considered as one of the main risk factors for seropositivity in pigs, especially in animals kept in outdoor facilities. This is due to the fact that only a few oocysts are sufficient to produce infection in pigs [1,36]. Furthermore, most farm workers do not use boots and coveralls or footbaths before entering stables, thus contributing to the introduction of the oocysts collected from the environment into the pig units. This could mask the influence of exposure to cats as a risk factor [36]. Therefore, the transmission of toxoplasmosis to pigs might rely on the presence of cats, either feral or domestic, on the pig farm. In America, the presence of cats on farms was confirmed to be an important risk factor for seropositivity of T. gondii infection in sows [52].
There is meager data regarding the epidemiology of T. gondii infection in cats in the study area. A recent report showed a very high seroprevalence (85.4%) of T. gondii and oocyst shedding (22.2%) in feral cats in Addis Ababa, Ethiopia, suggesting a high environmental contamination with oocysts which might also serve as a source of infection for other animals [8,12]. Studies have shown a high seroprevalence of T. gondii infection in pigs on farms with high soil contamination by T. gondii oocysts and presence of a high number of cats [53,54]. Therefore, the possible risk factors for pig infection with T. gondii in the present study area might be associated with exposure of pig farms to cats.
The final multivariable logistic model showed that the types of pig feed containing animal byproducts and extensive management systems are independent predictors of toxoplasmosis. Studies have indicated that the prevalence of T. gondii infection in pigs influenced by management systems may be as high as 68% in poorly managed non-confinement systems (free range pigs), where pigs are kept in properties with no or limited technological developments for effective sanitary measures. Prevalence may also vary strongly from one locality to another due to differences in certain ecological factors [1,55]. Earlier research done in the Netherlands reported a significantly higher risk of seropositivity for Toxoplasma antibodies in free range pigs than for those in an intensive pig unit [56,57]. In contrast, a study in Switzerland showed that conventional fattening of pigs under confined conditions and free-range pigs surprisingly exhibited the same prevalence (2.0%; 95% CI: 0.2–7.0%) [58]. A possible explanation for the high seropositivity of extensively managed pigs in the present study might be related to scavenging in areas contaminated with either cat fecal materials containing oocysts, or feeding on cadavers containing infective bradyzoites. Moreover, free-roaming behavior in extensively managed pigs favor contact with rodents and the probability of ingestion of infective cysts contained in their tissues [59,60].
In this study, the type of feed consumed by pigs was more likely to be a notable source of T. gondii infection as it frequently includes slaughter byproducts, which might contain tissue cysts. In addition, feed supplements, such as concentrates, forages, household leftovers, raw vegetables, and fruits might be contaminated with cat faeces containing oocysts.
Although presence of dogs on pig farms had no significant association with toxoplasmosis in the final model, it was significantly associated with T. gondii infection during univariable analysis. Many dogs were seen roaming around pig feeds (household leftovers, animal byproducts like cow whey, slaughterhouse leftovers). These dogs might contaminate pig feeds with oocysts of T. gondii from cat faeces. Although dogs do not produce T. gondii oocysts like cats, it has been suggested that outdoor dogs might contribute to Toxoplasma transmission in two ways: fur contamination and oocysts re-shedding near houses following ingestion of infected cat feaces [61,62]. Other studies also revealed that under laboratory conditions, dogs have been shown to eliminate viable oocysts after ingesting sporulated oocysts in cat feaces. Moreover, the fur of dogs that have come in contact with cat faeces may be a vector for transmission of oocysts to humans [63]. Data in US swine operations indicated that it is difficult to eliminate T. gondii infection from swine herds which allow access of cats or dogs to swine facilities [64].
Univariable logistic regression analysis of the present study revealed significantly higher seroprevalence (36.2%), (OR = 1.58; 95% CI: 1.02-2.43) in pigs of ≥ 12 months of age compared to those pigs <12 months of age. Similar findings were reported from elsewhere [37,47]. A recent study from Slovakia also identified that the seroprevalence of toxoplasmosis in sows (4.26%) was twice as high in slaughter pigs (2.06%) [65]. The relatively higher seroprevalence in pigs of ≥ 12 months of age as compared to pigs of < 12 months of age might be due to the longer contact time of older animals with a potentially infected environment containing T. gondii oocysts and/or tissue cysts [37].
Although pork is staple food widely eaten in many countries of the world, traditional Ethiopian food does not use any pork as most Ethiopians have historically adhered to Islam, the Ethiopian Orthodox Church, or Judaism, all of which prohibit eating pork. However, with increased population growth and urbanization, consumption of pork in becoming popular in urban areas among foreigners and a few Ethiopians (protestant). Generally, there is no raw pork consumption habit in Ethiopia but there are possibilities of unsafe handling and preparation of pork (e.g. humbuggers) leading to the consumption of undercooked smoked pork hence there is chance of acquiring T. gondii infection.
Conclusions
In conclusion, the study showed that T. gondii infection in pigs is widespread. Extensive management systems and pig feed types containing animal byproducts are independent predictors of T. gondii seropositivity. The high seroprevalence suggests contamination of the environment with T. gondii oocysts from freely moving cats on the premises of pigs and the likely role of pigs as a potential source of T. gondii infection for humans. Feeding of pigs with heat treated animal products and further studies to unravel the role of toxoplasmosis of pigs in causing reproductive failures and its zoonotic transmission are imperative.
Acknowledgements
The financial support of VLIR-UOS project no. “ZEIN 2010 PR 372” promotion of the PhD program in Veterinary Public Health at the Faculty of Veterinary Medicine, Belgium, and partial financial support by Maria Vitale grant No. RF 2007/RC 2009 from the Italian Ministry of Health are highly acknowledged. Special thanks also go to pig owners and attendants for their kind permission and assistance during sample collection. The authors are grateful to Margareta Langbacka Walker, advisor and trainer at the English Language Improvement Centre at Ambo University, for her valuable comments on the language aspects.
Abbreviations
- Masl
Meters above sea level
- °C
Degree celsius
- RPM
Revolutions per minute
- DAT
Direct agglutination test
- CI
Confidence interval
- IFAT
Indirect fluorescent antibody test
- ELISA
Enzyme linked immunosorbent assay
- MAT
Modified agglutination test
- OR
Odds ratio
- aOR
Adjusted odds ratio
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EZG conceived and designed the proposal, participated in the coordination and management of the study, collected, tested and analyzed the data and drafted the article. MMK and MA participated in sample collection, laboratory testing and drafting of the article. HA, MV and V di M participated in the study design and the edition of article. All authors have read and approved the final manuscript.
Contributor Information
Endrias Zewdu Gebremedhin, Email: endrias.zewdu@gmail.com.
Mulisa Megerssa Kebeta, Email: mulisam38@gmail.com.
Mebratu Asaye, Email: assayemebratu@yahoo.com.
Hagos Ashenafi, Email: hagos83@yahoo.com.
Vincenzo Di Marco, Email: dimarco.vince@gmail.com.
Maria Vitale, Email: marvitus@yahoo.com.
References
- 1.Dubey JP. Toxoplasmosis in pigs-The last 20 years. Vet Parasitol. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 2.FAO . Food and Agricultural Organization of the United Nations. 2005. [Google Scholar]
- 3.Dubey JP. Toxoplasmosis of Animals and Humans. 2. Boca Raton, Florida: CRC Press; 2010. pp. 1–313. [Google Scholar]
- 4.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2010;30:1217–58. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, et al. Fatal outbreak of human toxoplasmosis along the Maroni River. Epidemiol Parasitol Clin Infect Dis. 2007;45:88–95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 6.Central Statistics Authority (CSA) National statistics abstract. Addis Ababa, Ethiopia: Central Statistical Agency, Federal Democratic Republic of Ethiopia; 2007. [Google Scholar]
- 7.Teshale S, Dumètre A, Dardé ML, Merga B, Dorchies P. Serological survey of caprine toxoplasmosis in Ethiopia: prevalence and risk factors. Parasite. 2007;14:155–9. doi: 10.1051/parasite/2007142155. [DOI] [PubMed] [Google Scholar]
- 8.Dubey JP, Darrington C, Tiao N, Ferreira LR, Choudhary S, Molla B, et al. Isolation of viable Toxoplasma gondii from tissues and faeces of cats from Addis Ababa, Ethiopia. J Parasitol. 2013;99:56–8. doi: 10.1645/GE-3229.1. [DOI] [PubMed] [Google Scholar]
- 9.Dubey JP, Choudhary S, Tilahun G, Tiao N, Gebreyes WA, Zou X, et al. Genetic diversity of Toxoplasma gondii isolates from Ethiopian feral cats. Vet Parasitol. 2013;196:206–8. doi: 10.1016/j.vetpar.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Gebremedhin EZ, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet Res. 2013;9:117. doi: 10.1186/1746-6148-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebremedhin EZ, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Some risk factors for reproductive failures and contribution of Toxoplasma gondii infection in sheep and goats of central Ethiopia: A cross-sectional study. Res Vet Sci. 2013;95:894–900. doi: 10.1016/j.rvsc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Tiao N, Darrington C, Molla B, Saville WJA, Tilahun G, Kwok OCH, et al. An investigation into the seroprevalence of Toxoplasma gondii, Bartonella spp. feline immunodeficiency virus (FIV), and feline leukemia virus (FelV) cats from Addis Ababa. Epidemiol Infect. 2013;141:1029–33. doi: 10.1017/S0950268812001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilahun G, Tiao N, Ferreira LR, Oliveira S, Verma SK, Kwok OCH, et al. Seroprevalence of Toxoplasma gondii from free-range chicken (Gallus domesticus) from Addis Ababa, Ethiopia. J Parasitol. 2013;99:740–1. doi: 10.1645/12-25.1. [DOI] [PubMed] [Google Scholar]
- 14.Zewdu E, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res Vet Sci. 2013;94:43–8. doi: 10.1016/j.rvsc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Gebremedhin EZ, Gizaw G. Seroprevalence of Toxoplasma gondii infection in sheep and goats in three districts of Southern Nations, Nationalities and Peoples’ Region of Ethiopia. World Appl Sci J. 2014;31(11):1891–6. [Google Scholar]
- 16.Gebremedhin EZ, Tesfamaryam G, Yunus HA, Duguma R, Tilahun G, Di Marco V, et al. Seroepidemiology of Toxoplasma gondii infection in free range chickens (Gallus domesticus) of Central Ethiopia. Epidemiol Infect. 2014;24:1–10. doi: 10.1017/S0950268814000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebremedhin EZ, Yunus HA, Tesfamaryam G, Tessema TS, Dawo F, Terefe G, et al. First report of Toxoplasma gondii in camels (Camelus dromedarius) from Ethiopia: seroepidemiology and bioassay. BMC Vet Res. 2014;10:222. doi: 10.1186/s12917-014-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fikire Z, Tolosa T, Nigussie Z, Macias C, Kebede N. Prevalence and characterization of hydatidosis in animals slaughtered at Addis Ababa abattoir, Ethiopia. J Parasitol Vector Biol. 2012;4:1–6. [Google Scholar]
- 19.IPMS (Improving Productivity and Market Success) Ada’a-Liben Woreda pilot learning site diagnosis and program design. Addis Ababa, Ethiopia: ILRI (International Livestock Research Institute); 2004. pp. 24–32. [Google Scholar]
- 20.Ogato GS. The Quest for Mainstreaming Climate Change Adaptation into Urban Development Planning Of Ambo Town, Ethiopia. Am J Human Ecol. 2013;2:103–19. [Google Scholar]
- 21.Xiaolin MA. Pig husbandry strategies in a complex society, Central China. BIPPA. 2004;2(24):91–102. [Google Scholar]
- 22.Thrusfield M. Veterinary Epidemiology. 3. Oxford, UK: Blackwell Science Ltd; 2005. pp. 252–66. [Google Scholar]
- 23.Ayi I, Edu SAA, Apea-Kubi KA, Boamah D, Bosompem KM, Edoh D. Sero-epidemiology of toxoplasmosis amongst pregnant women in the greater Accra region of Ghana. Ghana Med J. 2009;43:107–14. doi: 10.4314/gmj.v43i3.55325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babaie J, Amiri S, Mostafavi E, Hassan N, Lotfi P, Rastaghi ARE, et al. Seroprevalence and Risk Factors for Toxoplasma gondii infection among pregnant women in Northeast Iran. CVI. 2013;20(11):1771–3. doi: 10.1128/CVI.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira KS, Franco RM, Leal DA. Transmission of toxoplasmosis (Toxoplasma gondii) by foods. Adv Food Nutr Res. 2010;60:1–19. doi: 10.1016/S1043-4526(10)60001-0. [DOI] [PubMed] [Google Scholar]
- 26.Lavikainen A. Human medical view on zoonotic parasites. Acta Vet Scand. 2010;52(1):S4. doi: 10.1186/1751-0147-52-S1-S4. [DOI] [Google Scholar]
- 27.Boughattas S, Bergaoui R, Essid R, Aoun K, Bouratbine A. Seroprevalence of Toxoplasma gondii infection among horses in Tunisia. Parasit Vectors. 2011;4:218. doi: 10.1186/1756-3305-4-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang N, Mu MY, Li HK, Long M, He JB. Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasit Vectors. 2012;5:237. doi: 10.1186/1756-3305-5-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/S0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 30.Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/AHR2005100. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay DS, Blagburn BL, Dubey JP. Coccidia and other protozoa. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DI, editors. Diseases of Swine. 8. Ames: Iowa State University Press; 1999. pp. 661–4. [Google Scholar]
- 32.Kim JH, Kang KI, Kang WC, Sohn JH, Jean TH, Park BK, et al. Porcine abortion outbreak associated with Toxoplasma gondii in Juju Island. Korea J Vet Sci. 2009;10:147–51. doi: 10.4142/jvs.2009.10.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aiello SE, Moses MA, Steigerwald MA, editors. The Merck Veterinary Manual. A handbook of diagnosis, therapy, and disease prevention and control for the veterinarian. 10. New Jersey., U.S.A: Merck & Co. Inc; 2012. [Google Scholar]
- 34.Kijlstra A, Meerburg B, Cornelissen J, de Craeye S, Vereijken P, Jongert E. The role of rodents and shrews in the transmission of Toxoplasma gondii to pig. Vet Parasitol. 2008;156:183–90. doi: 10.1016/j.vetpar.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Samico Fernandes EF, Samico Fernandes MF, Kim PC, de Albuquerque PP, de Souza Neto OL, de Santos A, et al. Prevalence of Toxoplasma gondii in Slaughtered Pigs in the State of Pernambuco, Brazil. J Parasitol. 2012;98:690–1. doi: 10.1645/GE-3032.1. [DOI] [PubMed] [Google Scholar]
- 36.Veronesi F, Ranucci D, Branciari R, Miraglia D, Mammoli R, Fioretti DP. Seroprevalence and risk factors for Toxoplasma gondii infection on finishing swine reared in the Umbria Region, Central Italy. Zoonoses Public Health. 2011;58:178–84. doi: 10.1111/j.1863-2378.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 37.Villari S, Vesco G, Petersen E, Crispo A, Buffolano W. Risk factors for toxoplasmosis in pigs bred in Sicily, Southern Italy. Vet Parasitol. 2009;161:1–8. doi: 10.1016/j.vetpar.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Klun I, Vujanić M, Yera H, Nikolić A, Ivović V, Bobić B, et al. Toxoplasma gondii infection in slaughter pigs in Serbia: seroprevalence and demonstration of parasites in blood. Vet Res. 2011;42:17. doi: 10.1186/1297-9716-42-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa S, Ajzenberg D, Canada N, Freire L, Costa JMC, Darde’ ML, et al. Biologic and molecular characterization of Toxoplasma gondii isolates from pigs from Portugal. Vet Parasitol. 2006;135:133–6. doi: 10.1016/j.vetpar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 40.El Moghazy FM, Kandil OM, Shaapan RM. Toxoplasma gondii: Comparison of some serological tests for antibody detection in sera of naturally infected pigs. World J Zool. 2011;6:204–8. [Google Scholar]
- 41.Fan CK, Su KE, Tsai YJ. Serological survey of Toxoplasma gondii infection among slaughtered pigs in Northwestern Taiwan. J Parasitol. 2004;90:653–4. doi: 10.1645/GE-177R. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Ruiqing L, Sun X, Shu F, Zhou Z, Nie K, et al. Seroprevalence of Toxoplasma gondii antibodies from slaughter pigs in Chongqing, China. Trop Anim Health Prod. 2012;44:685–7. doi: 10.1007/s11250-011-9965-3. [DOI] [PubMed] [Google Scholar]
- 43.Suaréz-Aranda F, Galisteo AJ, Jr, Hiramoto RM, Cardoso RPA, Meireles LR, Miguel O, et al. The prevalence and avidity of Toxoplasma gondii IgG antibodies in pigs from Brazil and Peru. Vet Parasitol. 2000;91:23–32. doi: 10.1016/S0304-4017(00)00249-1. [DOI] [PubMed] [Google Scholar]
- 44.Van Knapen F, Kremers AFT, Franchimont JH, Narucka U. Prevalence of antibodies to Toxoplasma gondii in cattle and swine in The Netherlands: towards an integrated control of livestock production. Vet Quart. 1995;17:87–91. doi: 10.1080/01652176.1995.9694539. [DOI] [PubMed] [Google Scholar]
- 45.Wyss R, Sager H, Muller N, Inderbitzin F, Ko¨nig M, Audige’ L, et al. Distribution of Toxoplasma gondii and Neospora caninum under aspects of meat hygiene. Aspekten Schweiz. Arch Tierheilk. 2000;142:95–108. [PubMed] [Google Scholar]
- 46.Huang CQ, Lin YY, Dai AL, Li XH, Yang XY, Yuan ZG, et al. Seroprevalence of Toxoplasma gondii infection in breeding sows in Western Fujian Province, China. Trop Anim Health Prod. 2010;42:115–8. doi: 10.1007/s11250-009-9393-9. [DOI] [PubMed] [Google Scholar]
- 47.Halova’ D, Mulcahy G, Rafter P, Turcˇekova’ L, Grant T, de Waal T. Toxoplasma gondii in Ireland: Seroprevalence and novel molecular detection method in sheep, pigs, deer and chickens. Zoonoses Public Health. 2012;60:168–73. doi: 10.1111/j.1863-2378.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 48.Heidari H, Gharekhani J, Tavoosidana GR. Role of toxoplasmosis in abortion of ewes in western Iran: a serological study. Sci Parasitol. 2013;14:99–103. [Google Scholar]
- 49.Gharekhani J. Serological study of Toxoplasma gondii infection in cattle from western Iran. Sci Parasitol. 2013;14:153–7. [Google Scholar]
- 50.Arko-Mensah J, Bosompem KM, Canacoo EA, Wastling JM, Akanmori BD. The seroprevalence of toxoplasmosis in pigs in Ghana. Acta Trop. 2000;76:27–31. doi: 10.1016/S0001-706X(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 51.Fajardo HV, D’ávila S, Bastos RR, Cyrino CD, Detoni ML, Garcia JL, et al. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, Southern Brazil. Parasit Vectors. 2013;6:191. doi: 10.1186/1756-3305-6-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmann T, Graham DH, Dahl E, Sreekumar C, Launer F, Corn JL, et al. Transmission dynamics of Toxoplasma gondii on a pig farm. Infect Genet Evol. 2003;3:135–41. doi: 10.1016/S1567-1348(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 53.Meerburg BG, Riel JWV, Cornelissen JB, Kijlstra A, Mul MF. Cats and goat whey associated with Toxoplasma gondii infection in pigs. Vector Borne Zoonotic Dis. 2006;6:266–74. doi: 10.1089/vbz.2006.6.266. [DOI] [PubMed] [Google Scholar]
- 54.Du F, Zhang Q, Yu Q, Hu M, Zhou Y, Zhao J. Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet Parasitol. 2012;187:53–6. doi: 10.1016/j.vetpar.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 55.Gamble HR, Brady RC, Dubey JP. Prevalence of Toxoplasma gondii infection in domestic pigs in the New England states. Vet Parasitol. 1999;82:129–36. doi: 10.1016/S0304-4017(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 56.Kijlstra A, Meerburg BG, Mul MF. Animal-friendly production systems may cause re-emergence of Toxoplasma gondii. Njas-Wageningen. J Life Sci. 2004;52:119–32. [Google Scholar]
- 57.Van der Giessen J, Fonville M, Bouwknegt M, Langelaar M, Vollema A. Seroprevalence of Trichinella spiralis and Toxoplasma gondii in pigs from different housing systems in The Netherlands. Vet Parasitol. 2007;148:371–4. doi: 10.1016/j.vetpar.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Berger-Schoch AE, Herrmann DC, Schares G, Müller N, Bernet D, Gottstein B, et al. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet Parasitol. 2011;177:290–7. doi: 10.1016/j.vetpar.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 59.Venturini MC, Bacigalupe D, Venturini L, Rambeaud M, Basso W, Unzaga JM, et al. Seroprevalence of Toxoplasma gondii in sows from slaughterhouses and in pigs from an indoor and an outdoor farm in Argentina. Vet Parasitol. 2004;124:161–5. doi: 10.1016/j.vetpar.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Thoma LF, de Glanville WA, Cook EA, Fèvre EM. The spatial ecology of free-ranging domestic pigs (Sus scrofa) in western Kenya. BMC Vet Res. 2013;9:46. doi: 10.1186/1746-6148-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frenkel JK, Parker BB. An apparent role of dogs in the transmission of Toxoplasma gondii: the probable importance of xenosmophilia. Ann NY Acad Sci. 1996;791:402–7. doi: 10.1111/j.1749-6632.1996.tb53546.x. [DOI] [PubMed] [Google Scholar]
- 62.Lee JY, Lee SE, Lee EG, Song KH. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res Vet Sci. 2008;85:125–7. doi: 10.1016/j.rvsc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Lindsay DS, Dubey JP, Butler JM, Blagburn BL. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet Parasitol. 1997;73:27–33. doi: 10.1016/S0304-4017(97)00048-4. [DOI] [PubMed] [Google Scholar]
- 64.Hu X, Kliebenstein J, Patton S, Zimmerman J, Hallam A, Roberts T, et al. Toxoplasma gondii in US swine operations: An assessment of management risk factors. Staff Paper No. 288, Iowa State University; 1997. 1–14.
- 65.Turčeková L, Antolová D, Reiterová K, Spišák F. Occurrence and genetic characterization of Toxoplasma gondii in naturally infected pigs. Acta Parasitol. 2013;58:361–6. doi: 10.2478/s11686-013-0154-6. [DOI] [PubMed] [Google Scholar]


