Abstract
Hexose phosphate is an important carbon source within the cytoplasm of host cells. Bacterial pathogens that invade, survive, and multiply within various host epithelial cells exploit hexose phosphates from the host cytoplasm through the hexose phosphate transport (HPT) system to gain energy and synthesize cellular components. In Escherichia coli, the HPT system consists of a two-component regulatory system (UhpAB) and a phosphate sensor protein (UhpC) that tightly regulate expression of a hexose phosphate transporter (UhpT). Although growing evidence suggests that Staphylococcus aureus also can invade, survive, and multiply within various host epithelial cells, the genetic elements involved in the HPT system in S. aureus have not been characterized yet. In this study, we identified and characterized the HPT system in S. aureus that includes the hptRS (a novel two-component regulatory system), the hptA (a putative phosphate sensor), and the uhpT (a hexose phosphate transporter) genes. The hptA, hptRS, and uhpT markerless deletion mutants were generated by an allelic replacement method using a modified pMAD-CM-GFPuv vector system. We demonstrated that both hptA and hptRS are required to positively regulate transcription of uhpT in response to extracellular phosphates, such as glycerol-3-phosphate (G3P), glucose-6-phosphate (G6P), and fosfomycin. Mutational studies revealed that disruption of the hptA, hptRS, or uhpT gene impaired the growth of bacteria when the available carbon source was limited to G6P, impaired survival/multiplication within various types of host cells, and increased resistance to fosfomycin. The results of this study suggest that the HPT system plays an important role in adaptation of S. aureus within the host cells and could be an important target for developing novel antistaphylococcal therapies.
INTRODUCTION
Staphylococcus aureus is a major human pathogen that causes a diverse array of diseases in nosocomial and community-acquired settings, ranging from superficial skin infections to severe diseases, such as endocarditis, septicemia, and necrotizing pneumonia (1). S. aureus adapts extremely well to various environmental conditions and hosts by regulating expression of colonization factors, such as microbial surface components recognizing adhesive matrix molecules (MSCRAMM), and virulence factors, such as cytotoxins, proteases, and superantigens. Expression of these factors is regulated by global transcriptional regulatory systems, such as sigma factors (σB), SarA, Rot, and MgrA, and two-component regulatory systems (TCSs) (2–5). A TCS is a signal transduction system in bacteria, consisting of a membrane-bound sensor histidine kinase and a corresponding cytoplasmic response regulator that enables bacteria to sense, respond, and adapt to a wide range of environmental stresses, such as oxygen, pH, and osmolarity, as well as antibiotic challenges. Within the various S. aureus strains for which the genome has been sequenced, 16 to 18 TCSs were identified (6). Of these TCSs, 12 TCSs have been characterized mainly with respect to their role in regulating expression of virulence factors and adaptation to environmental stress conditions, including AgrCA and SaeRS, which regulate virulence factors (3), ArlRS and LytSR, which regulate autolysis (7, 8), WalKR, GraRS, and VraRS, which regulate cell wall synthesis (9–11), HssRS, which regulates iron acquisition (12), NreBC, which regulates nitrogen sensing (13), SrrAB and AirRS, which regulate oxygen sensing (14, 15), and BraRS, which regulates bacitracin resistance (16). However, relatively little is known about the role of TCSs on nutritional challenges.
Sugar uptake is an essential process for microorganisms to gain energy and synthesize cellular compartments. The transport of sugar into bacterial cells is mediated by the phosphoenolpyruvate phosphotransferase system (PTS) (17) and by non-PTS mechanisms, such as the hexose phosphate transport (HPT) system.
Bacterial pathogens can survive and multiply within various host epithelial cells by exploiting nutrients available within the host cytoplasm, such as hexose phosphates, through the HPT system (18, 19). The HPT system has been well characterized in Escherichia coli, where it is composed of a two-component regulatory system (UhpAB) (18), a phosphate sensor protein (UhpC) (20), and a hexose phosphate transporter (UhpT) (21). UhpC is required to sense extracellular hexose phosphates, resulting in autophosphorylation of a conserved histidine residue in the cytoplasmic domain of UhpB. Subsequently, a phosphoryl group is transferred to a conserved aspartic acid residue in the response regulator domain of UhpA, which activates transcription of uhpT (22). UhpT is a hexose phosphate transporter that exchanges intracellular phosphate for extracellular hexose phosphate and fosfomycin (23).
Although there is growing evidence that S. aureus can survive and multiply within various types of host epithelial cells (24), little is known about the mechanism by which S. aureus adapts to intracellular conditions that are nutritionally limited to host cell metabolites. In this study, we characterized the HPT system in S. aureus, encoded by the SAUSA300_0216, SAUSA300_0217, SAUSA300_0218, and SAUSA300_0219 genes. We demonstrated that transcription of a hexose phosphate transporter, UhpT, encoded by SAUSA300_0216, is regulated by a novel TCS, HptRS, encoded by SAUSA300_0217 and SAUSA300_0218, and by a phosphate-sensing protein, HptA, encoded by SAUSA300_0219. We demonstrated that the HPT system is important to support intracellular survival/multiplication of S. aureus, where the carbon source is limited to hexose phosphate and affects the sensitivity to fosfomycin.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All strains used in this study are listed in Table 1. All S. aureus strains were cultured in Mueller-Hinton II (MH2) broth or agar (BBL Becton Dickinson) supplemented with chloramphenicol (10 μg/ml) when necessary. All E. coli DH5α strains were cultured in Luria-Bertani (LB) broth or agar (BBL Becton Dickinson) supplemented with ampicillin (100 μg/ml) when necessary.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| S. aureus | ||
| RN4220 | Cloning intermediate derived from 8325-4 | 45 |
| USA300 LAC | USA300 wild-type reference strain | |
| ΔHptA mutant | SAUSA300_0219, hptA deletion mutant | This study |
| ΔHptRS mutant | SAUSA300_0217 and 0218, hptR hptS double deletion mutant | This study |
| ΔUhpT mutant | SAUSA300_0216, uhpT deletion mutant | This study |
| ΔHptA-Comp mutant | LAC ΔHptA mutant complemented with pMK4-HptA | This study |
| ΔHptRS-Comp mutant | LAC ΔHptRS mutant complemented with pMK4-HptRS | This study |
| ΔUhpT-Comp mutant | LAC ΔHptA mutant complemented with pMK4-UhpT | This study |
| E. coli DH5α | Cloning intermediate | 46 |
| Plasmids | ||
| pMAD-CM-GFPuv | Allelic replacement temperature-sensitive vector | This study |
| pMK4 | E. coli-S. aureus shuttle vector | 26 |
| pMK4-HptA | pMK4 harboring complementation of the hptA deletion | This study |
| pMK4-HptRS | pMK4 harboring complementation of the hptR and hptS deletions | This study |
| pMK4-UhpT | pMK4 harboring complementation of the uhpT deletion | This study |
Construction of S. aureus deletion mutants.
The gene deletion mutants were generated by allelic replacement using a modified pMAD-CM temperature-sensitive shuttle vector system. To enhance the screening processes without an antibiotic selection marker, to thereby generate a markerless deletion mutant, the promoter region of the lactate dehydrogenase gene from Listeria monocytogenes was joined to the coding region of the GFPuv gene (Clontech) by overlapping PCR using primers listed in Table 2 and cloned between BglII and StuI sites in pMAD-CM (25), which resulted in pMAD-CM-GFPuv. To generate deletion mutants, the upstream and downstream flanking regions of hptA, hptRS, or uhpT were amplified from S. aureus LAC chromosomal DNA using primers listed in Table 2 and cloned between the BamHI/SalI and EcoRI/BglII sites, respectively, in pMAD-CM-GFPuv. The plasmids were constructed in E. coli DH5α and then transferred to S. aureus RN4220 prior to electroporation into S. aureus LAC by using electroporation, as described previously (25). Constructed strains were cultured on MH2 plates containing chloramphenicol at a nonpermissive plasmid replication temperature of 43°C overnight to promote the first recombination event. A single colony was transferred to MH2 broth and cultured at 37°C overnight to promote the second recombination event. The mutant candidate, showing the GFPuv-negative and chloramphenicol-sensitive phenotypes, was screened by PCR using the primers listed in Table 2 and DNA sequencing analysis. To complement the gene deletions, gene fragments from 500 bp upstream of the start codon containing the putative promoter regions along with each open reading frame were amplified by PCR using primers listed in Table 2 and cloned into the shuttle plasmid, pMK4 vector (26).
TABLE 2.
Primers used in the study
| Primer name | Primer sequence (5′→3′) | Source |
|---|---|---|
| Primers for deletion mutants | ||
| HptAupF | TCCTGTCGACGGATAAACGATTGATACAG | This study |
| HptAupR | TCAGACGCGTAAGGAGATATTAAGATTCGAGG | This study |
| HptAdnF | TTGAGAATTCTACTTGAGTAGATCCGTG | This study |
| HptAdnR | CACTCCCGGGCCGTTGAATATCTTGCCG | This study |
| HptAmF | TGTTTCTGACAACACACC | This study |
| HptRSupF | GCGCGGATCCGGTGTCAATATTGTAGGGAG | This study |
| HptRSupR | GCGCGTCGACCTAATTCAACAACATCAGCC | This study |
| HptRSdnF | GCGCGAATTCGTTTAGCCATATTATCTGCG | This study |
| HptRSdnR | GCGCAGATCTGGCATACAAACCTTATAGAC | This study |
| HptRSmF | GGCACTTGATAATTACAGG | This study |
| UhpTupF | AATCGGATCCAAGGCATTCCATTATCGG | This study |
| UhpTupR | ATGTGTCGACGAATCCTAAGTATCGGCC | This study |
| UhpTdnF | TTTTGAATTCCCTGGGAAATCCTGTTAT | This study |
| UhpTdnR | TTCTAGATCTCATAGAAAGCAACGATTCCT | This study |
| UhpTmF | ATCGGTGGTGCCATAGCA | This study |
| Primers for complementation | ||
| HptAF | GCGCGGATCCCATAGTCATTGTAGCATG | This study |
| HptAR | GCGCGTCGACGTCTATAAGGTTTGTATGCC | This study |
| HptRSF | GCGCGGATCCGGTGTCAATATTGTAGGGAG | This study |
| HptRSR | AAAGGTCGACAGTTGCTTCTGTTGGTCC | This study |
| UhpTF | GCGCGGATCCCGTACATATAAGAAATAC | This study |
| UhpTR | GCGCGTCGACCTCCCTACAATATTGACACC | This study |
| Primers for real-time PCR | ||
| RT-UhpTF | TGGATATTTTGTCGATGGACGTA | This study |
| RT-UhpTR | ACCCCGTTAAGTCCCCAAAG | This study |
| RT-GlpTF | TGATTGGGCGCCAGTCTACT | This study |
| RT-GlpTR | GGTCCACGACGACCTTTGAA | This study |
| RT-GlcUF | TCTTAATCGCACTTTTACCTGCTTT | This study |
| RT-GlcUR | GAGTGCGCCTAGCGTCGTA | This study |
| RT-BshAF | AGGCGTCGTTCCAATTGG | This study |
| RT-BshAR | CGACAAATCCAGTTTCACCATGT | This study |
| RT-MurAF | CGAAAATGCTGTTGTCGTTGA | This study |
| RT-MurAR | GCACAACCACCAGGCAATG | This study |
| RT-FosBF | GCGAATTTAAATATTGGCATCAGA | This study |
| RT-FosBR | CAGGGTCGGTAAAGTAAATTGATTG | This study |
Growth analysis.
Chemically defined medium (CDM) was prepared as described previously (27). Overnight cultures from MH2 broth were washed three times with MH2 broth or CDM supplemented with a single carbon source (glucose, glycerol-3-phosphate [G3P], or glucose-6-phosphate [G6P]; 0.5%, wt/vol) and adjusted to an optical density at 600 nm (OD60) of 0.01. Cells were cultured at 37°C with shaking at 200 rpm, and bacterial growth was monitored by measuring OD600 using a Genesys 20 spectrophotometer (Thermo Spectronic).
Quantitative real-time PCR (qRT-PCR).
To measure transcriptional changes in the uhpT, glpT, and glcU genes in response to various nutritional conditions, strains were cultured in various nutritional conditions as described above. To measure transcriptional changes in the genes related to fosfomycin resistance, overnight cultures from MH2 broth were washed three times and adjusted to an OD600 of 0.5 with MH2 broth or CDM supplemented with a single carbon source as described above in the presence of fosfomycin (128 μg/ml) for 6 h. Bacterial cells were harvested by centrifugation, and total RNA was isolated from the cell pellets using FastPrep (Thermo Fisher Scientific) and RNeasy kits (Qiagen), according to the manufacturers' recommendations.
For qRT-PCR, cDNA samples were generated from 1 μg of total RNA using SuperScriptase III (Invitrogen) according to the manufacturer's instructions. The qRT-PCR was performed using primers listed in Table 2, Power SYBR green master mix, and the ABI 7500 real-time PCR system (Applied Biosystems) according to the manufacturer's instructions. Real-time PCR data were analyzed using sequence detector system software (Applied Biosystems). The data were normalized by calculating the threshold cycle (CT) of the target minus the CT of the internal control (16S RNA and gyrB) (ΔCT). Relative quantification of the target gene was determined by the comparative CT method (ΔΔCT).
Intracellular survival and multiplication assay.
Human monocytic cells (THP-1) and alveolar epithelial cells (A549) were obtained from ATCC. Murine bone marrow-derived macrophages (BMDM) were prepared as previously described (28). Cell monolayers (∼80% confluence in 24-well tissue culture plates) were infected with bacteria at a multiplicity of infection (MOI) of 5 (5 bacteria per cell). After 30 min of infection, cells were washed and treated with cell culture medium containing gentamicin (100 μg/ml) to remove extracellular bacteria. After 30 min of gentamicin treatment, cells were washed and lysed, and the numbers of intracellular bacteria were determined by plate counting (t = 0). Parallel cultures were further maintained and harvested at the indicated time points (t = 3 h and t = 6 h). The percentage of surviving bacteria was calculated by the following formula: (the number of bacterial cells at t = 3 or t = 6/the number of bacteria at t = 0) × 100.
Antibiotic sensitivity assays.
The MIC of fosfomycin was determined using a fosfomycin epsilometer test (Etest; bioMérieux) according to the manufacturer's instructions.
Statistical analysis.
Statistical significance was analyzed with the Student t test using GraphPad Prism software (GraphPad).
RESULTS
Identification of a putative hexose phosphate transport system in S. aureus.
Genome sequence analysis showed that genes encoding a hexose phosphate transporter, UhpT (SAUSA300_216), a putative histidine kinase (SAUSA300_217), a response regulator protein (SAUSA300_218), and a putative iron compound binding protein (SAUSA300_0219) are highly conserved in all sequenced S. aureus strains, but these genes have not been characterized yet (Fig. 1A). In silico analysis showed that the SAUSA300_216 gene encodes a putative 458-amino-acid transmembrane protein with a major facilitator superfamily 1 (MFS_1) domain showing 51% identities and 67% positive matching with the UhpT in E. coli. The SAUSA300_0217 gene encodes a putative 252-amino-acid protein with a response regulator receiver domain at the N terminus and a helix-turn-helix domain at the C terminus, which are typically associated with a response regulator in TCSs (29). The SAUSA300_0218 gene encodes a putative 518-amino-acid protein with a histidine kinase domain and a histidine kinase-type ATPase catalytic (HATPase_c) domain at the C terminus, which are typically associated with a sensor protein in TCSs (29). The SAUSA300_0219 gene encodes a putative 323-amino-acid protein with a bacterial extracellular solute binding protein 2 (SBP II) domain, which potentially can interact with a phosphonate (30). These results suggested that the SAUSA300_216, 217, 218, and 219 genes may represent the HPT system in S. aureus, similar to uhpABCT in E. coli. Based on this assumption, we refer here to this putative TCS (SAUSA300_0218 and 0217) as the hexose phosphate transporter regulation system (HptRS), a putative iron compound binding protein (SAUSA300_0219) as HptA, and a hexose phosphate transporter as UhpT (SAUSA300_216).
FIG 1.
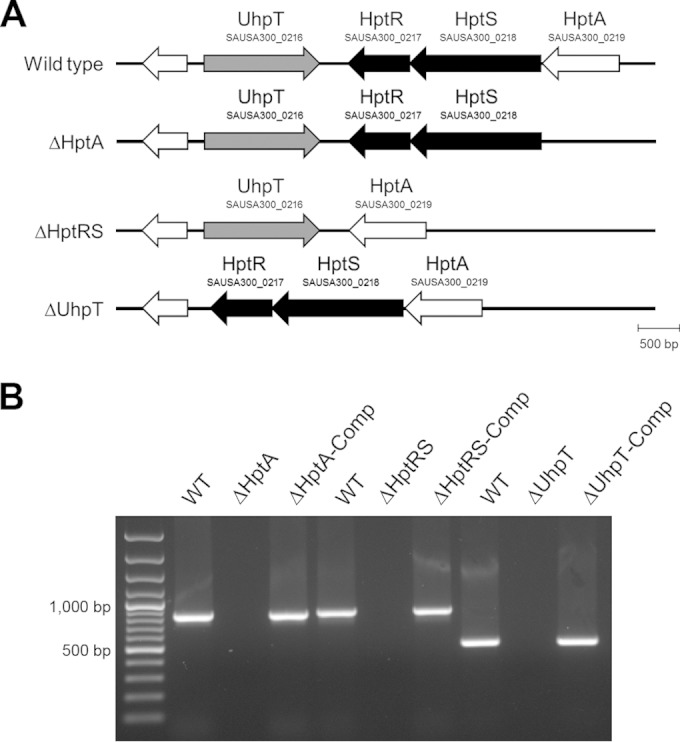
Identification of a putative hexose phosphate transport system in S. aureus. (A) A schematic map of the genomic organization of the hexose phosphate transport system in S. aureus LAC and its mutant derivatives; (B) a PCR confirmation of a deletion mutant of HptA (ΔHptA mutant), HptRS (ΔHptRS mutant), and UhpT (ΔUhpT mutant) and the corresponding complemented strains, pMK4-HptA, pMK4-HptRS, and pMK4-UhpT, respectively.
The HPT system is required for growth under nutritional conditions specifically limited to G6P.
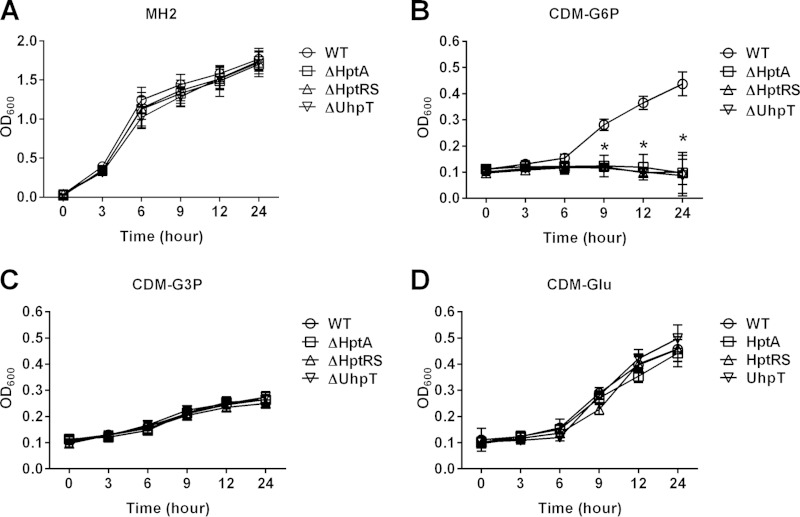
To determine the function of HptA, HptRS, and UhpT, we generated deletion mutants of HptA (ΔHptA mutant), HptRS (ΔHptRS mutant), and UhpT (ΔUhpT mutant) by an allelic replacement method using a modified temperature-sensitive pMAD-CM-GFPuv vector system, an efficient approach for generating and screening a markerless deletion mutant, and a corresponding complement strain using pMK4, in the S. aureus LAC strain background (Fig. 1B). To analyze the function of HptA, HptRS, and UhpT in carbohydrate metabolism, the growth of the wild-type, mutants, and complemented strains in various nutritional conditions was measured. When cultured in MH2 broth, the growth of wild-type and mutant strains was not affected (Fig. 2A). In contrast, when cultured in CDM supplemented with glucose-6-phosphate (G6P) as a sole carbon source, the growth of the ΔHptA, ΔHptRS, and ΔUhpT strains was significantly inhibited compared to that of the wild type (Fig. 2B), and the complemented mutant strains restored the growth (see Fig. S2 in the supplemental material). When cultured in CDM supplemented with glucose or glycerol-3-phosphate (G3P), the growth of the wild type and mutants was not affected (Fig. 2C and D). These results indicated that the hptA, hptRS, and uhpT genes are required for growth under nutrition-limited conditions, especially when G6P is the sole carbon source.
FIG 2.
Role of the HPT system in growth under nutrition specifically limited to glucose-6-phosphate. Results shown are the growth kinetics of the wild-type, ΔHptA, ΔHptRS, and ΔUhpT strains in MH2 (A) or in chemically defined medium (CDM) supplemented with G6P (B), G3P (C), or glucose (Glu) (D) as the sole carbon source. Results shown are combined from triplicate measurements from three independent experiments. The asterisk indicates statistical difference from the wild type at P values of <0.001.
The hptA and hptRS genes are required for transcriptional activation of uhpT in response to extracellular G6P or G3P as the sole carbon source.
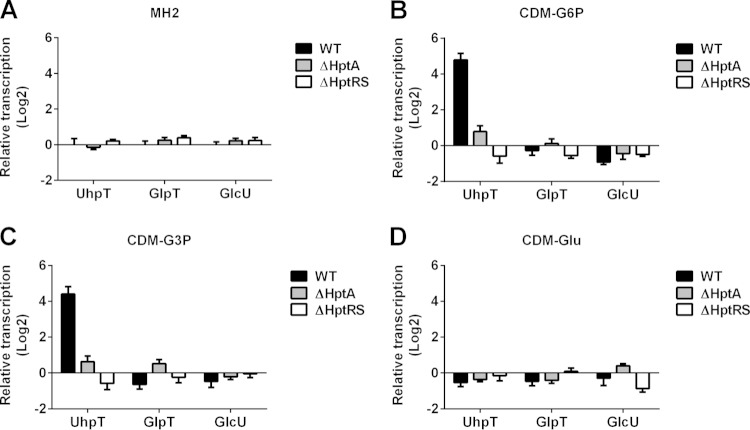
To determine whether the hptA and hptRS genes regulate transcription of the uhpT gene, as in E. coli, we determined the transcriptional profiles of the uhpT gene in various nutritional conditions by using qRT-PCR. When cultured in MH2 broth, transcription of the uhpT gene was not significantly different in the wild-type, mutant, and complemented strains (Fig. 3A; see also Fig. S1A in the supplemental material). In contrast, when cultured in CDM supplemented with G6P as the sole carbon source, transcription of the uhpT gene in the wild-type and complemented strains significantly increased (approximately 18-fold), but not in the ΔHptA or ΔHptRS strains, compared to the result obtained from the MH2 culture (Fig. 3B; see also Fig. S1B). Interestingly, when cultured in CDM supplemented with G3P, transcription of the uhpT gene in the wild-type strain also significantly increased (Fig. 3C; see also Fig. S1C). When cultured in CDM supplemented with glucose as the sole carbon source, no significant changes were observed (Fig. 3D; see also Fig. S1D). To further analyze the role of HptA and HptRS on other carbohydrate translocases, such as GlcU and GlpT, translocases for glucose and G3P, respectively, transcription of these genes was determined but showed no differences (Fig. 3A to D). Combined with the in silico analysis and the growth results, these results suggest that HptA is a phosphate sensor protein that activates HptRS TCS, resulting in transcriptional activation of the uhpT gene; thus, HptA and HptRS represent the HPT system in S. aureus.
FIG 3.
Role of HptA and HptRS in transcriptional regulation of the UhpT in response to extracellular G3P and G6P. Transcription of UhpT in the wild-type, ΔHptA, and ΔHptRS strains, cultured in MH2 broth (A) or in CDM supplemented with G6P (B), G3P (C), or glucose (Glu) (D) as the sole carbon source, was measured using qRT-PCR. Results shown are combined from triplicate measurements from two independent experiments.
The hptRS gene is required for intracellular survival and multiplication of S. aureus.
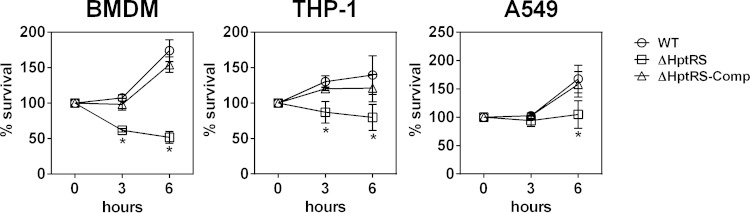
Since hexose phosphates are important carbon sources within the host cell cytosol, we hypothesized that any defect in the HPT system would negatively affect intracellular survival and multiplication of S. aureus. As shown in Fig. 4, the intracellular survival and multiplication of the ΔHptRS mutant in murine bone marrow-derived macrophages (BMDM), human monocytic cells (THP-1), and human alveolar epithelial cells (A549) were clearly impaired compared to those of the wild-type and complemented strains. These results suggest that uptake of hexose phosphate by the HPT system is important for intracellular survival and multiplication of S. aureus.
FIG 4.
Role of HptRS in intracellular survival/multiplication of S. aureus. A cell monolayer of murine bone marrow-derived macrophages (BMDM), human monocytic cells (THP-1), and human alveolar epithelial cells (A549) was infected with the wild-type or ΔHptRS strains at an MOI of 5. After 30 min of infection, extracellular bacteria were removed by gentamicin treatment, and survival/multiplication of S. aureus within the host cells were measured at the indicated time points. Results shown are combined from triplicate measurements from three independent experiments. The asterisk indicates statistical difference from the wild type at P values of <0.05.
The hptRS deletion mutant exhibits fosfomycin resistance in S. aureus.
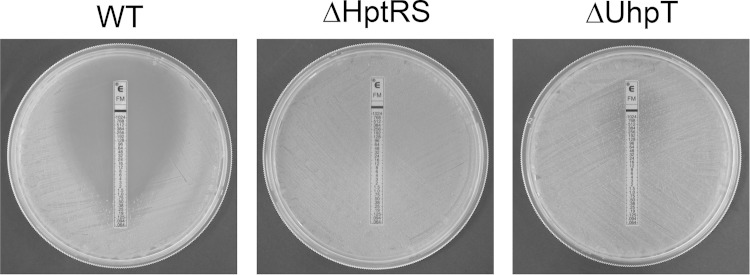
Fosfomycin is a bactericidal agent that acts by inhibiting bacterial cell wall biosynthesis by inactivating the enzyme UDP-N-acetylglucosamine-3-enolpyruvyltransferase, also known as MurA (31). Since fosfomycin requires UhpT gene expression to enter bacterial cells (32), the fosfomycin MIC of the wild-type, ΔHptRS, and ΔUhpT strains was determined using an Etest. As shown in Fig. 5, the fosfomycin MIC of the wild-type LAC strain was found to be 0.5 to 1.5 μg/ml. In contrast, the ΔHptRS and ΔUhpT strains were highly resistant to fosfomycin (>1,024 μg/ml). Both complemented mutant strains exhibited restored sensitivity to fosfomycin (1.0 to 2.5 μg/ml).
FIG 5.
Role of HptRS and UhpT in fosfomycin sensitivity. The fosfomycin MIC of the wild-type, ΔHptRS, and ΔUhpT strains was determined using the Etest by following the manufacturer's instructions.
Resistance to fosfomycin could be induced by (i) the impaired uptake of fosfomycin through UhpT and GlpT (33), (ii) overexpression or mutation in MurA (33), (iii) biochemical modifications of fosfomycin (bacillithiol conjugation) by FosB (34), and (iv) impaired bacillithiol synthesis by downregulation of the bacillithiol synthesis enzyme, BshA (35). It is noteworthy that the wild-type LAC strain is highly sensitive to the fosfomycin, although it harbors both the fosB and bshA genes. To understand the effect of HptRS on the genes related to fosfomycin resistance (glpT, uhpT, murA, fosB, and bshA) and how the wild-type LAC strain is sensitive to fosfomycin, transcription of these genes in response to an exposure to fosfomycin was measured using qRT-PCR. When cultured in MH2 broth, transcription of the glpT, uhpT, murA, fosB, and bshA genes was not different among the wild-type, ΔHptRS, and complemented strains (Fig. 6A). However, when exposed to a high level of fosfomycin (128 μg/ml) for 6 h, transcription of the glpT, uhpT, murA, and fosB genes increased (2- to 4-fold) but that of the bshA gene was not changed in the wild-type and complemented strains, compared to the data obtained from the wild-type strain cultured in MH2 broth without fosfomycin (Fig. 6B). On the contrary, transcription of the glpT, uhpT, murA, fosB, and bshA genes decreased in the ΔHptRS strain (Fig. 6B). These results suggested that, in the wild-type strain, a transcriptional increase of the glpT and uhpT genes without a transcriptional increase of the bshA gene resulted in an increased fosfomycin uptake but not a sufficient supply of bacillithiol, thereby making the bacteria sensitive to fosfomycin. In contrast, in the ΔHptRS strain, a transcriptional decrease of the glpT and uhpT genes resulted in a decreased fosfomycin uptake, thereby causing resistance to fosfomycin.
FIG 6.
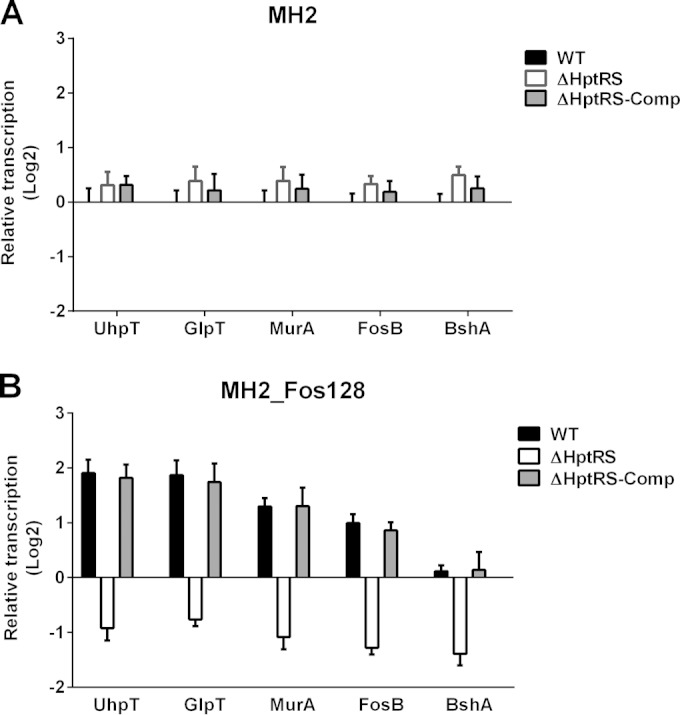
Effect of an exposure to fosfomycin on transcription of genes related to fosfomycin resistance. The wild-type and ΔHptRS strains were grown in MH2 broth (A) or in MH2 broth supplemented with a high level of fosfomycin (128 μg/ml) for 6 h (B). Transcription of genes related to fosfomycin resistance was measured using qRT-PCR. Results shown are combined from triplicate measurements from two independent experiments.
DISCUSSION
For successful survival/multiplication within host cells, invading pathogens must adapt to the available nutrients and other physiological conditions, such as pH, temperature, and oxygen, existing in these cells. Within host cells, glucose and related metabolic derivatives, such as G3P, G6P, pyruvate, lactate, and glycerol, are the most important carbon sources (36). The transport and use of nutrients by invading pathogens are mediated by multiple specific transport systems and metabolic pathways controlled by both global and specific transcriptional regulators (37). Differential gene expression profile (DGEP) analysis of Listeria monocytogenes and Shigella flexneri grown in host epithelial cells or macrophages, compared with laboratory-defined medium (38–40), showed that upregulation of genes involved in uptake, such as glpT and uhpT, in catabolism of G3P and G6P, such as glpF, glpK, glpD, and dhaK, and in downregulation of glycolysis genes suggested the importance of G3P and G6P for intracellular growth.
In this study, we identified and characterized the HPT system in S. aureus. We demonstrated that the disruption of the hptA, hptRS, and uhpT genes impaired the growth under nutrition-limited conditions when the available carbon source was limited to G6P but not to glucose or MH2 broth. Similarly, transcription of the uhpT gene in the wild-type strain was increased when phosphate substrates, such as G3P, G6P, and fosfomycin, were present. The disruption of the hptA or hptRS gene abolished transcriptional activation of the uhpT gene. These results demonstrate that UhpT is the only transporter for G6P in S. aureus and that transcriptional activation of uhpT by the HPT is required for G6P or fosfomycin uptake. In combination with the in silico analysis data, these results strongly suggest that the HPT system in S. aureus consists of HptA, HptRS, and UhpT, in which HptA senses extracellular phosphates, such as G3P, G6P, and fosfomycin, and activates HptRS, resulting in transcriptional activation of the uhpT gene, similar to the HPT system described in E. coli (22).
We also demonstrated that the disruption of the hptRS genes impaired survival/multiplication of S. aureus within various types of host cells, possibly due to the lack of transcriptional activation of the uhpT gene, resulting in an impaired uptake of hexose phosphates. In agreement with our findings, previous studies demonstrated that a high risk of S. aureus respiratory infection is correlated with an increased glucose concentration in blood and airway spaces (41). Another recent study demonstrated that S. aureus, upon internalization in A549 cells, highly increased transcription of transporters of inorganic ions, nucleotides, and carbohydrates, including UhpT (42). These results suggest that hexose phosphates are important nutritional resources within the host cytoplasm for survival/multiplication of S. aureus, and the HPT system plays an important role in this process.
Fosfomycin is a broad-spectrum bactericidal antibiotic in clinical use against both Gram-positive and -negative bacteria (43). It acts as a phosphoenolpyruvate (PEP) analogue, which inhibits the activity of MurA, preventing the formation of UDP-N-acetylglucosamine-3-O-enolpyruvate, a peptidoglycan precursor, from UDP-GlcNAc, which is the first step in the peptidoglycan biosynthesis pathway (31). Fosfomycin can be administered in a high dose since it has very few side effects in humans and is excreted from the body without being metabolized. Because of the unique mechanism of its action and advantages, fosfomycin showed clinical usefulness for treating osteomyelitis, endocarditis, and necrotizing pneumonia caused by multidrug-resistant S. aureus, in combination with daptomycin, rifampin, and vancomycin, exerting synergistic effects (44). Fosfomycin uptake is essential for antibiotic activity, and intrinsic resistance to the antibiotic in some pathogenic species is caused by either mutations in transporter genes or the lack of transport. While fosfomycin is transported into E. coli through both UhpT and GlpT, it has been reported that GlpT is the only fosfomycin transporter in P. aeruginosa, due to the absence of the uhpT gene on its chromosome (32). Consequently, a dysregulation of either UhpT or GlpT confers increased fosfomycin resistance in these species. Consistent with these findings, our results also showed that a disruption of HptA, HptRS, or UhpT resulted in a high level of fosfomycin resistance, indicating that UhpT is the main transporter for taking up fosfomycin in S. aureus LAC. Furthermore, in the wild-type strain, transcriptional activation of uhpT without sufficient activation of the bshA gene, which supplies substrates for the fosfomycin modification enzyme (FosB), resulted in high sensitivity to fosfomycin. A recent study demonstrated that a disruption of the bshA gene, which is responsible for conjugating l-malic acid to UDP-GlcNAc, highly increases transcription of UhpT (∼20-fold), suggesting the presence of interactions between the HPT system and the intracellular UDP-GlcNAc level (35).
In summary, we identified and characterized the hptRS regulon (hptA-hptRS-uhpT) encoding major components for the HPT system in S. aureus. The staphylococcal HPT system is important for intracellular survival/multiplication by sensing extracellular phosphate substrates and triggering an adaptive response to take up a particular carbon source. Given the fact that HptRS is highly responsive to extracellular phosphates that activate transcription of the uhpT gene, the main transporter for fosfomycin, the HPT system might be a useful target for developing novel antistaphylococcal therapies.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grant 1P20GM103646-01A from the NIGMS Center for Biomedical Research Excellence in Pathogen-Host Interactions, an ORGS MSU-CVM internal grant, and a QIA international research grant to K.S.S.
We thank Stephen Pruett for revising the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.03109-14.
REFERENCES
- 1.DeLeo FR, Chambers HF. 2009. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest 119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A 89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. 1995. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet 248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 4.McNamara PJ, Milligan-Monroe KC, Khalili S, Proctor RA. 2000. Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J Bacteriol 182:3197–3203. doi: 10.1128/JB.182.11.3197-3203.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kullik II, Giachino P. 1997. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol 167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 6.Ibarra JA, Perez-Rueda E, Carroll RK, Shaw LN. 2013. Global analysis of transcriptional regulators in Staphylococcus aureus. BMC Genomics 14:126. doi: 10.1186/1471-2164-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier B, Klier A, Rapoport G. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol 41:247–261. doi: 10.1046/j.1365-2958.2001.02515.x. [DOI] [PubMed] [Google Scholar]
- 8.Brunskill EW, Bayles KW. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol 178:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meehl M, Herbert S, Gotz F, Cheung A. 2007. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother 51:2679–2689. doi: 10.1128/AAC.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardete S, Wu SW, Gill S, Tomasz A. 2006. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob Agents Chemother 50:3424–3434. doi: 10.1128/AAC.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaune A, Poupel O, Mallet A, Coic YM, Msadek T, Dubrac S. 2011. Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PLoS One 6:e17054. doi: 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, Skaar EP. 2007. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 1:109–119. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlag S, Fuchs S, Nerz C, Gaupp R, Engelmann S, Liebeke M, Lalk M, Hecker M, Gotz F. 2008. Characterization of the oxygen-responsive NreABC regulon of Staphylococcus aureus. J Bacteriol 190:7847–7858. doi: 10.1128/JB.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol 183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun F, Ji Q, Jones MB, Deng X, Liang H, Frank B, Telser J, Peterson SN, Bae T, He C. 2012. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in Staphylococcus aureus. J Am Chem Soc 134:305–314. doi: 10.1021/ja2071835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. 2011. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol Microbiol 81:602–622. doi: 10.1111/j.1365-2958.2011.07735.x. [DOI] [PubMed] [Google Scholar]
- 17.Postma PW, Lengeler JW, Jacobson GR. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Island MD, Wei BY, Kadner RJ. 1992. Structure and function of the uhp genes for the sugar phosphate transport system in Escherichia coli and Salmonella typhimurium. J Bacteriol 174:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chico-Calero I, Suarez M, Gonzalez-Zorn B, Scortti M, Slaghuis J, Goebel W, Vazquez-Boland JA. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc Natl Acad Sci U S A 99:431–436. doi: 10.1073/pnas.012363899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Island MD, Kadner RJ. 1993. Interplay between the membrane-associated UhpB and UhpC regulatory proteins. J Bacteriol 175:5028–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weston LA, Kadner RJ. 1988. Role of uhp genes in expression of the Escherichia coli sugar-phosphate transport system. J Bacteriol 170:3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhamme DT, Arents JC, Postma PW, Crielaard W, Hellingwerf KJ. 2001. Glucose-6-phosphate-dependent phosphoryl flow through the Uhp two-component regulatory system. Microbiology 147:3345–3352. [DOI] [PubMed] [Google Scholar]
- 23.Sonna LA, Ambudkar SV, Maloney PC. 1988. The mechanism of glucose 6-phosphate transport by Escherichia coli. J Biol Chem 263:6625–6630. [PubMed] [Google Scholar]
- 24.Fraunholz M, Sinha B. 2012. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. 2008. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MA, Yasbin RE, Young FE. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Burd H, Liu J, Almaas E, Wiest O, Barabasi AL, Oltvai ZN, Kapatral V. 2009. Comparative genome-scale metabolic reconstruction and flux balance analysis of multiple Staphylococcus aureus genomes identify novel antimicrobial drug targets. J Bacteriol 191:4015–4024. doi: 10.1128/JB.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weischenfeldt J, Porse B. 2008. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008:pdb prot5080. doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- 29.Jung K, Fried L, Behr S, Heermann R. 2012. Histidine kinases and response regulators in networks. Curr Opin Microbiol 15:118–124. doi: 10.1016/j.mib.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Tam R, Saier MH Jr. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev 57:320–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown ED, Vivas EI, Walsh CT, Kolter R. 1995. MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J Bacteriol 177:4194–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castaneda-Garcia A, Rodriguez-Rojas A, Guelfo JR, Blazquez J. 2009. The glycerol-3-phosphate permease GlpT is the only fosfomycin transporter in Pseudomonas aeruginosa. J Bacteriol 191:6968–6974. doi: 10.1128/JB.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson MK, Keithly ME, Goodman MC, Hammer ND, Cook PD, Jagessar KL, Harp J, Skaar EP, Armstrong RN. 2014. Structure and function of the genomically encoded fosfomycin resistance enzyme, FosB, from Staphylococcus aureus. Biochemistry 53:755–765. doi: 10.1021/bi4015852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AA, Sharma SV, Strankman AW, Duran SR, Rawat M, Hamilton CJ. 2013. Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus. Biochem J 451:69–79. doi: 10.1042/BJ20121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posada AC, Kolar SL, Dusi RG, Francois P, Roberts AA, Hamilton CJ, Liu GY, Cheung A. 2014. Importance of bacillithiol in the oxidative stress response of Staphylococcus aureus. Infect Immun 82:316–332. doi: 10.1128/IAI.01074-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SA, Palmer KL, Whiteley M. 2008. Revisiting the host as a growth medium. Nat Rev Microbiol 6:657–666. doi: 10.1038/nrmicro1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucchini S, Liu H, Jin Q, Hinton JC, Yu J. 2005. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun 73:88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph B, Mertins S, Stoll R, Schar J, Umesha KR, Luo Q, Muller-Altrock S, Goebel W. 2008. Glycerol metabolism and PrfA activity in Listeria monocytogenes. J Bacteriol 190:5412–5430. doi: 10.1128/JB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, Domann E, Chakraborty T, Hain T. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect Immun 74:1323–1338. doi: 10.1128/IAI.74.2.1323-1338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garnett JP, Braun D, McCarthy AJ, Farrant MR, Baker EH, Lindsay JA, Baines DL. 2014. Fructose transport-deficient Staphylococcus aureus reveals important role of epithelial glucose transporters in limiting sugar-driven bacterial growth in airway surface liquid. Cell Mol Life Sci doi: 10.1007/s00018-014-1635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garzoni C, Francois P, Huyghe A, Couzinet S, Tapparel C, Charbonnier Y, Renzoni A, Lucchini S, Lew DP, Vaudaux P, Kelley WL, Schrenzel J. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8:171. doi: 10.1186/1471-2164-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry AL, Brown SD. 1995. Antibacterial spectrum of fosfomycin trometamol. J Antimicrob Chemother 35:228–230. doi: 10.1093/jac/35.1.228. [DOI] [PubMed] [Google Scholar]
- 44.Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A. 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 56:4511–4515. doi: 10.1128/AAC.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RG, Walker DC, McInnes RR. 1993. E. coli host strains significantly affect the quality of small scale plasmid DNA preparations used for sequencing. Nucleic Acids Res 21:1677–1678. doi: 10.1093/nar/21.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.