Abstract
Tuberculosis is a chronic bacterial disease with a complex pathogenesis. An effective immunity against Mycobacterium tuberculosis requires both the innate and adaptive immune responses, including proper T helper (Th) type 1 cell function. FURIN is a proprotein convertase subtilisin/kexin (PCSK) enzyme, which is highly expressed in Th1 type cells. FURIN expression in T cells is essential for maintaining peripheral immune tolerance, but its role in the innate immunity and infections has remained elusive. Here, we utilized Mycobacterium marinum infection models in zebrafish (Danio rerio) to investigate how furin regulates host responses against mycobacteria. In steady-state furinAtd204e/+ fish reduced furinA mRNA levels associated with low granulocyte counts and elevated Th cell transcription factor expressions. Silencing furin genes reduced the survival of M. marinum-infected zebrafish embryos. A mycobacterial infection upregulated furinA in adult zebrafish, and infected furinAtd204e/+ mutants exhibited a proinflammatory phenotype characterized by elevated tumor necrosis factor a (tnfa), lymphotoxin alpha (lta) and interleukin 17a/f3 (il17a/f3) expression levels. The enhanced innate immune response in the furinAtd204e/+ mutants correlated with a significantly decreased bacterial burden in a chronic M. marinum infection model. Our data show that upregulated furinA expression can serve as a marker for mycobacterial disease, since it inhibits early host responses and consequently promotes bacterial growth in a chronic infection.
INTRODUCTION
Tuberculosis (TB) is an epidemic infectious disease caused by the mycobacterial species Mycobacterium tuberculosis (1, 2). Circa 13% of the individuals with active TB were simultaneous carriers of the human immunodeficiency virus (HIV), and almost one-third of TB-associated deaths occurred among HIV+ patients, demonstrating the critical role of cluster of differentiation 4 (CD4+) T lymphocyte-mediated immunity in the control of M. tuberculosis infection (3, 4). More specifically, the adaptive immunity against TB is primarily mediated by T helper (Th) type 1 cells, as is suggested by the gene expression profile upon infection (5), as well as the infection-induced mortality of gamma interferon-deficient (6, 7) and interleukin-12 (IL-12)-deficient (8) mice.
The proprotein convertase subtilisin/kexin (PCSK) enzymes are a family of serine endoproteases with nine members in humans: PCSK1 and -2, FURIN, PCSK4 to -7, membrane-bound transcription factor peptidase site 1 (MBTPS1), and PCSK9 (9). Typically, PCSKs convert precursor proteins (proproteins) into their biologically active forms by cleaving them at specific target motifs made up of the basic amino acids lysine and arginine (9, 10). FURIN was the first identified mammalian PCSK and is present in vertebrates and many invertebrates (11, 12). A series of in vitro experiments have suggested a central role for FURIN in host defense because it proteolytically activates several immunoregulatory proproteins, such as membrane-inserted matrix metallopeptidase 14 (13) and integrins (9), as well as tumor necrosis factor (TNF) and transforming growth factor beta (TGFB) family cytokines (e.g., the TNF superfamily, member 13b, and TGFB1) (12). In addition, infectious agents, including bacterial toxins (anthrax) and viral proteins (HIV gp160), are processed by FURIN (12).
Previously, we and others have shown that FURIN is predominantly expressed in Th1 cells and that FURIN expression is induced in activated CD4+ T lymphocytes and myeloid cells (14–17). Our functional analyses using mice with a tissue-specific deletion of Furin in T cells (CD4cre-furfl/fl) further demonstrated that FURIN is essential for the adequate maturation of pro-TGFB1 and for T regulatory (Treg) cell-mediated immune suppression in vivo (18). The breakage of peripheral immune tolerance in CD4cre-furfl/fl mice resulted in an age-related progression of a systemic autoimmune disease characterized by excessive numbers of overtly activated CD4+ and CD8+ T cells and an increase in proinflammatory cytokine production. In line with the critical role of FURIN in immune suppression, the administration of exogenous recombinant FURIN can alleviate autoimmunity in an experimental arthritis model (19). Notably, as a germ line Furin gene knockout (KO) in mice is lethal during embryonic development (20), the systemic role of FURIN in immune regulation and infections is still poorly understood.
Zebrafish (Danio rerio) is a small nonmammalian vertebrate model organism, with humoral and cellular components of the innate and adaptive immune systems similar to those of humans (21–24). Mycobacterium marinum, a close relative of M. tuberculosis, is a natural zebrafish pathogen and causes a mycobacterial disease, which shares the main pathological and histological features of human TB (25, 26). Consequently, an M. marinum infection in fish is considered a relevant, cost-effective and ethical tool for studying the human mycobacterial disease. Both embryo and adult zebrafish infection models are now well established; while embryos can be used to specifically investigate innate immune responses (27, 28), the adult model enables the study of a chronic progressive mycobacterial infection, as well as spontaneous latency (25, 29).
Genetic variation affects TB susceptibility in humans. To study mutant phenotypes of selected host genes, a large collection of mutant zebrafish strains is available (Zebrafish Mutation Project, Wellcome Trust Sanger Institute, Cambridge, United Kingdom). Zebrafish has two FURIN orthologs: furinA and furinB. furinA, like the mammalian FURIN gene, has a critical, nonredundant role in organism development (30). Here, we have silenced the expression of furin genes in developing fish and used a furinAtd204e/+ mutant zebrafish strain to study how FurinA regulates the development of adult zebrafish immune cells and the host response against mycobacteria.
MATERIALS AND METHODS
Zebrafish lines and maintenance.
Nine- to 16-month-old zebrafish were used in the adult experiments. furinAtd204e mutation-bearing zebrafish in an AB genetic background (Zebrafish Information Network [ZFIN] ID: ZDB-GENO-080606-310) were purchased from the Zebrafish International Resource Center (Oregon). The genotypes of the furinAtd204e/+ mutant zebrafish and their wild-type (WT) siblings were confirmed by sequencing (30). The fish were kept in a standardized flowthrough system (Aquatic Habitats, Florida, USA) with a light/dark cycle of 14 h and 10 h and fed with SDS 400 food twice a day. Until 7 days postfertilization (dpf), embryos were grown according to standard protocols in embryo medium (E3) at 28.5°C. The zebrafish housing, care, and all experiments have been approved by the National Animal Experiment Board of Finland (permits LSLH-2007-7254/Ym-23, ESAVI/6407/04.10.03/2012, ESAVI/733/04.10.07/2013, ESAVI/2267/04.10.03/2012, and ESAVI/8125/04.10.07/2013).
Flow cytometry.
Zebrafish were euthanized in a 0.04% 3-aminobenzoic acid ethyl ester anesthetic (pH 7.0; Sigma-Aldrich, Missouri, USA), and kidneys were isolated and homogenized into a single-cell suspension of phosphate-buffered saline with 0.5% fetal bovine serum (Gibco/Invitrogen, California, USA). Relative amounts of blood cell precursors, erythrocytes, granulocytes, and lymphocytes were determined by flow cytometry in steady-state (uninfected) furinAtd204e/+ mutants and WT controls by using a FACSCanto II (Becton Dickinson, New Jersey, USA). The data were analyzed with the FlowJo program (v7.5; Tree Star, Inc., Oregon, USA). Hematopoietic cell types were identified based on granularity (side scatter [SSC]) and particle size (forward scatter [FSC]) (31). Granulocytes and lymphocytes for furinA expression analyses were purified from WT AB zebrafish kidneys by using flow cytometric sorting with a FACSAria I apparatus (Becton Dickinson).
Experimental infections in adult zebrafish.
M. marinum (ATCC 927 strain) was cultured and the inoculation performed as described previously (25). In brief, the zebrafish were anesthetized with 0.02% 3-aminobenzoic acid ethyl ester and various amounts of M. marinum, together with 0.3 mg/ml phenol red (Sigma-Aldrich), were injected intraperitoneally (i.p.) using an Omnican 100 (30-gauge) insulin needle (Braun, Melsungen, Germany). The M. marinum CFU used in the infections were verified by plating serial dilutions on 7H10 agar plates. Infected fish were tracked daily, and humane endpoint criteria of the national ethical board were monitored.
MO and M. marinum coinjections.
Oligonucleotide sequences for furinA and furinB gene silencing morpholinos (MOs) and the injection protocol have been previously described (32). The injection volume was set to 2 nl, and 0.25 pmol of both furinA and furinB MOs or 0.5 pmol of RC MO was used. M. marinum was simultaneously coinjected into the yolk sac, and 2% polyvinylpyrrolidone was used as a carrier solution in the suspension (27, 33). Survival was analyzed daily with a visual inspection with an Olympus IX71 microscope.
Histology.
The presence of M. marinum in infected adult zebrafish was verified with a histological analysis and Ziehl-Neelsen staining (25, 34). Uninfected controls were included to exclude background mycobacterial infection. Sections were visualized with an Olympus BX51 microscope and Olympus ColorView IIIu camera using a ×100 magnification or with a fully automated Objective Imaging Surveyor virtual slide scanner (Objective Imaging, Cambridge, United Kingdom). Digitization of scanned sample sections was done at a resolution of 0.4 μm per pixel using a 20× Plan Apochromatic microscope objective, and image data were converted to JPEG2000 format as described previously (35).
qRT-PCR.
RNA and/or DNA was isolated from kidneys, lymphocytes, granulocytes, and the tissue homogenates of organs in the abdominal cavity using an RNeasy RNA purification kit (Qiagen, Hilden, Germany) or with an RNA-DNA coextraction method for TRIreagent (Molecular Research Center, Ohio, USA). The relative mRNA levels of target genes were quantified from cDNA with quantitative real-time PCR (qRT-PCR). The reverse transcription was done with an iScript Select cDNA synthesis kit (Bio-Rad, California, USA). Maxima SYBR green qPCR master mix (Fermentas, Burlington, Canada) and a CFX96 qPCR machine (Bio-Rad) were used. Primer sequences and ZFIN identification codes for the qRT-PCR-analyzed genes are listed in Table S1 in the supplemental material. The expression of target genes was normalized to the expression of eukaryotic translation elongation factor 1 alpha 1, like 1 (eef1a1l1 or ef1a) (36). Whenever the RNA-DNA coextraction method was used, the total DNA was isolated simultaneously with the RNA to quantify the M. marinum load in the fish with qRT-PCR (25). The results were analyzed with the Bio-Rad CFX Manager software v1.6 (Bio-Rad). No template control samples (H2O) were included in all experiments to monitor contamination. Melting curve analyses, followed by 1.5% agarose (Bioline, London, United Kingdom) gel electrophoresis, were done to validate the qRT-PCR products of the target genes.
Statistical analysis.
Statistical analyses were performed with the Prism v5.02 program (GraphPad Software, Inc., California, USA). A log-rank (Mantel-Cox) test was used in the survival experiments and a nonparametric Mann-Whitney analysis in the flow cytometry and qRT-PCR experiments. P values of <0.05 were considered significant.
RESULTS
furinA is upregulated in a mycobacterial infection and it controls granulopoiesis and Th cell transcription factor expression.
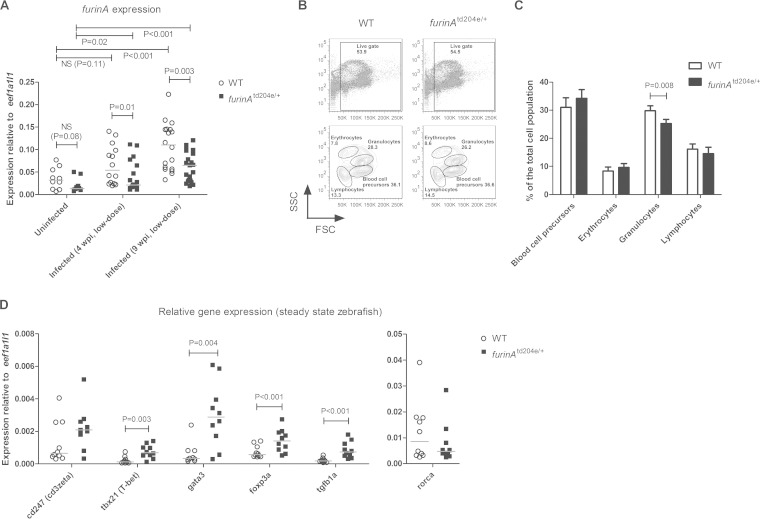
In the furinAtd204e mutant fish, a specific thymidine (T)-to-adenosine (A) splice site mutation results in a skipped exon 9 during the transcription of the furinA gene (see Fig. S1 in the supplemental material) (30). This leads to a loss-of-function FurinA mutant protein and enables the design of qRT-PCR primers, which can be used to specifically quantify native furinA mRNA molecules. In accordance with the developmental lethality of other homozygous furinA zebrafish mutants (>98% lethality of furinAtg419/tg419 mutant) (30), no homozygous furinAtd204e/td204e mutant fish could be obtained in our fish crosses (up to ∼450 genotyped fish), suggesting that in homozygous form this allele is also lethal. In contrast, the heterozygous furinAtd204e/+ mutants were born in normal Mendelian ratios and did not show signs of developmental defects or spontaneous autoimmunity. First, to determine the effect of a heterozygous furinAtd204e mutation on mRNA levels, furinA expression in uninfected and M. marinum-infected adult fish (4 and 9 weeks postinfection [wpi], low dose; 34 ± 10 CFU) was quantified with qRT-PCR (Fig. 1A). Previously, in vitro analyses have shown that FURIN expression is upregulated as a result of CD4+ T cell activation and in lipopolysaccharide (LPS)-stimulated CD14+ myeloid cells (14, 15). In accordance with this, the M. marinum infection caused an induction in furinA mRNA expression in both furinAtd204e/+ (1.6-fold at 4 wpi, P = 0.02, and 4.9-fold at 9 wpi, P < 0.001) and WT zebrafish (1.7-fold at 4 wpi, not significant [NS], P = 0.11; 3.4-fold at 9 wpi, P < 0.001) demonstrating that immune activation in vivo upregulates this convertase. Furthermore, in the infected groups, furinAtd204e/+ fish had on average 39% (P = 0.01) and 43% less (P = 0.003) furinA mRNA compared to WT controls at 4 and 9 wpi, respectively. A similar trend was also observed in uninfected zebrafish with a 44% decrease in furinA expression (NS, P = 0.08). Put together, the data indicate that furinA is upregulated in response to a mycobacterial infection, and that the furinAtd204e/+ zebrafish can be used to explore the functional role of this PCSK in a mycobacterial infection in vivo.
FIG 1.
furinA expression is reduced in furinAtd204e/+ zebrafish and associates with decreased granulocyte counts, as well as altered T helper cell subtype transcription factor expression. (A) Relative furinA expression was measured in uninfected (n = 10) and M. marinum-infected (at 4 and 9 wpi, low-dose, n = 13 to 21) furinAtd204e/+ mutant adult zebrafish and WT controls with qRT-PCR. Samples were run as technical duplicates. (B and C) The relative percentages of blood cell precursors, erythrocytes, granulocytes, and lymphocytes were determined in the kidneys of steady-state (uninfected) furinAtd204e/+ mutants and WT zebrafish (n = 5 in both groups) with flow cytometry, based on granularity (SSC) and cell size (FSC). Representative flow cytometry plots are shown in panel B. Gated populations are outlined, and the cell counts inside the gates are given as the percentages of the total viable cell population. The average relative percentages of different hematopoietic cell populations in mutants and controls are plotted in panel C (error bars indicate the standard deviations). (D) Relative expressions of different Th cell-associated genes (cd247, tbx21, gata3, foxp3a, and rorca), as well as tgfb1a, were quantified in furinAtd204e/+ mutants and WT controls (n = 10 in both groups) with qRT-PCR. Gene expressions in panels A and D were normalized to eef1a1l1 expression and represented as a scatter dot plot and median. In panel A, a one-tailed Mann-Whitney test was used in the statistical comparison of differences between furinAtd204e/+ zebrafish and WT controls, and a two-tailed Mann-Whitney test was used in panels C and D, as well as in the comparisons between uninfected and infected experimental groups in panel A.
The development of hematopoietic cells in zebrafish is highly similar to that in humans (31, 37). To assess the effect of the reduced furinA expression on hematopoiesis in the furinAtd204e/+ fish, we studied their blood cell composition with flow cytometry (Fig. 1B and C) (31). The flow cytometric analysis revealed no marked differences in blood cell precursor, erythrocyte or lymphocyte populations in furinAtd204e/+ zebrafish compared to WT controls. However, the amount of granulocytes in furinAtd204e/+ fish was significantly decreased, by an average of 15.4% (P = 0.008), compared to controls, indicating a role for FurinA in granulopoiesis.
Previously, we showed that FURIN is critical for normal mammalian Th polarization and CD4+ Treg cell function; CD4cre-furfl/fl mice have abnormally large effector CD4+ and Treg cell populations accompanied with an excessive production of Th1 and Th2 cytokines (15, 18). To address whether FurinA regulates the generation of Th subsets in zebrafish, we assessed the expression of different T cell markers (CD247 antigen; cd247, T-box 21; tbx21, gata3, forkhead box P3a; foxp3a, retinoic acid receptor-related orphan receptor C a; rorca) in furinAtd204e/+ mutants and WT controls (Fig. 1D). As in T cell-specific FURIN conditional KO (cKO) mice, the mRNA levels of Th1, Th2, and the Treg cell markers tbx21 (T-bet, P = 0.003), gata3 (P = 0.004), and foxp3a (P < 0.001) were elevated in furinAtd204e/+ zebrafish. In contrast, there was no significant difference in the expression of the Th17 cell marker rorca between furinAtd204e/+ and WT zebrafish, which is in line with the normal IL-17 production previously observed in CD4cre-furfl/fl mice (18).
TGFB1 directly induces Furin expression in rodents, which is a prerequisite for its functional maturation and anti-inflammatory function (18, 38). Consequently, the autoimmune phenotype of CD4cre-furfl/fl mice can be chiefly attributed to a lack of bioavailable, T cell-produced TGFB1. In our present study, zebrafish FurinA was found to regulate tgfb1a expression in vivo (Fig. 1D), which could result from an attempt to compensate for the defective maturation of the Tgfb1a cytokine by increasing the efficiency of tgfb1a transcription.
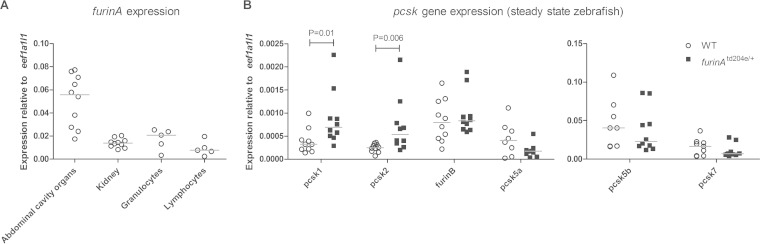
The quantification of the furinA mRNA expression in WT zebrafish demonstrated that it is expressed in both innate and adaptive immune cells (Fig. 2A), which is in line with the previously reported ubiquitous expression pattern of FURIN orthologues in vertebrates (9, 32). In mammals, the first seven PCSK enzymes have been demonstrated to exhibit a significant functional redundancy and shared substrate molecules, which interferes with the interpretation of a PCSK specific phenotype (39). Therefore, we next addressed the expression of the zebrafish pcsk genes (pcsk1, pcsk2, furinB, pcsk5a, pcsk5b, and pcsk7) in furinAtd204e/+ mutants and WT controls (Fig. 2B). The pcsk genes furinB, pcsk5a, pcsk5b, and pcsk7 showed comparable expression levels between furinAtd204e/+ and WT zebrafish, whereas pcsk1 and pcsk2 were significantly upregulated in the furinAtd204e/+ fish (P = 0.01 and P = 0.006, respectively), which theoretically could partially compensate for the effect of reduced furinA expression.
FIG 2.
Expression of zebrafish pcsk genes in furinAtd204e/+ mutants and WT controls. (A) Relative furinA expression was measured with qRT-PCR in the tissue homogenates of organs in the abdominal cavity (n = 10) and kidney (n = 10) as well as in purified granulocytes (n = 5) and lymphocytes (n = 5) isolated from steady-state WT AB zebrafish. Samples were run as technical duplicates. (B) The relative expressions of zebrafish pcsk genes (pcsk1, pcsk2, furinB, pcsk5a, pcsk5b, and pcsk7) were quantified in the tissue homogenates of organs in the abdominal cavities of steady-state adult furinAtd204e/+ mutant (n = 10) and WT (n = 8 to 10) zebrafish by using qRT-PCR. Gene expressions were normalized to eef1a1l1 expression and are represented as a scatter dot plot and median. A two-tailed Mann-Whitney test was used in the statistical comparison of differences.
Furin regulates the survival of M. marinum-infected zebrafish embryos.
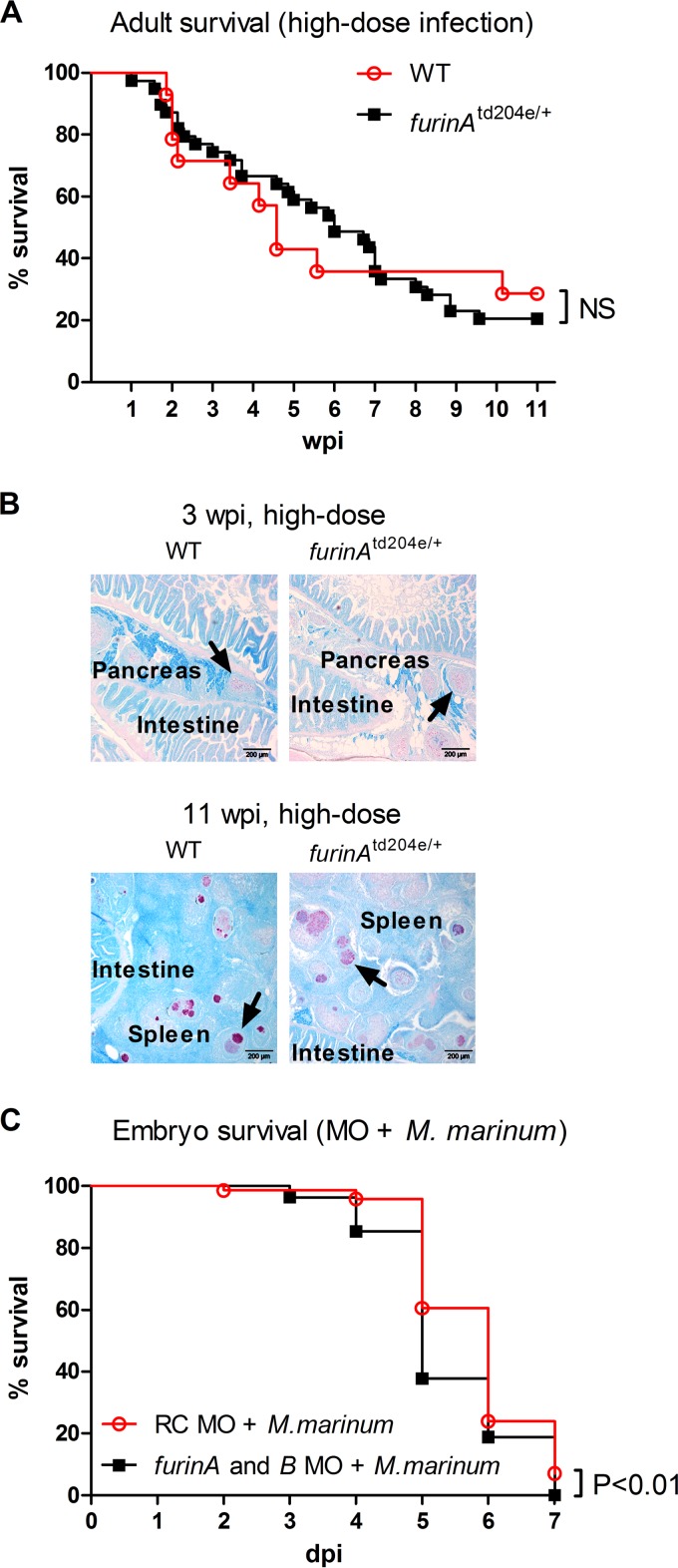
Whereas upregulated T cell gene expression in furinAtd204e/+ zebrafish indicates enhanced immune responses, granulopenia can result in immunodeficiency. To study the net effect of FurinA on mycobacterial host defense in adult zebrafish, we infected furinAtd204e/+ and WT zebrafish with a high-dose of M. marinum (8,300 ± 1,800 CFU) and followed their survival for 11 weeks (Fig. 3A). WT fish exhibited ca. 60% mortality during the first 5 weeks and about one-third of them were alive at the study endpoint (Fig. 3A). furinAtd204e/+ mutants showed similar lethality, and no statistical difference in gross survival between mutant and WT fish could be detected. In addition, a histopathological examination revealed that the two fish groups had similarly organized granulomas at both 3 and 11 wpi, and there were no obvious differences in the numbers of granulomas (Fig. 3B). Uninfected WT and furinAtd204e/+ zebrafish controls did not show background mycobacteriosis in a Ziehl-Neelsen staining (data not shown).
FIG 3.
Role of furin in zebrafish survival during M. marinum infection. (A) The survival of adult furinAtd204e/+ (n = 39) and WT (n = 14) zebrafish was monitored for 11 weeks after an experimental high-dose M. marinum inoculate. The data were collected from a single experiment. (B) M. marinum granulomas in adult WT and furinAtd204e/+ zebrafish infected with a high-dose bacterial inoculate were identified with Ziehl-Neelsen staining at 3 wpi (17,300 ± 6,900 CFU) and 11 wpi (8,300 ± 1,800 CFU). Representative images from 4 to 10 individuals per group are shown. Typical granulomas are indicated with arrows. (C) Zebrafish embryos were microinjected before the four-cell stage with RC (n = 71) or both furinA and furinB MOs (n = 82) and M. marinum (131 ± 125 CFU). At 1 dpf, embryos were screened to identify successfully injected embryos, and survival was monitored up until 7 dpf. Collated data from two separate experiments with 30 and 41 embryos in the RC MO groups and 27 and 55 embryos in the furinA and furinB MO groups are shown. In panels A and C, a log-rank (Mantel-Cox) test was used for the statistical comparison of differences.
Morpholino (MO)-based expression silencing in developing zebrafish embryos can be used to study a gene's function specifically in innate immune responses (22, 40). Since furinA regulated the granulopoiesis, we addressed its role in innate immunity by inhibiting the expressions of furinA and furinB in the embryonic M. marinum infection model (27, 32, 33). Infecting either control (random control MO injected [RC]) or the double furin gene knockdown embryos with M. marinum (131 ± 125 CFU) resulted in substantial lethality by 7 days postinfection (dpi; 93 and 100%, respectively, Fig. 3C). The survival of infected furinA+B morphants was, however, significantly reduced compared to controls (furinA+B versus RC, P < 0.01). Notably, as FurinA is essential for zebrafish development the increased lethality of M. marinum-infected furin morphant embryos could result from general developmental defects.
FurinA inhibits the early expression of proinflammatory cytokine genes in a mycobacterial infection.
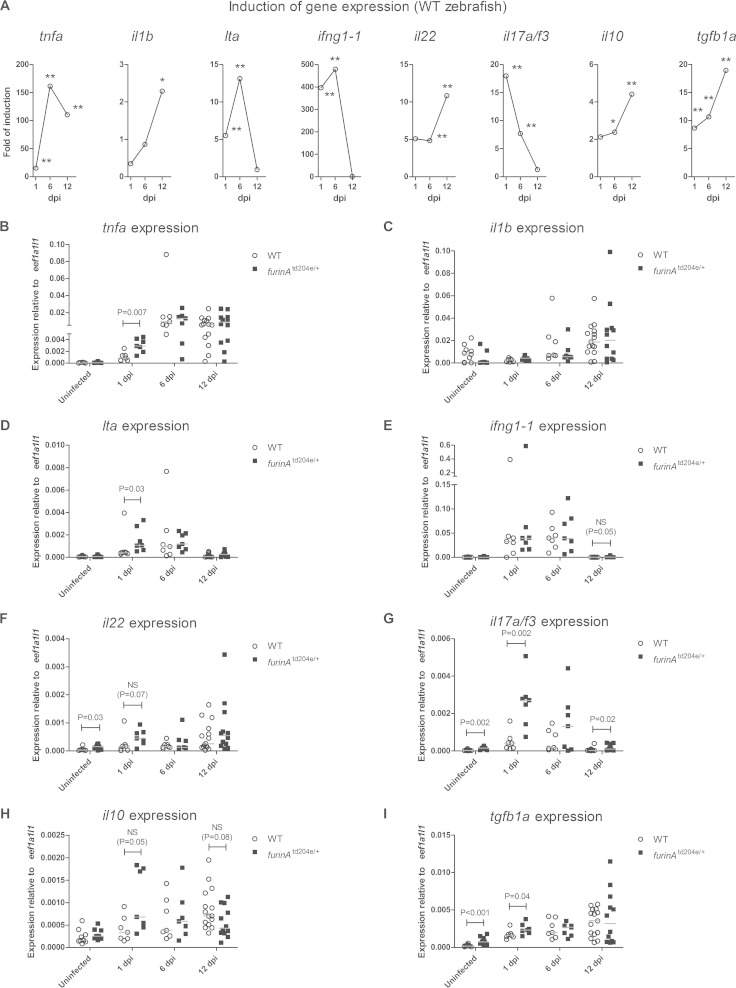
The containment of a mycobacterial disease is critically dependent on adaptive Th1 type responses but also on adequate innate immune responses. The significance of the innate immunity is perhaps best illustrated by an increased susceptibility to TB in patients receiving anti-TNF neutralizing antibodies and an association of human Toll-like receptor polymorphisms with an increased disease risk (4, 41, 42). FURIN can process target molecules that are important in innate immunity in vitro (e.g., TNF converting enzyme and Toll-like receptor 7) (43, 44), but whether it also regulates innate immune responses in infections in vivo has not been addressed. We next analyzed the early immune response against M. marinum by measuring the cytokine gene expression in furinAtd204e/+ fish and WT controls. Both furinAtd204e/+ and WT adult zebrafish were infected with a high dose of M. marinum (10,300 ± 3,300 CFU) and a qRT-PCR expression analysis of both proinflammatory (tnfa, il1b, lta, ifng1-1, il22, and il17a/f3) and anti-inflammatory (il10 and tgfb1a) cytokine genes was performed at 1, 6, and 12 dpi (Fig. 4).
FIG 4.
FurinA attenuates the early expression of proinflammatory cytokine genes in an experimental high-dose mycobacterial infection. The relative expression of proinflammatory cytokine genes (tnfa, il1b, lta, ifng1-1, il22, and il17a/f3) and anti-inflammatory cytokine genes (il10 and tgfb1a) was determined in adult furinAtd204e/+ (n = 7 to 12) and WT (n = 7 to 15) zebrafish with qRT-PCR after a high dose of an M. marinum inoculate at 1, 6, and 12 dpi. (A) Fold gene expression induction median shown for all of the aforementioned genes in infected WT zebrafish. The fold induction was normalized to the gene expression median in uninfected zebrafish. *, P < 0.05; **, P < 0.01. (B to I) Relative gene expression in furinAtd204e/+ and WT zebrafish represented as a scatter dot plot and median. Note the different scales of the y axes and the divided y axis in panels B and E. Gene expressions were normalized to eef1a1l1 expression. At 1 and 6 dpi, samples were run as technical duplicates and uninfected, as well as 12-dpi, samples once. A two-tailed Mann-Whitney test was used in the statistical comparison of differences.
An analysis of the kinetics of the cytokine gene induction in WT fish (Fig. 4A) demonstrated that the expression levels of tnfa, lta, and ifng1-1 were significantly upregulated upon M. marinum infection already at 1 dpi (15.1-, 5.5-, and 396.4-fold, respectively), with rising kinetics until 6 dpi (161.4-, 13.1-, and 478.8-fold, respectively). At 12 dpi, the induction of tnfa had declined to 110.3-fold, whereas lta and ifng1-1 expressions had returned to their baseline levels. il17a/f3 was also significantly induced at 1 dpi (18.0-fold), but its expression decreased during the following days (6 dpi, 7.7-fold; 12 dpi, baseline expression). In contrast, both il1b and il22 showed a delayed expression pattern by peaking at 12 dpi (il1b, 2.3-fold; il22, 10.8-fold). The induction of the anti-inflammatory cytokine genes il10 and tgfb1a was evident already by day 6 postinfection (il10, 2.4-fold; tgfb1a, 10.7-fold), and the expression of both genes was even more pronounced at 12 dpi (4.4- and 19.0-fold, respectively). In conclusion, an M. marinum infection in zebrafish results in an enhancement in the levels of various macrophage, natural killer cell, γδ T cell, and lymphoid tissue inducer cell-associated cytokines already during the first 12 days after infection, indicating an efficient activation of pro- and anti-inflammatory processes.
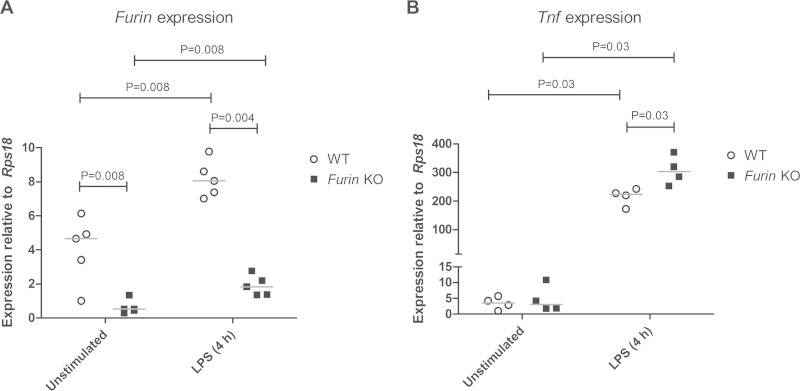
To determine how FurinA contributes to the early cytokine levels induced by M. marinum, we compared the expression of the aforementioned cytokine genes in infected furinAtd204e/+ and WT zebrafish (Fig. 4B to I). furinAtd204e/+ mutants showed a significantly higher relative expression of the proinflammatory cytokine genes tnfa (P = 0.007), lta (P = 0.03), and il17a/f3 (P = 0.002) at 1 dpi compared to WT fish. Interestingly, the inherent relative upregulation of tgfb1a in furinAtd204e/+ mutants was completely abolished by the 12th postinfective day, and this was accompanied by a relative reduction in il10 gene expression. The low furinA expression also associated with a sustained upregulation of the il17a/f3 cytokine gene. Collectively, these results could indicate that inflammation-accelerating innate cytokine responses dominate in M. marinum-infected furinAtd204e/+ mutant fish. To demonstrate that FURIN attenuates proinflammatory responses specifically in innate immune cells, we used cultured macrophages from WT and LysMcre-furfl/fl mice (Fig. 5) (45, 46). In these experiments we saw that in activated macrophages reduced Furin mRNA levels (77% decrease, P = 0.004) are associated with significantly upregulated transcription of the proinflammatory cytokine gene Tnf (P = 0.03).
FIG 5.
Reduced Furin expression is associated with an upregulated expression of Tnf in activated mouse macrophages. Bone marrow-derived macrophages were cultured from Furin KO (LysMcre-furfl/fl) and WT littermate mice (n = 4 to 5) as described previously (46). The relative expressions of Furin (Ensembl ID ENSMUSG00000030530) (A) and Tnf (Ensembl ID ENSMUSG00000024401) (B) were determined in unstimulated and LPS-stimulated (4 h) samples with qRT-PCR. Gene expressions were normalized to ribosomal protein S18 (Rps18, Ensembl ID ENSMUSG00000008668) expression and are represented as a scatter dot plot and median. A two-tailed Mann-Whitney test was used in the statistical comparison of differences. The qRT-PCR primers used for the mouse genes were as follows: Furin, 5′-CAGAAGCATGGCTTCCACAAC-3′ and 5′-TGTCACTGCTCTGTGCCAGAA-3′; Tnf, 5′-CTTCTGTCTACTGAACTTCGGG-3′ and 5′-CAGGCTTGTCACTCGAATTTTG-3′; and Rps18, 5′-GTGATCCCTGAGAAGTTCCAG-3′ and 5′-TCGATGTCTGCTTTCCTCAAC-3′.
furinAtd204e/+ mutants have decreased bacterial burden and cd247 expression in a chronic M. marinum infection model.
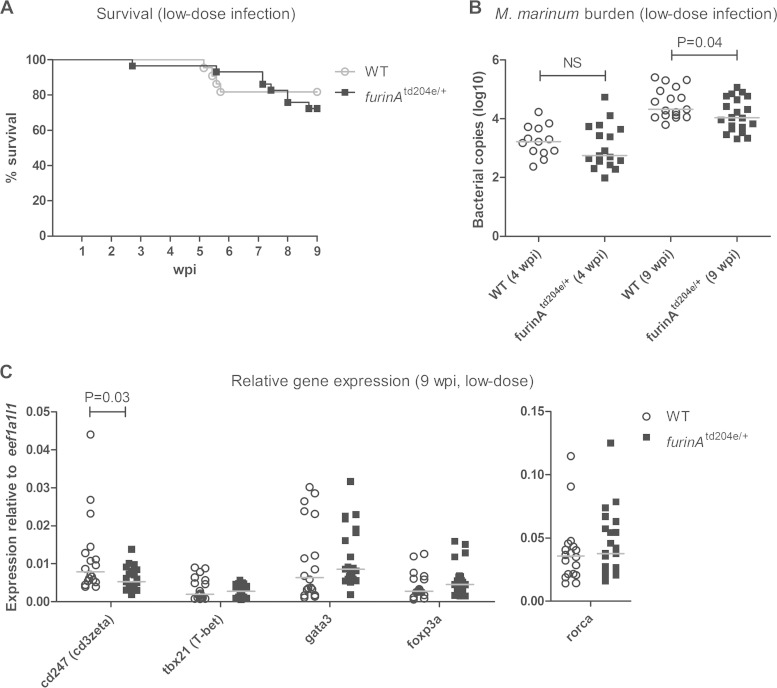
We have recently established a model for studying a latent mycobacterial infection in adult zebrafish (25). A low-dose i.p. M. marinum inoculate (∼35 CFU) results in static bacterial burdens, a constant number of granulomas, and low mortality. We thus utilized this model to investigate how FurinA contributes to the adaptive mycobacterial immunity and the development of mycobacterial latency. When furinAtd204e/+ mutant and WT control fish were infected with small amounts of M. marinum (46 ± 8 CFU) and survival was monitored for 9 weeks, statistically significant difference between the groups could not be observed (Fig. 6A). However, mycobacterial quantification revealed a trend of smaller bacterial amount in the furinAtd204e/+ mutants at 4 wpi (34 ± 10 CFU, NS) (Fig. 6B), but significantly reduced M. marinum copy numbers from the internal organ isolates of infected furinAtd204e/+ zebrafish compared to WT fish at 9 wpi (1.9-fold reduction, P = 0.04) (Fig. 6B). On average, bacterial copy number medians at 9 wpi were 11,000 (13 copies in 100 ng of zebrafish DNA) in furinAtd204e/+ mutants and 21,000 (50 copies in 100 ng of zebrafish DNA) in WT zebrafish, which suggests that furinA inhibits host responses in chronic mycobacterial infection.
FIG 6.
Downregulation of furinA expression decreases the M. marinum burden and the T cell marker cd247 mRNA level in an experimental low-dose mycobacterial infection. A latent mycobacterial infection was induced with a low-dose M. marinum inoculate. (A) Survival of adult furinAtd204e/+ (n = 29) and WT (n = 22) zebrafish was monitored for 9 weeks. A log-rank (Mantel-Cox) test was used for the statistical comparison of differences. The data were collected from a single experiment. (B) The M. marinum burden of furinAtd204e/+ mutants (n = 17 to 20) and WT controls (n = 13 to 18) was quantified with DNA qRT-PCR at 4 and 9 wpi. Bacterial load is represented as the median of total bacterial copies (log10). M. marinum quantifications were run as technical duplicates. (C) The relative expression of Th cell markers (cd247, tbx21, gata3, foxp3a, and rorca) was quantified with qRT-PCR in furinAtd204e/+ mutants (n = 18 to 21) and WT controls (n = 18) at 9 wpi. Gene expressions were normalized to eef1a1l1 expression and represented as a scatter dot plot and median. Expression analyses were run as technical duplicates. In panels B and C, a two-tailed Mann-Whitney test was used in the statistical comparison of differences.
The reduced mycobacterial load in latency could be a result of the upregulation of proinflammatory cytokines upon the M. marinum infection in furinAtd204e/+ mutant fish (Fig. 4B to G) but also a consequence of inherently accelerated T cell responses (Fig. 1D). To evaluate the T cell responses in latency, we quantified the relative expression of a general T cell marker cd247 (cd3zeta) and Th cell subtype-associated transcription factors (tbx21, gata3, foxp3a, and rorca) in furinAtd204e/+ mutant and control fish at both 4 and 9 wpi (see Fig. S2 in the supplemental material and Fig. 6C). As expected, an infection-induced upregulation of these genes was seen in both WT and mutant zebrafish (at 4 wpi, 2.9- to 23.9-fold and 1.5- to 8.1-fold, respectively, and at 9 wpi, 4.2- to 19.0-fold and 2.5- to 8-fold, respectively), suggesting T cell activation. Interestingly, at 9 wpi, cd247 expression was significantly lower in infected furinAtd204e/+ mutants compared to WT controls (P = 0.03), whereas the expression of the Th subset-associated transcription factors tbx21, gata3, foxp3a, and rorca did not differ between infected furinAtd204e/+ fish and controls. In addition, the expression levels of innate immunity cytokine genes (tnfa, il1b, il10, tgfb1a, lta, ifng1-1, and il17a/f3) were found to be similar between furinAtd204e/+ and WT fish (see Fig. S2 and S3 in the supplemental material), indicating that innate immune cell activity during a chronic M. marinum infection is FurinA independent.
Together, the reduced relative expression of cd247 at 9 wpi and loss of upregulation of tbx21, gata3, and foxp3a in furinAtd204e/+ zebrafish compared to WT controls indicate that FurinA enhances T cell responses in a mycobacterial infection. However, furinAtd204e/+ mutants had lower M. marinum copy numbers, which suggests that FurinA also inhibits antimycobacterial host responses.
DISCUSSION
Despite intensive studies, our understanding of the pathogenesis and host immunity of TB is still incomplete. We found that furinA expression is upregulated upon M. marinum infection and that inhibiting furin genes in developing zebrafish reduces the survival of infected embryos. An analysis of furinAtd204e/+ mutant adult zebrafish demonstrated that FurinA regulates the development of granulocytes and the expression of Th subset-associated genes in steady-state fish. When furinAtd204e/+ mutant fish were infected with a high dose of M. marinum, reduced furinA mRNA levels were found to correlate with an enhanced expression of the proinflammatory cytokine genes tnfa, lta, and il17a/f3. In contrast, experiments using a latent mycobacterial infection model showed that infected furinAtd204e/+ mutants have lowered expression levels of the T cell marker gene cd247 (cd3zeta) compared to controls. The net effect of the reduced furinA expression in adult zebrafish was a significant decrease in M. marinum copy numbers in a low-dose infection model, suggesting that FurinA attenuates protective host responses against mycobacteria.
Through catalyzing the endoproteolytic cleavage of target molecules, PCSK enzymes regulate the maturation of host defense factors, as well as the activity of invading pathogens (9, 10). In vitro analyses have demonstrated that PCSK enzymes have significantly overlapping biochemical functions in substrate processing, and therefore genetic inactivation of PCSKs is instrumental for decoding their specific biological roles (9, 10, 47). We have previously characterized the expression of seven pcsk genes in developing embryos and multiple adult zebrafish tissues (32). Two orthologous genes of mammalian FURIN, furinA and furinB (30), were found to be ubiquitously expressed, and biochemical analyses showed that FurinA, but not FurinB, is able to proteolytically process pro-Tgfb1a, suggesting that FurinA is the corresponding biological equivalent for human FURIN (32). Germ line Furin KO mice die on day 11 of embryogenesis due to severe developmental defects in ventral closure, as well as in heart tube fusion and looping (20). Accordingly, we could not identify any homozygous adult furinAtd204e/td204e fish, indicating that FurinA has a specific, nonredundant function also during zebrafish development. Importantly, however, in its heterozygous form, the adult furinAtd204e allele did not interfere with normal development but reduced the levels of furinA mRNA. This in turn allowed the use of adult furinAtd204e/+ mutants in the experiments to assess how furinA expression regulates host responses. Interestingly, furinA downregulation in furinAtd204e/+ mutant zebrafish upregulated pcsk1 and pcsk2 expression, which implies an attempt to compensate for the reduced FurinA activity. In mammals, PCSK1 and PCSK2 have restricted gene expression patterns, function chiefly in neuroendocrine tissues, and are not able to compensate for FURIN during development (9, 10). However, the lack of PCSK1 was recently found to associate with a proinflammatory phenotype and increased lethality in LPS-induced septic shock in mice (48). Consequently, the elevated pcsk1 expression in zebrafish could theoretically also attenuate inflammation in zebrafish and thus partially mask the specific immunoregulatory function of FurinA.
It is well established that protective immunity against TB is mediated by both innate and adaptive immune responses. As in mammals, the cells of the zebrafish immune system include lymphocytes, neutrophils, and macrophages (49), as well as dendritic cells (50), eosinophils (51, 52), human mast cell-like cells (53), and natural killer cells (54). Our flow cytometric analyses of furinAtd204e/+ mutant fish kidneys (the primary site of hematopoiesis in fish) showed normal numbers of lymphocytes, blood cell precursors, and erythrocytes, but low granulocyte counts, indicating that FurinA promotes granulopoiesis. Granulocyte maturation is regulated through a complex network of protein mediators (55), some of which are known substrates for PCSKs (12). For example, granulocyte development is disrupted in mice deficient in integrin alpha 9 (56). Also, functional NOTCH signaling promotes entry into granulopoiesis (57), whereas conditional inactivation of TNF converting enzyme increases granulopoiesis (58). Deciphering the detailed molecular mechanisms by which FurinA regulates granulocyte development, however, would require the spatiotemporal identification of its specific substrates using proteomics analyses, followed by characterizing the function of the substrates in zebrafish.
Although the Th1 type cell immune response is crucial in adaptive immunity against TB (5–8), other Th lymphocyte subsets, including Th2, Th17, and Treg cells, also regulate the magnitude of the host defense and survival (59–62). We have previously shown that FURIN is dispensable for T cell development in mice but that it plays a role in CD4+ T cell activation and polarization (15, 18). When we characterized the expression of Th cell subtype transcription factors in steady-state zebrafish, we found that decreased furinA expression associated with the upregulation of tbx21 (a Th1 cell marker), gata3 (a Th2 cell marker), and foxp3a (a Treg cell marker) expression, suggesting an increase in Th1, Th2, and Treg cell counts in the furinAtd204e/+ mutants. These findings are in line with the previously reported hyperproduction of both Th1 and Th2 hallmark cytokines and increased Treg cell numbers in FURIN T cell cKO mice (18) but also indicate that reduced FURIN expression (and not only the lack of it) can accelerate Th1 and Th2 responses. In contrast, aging furinAtd204e/+ mutants did not develop overt autoimmunity, which demonstrates that the residual furinA expression, accompanied with elevated tgfb1a mRNA levels, is sufficient for maintaining adequate peripheral immune tolerance in steady state.
To assess how granulopenia and altered Th subtype gene expressions in furinAtd204e/+ mutants might contribute to the host defense against mycobacteria, adult zebrafish were infected i.p. with M. marinum inoculates. furinAtd204e/+ mutants exhibited similar gross survival, and statistically significant differences could not be observed. In contrast, inhibiting furin genes during development associated with significantly reduced survival of M. marinum-infected embryos. Albeit these findings could be indicative of either immunodeficiency or an unnecessarily strong host response in the lack of Furin, they need to be interpreted cautiously. The expression of furinA is critical for zebrafish development, and survival differences in furinA+B morphant fish could simply result from “failure to thrive.” Therefore, we chose to use adult furinAtd204e/+ fish to address how furinA regulates the innate immune responses in M. marinum infection (30). After a high-dose mycobacterial infection, lower furinA mRNA expression levels resulted in a proinflammatory phenotype characterized by enhanced early expression of tnfa, lta, and il17a/f3 but declining expression levels of the anti-inflammatory cytokine genes il10 and tgfb1a. Previously, TNF and IL-17 have been linked to a protective, innate immunity against TB (61, 63), and an LTA polymorphism has been associated with susceptibility to the disease (64). The role of Tnfa appears, however, complicated; Roca and Ramakrishnan recently showed that either deficient or excess production of this cytokine accelerates TB pathogenesis through reduced microbicidal activity of macrophages or programmed necrosis of macrophages, respectively (65). Since furinA downregulation causes a proinflammatory phenotype, FurinA deficiency could be beneficial for protection by increasing the early microbicidal activity of innate cells through upregulated Tnfa levels. In addition, both furinAtd204e/+ mutants and controls showed well-organized granulomas and no free bacteria in Ziehl-Neelsen staining, which suggests relatively normal macrophage function also in controlling the high bacterial loads in the chronic phase.
We have previously shown that infecting zebrafish with a low M. marinum dose (∼35 CFU) results in a nonprogressive mycobacterial disease that can be reactivated by gamma irradiation (25). In this model, the host survival and the latent state of infection both depend on functional adaptive immune responses and normal lymphocyte numbers. The determination of the mycobacterial burden in latency revealed that reduced furinA expression associated with significantly decreased M. marinum copy numbers, and this could not be explained by elevated T cell responses. Specifically, we noticed that furinAtd204e/+ mutant fish actually expressed lower levels of the general T cell marker gene cd247 (cd3zeta) and that the overexpression of Th1/2, as well as Treg marker genes in steady-state mutants, was completely abolished in the chronically infected furinAtd204e/+ zebrafish. How furinA downregulation affects these responses is not clear but would require a careful kinetic analysis of marker gene expression levels. In summary, we can conclude that a reduction in systemic furinA expression associates with enhanced host responses to mycobacteria in zebrafish.
A challenge in TB diagnostics is to specifically identify the activation of latent infection. The present means, such as the tuberculin skin test, the interferon gamma release assay (IGRA), and a chest X-ray, can only reveal the presence of TB-associated memory cells and tissue damage, but there are no markers available for the detection of mycobacterial growth in the host in the clinic. Our data show that furinA/FURIN expression is upregulated in the host in response to a mycobacterial infection and the Th1 hallmark cytokine IL-12 (15). FURIN is also secreted from macrophages in response to LPS activation (14), and it can be measured from serum (66). Therefore, in the future it will be interesting to assess whether serum FURIN levels can be used as an infection biomarker to mirror mycobacterial growth and the activation of Th1 type immune responses. Furthermore, PCSK inhibitors have relatively recently been suggested as drugs for cancer and infectious diseases (9, 10, 67). Blocking FURIN also associates with accelerated immune responses, as shown by the spontaneous development of autoimmunity in T cell-specific FURIN cKO mice and by the prevention of experimental arthritis upon recombinant FURIN administration (18, 19). Our results here demonstrate that diminished furinA expression reduces mycobacterial loads in a latent infection model, which suggests that PCSK inhibitors could potentially be used to harness also TB. Adverse effects, such as autoimmunity and developmental defects in stem cells, may pose a significant clinical problem. Investigating the molecular mechanisms by which FURIN regulates mycobacterial immunity further may help us find specific target molecules for future drug development.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the Jane and Aatos Erkko Foundation (M. Rämet), Academy of Finland (projects 128623 and 135980 [M. Pesu], 139225 [M. Rämet], and 121003 [M. Parikka]), a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme (M. Pesu), the Emil Aaltonen Foundation (M. Pesu and S.-K. Harjula), the Sigrid Jusélius Foundation (M. Pesu and M. Rämet), The Tampere Tuberculosis Foundation (M. Pesu, M. Rämet, M. Parikka, S.-K. Harjula, and M. Hammarén), Competitive Research Funding of the Tampere University Hospital (grants 9M080, 9N056, and 9S051 [M. Pesu], 9M093 [M. Rämet], and 9NO52 [M. Parikka]), the Foundation of the Finnish Anti-Tuberculosis Association (S.-K. Harjula, M. Hammarén, and M. Parikka), the University of Tampere Doctoral Programme in Biomedicine and Biotechnology (M. Ojanen, M. Hammarén, and Z. Cordova), the City of Tampere (S.-K. Harjula), and the Orion-Farmos Research Foundation (M. Hammarén). The zebrafish work was carried out at the University of Tampere core facility supported by Biocenter Finland, the Tampere Tuberculosis Foundation, and the Emil Aaltonen Foundation. The authors declare no commercial or financial conflict of interest.
We thank Sanna Hämäläinen, Kaisa Oksanen, Leena Mäkinen, Hannaleena Piippo, Jenna Ilomäki, and Annemari Uusimäki for technical assistance and Jorma Isola for his help in performing virtual microscopy with the University of Tampere core facility equipment.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.03135-14.
REFERENCES
- 1.Butler D. 2000. New fronts in an old war. Nature 406:670–672. doi: 10.1038/35021291. [DOI] [PubMed] [Google Scholar]
- 2.Zumla A, Raviglione M, Hafner R, von Reyn CF. 2013. Tuberculosis. N Engl J Med 368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 3.North R, Jung Y. 2004. Immunity to tuberculosis. Annu Rev Immunol 22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 4.Philips JA, Ernst JD. 2012. Tuberculosis pathogenesis and immunity. Annu Rev Pathol 7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AM, Mayer-Barber KD, Sher A. 2011. Role of innate cytokines in mycobacterial infection. Mucosal Immunol 4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, Magram J, Ferrante J, Orme IM. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidah NG, Prat A. 2012. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 10.Artenstein AW, Opal SM. 2011. Proprotein convertases in health and disease. N Engl J Med 365:2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- 11.Fuller RS, Brake AJ, Thorner J. 1989. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science 246:482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remacle AG, Rozanov DV, Fugere M, Day R, Strongin AY. 2006. Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene 25:5648–5655. doi: 10.1038/sj.onc.1209572. [DOI] [PubMed] [Google Scholar]
- 14.Turpeinen H, Raitoharju E, Oksanen A, Oksala N, Levula M, Lyytikainen LP, Jarvinen O, Creemers JW, Kahonen M, Laaksonen R, Pelto-Huikko M, Lehtimaki T, Pesu M. 2011. Proprotein convertases in human atherosclerotic plaques: the overexpression of FURIN and its substrate cytokines BAFF and APRIL. Atherosclerosis 219:799–806. doi: 10.1016/j.atherosclerosis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Pesu M, Muul L, Kanno Y, O'Shea JJ. 2006. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood 108:983–985. doi: 10.1182/blood-2005-09-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meissner F, Scheltema RA, Mollenkopf HJ, Mann M. 2013. Direct proteomic quantification of the secretome of activated immune cells. Science 340:475–478. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- 17.Lund RJ, Chen Z, Scheinin J, Lahesmaa R. 2004. Early target genes of IL-12 and STAT4 signaling in th cells. J Immunol 172:6775–6782. doi: 10.4049/jimmunol.172.11.6775. [DOI] [PubMed] [Google Scholar]
- 18.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O'Shea JJ. 2008. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Ah Kioon MD, Lalou C, Larghero J, Launay JM, Khatib AM, Cohen-Solal M. 2012. Protective role of systemic furin in immune response-induced arthritis. Arthritis Rheum 64:2878–2886. doi: 10.1002/art.34523. [DOI] [PubMed] [Google Scholar]
- 20.Roebroek AJ, Umans L, Pauli IG, Robertson EJ, van Leuven F, Van de Ven WJ, Constam DB. 1998. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development 125:4863–4876. [DOI] [PubMed] [Google Scholar]
- 21.Meeker ND, Trede NS. 2008. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol 32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan C, Kim CH. 2008. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol 25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Lohi O, Parikka M, Ramet M. 2013. The zebrafish as a model for paediatric diseases. Acta Paediatr 102:104–110. doi: 10.1111/j.1651-2227.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LY, Nie L, Zhu G, Xiang LX, Shao JZ. 2013. Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol 39:39–62. doi: 10.1016/j.dci.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Parikka M, Hammaren MM, Harjula SK, Halfpenny NJ, Oksanen KE, Lahtinen MJ, Pajula ET, Iivanainen A, Pesu M, Ramet M. 2012. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog 8:e1002944. doi: 10.1371/journal.ppat.1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamm LM, Brown EJ. 2004. Mycobacterium marinum: the generalization and specialization of a pathogenic Mycobacterium. Microbes Infect 6:1418–1428. doi: 10.1016/j.micinf.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho R, de Sonneville J, Stockhammer OW, Savage ND, Veneman WJ, Ottenhoff TH, Dirks RP, Meijer AH, Spaink HP. 2011. A high-throughput screen for tuberculosis progression. PLoS One 6:e16779. doi: 10.1371/journal.pone.0016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. 2002. Real-time visualization of Mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17:693–702. doi: 10.1016/S1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- 29.Prouty MG, Correa NE, Barker LP, Jagadeeswaran P, Klose KE. 2003. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol Lett 225:177–182. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- 30.Walker MB, Miller CT, Coffin Talbot J, Stock DW, Kimmel CB. 2006. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol 295:194–205. doi: 10.1016/j.ydbio.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Langenau DM, Ferrando AA, Traver D, Kutok JL, Hezel JP, Kanki JP, Zon LI, Look AT, Trede NS. 2004. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci U S A 101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turpeinen H, Oksanen A, Kivinen V, Kukkurainen S, Uusimaki A, Ramet M, Parikka M, Hytonen VP, Nykter M, Pesu M. 2013. Proprotein convertase subtilisin/kexin type 7 (PCSK7) is essential for the zebrafish development and bioavailability of transforming growth factor β1a (TGFβ1a). J Biol Chem 288:36610–36623. doi: 10.1074/jbc.M113.453183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benard EL, van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH. 2012. Infection of zebrafish embryos with intracellular bacterial pathogens. J Vis Exp 61:3781. doi: 10.3791/3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oksanen KE, Halfpenny NJ, Sherwood E, Harjula SK, Hammaren MM, Ahava MJ, Pajula ET, Lahtinen MJ, Parikka M, Ramet M. 2013. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine 31:5202–5209. doi: 10.1016/j.vaccine.2013.08.093. [DOI] [PubMed] [Google Scholar]
- 35.Tuominen VJ, Isola J. 2009. The application of JPEG2000 in virtual microscopy. J Digital Imaging 22:250–258. doi: 10.1007/s10278-007-9090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang R, Dodd A, Lai D, McNabb WC, Love DR. 2007. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin 39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellett F, Lieschke GJ. 2010. Zebrafish as a model for vertebrate hematopoiesis. Curr Opin Pharmacol 10:563–570. doi: 10.1016/j.coph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG. 2001. Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol 158:305–316. doi: 10.1016/S0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, Essalmani R, Prat A. 2008. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol 40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Nasevicius A, Ekker SC. 2000. Effective targeted gene “knockdown” in zebrafish. Nat Genet 26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. 2012. Mycobacterium tuberculosis: immune evasion, latency, and reactivation. Immunobiology 217:363–374. doi: 10.1016/j.imbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Velez DR, Wejse C, Stryjewski ME, Abbate E, Hulme WF, Myers JL, Estevan R, Patillo SG, Olesen R, Tacconelli A, Sirugo G, Gilbert JR, Hamilton CD, Scott WK. 2010. Variants in Toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet 127:65–73. doi: 10.1007/s00439-009-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. 2012. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science 335:225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hipp M, Shepherd D, Gileadi U, Aichinger M, Kessler B, Edelmann M, Essalmani R, Seidah N, Reis e Sousa C, Cerundolo V. 2013. Processing of human Toll-like receptor 7 by furin-like proprotein convertases is required for its accumulation and activity in endosomes. Immunity 39:711–721. doi: 10.1016/j.immuni.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 46.Taverniti V, Stuknyte M, Minuzzo M, Arioli S, De Noni I, Scabiosi C, Cordova ZM, Junttila I, Hamalainen S, Turpeinen H, Mora D, Karp M, Pesu M, Guglielmetti S. 2013. S-layer protein mediates the stimulatory effect of Lactobacillus helveticus MIMLh5 on innate immunity. Appl Environ Microbiol 79:1221–1231. doi: 10.1128/AEM.03056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turpeinen H, Ortutay Z, Pesu M. 2013. Genetics of the first seven proprotein convertase enzymes in health and disease. Curr Genomics 14:453–467. doi: 10.2174/1389202911314050010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Refaie S, Gagnon S, Gagnon H, Desjardins R, D'Anjou F, D'Orleans-Juste P, Zhu X, Steiner DF, Seidah NG, Lazure C, Salzet M, Day R. 2012. Disruption of proprotein convertase 1/3 (PC1/3) expression in mice causes innate immune defects and uncontrolled cytokine secretion. J Biol Chem 287:14703–14717. doi: 10.1074/jbc.M111.323220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renshaw SA, Trede NS. 2012. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech 5:38–47. doi: 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin AF, Xiang LX, Wang QL, Dong WR, Gong YF, Shao JZ. 2009. The DC-SIGN of zebrafish: insights into the existence of a CD209 homologue in a lower vertebrate and its involvement in adaptive immunity. J Immunol 183:7398–7410. doi: 10.4049/jimmunol.0803955. [DOI] [PubMed] [Google Scholar]
- 51.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. 2001. Myelopoiesis in the zebrafish, Danio rerio. Blood 98:643–651. doi: 10.1182/blood.V98.3.643. [DOI] [PubMed] [Google Scholar]
- 52.Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, Banuelos K, Romo-Fewell O, Aroian RV, Traver D. 2010. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116:3944–3954. doi: 10.1182/blood-2010-03-267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobson JT, Seibert J, Teh EM, Da'as S, Fraser RB, Paw BH, Lin TJ, Berman JN. 2008. Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood 112:2969–2972. doi: 10.1182/blood-2008-03-145011. [DOI] [PubMed] [Google Scholar]
- 54.Yoder JA, Turner PM, Wright PD, Wittamer V, Bertrand JY, Traver D, Litman GW. 2010. Developmental and tissue-specific expression of NITRs. Immunogenetics 62:117–122. doi: 10.1007/s00251-009-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. 2000. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia 14:973–990. doi: 10.1038/sj.leu.2401808. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Huang X, Atakilit A, Zhu Q, Corey SJ, Sheppard D. 2006. The integrin α9β1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity 25:895–906. doi: 10.1016/j.immuni.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Tan-Pertel HT, Walker L, Browning D, Miyamoto A, Weinmaster G, Gasson JC. 2000. Notch signaling enhances survival and alters differentiation of 32D myeloblasts. J Immunol 165:4428–4436. doi: 10.4049/jimmunol.165.8.4428. [DOI] [PubMed] [Google Scholar]
- 58.Horiuchi K, Kimura T, Miyamoto T, Miyamoto K, Akiyama H, Takaishi H, Morioka H, Nakamura T, Okada Y, Blobel CP, Toyama Y. 2009. Conditional inactivation of TACE by a Sox9 promoter leads to osteoporosis and increased granulopoiesis via dysregulation of IL-17 and G-CSF. J Immunol 182:2093–2101. doi: 10.4049/jimmunol.0802491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. 2007. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med 204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Zhou B, Li M, Deng Q, Wu X, Le X, Wu C, Larmonier N, Zhang W, Zhang H, Wang H, Katsanis E. 2007. CD4+ CD25+ FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol 123:50–59. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 62.Hammaren MM, Oksanen KE, Nisula HM, Luukinen BV, Pesu M, Ramet M, Parikka M. 2014. Adequate Th2-type response associates with restricted bacterial growth in latent mycobacterial infection of zebrafish. PLoS Pathog 10:e1004190. doi: 10.1371/journal.ppat.1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clay H, Volkman HE, Ramakrishnan L. 2008. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Elorriaga G, Carrillo-Montes G, Mendoza-Aguilar M, Gonzalez-Bonilla C. 2010. Polymorphisms in tumor necrosis factor and lymphotoxin A in tuberculosis without and with response to treatment. Inflammation 33:267–275. doi: 10.1007/s10753-010-9181-8. [DOI] [PubMed] [Google Scholar]
- 65.Roca FJ, Ramakrishnan L. 2013. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar A, Singh S, Ahirwar SK, Nath G. 2014. Proteomics-based identification of plasma proteins and their association with the host-pathogen interaction in chronic typhoid carriers. Int J Infect Dis 19:59–66. doi: 10.1016/j.ijid.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker GL, Lu Y, Hardes K, Strehlow B, Levesque C, Lindberg I, Sandvig K, Bakowsky U, Day R, Garten W, Steinmetzer T. 2012. Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases. J Biol Chem 287:21992–22003. doi: 10.1074/jbc.M111.332643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.