Abstract
The dorsal cochlear nucleus (DCN) is one of the first stations within the central auditory pathway where the basic computations underlying sound localization are initiated and heightened activity in the DCN may underlie central tinnitus. The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT), is associated with many distinct behavioral or cognitive states, and serotonergic fibers are concentrated in the DCN. However, it remains unclear what is the function of this dense input. Using a combination of in vitro electrophysiology and optogenetics in mouse brain slices, we found that 5-HT directly enhances the excitability of fusiform principal cells via activation of two distinct 5-HT receptor subfamilies, 5-HT2A/2CR (5-HT2A/2C receptor) and 5-HT7R (5-HT7 receptor). This excitatory effect results from an augmentation of hyperpolarization-activated cyclic nucleotide-gated channels (Ih or HCN channels). The serotonergic regulation of excitability is G-protein-dependent and involves cAMP and Src kinase signaling pathways. Moreover, optogenetic activation of serotonergic axon terminals increased excitability of fusiform cells. Our findings reveal that 5-HT exerts a potent influence on fusiform cells by altering their intrinsic properties, which may enhance the sensitivity of the DCN to sensory input.
Keywords: auditory, serotonin, tinnitus
Introduction
The serotonergic system modulates diverse physiological and behavioral functions, such as sleep, feeding, nociception, mood, and emotions (Lucki, 1998). Serotonergic dysfunction has been implicated in a variety of psychiatric disorders, including depression, anxiety, schizophrenia, Parkinson's disease, and Alzheimer disease (Meltzer et al., 1998; Jones and Blackburn, 2002; Huot et al., 2011). The majority of serotonergic neurons are found in the dorsal and medial raphe nuclei, sending widespread projections to many brain regions including the auditory system (Dahlström and Fuxe, 1964; Steinbusch, 1981; Descarries et al., 1982), with dense innervation in the cochlear nucleus (Steinbusch, 1981; Thompson and Thompson, 2001). Although the physiological function of 5-HT in the auditory system is unclear, it may differentially modulate the response to simple and complex sounds such as vocalizations (Ebert and Ostwald, 1992; Revelis et al., 1998; Hurley and Pollak, 1999, 2005; Hurley and Hall, 2011; Wood et al., 2013). Dysfunction of the serotonergic system is implicated in the generation or perception of tinnitus (Marriage and Barnes, 1995; Simpson and Davies, 2000; Salvinelli et al., 2003; Caperton and Thompson, 2010). Moreover, it has been suggested that selective 5-HT reuptake inhibitors (SSRIs) often used in the treatment of depression and anxiety disorders (Stark et al., 1985; Wong et al., 1995), might be also used to treat tinnitus (Shemen, 1998; Folmer and Shi, 2004; Fornaro and Martino, 2010; Oishi et al., 2010; Baldo et al., 2012). Therefore, understanding the normal physiological effects of 5-HT in auditory system may provide insight into normal brain function and suggest new approaches in the treatment of tinnitus.
The role of 5-HT in auditory system may be of particular interest in the dorsal cochlear nucleus (DCN), where auditory and multisensory signals converge at fusiform principal cells. Among proposed functions of the DCN is sound source localization and orientation to sounds of interest (Sutherland et al., 1998; Imig et al., 2000; May, 2000; Oertel and Young, 2004), and is a proposed site of central tinnitus generation and modulation (Levine, 1999; Brozoski et al., 2002, 2012; Kaltenbach et al., 2004; Wang et al., 2009; Middleton et al., 2011; Dehmel et al., 2012; Koehler and Shore, 2013; Li et al., 2013; Luo et al., 2014). Moreover, the DCN receives a dense serotonergic innervation, originating predominantly from the dorsal and medial raphe nuclei (Parent et al., 1981; Steinbusch, 1981; Willard et al., 1984; Klepper and Herbert, 1991; Thompson et al., 1994, 1995; Hurley and Thompson, 2001; Thompson and Thompson, 2001), and contains multiple subtypes of 5-HT receptors, including 5-HT1AR, 5-HT2AR, and 5-HT2CR (Pazos et al., 1985; Thompson et al., 1994; Wright et al., 1995; Cornea-Hébert et al., 1999; Thompson and Wiechmann, 2002), and measureable levels of 5-HT (Cransac et al., 1995). Yet, despite the potential physiological functions of serotonergic raphe-DCN pathway, it is unclear what 5-HT does in the DCN. Here, we found that 5-HT activates 5-HT2A/2CR and 5-HT7R, and thereby exerts excitatory control of fusiform cells by altering their intrinsic properties; thus, 5-HT regulates the output of a primary auditory nucleus.
Materials and Methods
Slice preparation.
All procedures were approved by the Oregon Health and Science University's IACUC. C57BL/6J wild-type mice of both sexes at P16–P49 were used for the majority of the experiments. For optogenetic experiments, the mice were B6; SJL-Tg (Tph2-COP4*H13R/EYFP)5Gfng/J (Tph2-ChR2-YFP, JAX stock #014555; Zhao et al., 2011). Mice were anesthetized by isoflurane inhalation and then decapitated. Brains were quickly removed and placed in a vibratome (Leica). Coronal slices containing the DCN (260–300 μm) were prepared in an ice-cold cutting solution containing the following (in mm): 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, and bubbled with 95% O2/5% CO2, and then the slices were maintained for 1 h in warm (∼33°C) ACSF solution containing the following (in mm): 130 NaCl, 2.1 KCl, 1.7 CaCl2, 1.0 MgSO4, 1.2 KH2PO4, 20 NaHCO3, 3 Na-HEPES, 11 glucose; bubbled with 95% O2/5% CO2, 300–310 mOsm. After 1 h recovery, slices were maintained in the same solution at room temperature (∼22°C) until recording.
Electrophysiology.
Slices were transferred into a recording chamber, and continuously perfused with ∼33°C 95% O2/5% CO2 oxygenated ACSF at ∼2 ml/min. The DCN neurons were visualized by Dodt contrast optics with a 60× water-immersion objective on the stage of an upright microscope (Olympus, BX51W). Fusiform and cartwheel cells were identified based on their location, morphology and electrophysiological properties (Manis et al., 1994; Zhang and Oertel, 1994; Golding and Oertel, 1997; Tzounopoulos et al., 2004). Synaptic transmission was blocked in most experiments by adding 10 μm NBQX, 5 μm R-CPP, 1 μm strychnine, and 10 μm SR95531. For whole-cell recordings, pipettes were filled with a solution containing the following (in mm): 113 K-gluconate, 9 HEPES, 2.75 MgCl2, 1.75 MgSO4, 0.1 EGTA, 14 Tris2-phosphocreatine, 4 Na2-ATP, 0.3 Tris-GTP; osmolality adjusted to ∼290 mOsm with sucrose, pH adjusted to 7.25 with KOH. The membrane potential values are corrected for a 10 mV junction potential. For loose cell-attached recordings, pipettes were filled with a normal ACSF solution. Patch pipettes (3–5 MΩ) were pulled from borosilicate glass (WPI). For all voltage-clamp experiments, series resistance (<20 MΩ) was compensated by 65–80% and membrane potential was held constant at −70 mV except for experiments shown in Figure 4. Experiments were excluded if series resistance varied >20% over the course of the recording. In current-clamp recordings, the pipette capacitance was canceled and bridge balance was maintained.
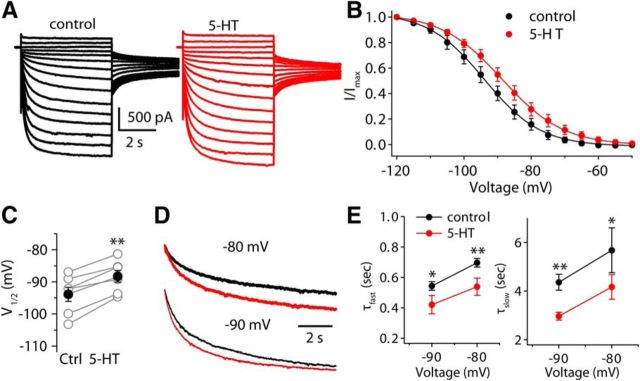
Figure 4.
5-HT positively shifts the activation curve for the Ih and reduces the time constant for Ih activation. A, Sample current traces from a fusiform cell before and after applying 5-HT. Currents were evoked with a series of 5 s voltage steps from −120 to −50 mV, in 5 mV increments, from a holding potential of −60 mV, and followed by a test pulse to −75 mV. B, Mean normalized tail currents are plotted as a function of voltage steps and fit with the Boltzmann equation (n = 7). Tail currents were measured at −75 mV in the presence of Ba2+ to eliminate the contamination of Kir. C, Pooled data showing the effects of 5-HT on V1/2. Open symbols represent V1/2 of individual neurons, and filled symbols represent the mean of V1/2. D, Superimposition of current traces evoked by 10 s steps to −80 and −90 mV from a holding potential of −60 mV before and after applying 5-HT. E, Pooled data of time constants for Ih activation in the absence and presence of 5-HT (n = 4) at two voltages. Error bars are ± SEM.
For microiontophoresis, methods were similar to a previous study (Perrier and Cotel, 2008): a micropipette (40–80 MΩ) was filled with 50–100 mm serotonin hydrochloride dissolved in 165 mm NaCl, and the pH adjusted to 4.5 using HCl. Retaining current of −20 nA were applied to micropipette to reduce drug leakage between ejection periods. 5-HT was ejected by applying positive currents (+40–80 nA) for 3–5 s. In control experiments, iontophoresis of drug carrier saline (165 mm NaCl) alone failed to evoke any detectable currents using the same iontophoresis current level as using those to eject 5-HT. The micropipette was positioned close to the soma of the recorded neurons.
Optogenetic stimulation was performed as previously described (Apostolides and Trussell, 2013). Briefly, wide-field activation of ChR2 was achieved with blue light from a LED (470 nm) transmitted through the fluorescent light path of the microscope. Photostimulation was delivered at 3 min intervals, and the maximal light intensity reaching the brain slice was ∼15 mW/mm2.
Data acquisition and analysis.
Data were collected using a Multiclamp 700B amplifier and pClamp 10 software (Molecular Devices). Signals were digitized at 10–50 kHz with a Digidata 1322A (Molecular Devices) and low-pass filtered at 1–10 kHz for offline analysis.
To assess the effects of 5-HT on activation parameters of Ih, an activation curve was obtained by fitting averaged normalized Ih currents with a Boltzmann equation of the form I/Imax = 1/[1 + exp(Vm − V1/2)/s] in which I/Imax is the normalized current, Vm is the membrane potential, V1/2 is the potential at half-maximal conductance and s is the slope factor. The time constant of Ih activation was determined by fitting the currents evoked by a step to −80 or −90 mV potential with a double-exponential function of the form: It = Iss + Ifast × exp(−t/τfast) + Islow × exp(−t/τslow), where It is the current amplitude at time t, Iss is the steady-state current at a given potential, Ifast and Islow denote the amplitude of the slow and fast current components, and τfast and τslow are the corresponding time constants for Ih activation.
All data are presented as mean ± SEM unless specified otherwise, and statistical significance was assessed using Student's t tests as appropriate; *p < 0.05, **p < 0.01 and ***p < 0.001.
Immunohistochemistry.
Tph2-ChR2 transgenic mice (P20–P30) were deeply anesthetized with isoflurane and were transcardially perfused with warm (∼38C°) PBS, pH 7.4, followed by ice-cold 4% paraformaldehyde (PFA) in PBS. After perfusion, mouse brains were dissected out and postfixed in 4% PFA overnight at 4°C. The brains were rinsed thoroughly in PBS, pH 7.4, embedded in 4% agar, and sliced into 30 μm coronal sections using a vibratome (Leica VT1000S). Next, sections were washed in PBS for 30 min and subsequently permeabilized in 1% Triton X-100 in PBS for 1 h. After again being washed in PBS for 30 min, sections were incubated for 30 min in blocking solution consisting 2% fish gelatin in PBS. The sections were then incubated with anti-GFP AlexaFluor 488-conjugated antibody (10 μg/ml; Invitrogen) in blocking solution overnight at 4°C. After being washed in PBS for 30 min, sections were postfixed in 4% PFA for 1 h. After being washed again in PBS, sections were then mounted on slides and coverslipped with mounting medium. Images were acquired using laser-scanning confocal microscopy (Olympus FV1000).
Reagents.
5-HT was applied by either bath application or iontophoresis, and all other drugs were applied either in the bath or through the recording pipette. NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide), R-CPP ((R)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid), SR95531 (Gabazine), and TTX (tetrodotoxin) were obtained from Abcam. 5-HT (serotonin hydrochloride), ketanserin, MDL-11939, SB-242084, α-methyl-5-HT, SB-269970, WAY-100135, ZD7288, SQ22536, 8-Br-cAMP, PKC19–31, genistein, PP1 (1-(1,1-dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine), and PP2 (3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine) were purchased from Tocris Bioscience. All other drugs and chemicals were from Sigma-Aldrich.
Results
5-HT regulates excitability of fusiform cells
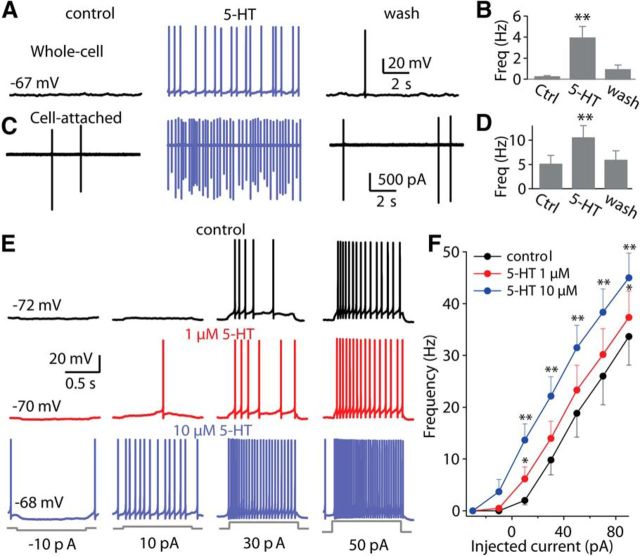
To explore the effects of 5-HT in DCN, whole-cell and loose cell-attached recordings were obtained from acute slices of mouse brainstem containing DCN. Under current-clamp, bath application of 10 μm 5-HT produced a significant depolarization of the resting membrane potential (control: −68.1 ± 1.5 mV, 5-HT: −63.0 ± 1.4 mV; p < 0.001, paired t test, n = 8). This depolarization led to enhancement of spontaneous spike activity (control: 0.2 ± 0.1 Hz, 5-HT: 3.9 ± 1.1 Hz; p < 0.01, n = 10; Fig. 1A,B). These (and subsequent) experiments were performed in the presence of blockers of ionotropic glutamate receptors, GABAA receptors, and glycine receptors, except as otherwise noted, and thus the excitatory actions of 5-HT are likely to be postsynaptic in nature. Moreover, the depolarization produced by 5-HT persisted in the presence of 1 μm TTX or blockers of synaptic transmission, again indicating a postsynaptic mechanism of 5-HT on fusiform cells. The 5-HT-elicited increase in firing rate was reversed by returning the membrane potential to control levels with a negative bias current (data not shown), indicating that it was the 5-HT-induced depolarization that initiated spike firing, probably at a region at or close to the cell body. To examine the nature of 5-HT actions without any disturbance to the intracellular environment, loose cell-attached voltage-clamp recordings were performed in fusiform cells. As with whole-cell recording, 5-HT (10 μm) significantly increased the spike rate (baseline: 5.2 ± 1.7 Hz, 5-HT, 10.6 ± 2.4 Hz; p < 0.01, n = 12; Fig. 1C,D). To examine how 5-HT affects the sensitivity of fusiform cells to current stimuli, a series of 1 s current steps were injected into fusiform cells, starting with −30 pA and incrementing by 20 pA, and spike rate at each current level was measured with and without 5-HT. These experiments showed that 5-HT shifted the input-output relation to the left in a dose-dependent manner (Fig. 1E,F). To determine the specificity of this effect we also looked at the action of 5-HT on cartwheel interneurons, which provide potent inhibition to fusiform cells, and found that no detectable effects on spike rate or membrane potential (data not shown).
Figure 1.
5-HT directly enhances the excitability of fusiform cells. A, C, 5-HT depolarized the membrane potential and increased spontaneous spike rate under whole-cell (A) and cell-attached (C) recordings. B, D, 5-HT significantly increased spontaneous spike rate both under whole-cell (n = 10) and cell-attached (n = 12) conditions. E, F, Current-clamp recordings from a fusiform cell (control: black traces; in the presence of 1 and 10 μm 5-HT: red and blue traces, respectively), showing responses to increasing current injections into soma (starting with −30 pA and increasing by 20 pA, 1 s duration). E, Representative traces of action potential firing elicited by somatic injection of current pulses illustrating the effects of 1 or 10 μm 5-HT. F, Firing frequency as a function of injection current amplitude (n = 8). Negative bias current (∼−20 pA under the threshold of action potential) was used to prevent or reduce spontaneous firing. Error bars represent ± SEM; *p < 0.05, **p < 0.01 (paired t test, unless indicated otherwise).
5-HT-evoked response is mediated by 5-HT2A/2CR and 5-HT7R
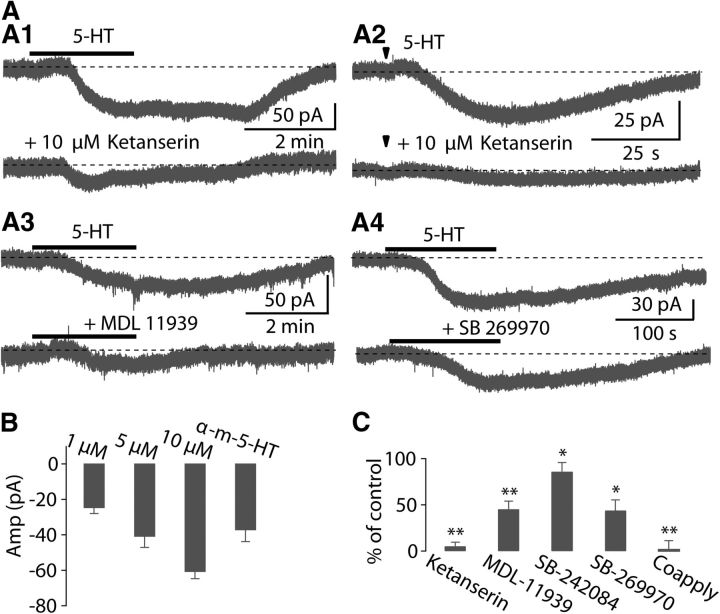
Consistent with the current-clamp data, fusiform cells voltage-clamped at holding potentials of −70 mV or close to the resting membrane potential, responded to bath application (1, 5, or 10 μm) or iontophoresis (50 mm) of 5-HT with a slow, steady, and reversible inward current (Fig. 2A,B). The pharmacological identification of 5-HT receptor subtypes mediating the excitatory effects of 5-HT was investigated by using specific agonists and antagonists for 5-HT receptors in voltage-clamped cells. Previous studies have shown that 5-HT can produce a slow depolarization of the resting membrane potential by activation of 5-HT2R (5-HT2 receptor) in cortical neurons (Pierce and Peroutka, 1990; Araneda and Andrade, 1991; Tanaka and North, 1993; Zhang, 2003). Moreover, 5-HT2AR and 5-HT2CR are present in cochlear nucleus (Pazos et al., 1985; Thompson et al., 1994; Wright et al., 1995; Cornea-Hébert et al., 1999), We therefore asked whether these receptors mediate the 5-HT response in DCN. Application of the 5-HT2AR antagonist ketanserin at 10 μm largely blocked the inward current elicited by bath application (10 μm) or iontophoresis (50 mm) of 5-HT (control: −60.2 ± 9.5 pA, ketanserin: −2.2 ± 2.6 pA; p < 0.001, n = 9; Fig. 2A1,A2,C), suggesting that the inward current may be mediated by 5-HT2AR. However, 2 μm ketanserin only partially blocked the response (data not shown), and a similar partial block was achieved with the selective 5-HT2AR antagonist MDL-11939 (2 μm; 45.3 ± 8.6% of control, p < 0.05, n = 11; Fig. 2A3,C). Moreover, the 5-HT2CR antagonist SB-242084 (2 μm) slightly suppressed the 5-HT response (86.0 ± 7.9% of control, p < 0.05 n = 6; Fig. 2C). Additionally, application of 25 μm α-methyl-5-HT, a selective agonist of 5-HT2R, induced an inward current (−37.0 ± 6.8 pA; n = 4; Fig. 2B) similar to that of 5-HT. These data suggest that a combination of 5-HT2A/2CRs contribute to the 5-HT-evoked response.
Figure 2.
5-HT2A/2CR and 5-HT7R are largely responsible for the inward current produced by 5-HT. A, Under voltage-clamp, representative traces of slow inward currents elicited by bath application of 10 μm or iontophoresis of 50 mm 5-HT were almost completely abolished by 10 μm ketanserin (A1, A2), partially blocked by 2 μm MDL-11939 (A3), or 1 μm SB-269970 (A4). Iontophoresis of 5-HT indicated by arrowhead. In this figure and following figures, gray dashed lines denote basal holding currents or the resting membrane potential before application of 5-HT. B, Summary of currents evoked by 5-HT (1 μm, n = 7; 5 μm, n = 7; 10 μm, n = 35), and α-methyl-5-HT (25 μm, n = 4). C, Summary of effects of ketanserin (n = 9), MDL-11939 (n = 11), SB-242084 (n = 6), SB-269970 (n = 6), and coapplication of SB-269970 plus MDL-11939 (n = 4) on the 5-HT-induced current. Error bars are ± SEM.
However, the inward current was not fully blocked by the 5-HT2AR antagonist MDL-11939 plus 5-HT2CR antagonist SB-242084 (31.5 ± 13.9% of control, p < 0.05, n = 3). Previous studies have shown that ketanserin also exhibits affinity for 5-HT7R (5-HT7 receptor) (Shen et al., 1993; Jasper et al., 1997; Adham et al., 1998). Because ketanserin at high concentration (10 μm) could almost completely block the 5-HT-evoked response, we wondered whether ketanserin was also blocking 5-HT7R response to 5-HT. Indeed, it is well established that activation of 5-HT7R can mediate a depolarizing effect on some central neurons (Cardenas et al., 1999; Chapin and Andrade, 2001a, b; Béïque et al., 2004). Therefore, we examined the effects of SB-269970, a potent and selective antagonist for 5-HT7R, on the 5-HT-evoked current. We found that SB-269970 (1 μm) partially suppressed the 5-HT-evoked current (43.9 ± 11.3% of control; p < 0.01, n = 6; Fig. 2A4,C). In addition, coapplication of SB-269970 and MDL-11939 completely blocked the 5-HT current (2.0 ± 9.4% of control; p < 0.01, n = 4; Fig. 2C). These data suggest that the 5-HT response results from the coactivation of 5-HT2A/2CR and 5-HT7R. Immunochemical studies have shown that 5-HT1AR is also expresses in the cochlear nucleus (Pazos et al., 1985; Wright et al., 1995; Thompson and Wiechmann, 2002), although it is unclear in what cell types. However, WAY-100135 (10 μm), a selective antagonist for 5-HT1AR, did not affect the 5-HT current (100.6 ± 15.1% of the control; p > 0.05, n = 3), suggesting that 5-HT1AR are not involved in the effects of 5-HT on fusiform cells. Overall, these results indicate that activation of both 5-HT2A/2CR and 5-HT7R result in excitation of fusiform cells.
HCN channels are the downstream targets of serotonergic signaling
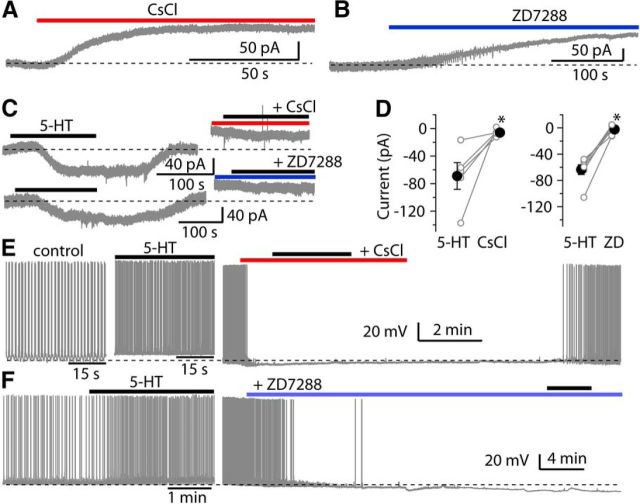
Fusiform cells express a wide array of ion channels, including Na+, Ca2+, Kir, KA, KCNQ, and HCN channels, the latter being the channel subtype that generates the Ih current (Hirsch and Oertel, 1988; Harasztosi et al., 1999; Kanold and Manis, 1999; Molitor and Manis, 2003; Pál et al., 2003; Leao et al., 2012). To determine what ion channels are responsible for the 5-HT response, the effects of blockers of Na+, Ca2+, K+, and HCN channels were tested on the 5-HT-induced current. Of these blockers, TTX, Cd2+, Ba2+, and XE991 failed to reduce the 5-HT-induced current (data not shown). However, Cs+ and ZD7288, selective blockers of HCN channels, completely inhibited the 5-HT response (Fig. 3). Moreover, bath application of either 2 mm Cs+ or 10 μm ZD7288 alone induced an outward shift of holding current under voltage-clamp (Fig. 3A,B). Because 5-HTR (5-HT receptor) antagonists by themselves had no effect on holding current, this result indicates that HCN channels are active partially at rest, and indeed contribute to the resting membrane potential and regulate the excitability of fusiform cells, consistent with the previous studies (Pál et al., 2003; Apostolides and Trussell, 2014). We further compared the inward current evoked by 5-HT before and after application of 2 mm Cs+ or 10 μm ZD7288. The inward current evoked by 5-HT was completely blocked by Cs+ (control: −68.8 ± 19.5 pA, Cs+: −5.7 ± 2.3 pA; p < 0.05, n = 5; Fig. 3C,D) or ZD7288 (control: −64.3 ± 8.0 pA, 10 min after applying ZD7288: −2.2 ± 3.0 pA; p < 0.001, n = 7). Similarly, under current-clamp conditions, bath application of either Cs+ or ZD7288 alone caused a hyperpolarization of the membrane potential, and blocked spontaneous spiking firing, and also abolished the effects of 5-HT on the membrane potential or spike activity (Fig. 3E,F). These data indicate that HCN channels are the primary downstream targets for these observed excitatory actions of 5-HT on fusiform cells.
Figure 3.
HCN channels are important determinants of intrinsic excitability and the downstream targets of 5-HT-induced response. A, B, Representative traces showing that bath application of 2 mm Cs+ or 10 μm ZD7288 induced an outward shift of holding current in fusiform cells. C, Response to 10 μm 5-HT was blocked by HCN channel blockers 2 mm Cs+ or 10 μm ZD7288, (top and bottom traces, respectively). D, Summary of the effects of Cs+ (n = 5) and ZD7288 (n = 7) on the 5-HT evoked current. Open symbols represent 5-HT currents of individual neurons, and filled symbols represent the mean of 5-HT currents. E, Under current-clamp, representative traces from a fusiform cell showing 5-HT increased the spike firing (left trace: control; middle trace: 5-HT application), and application of Cs+ produces a hyperpolarization of membrane potential and also suppressed the increase in the spike firing by 5-HT (right trace). F, Similarly, representative traces from another fusiform cell showing that 5-HT increased the spike firing (left trace), and application of ZD7288 produced a hyperpolarization of membrane potential and also suppressed the increase in the spike firing by 5-HT (right trace). Error bars are ± SEM.
We next explored how 5-HT affected the biophysical properties of Ih by measuring the voltage dependence of Ih from the amplitude of tail current relaxations. The experiments were performed in ACSF that contains 1 μm TTX and 200 μm Ba2+, to block Na+ channels and inward rectifier K+ channels. Activation curves for Ih were constructed by applying a series of voltage steps from a holding potential at −60 mV to various levels to activate Ih, and then activation voltage steps were followed by a test pulse to −75 mV with minimal contamination of the tail currents. The amplitude of tail current is proportional to the level of Ih conductance for a given prepulse voltage step. Activation curves for Ih were obtained by plotting tail current amplitudes against initial step potential, and then fitting with a Boltzmann function (see Materials and Methods). Application of 10 μm 5-HT resulted in a 5.6 mV positive shift of the activation curve of Ih on the voltage axis (control: −93.9 ± 2.2 mV, 5-HT: −88.3 ± 1.8 mV; p < 0.05, t test, n = 7; Fig. 4A–C) without significantly affecting the amplitude of maximal tail current (control: −418.1 ± 84.7 pA, 5-HT: −439.8 ± 96.6; p > 0.05, n = 7), suggesting that the enhancement of Ih resulted from a shift in the activation properties of the Ih, but not from an increase in the maximal level Ih activation. Accompanying this shift in the activation curve of Ih was a reduction in the time constant for Ih activation at any given potential by 5-HT. We characterized this effect of 5-HT by applying a 10 s voltage step to fully activate the Ih at −80 mV and −90 mV, and then comparing the time constant of Ih before and after 5-HT application. The activation time course of Ih was significantly faster after applying 5-HT at holding potentials of −80 mV (τfast control: 695.7 ± 28.3 ms, 5-HT: 538.8 ± 55.7 ms, p < 0.01; τslow control: 5.7 ± 0.9 s, 5-HT: 4.2 ± 0.5 s, p < 0.05; n = 4; Fig. 4D,E), or −90 mV (τfast control: 544.2 ± 27.6 ms, 5-HT: 421.6 ± 58.6 ms, p < 0.05; τslow control: 4.4 ± 0.3 s, 5-HT: 3.0 ± 0.2 s, p < 0.01; n = 4). These results indicate that excitation of fusiform cells by 5-HT is accompanied by an acceleration of HCN channel gating and shift in voltage sensitivity.
Serotonergic regulation of excitability is G-protein-dependent and involves cAMP and Src kinase signaling pathways
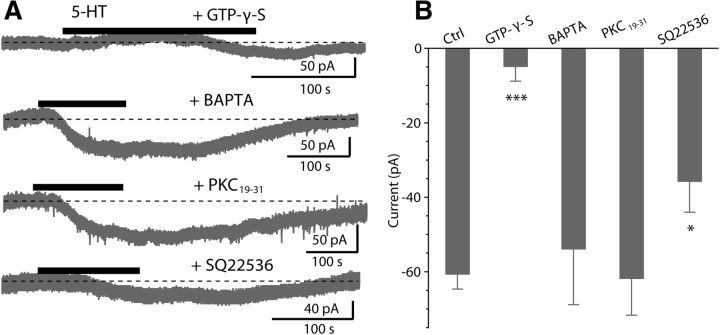
We next determined what signaling pathways couple 5-HTR to Ih. Both 5-HT2A/2CR and 5-HT7R are G-protein-coupled receptors (GPCRs). To confirm that G-proteins are required for the 5-HT-induced response, we replaced the GTP in the internal recording solution with 1.5 mm GTP-γ-S, a nonhydrolysable GTP analog that should alter G-proteins signaling and potentially disrupt the ability of 5-HT2A/2CR and 5-HT7R to evoke an inward current. Indeed, in the presence of GTP-γ-S, 5-HT failed to induce an inward current (−4.8 ± 4.0 pA, n = 7; Fig. 5A,B), suggesting that the 5-HT-evoked current is mediated by a G-protein-dependent pathway.
Figure 5.
A G-protein-dependent transduction mechanism. A, Thirty minutes after establishing the whole-cell configuration, application of 10 μm 5-HT evoked a response with intracellular perfusions of 1.5 mm GTP-γ-S, 10 mm BAPTA, 10 μm PKC19–31 and 1 mm SQ22536. B, Pooled data for the 5-HT-evoked current under normal internal recording solution (n = 35) or during intracellular dialysis of GTP-γ-S (n = 7), BAPTA (n = 5), PKC19–31 (n = 6), and SQ22536 (n = 5). Bars represent mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired t test).
5-HT2A/2CR are known to couple to Gq to activate PLC (phospholipase C), leading to the release of IP3 (inositol-1,4,5-trisphosphate) and DAG (diacylglycerol; Hoyer et al., 1994). PLC-mediated IP3 might initiate the intracellular Ca2+ release from intracellular endoplasmic reticulum stores, and increase Ca2+ could enhance the Ih (Lüthi and McCormick, 1999). To test this possibility, we determined the effects of intracellular BAPTA on the 5-HT-induced current. When recorded cells were dialyzed with 10 mm BAPTA, 5-HT still induced a robust inward current (−53.8 ± 15.0 pA, p > 0.05, unpaired t test, n = 5; Fig. 5A,B) compared with the control conditions (−60.6 ± 4.1 pA, n = 35), suggesting a Ca2+ independent pathway. In addition, PLC-mediated DAG might activate the protein kinase C (PKC), and PKC activation could affect the Ih (He et al., 2014). To determine the role of PKC, we included a PKC inhibitor peptide PKC19–31 in the recording pipette and waited >30 min after an establishing whole-cell recording, a procedure that effectively blocks PKC activity in other DCN neurons (Bender et al., 2010). However, intracellular dialysis with 10 μm PKC19–31 failed to block the 5-HT-evoked current (−61.7 ± 9.9 pA, p > 0.05, unpaired t test, n = 6; Fig. 5A,B), compared with the current recorded with normal internal solutions (−60.6 ± 4.1 pA, n = 35), suggesting that PLC/PKC signaling pathway is not involved in 5-HT2A/2CR signaling. Altogether, these data suggest that serotonergic signaling in fusiform cells does not require a PLC-mediated pathway.
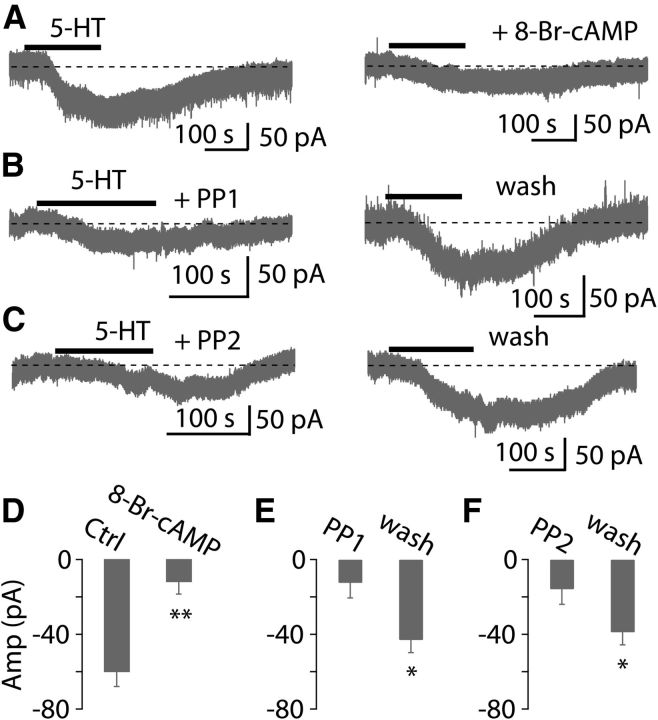
5-HT7R is known to couple to Gs, and stimulation of Gs leads to activation of adenylyl cyclase and consequently an increase in intracellular cAMP (Hoyer et al., 1994). In addition, it is well known that Ih is often sensitive to intracellular cAMP (Banks et al., 1993; He et al., 2014). Thus, we asked whether intracellular cAMP signaling via activation of 5-HT7R is involved in the augmentation of Ih. To test this possibility, we examined the effects of inhibitors and activators of the cAMP pathway on the 5-HT current. First, we included in the recording pipette an inhibitor of adenylyl cyclase SQ22536 (1 mm). Under these conditions, the 5-HT current was partially attenuated (−35.7 ± 8.4 pA, p < 0.05, unpaired t test, n = 5; Fig. 5A,B), compared with control conditions (−60.6 ± 4.1 pA, n = 35), suggesting a role for adenylyl cyclase. To further investigate whether cAMP is responsible for the effects of 5-HT on the fusiform cells, we examined the effects of a membrane-permeable cAMP analog 8-Br-cAMP, an adenylate cyclase activator, on the 5-HT-evoked current. Bath application of 8-Br-cAMP (0.5–1.0 mm) induced an inward current in most cells recorded, as expected for an activator of Ih, and moreover resulted in a large reduction of the 5-HT-induced current (control: −59.8 ± 8.2 pA, 20 min after applying 8-Br-cAMP: −11.8 ± 6.8 pA; p < 0.01, n = 8; Fig. 6A,D). These results are consistent with the idea that cAMP signaling pathway is partially involved in the excitatory effects of 5-HT on fusiform cells through activation of 5-HT7R.
Figure 6.
cAMP and Src kinase activities are involved in the serotonergic signaling. A, Left, Application of 10 μm 5-HT induced an inward current. Right, Twenty minute bath application of 1 mm 8-Br-cAMP, a membrane-permeable cAMP analog, largely blocked this inward current. B, C, Left, Preincubation of PP1 or PP2, selective Src kinase inhibitors, for >1 h, application of 10 μm 5-HT induced a small inward current. Right, After 1 h washout of PP1 and PP2 (20 μM), application of 5-HT induced a larger inward current. D–F, Pooled data showing the 5-HT-evoked current in the presence and absence of 8-Br-cAMP (n = 8), PP1 (n = 7), and PP2 (n = 6). Bars are mean ± SEM.
Previous studies have shown that Src tyrosine kinase activity is involved in 5-HT2A/2CR signaling (González-Maeso et al., 2007; Lu et al., 2008; Schmid and Bohn, 2010; Bigford et al., 2012; Sung et al., 2013), and that Src tyrosine kinase activity could modulate HCN channels (Zong et al., 2005; Arinsburg et al., 2006; Li et al., 2008). To examine the possible contribution of Src tyrosine activity to 5-HT signaling in DCN, we assessed the effects of genistein, PP1, and PP2, selective inhibitors for Src kinase, on the 5-HT-induced inward current. Bath application of 30 μM genistein, a general inhibitor for Src kinase, partially suppressed the 5-HT-evoked current (74.6 ± 15.5% of control; p < 0.05, n = 5), suggesting that Src tyrosine kinase activity may be involved in the excitatory effects of 5-HT on fusiform cells. Further evidence consistent with this conclusion comes from the effects of PP1 and PP2 on the 5-HT-induced current. Slices were incubated with either PP1 or PP2 (both 20 μM), the selective inhibitors for Src family kinases, before application of 5-HT. Preincubation of PP1 or PP2 for >1 h reversibly blocked the inward current evoked by 5-HT (PP1 preincubation: −12.4 ± 3.6 pA, 1 h after wash of PP1: −43.0 ± 3.7 pA; p < 0.05, n = 7; PP2 preincubation: −15.8 ± 3.6 pA, 1 h after wash of PP2: −38.9 ± 9.7 pA; p < 0.05, n = 6; Fig. 6B–C,E–F). Together, these data suggest that serotonergic regulation of neuronal excitability of fusiform cells is G-protein-dependent, and involves cAMP and Src signaling-dependent pathways through activation of 5-HT7R and 5-HT2A/2CR, thereby enhancing resting activation of Ih.
Stimulation of serotonergic afferents regulates excitability of fusiform cells
While the data so far demonstrate that exogenous 5-HT enhances the excitability of DCN neurons, it is important to ask whether endogenous transmitter released from serotonergic afferent fibers has actions similar to that of exogenous 5-HT. To test this, we took advantage of a Tph2-ChR2-YFP mouse line in which the light activated cation channel ChR2 is expressed selectively in serotonergic neurons (Zhao et al., 2011). We first confirmed that ChR2-EYFP-positive fibers were present in the DCN by labeling sectioned material with a fluorescent antibody which recognizes EYFP (Zhao et al., 2011; see Materials and Methods). Fibers were abundantly present in the cell body and deep layers of the DCN, but nearly absent from the molecular layer (Fig. 7A,B). A similar pattern of serotonergic innervation of the DCN observed in the Mexican free-tailed bat, labeled fibers are much higher density in fusiform cell layer than in the molecular layer (Hurley and Thompson, 2001). In contrast, fibers are densely concentrated in the molecular layer but less heavily in the fusiform cell layer in rat, cat, guinea pig, and opossum (Willard et al., 1984; Klepper and Herbert, 1991; Thompson et al., 1995; Thompson and Thompson, 2001), suggesting species differences in fiber distribution. Interestingly, label was also absent in the cerebellar molecular layer but apparent in the granule cell region (Fig. 7A), which is consistent with the observations in some species (Dieudonné, 2001). We next examined whether optical simulation of serotonergic axonal terminals in brain slices could produce a response in voltage-clamped fusiform cells. Photostimulation with a single 5 ms blue light pulse did not induce detectable responses; however, tetanic photostimulation (20, 50, or 100 Hz, 5 ms light pulses for 10–20 s) resulted in a slow inward current in a significant fraction of fusiform cells tested (33.3%, 18 of 54 cells; 10 μm fluoxetine was added into ACSF solutions to decrease rate of 5-HT clearance in some experiments). This result is consistent with the observation that the 5-HT release by activation of ChR2 depends on the duration of light exposure and light frequency (Dankoski and Wightman, 2013; Dugué et al., 2014; Miyazaki et al., 2014). This inward current was markedly reduced by 10 μm ketanserin (light stimulus: −30.1 ± 5.3 pA, ketanserin: −4.5 ± 3.6 pA; p < 0.05, n = 4; Fig. 8A,B), suggesting that the light-evoked slow responses were mediated by serotonergic transmission. These data also suggest that serotonergic transmission is intrinsically slow, consistent with a idea that 5-HT mostly acts via a volume transmission mode in the CNS (Bunin and Wightman, 1998; Ridet and Privat, 2000; Dieudonné, 2001). Consistent with this idea, nonjunctional varicosities have been observed in the DCN (Thompson et al., 1995). More importantly, in some cases, using current-clamp recording, tetanic photostimulation depolarized the cells sufficient to trigger or increases spontaneous spike firing (Fig. 8C). To further explore the effect of serotonergic axon stimulation on the excitability of fusiform cells, action potentials were elicited by injecting a series of current steps (−10 to +50 pA, 500–1000 ms, ΔI = 20 pA) before and after light stimulus. Photostimulation increased the firing rate upon 10 or 30 pA depolarizing steps in five of eight cells tested (10 pA baseline: 3.2 ± 1.0 Hz, light stimulus: 5.9 ± 1.0 Hz; 30 pA baseline: 9.6 ± 1.7 Hz, light stimulus: 14.2 ± 2.5 Hz; p < 0.05, n = 5; Fig. 8D,E). Thus, these data suggest that 5-HT is released from serotonergic fibers by optical stimulation in the DCN and can regulate the excitability of fusiform cells in a manner similar to that of exogenous 5-HT.
Figure 7.
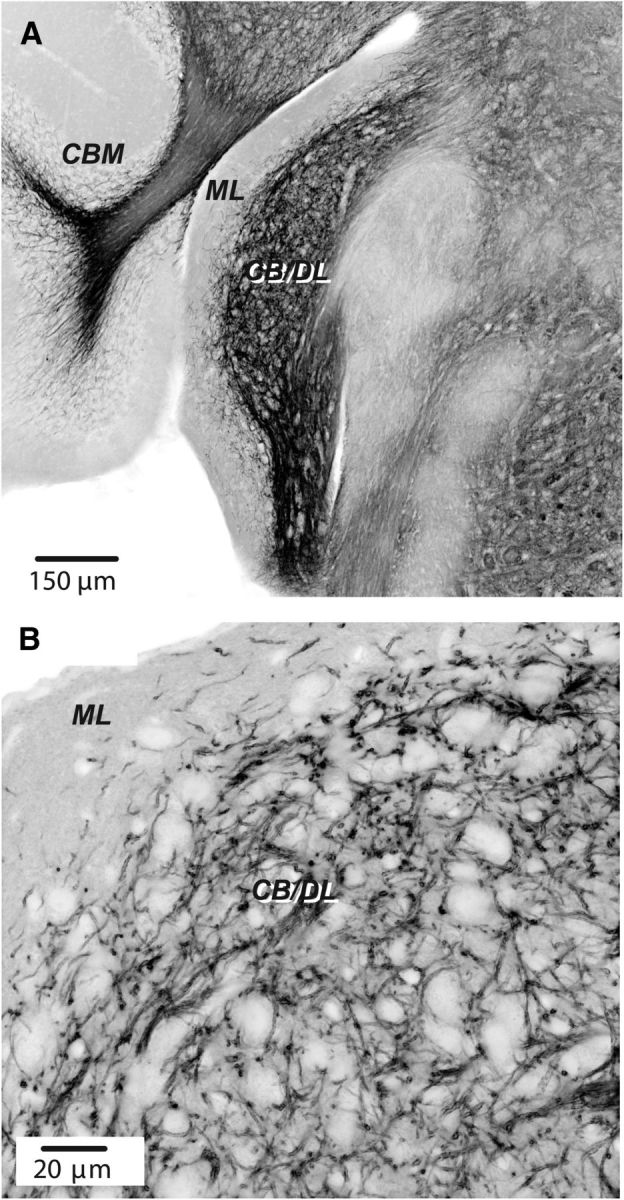
Expression of ChR2 in DCN in a Tph2-ChR2-EYFP mouse line. A, Section showing DCN and adjoining structures. Image printed in negative shows fluorescent labeling using antibody labeling for EYFP. ML, Molecular layer of DCN; CB/DL, fusiform cell body and deep layers; CBM, cerebellar cortex. Confocal image taken with a 10× objective. B, Labeling in DCN imaged using a 60× oil-immersion objective. Confocal image stack of four planes. Note preponderance of labeling up to the cell body layer and relative absence in the molecular layer.
Figure 8.
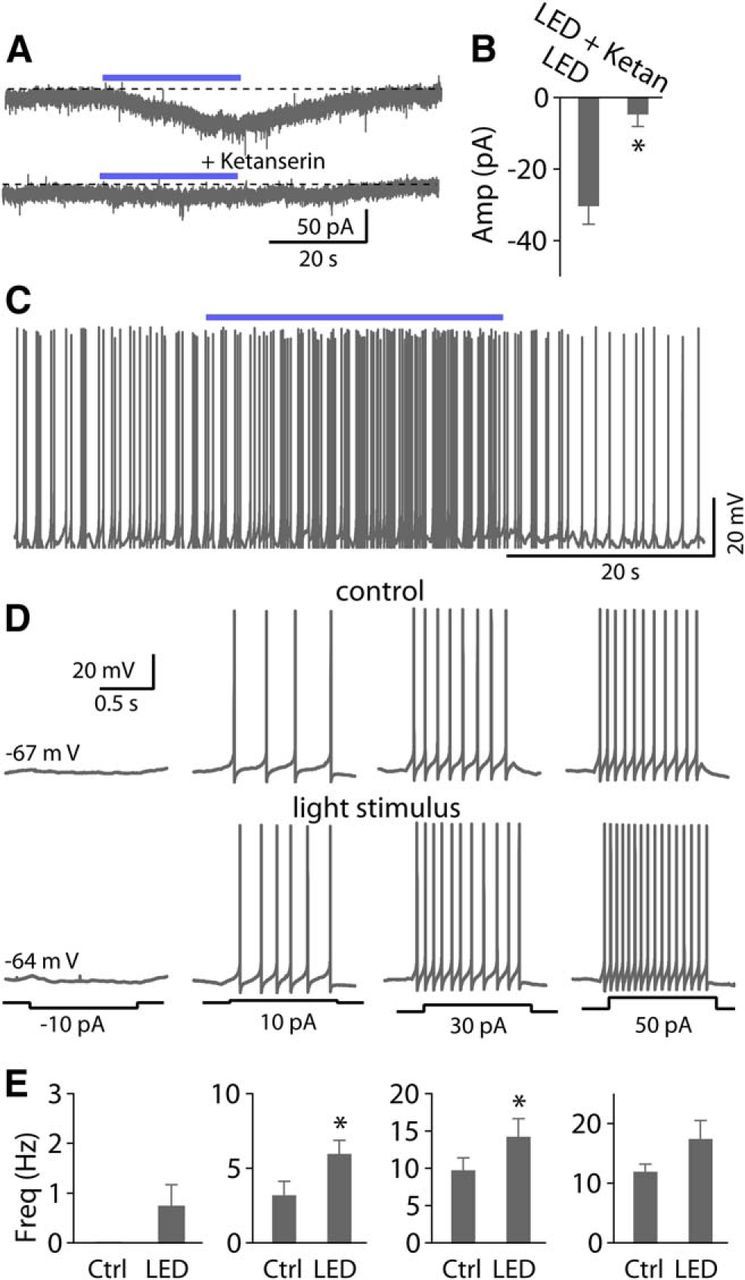
Photostimulation of serotonergic axons expressing ChR2 increases spike firing in fusiform cells. A, Sustained photostimulation (blue light pulse with 5 ms duration) elicited an inward current. Top trace, An inward current evoked by a 30 s train of light pulses at 100 Hz. Bottom trace, This current was blocked by 10 μm ketanserin. B, Pooled data show photostimulation-evoked currents were largely blocked by ketanserin (n = 4). C, Example traces from a fusiform cell show that tetanic photostimulation increases the firing rate. D, Representative traces of action potential firing elicited by somatic injection of current pulses (−10, 10, 30, and 50 pA, 1 s duration) illustrating the effects of photostimulation of serotonergic fibers. E, Pooled data show photostimulation of serotonergic fibers significantly increase the firing rate at 10 or 30 pA depolarizing steps (n = 5).
Discussion
Serotonergic regulation of fusiform cells
The DCN is composed of multiple cell types distributed in different sensory processing domains (Oertel and Young, 2004); knowing which cells are affected by 5-HT, and how, may allow the functional role of 5-HT to emerge. Serotonergic fibers densely innervate the cell layer that contains fusiform somata, and the molecular and deep layers that contain the dendrites of fusiform cells (Klepper and Herbert, 1991; Thompson et al., 1995; Hurley and Thompson, 2001; Thompson and Thompson, 2001). We found that after blocking fast synaptic inputs (glutamate, GABA, and glycine), exogenous or endogenous 5-HT depolarized fusiform cells and increased spontaneous spike rate, indicating a direct action on fusiform cell excitability. Therefore, fusiform principal cells in the DCN are a target of serotonergic raphe fibers. Although 5-HT regulates the excitability of fusiform cells by altering their intrinsic properties, it remains to be seen whether 5-HT also modulates synaptic function. Fusiform cells receive excitatory inputs from parallel fibers, auditory nerve and possibly T-stellate cells in the ventral cochlear nucleus (VCN; Oertel et al., 2011). Given that serotonergic fibers also strongly innervate the granule cell domain and VCN (Klepper and Herbert, 1991; Thompson et al., 1995; Thompson and Thompson, 2001), and that T-stellate cells are sensitive to 5-HT (Oertel et al., 2011), 5-HT may also modulate excitatory inputs to fusiform cells. Strong labeling for serotonergic fibers is observed in the DCN region containing inhibitory vertical cells. As serotonergic inputs could potentially contact vertical cells, it will be important to investigate whether 5-HT regulates the activity of vertical cells and their feedforward inhibition to fusiform cells.
Our data indicate that 5-HT2A/2CR and 5-HT7R mediate the effects of 5-HT on fusiform cells. The 5-HT response was partially blocked by the selective 5-HT2AR antagonist MDL-11939, slightly suppressed by 5-HT2CR antagonist SB-242084, and partially mimicked by the 5-HT2R agonist α-methyl-5-HT, indicating that the 5-HT response was partially mediated by some combination of 5-HT2A/2CR. Although ketanserin at high concentration (10 μm) almost completely blocked the response, it may also block 5-HT7R (Adham et al., 1998), suggesting that 5-HT7R may contribute to the 5-HT response. Indeed, the 5-HT-response was partially blocked by the 5-HT7R antagonist SB-269970, and the remaining currents was suppressed by MDL-11939. In addition, our data show that 8-Br-cAMP partially mimicked and occluded the 5-HT-evoked response, consistent with studies showing that 5-HT7R depolarizes neurons through activation of adenylyl cyclase and increase in cAMP (Hoyer et al., 1994). All these data suggest that the effects of 5-HT on fusiform cells are mediated probably by coactivation of 5-HT2A/2CR and 5-HT7R in individual neurons. In support, 5-HT2AR and 5-HT2CR are present in the DCN (Pazos et al., 1985; Thompson et al., 1994; Wright et al., 1995; Cornea-Hébert et al., 1999), and labeling of large, presumptive fusiform cells is apparent in DCN of Htr7-EGFP mice (www.gensat.org). Similar coexpression of 5-HT2AR and 5-HT7R in individual cells has been observed in other central neurons (Béïque et al., 2004; Bonsi et al., 2007).
Ionic mechanism and signal transduction pathways
Despite the multiplicity of receptors involved in the 5-HT response, HCN channels are likely to be entirely responsible for the resulting enhancement of fusiform cell excitability. Our data show that Ih is involved in setting resting potential and ongoing spontaneous firing. Pharmacological block of Ih with Cs+ or ZD7288 was sufficient to abolish the 5-HT response. 5-HT shifts positively the activation curve for Ih without altering maximal activation, thus activating more channels when the cell is at rest. Finally, 5-HT decreased activation time constants for Ih. Because Ih time constants increase with depolarization (Chen et al., 2001), a uniform decrease in time constants implies a rightward shift in the activation curve. Together, Ih is the primary downstream target of 5-HT in fusiform cells, as seen in several other regions of the CNS (Bobker and Williams, 1989; Pape and McCormick, 1989; Takahashi and Berger, 1990; Larkman and Kelly, 1992; Cardenas et al., 1999).
The actions of 5-HT were G-protein-dependent, probably engaging cAMP and Src kinase signaling pathways. 5-HT responses were abolished by GTP-γ-S, which arrests G-protein function. Furthermore, the adenylyl cyclase inhibitor SQ22536 partially blocked the 5-HT response. 8-Br-cAMP evoked an inward current and occluded the 5-HT response. Moreover, 5-HT's effects on Ih channel gating mirrored the effects of cAMP on these channels (Chen et al., 2001). Accordingly, we speculate that 5-HT7R could stimulate adenylate cyclase to upregulate intracellular cAMP. Increased cAMP may act directly on HCN, although an additional route involving phosphorylation cannot be excluded here. Intracellular dialysis of BAPTA or PKC19–31 did not affect the 5-HT current, excluding PLC/IP3/Ca2+ and PLC/PKC signaling involvement. Genistein, a tyrosine kinase inhibitor, partially blocked the 5-HT response, and preincubation of the Src kinase inhibitors PP1 or PP2 inhibited the 5-HT response. Thus, activation of 5-HT2A/2CR may also lead to increased Src kinase activity, and thereby enhance the Ih. Notably, Src kinase signaling pathways have been shown to modulate Ih in a manner similar to 5-HT and cAMP (Li et al., 2008; He et al., 2014). All these data suggest that serotonergic signaling in fusiform cells may be mediated by both multiple receptors and multiple signaling pathways.
Functional implications
Given the importance of the DCN in auditory function and tinnitus pathophysiology, the serotonergic regulation of neuronal excitability of the primary output neurons may have important outcomes.
Activation of the serotonergic raphe-DCN pathway could increase excitability and reduce acoustic thresholds of fusiform cells. It may be that this pathway functions as a “gain-setter” in controlling DCN output. Serotonergic neurons in the raphe nuclei fire at 3–5 Hz in quiet, awake animals (Trulson and Jacobs, 1979; Trulson and Trulson, 1982a; Rasmussen et al., 1986). In addition, the raphe-DCN pathway may be regulated by sensory inputs and behavioral state (Trulson and Trulson, 1982b). The serotonergic system receives direct input from inferior colliculus (Pollak Dorocic et al., 2014), and acoustic stimuli activate the serotonergic system (Trulson and Trulson, 1982b; Cransac et al., 1998), suggesting that acoustic stimuli might regulate the serotonergic raphe-DCN pathway. Increased activity of serotonergic neurons is associated with behavioral arousal. Thus, 5-HT release in response to sensory stimuli or behavioral events might dynamically regulate the excitability and acoustic threshold of fusiform neurons by modulating their membrane properties.
Aberrant serotonergic transmission at one or more levels in central auditory pathways might play a role in the pathogenesis of tinnitus. Although the mechanisms underlying tinnitus remain unclear, spontaneous hyperactivity in DCN fusiform cells is associated with tinnitus (Kaltenbach et al., 2005; Kaltenbach and Godfrey, 2008; Baizer et al., 2012). Our data show that 5-HT increases excitability of fusiform cells, and may lead to enhanced downstream excitation, consistent with previous in vivo studies suggesting that 5-HT2R and 5-HT7R play a role in the regulation of auditory network excitability (Bourson et al., 1997; Brennan et al., 1997; Applegate and Tecott, 1998; Holmes et al., 1998; Oliveira and Zatz, 1999). Thus, serotonergic dysfunction (e.g., upregulation of 5-HT levels, 5-HTR density or receptor sensitivity to 5-HT) in DCN might contribute to the generation and maintenance of tinnitus (Marriage and Barnes, 1995; Simpson and Davies, 2000; Caperton and Thompson, 2010; Hurley and Hall, 2011; Baizer et al., 2012). Accordingly, upregulation of extracellular 5-HT levels in DCN increases with noise exposure (Cransac et al., 1998), which can cause tinnitus (Kaltenbach, 2011). Notably, in spinal cord, 5-HT2R density and sensitivity to 5-HT can be upregulated following spinal injury (Murray et al., 2010; Husch et al., 2012). It remains to be investigated whether such modification of serotonergic activity could be induced following cochlear damage. Moreover, SSRIs are commonly used to treat tinnitus in patients with and without depression (Parnes, 1997; Andersson and McKenna, 1998; Folmer and Shi, 2004; Robinson et al., 2007; Fornaro and Martino, 2010; Baldo et al., 2012); however, the success of such treatments are inconsistent, and some patients reported a worsening of tinnitus. Acute treatment of SSRIs should increase extracellular concentrations of 5-HT, which based on our study, may then activate 5-HT2A/2CR and 5-HT7R and enhance DCN output. Responses to pharmaceutical agents may change as transmitter and receptor levels accommodate to the treatment. Thus, activation of 5-HT receptors in fusiform cells may be associated with the onset of a therapeutic response, indeed some patients report tinnitus after beginning SSRIs regiment (Robinson, 2007; Robinson et al., 2007). Perhaps therapeutic action of SSRIs on tinnitus results from secondary plasticity in 5-HT signaling (e.g., downregulation of 5-HT receptor) induced by boosting serotonergic activity after chronic SSRIs treatment. Indeed, it is well established that a decrease in 5-HT2A/2CR density can be produced by chronic administration of 5-HT and other 5-HT receptor agonists (Buckholtz et al., 1988; Eison et al., 1989; Anji et al., 2000), as well as antidepressants (Eison and Mullins, 1996). Thus, a long-term reduction in 5-HT2R density by chronic treatment with SSRIs might play a role in the therapeutic effects of SSRIs in tinnitus. The current study showing the action of 5-HT at a cellular level in the DCN may provide a basis for future studies exploring how SSRIs affect tinnitus.
Footnotes
This work was supported by NIH Grants NS028901, DC004450 (L.O.T.), Hearing Health Foundation (Z.-Q.T.), and Tartar Trust Fellowship (Z.-Q.T.). We thank Drs Daniel Yaeger and Pierre F. Apostolides for preliminary observations that led us to investigate the serotonergic modulation in DCN; Dr Carolina Borges-Merjane for immunohistochemistry; Drs Hai Huang, Hsin-Wei Lu, Pierre F. Apostolides, and Daniel Yaeger for technical advice; Ruby Larisch and Michael Bateschell for help with mouse colony management and genotyping; all members of the Trussell lab for helpful discussions; and Dr John T. Williams for critical comments on the paper.
The authors declare no competing financial interests.
References
- Adham N, Zgombick JM, Bard J, Branchek TA. Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J Pharmacol Exp Ther. 1998;287:508–514. [PubMed] [Google Scholar]
- Andersson G, McKenna L. Tinnitus masking and depression. Audiology. 1998;37:174–182. doi: 10.3109/00206099809072971. [DOI] [PubMed] [Google Scholar]
- Anji A, Kumari M, Sullivan Hanley NR, Bryan GL, Hensler JG. Regulation of 5-HT(2A) receptor mRNA levels and binding sites in rat frontal cortex by the agonist DOI and the antagonist mianserin. Neuropharmacology. 2000;39:1996–2005. doi: 10.1016/S0028-3908(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Rapid, activity-independent turnover of vesicular transmitter content at a mixed glycine/GABA synapse. J Neurosci. 2013;33:4768–4781. doi: 10.1523/JNEUROSCI.5555-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Control of interneuron firing by subthreshold synaptic potentials in principal cells of the dorsal cochlear nucleus. Neuron. 2014;83:324–330. doi: 10.1016/j.neuron.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate CD, Tecott LH. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol. 1998;154:522–530. doi: 10.1006/exnr.1998.6901. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-B. [DOI] [PubMed] [Google Scholar]
- Arinsburg SS, Cohen IS, Yu HG. Constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J Cardiovasc Pharmacol. 2006;47:578–586. doi: 10.1097/01.fjc.0000211740.47960.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizer JS, Manohar S, Paolone NA, Weinstock N, Salvi RJ. Understanding tinnitus: the dorsal cochlear nucleus, organization and plasticity. Brain Res. 2012;1485:40–53. doi: 10.1016/j.brainres.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo P, Doree C, Molin P, McFerran D, Cecco S. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2012;9:CD003853. doi: 10.1002/14651858.CD003853.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA, Smith PH. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol. 1993;70:1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Ford CP, Trussell LO. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron. 2010;68:500–511. doi: 10.1016/j.neuron.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigford GE, Chaudhry NS, Keane RW, Holohean AM. 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-d-aspartate GluN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J Biol Chem. 2012;287:11049–11059. doi: 10.1074/jbc.M111.277806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989;2:1535–1540. doi: 10.1016/0896-6273(89)90041-X. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32:1840–1854. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- Bourson A, Kapps V, Zwingelstein C, Rudler A, Boess FG, Sleight AJ. Correlation between 5-HT7 receptor affinity and protection against sound-induced seizures in DBA/2J mice. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:820–826. doi: 10.1007/PL00005123. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nat Genet. 1997;16:387–390. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol. 2012;13:55–66. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz NS, Zhou DF, Freedman DX. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1988;42:2439–2445. doi: 10.1016/0024-3205(88)90342-6. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caperton KK, Thompson AM. Activation of serotonergic neurons during salicylate-induced tinnitus. Laryngoscope. 2010;120:S203. doi: 10.1002/lary.21670. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus: I. Pharmacological characterization. J Pharmacol Exp Ther. 2001a;297:395–402. [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus: II. Involvement of the hyperpolarization-activated current I(h) J Pharmacol Exp Ther. 2001b;297:403–409. [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(SICI)1096-9861(19990628)409:2<187::AID-CNE2>3.0.CO%3B2-P. [DOI] [PubMed] [Google Scholar]
- Cransac H, Cottet-Emard JM, Pequignot JM, Peyrin L. Monoamines (noradrenaline, dopamine, serotonin) in the rat cochlear nuclei: endogenous levels and turnover. Hear Res. 1995;90:65–71. doi: 10.1016/0378-5955(95)00147-X. [DOI] [PubMed] [Google Scholar]
- Cransac H, Cottet-Emard JM, Hellström S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear Res. 1998;118:151–156. doi: 10.1016/S0378-5955(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dankoski EC, Wightman RM. Monitoring serotonin signaling on a subsecond time scale. Front Integr Neurosci. 2013;7:44. doi: 10.3389/fnint.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus–possible basis for tinnitus-related hyperactivity? J Neurosci. 2012;32:1660–1671. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Dieudonné S. Serotonergic neuromodulation in the cerebellar cortex: cellular, synaptic, and molecular basis. Neuroscientist. 2001;7:207–219. doi: 10.1177/107385840100700306. [DOI] [PubMed] [Google Scholar]
- Dugué GP, Lörincz ML, Lottem E, Audero E, Matias S, Correia PA, Léna C, Mainen ZF. Optogenetic recruitment of dorsal raphe serotonergic neurons acutely decreases mechanosensory responsivity in behaving mice. PloS One. 2014;9:e105941. doi: 10.1371/journal.pone.0105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci Lett. 1992;145:51–54. doi: 10.1016/0304-3940(92)90201-H. [DOI] [PubMed] [Google Scholar]
- Eison AS, Mullins UL. Regulation of central 5-HT2A receptors: a review of in vivo studies. Behav Brain Res. 1996;73:177–181. doi: 10.1016/0166-4328(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Yocca FD, Gianutsos G. Effects of imipramine and serotonin-2 agonists and antagonists on serotonin-2 and beta-adrenergic receptors following noradrenergic or serotonergic denervation. Life Sci. 1989;44:1419–1427. doi: 10.1016/0024-3205(89)90400-1. [DOI] [PubMed] [Google Scholar]
- Folmer RL, Shi YB. SSRI use by tinnitus patients: interactions between depression and tinnitus severity. Ear Nose Throat J. 2004;83:107–108. 110–112. [PubMed] [Google Scholar]
- Fornaro M, Martino M. Tinnitus psychopharmacology: a comprehensive review of its pathomechanisms and management. Neuropsychiatr Dis Treat. 2010;6:209–218. doi: 10.2147/ndt.s10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78:248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Harasztosi C, Forsythe ID, Szûcs G, Stanfield PR, Rusznák Z. Possible modulatory role of voltage-activated Ca(2+) currents determining the membrane properties of isolated pyramidal neurones of the rat dorsal cochlear nucleus. Brain Res. 1999;839:109–119. doi: 10.1016/S0006-8993(99)01723-0. [DOI] [PubMed] [Google Scholar]
- He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014;112:1–23. doi: 10.1016/j.pneurobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Oertel D. Intrinsic properties of neurones in the dorsal cochlear nucleus of mice, in vitro. J Physiol. 1988;396:535–548. doi: 10.1113/jphysiol.1988.sp016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genet. 1998;7:1507–1509. doi: 10.1093/hmg/7.9.1507. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson's disease. Prog Neurobiol. 2011;95:163–212. doi: 10.1016/j.pneurobio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Hall IC. Context-dependent modulation of auditory processing by serotonin. Hear Res. 2011;279:74–84. doi: 10.1016/j.heares.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. J Comp Neurol. 2001;435:78–88. doi: 10.1002/cne.1194. [DOI] [PubMed] [Google Scholar]
- Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N, Harris-Warrick RM. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012;32:13145–13154. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Bibikov NG, Poirier P, Samson FK. Directionality derived from pinna-cue spectral notches in cat dorsal cochlear nucleus. J Neurophysiol. 2000;83:907–925. doi: 10.1152/jn.2000.83.2.907. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b) Br J Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–568. doi: 10.1016/S0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol. 2008;17:S148–S161. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206:200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. J Neurosci. 1999;19:2195–2208. doi: 10.1523/JNEUROSCI.19-06-02195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-H. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013;33:19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol. 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol. 2012;107:3008–3019. doi: 10.1152/jn.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20:351–362. doi: 10.1016/S0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- Li CH, Zhang Q, Teng B, Mustafa SJ, Huang JY, Yu HG. Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am J Physiol Cell Physiol. 2008;294:C355–C362. doi: 10.1152/ajpcell.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110:9980–9985. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Alioua A, Kumar Y, Kundu P, Eghbali M, Weisstaub NV, Gingrich JA, Stefani E, Toro L. c-Src tyrosine kinase, a critical component for 5-HT2A receptor-mediated contraction in rat aorta. J Physiol. 2008;586:3855–3869. doi: 10.1113/jphysiol.2008.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/S0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Luo H, Pace E, Zhang X, Zhang J. Blast-induced tinnitus and spontaneous firing changes in the rat dorsal cochlear nucleus. J Neurosci Res. 2014;92:1466–1477. doi: 10.1002/jnr.23424. [DOI] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. Modulation of a pacemaker current through Ca(2+)-induced stimulation of cAMP production. Nat Neurosci. 1999;2:634–641. doi: 10.1038/10189. [DOI] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J Comp Neurol. 1994;348:261–276. doi: 10.1002/cne.903480208. [DOI] [PubMed] [Google Scholar]
- Marriage J, Barnes NM. Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction? J Laryngol Otol. 1995;109:915–921. doi: 10.1017/s0022215100131676. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res. 2000;148:74–87. doi: 10.1016/S0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF., 3rd Serotonin in aging, late-life depression, and Alzheimer's disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Dendritic Ca2+ transients evoked by action potentials in rat dorsal cochlear nucleus pyramidal and cartwheel neurons. J Neurophysiol. 2003;89:2225–2237. doi: 10.1152/jn.00709.2002. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R. The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res. 2011;276:61–69. doi: 10.1016/j.heares.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N, Kanzaki S, Shinden S, Saito H, Inoue Y, Ogawa K. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15:187–193. doi: 10.1159/000251916. [DOI] [PubMed] [Google Scholar]
- Oliveira JR, Zatz M. The study of genetic polymorphisms related to serotonin in Alzheimer's disease: a new perspective in a heterogenic disorder. Braz J Med Biol Res. 1999;32:463–467. doi: 10.1590/S0100-879X1999000400014. [DOI] [PubMed] [Google Scholar]
- Pál B, Pór A, Szucs G, Kovács I, Rusznák Z. HCN channels contribute to the intrinsic activity of cochlear pyramidal cells. Cell Mol Life Sci. 2003;60:2189–2199. doi: 10.1007/s00018-003-3187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain: a radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Parnes SM. Current concepts in the clinical management of patients with tinnitus. Eur Arch Otorhinolaryngol. 1997;254:406–409. doi: 10.1007/BF02439968. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain: II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Cotel F. Serotonin differentially modulates the intrinsic properties of spinal motoneurons from the adult turtle. J Physiol. 2008;586:1233–1238. doi: 10.1113/jphysiol.2007.145706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Antagonist properties of d-LSD at 5-hydroxytryptamine2 receptors. Neuropsychopharmacology. 1990;3:503–508. [PubMed] [Google Scholar]
- Pollak Dorocic I, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Strecker RE, Jacobs BL. Single unit response of noradrenergic, serotonergic and dopaminergic neurons in freely moving cats to simple sensory stimuli. Brain Res. 1986;369:336–340. doi: 10.1016/0006-8993(86)90546-9. [DOI] [PubMed] [Google Scholar]
- Revelis J, Thompson AM, Britton BH, Thompson GC. Effects of para-chlorophenylalanine (pCPA) on the bush baby auditory brainstem response. Hear Res. 1998;116:119–130. doi: 10.1016/S0378-5955(97)00210-4. [DOI] [PubMed] [Google Scholar]
- Ridet I, Privat A. Volume transmission. Trends Neurosci. 2000;23:58–59. doi: 10.1016/S0166-2236(99)01523-4. [DOI] [PubMed] [Google Scholar]
- Robinson S. Antidepressants for treatment of tinnitus. Prog Brain Res. 2007;166:263–271. doi: 10.1016/S0079-6123(07)66024-5. [DOI] [PubMed] [Google Scholar]
- Robinson SK, Viirre ES, Stein MB. Antidepressant therapy in tinnitus. Hear Res. 2007;226:221–231. doi: 10.1016/j.heares.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Salvinelli F, Casale M, Paparo F, Persico AM, Zini C. Subjective tinnitus, temporomandibular joint dysfunction, and serotonin modulation of neural plasticity: causal or casual triad? Med Hypotheses. 2003;61:446–448. doi: 10.1016/S0306-9877(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemen L. Fluoxetine for treatment of tinnitus. Otolaryngol Head Neck Surg. 1998;118:421. doi: 10.1016/S0194-5998(98)70332-8. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Simpson JJ, Davies WE. A review of evidence in support of a role for 5-HT in the perception of tinnitus. Hear Res. 2000;145:1–7. doi: 10.1016/S0378-5955(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Stark P, Fuller RW, Wong DT. The pharmacologic profile of fluoxetine. J Clin Psychiatry. 1985;46:7–13. [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Sung DJ, Noh HJ, Kim JG, Park SW, Kim B, Cho H, Bae YM. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive KV channels via the 5-HT2A receptor and Src tyrosine kinase. Exp Mol Med. 2013;45:e67. doi: 10.1038/emm.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB. Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res. 1998;120:86–108. doi: 10.1016/S0378-5955(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Serotonin projection patterns to the cochlear nucleus. Brain Res. 2001;907:195–207. doi: 10.1016/S0006-8993(01)02483-0. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Wiechmann AF. 5-HT(1A) receptor subtype mRNA expression in cochlear nucleus. Hear Res. 2002;164:77–81. doi: 10.1016/S0378-5955(01)00413-0. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngol Head Neck Surg. 1994;110:93–102. doi: 10.1016/S0194-5998(94)70797-9. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Moore KR, Thompson GC. Distribution and origin of serotoninergic afferents to guinea pig cochlear nucleus. J Comp Neurol. 1995;351:104–116. doi: 10.1002/cne.903510110. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson VM. Activity of nucleus raphe pallidus neurons across the sleep-waking cycle in freely moving cats. Brain Res. 1982a;237:232–237. doi: 10.1016/0006-8993(82)90572-8. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson VM. Differential effects of phasic auditory and visual stimuli on serotonergic neurons in the nucleus raphe dorsalis and nucleus raphe pallidus in freely moving cats. Neurosci Lett. 1982b;32:137–142. doi: 10.1016/0304-3940(82)90263-4. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FH, Ho RH, Martin GF. The neuronal types and the distribution of 5-hydroxytryptamine and enkephalin-like immunoreactive fibers in the dorsal cochlear nucleus of the North American opossum. Brain Res Bull. 1984;12:253–266. doi: 10.1016/0361-9230(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-O. [DOI] [PubMed] [Google Scholar]
- Wood WE, Roseberry TK, Perkel DJ. HTR2 receptors in a songbird premotor cortical-like area modulate spectral characteristics of zebra finch song. J Neurosci. 2013;33:2908–2915. doi: 10.1523/JNEUROSCI.4291-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J Neurophysiol. 1994;71:914–930. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]
- Zhang ZW. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci. 2003;23:3373–3384. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, Li R, Mistrik P, Gerstner A, Much B, Baumann L, Michalakis S, Zeng R, Chen Z, Biel M. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem. 2005;280:34224–34232. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]