Abstract
Brucella species can cause brucellosis, a zoonotic disease that causes serious livestock economic losses and represents a public health threat. The mechanism of virulence of Brucella spp. is not yet fully understood. Therefore, it is crucial to identify new molecules that serve as virulence factors to better understand this host-pathogen interplay. Here, we evaluated the role of the Brucella membrane fusogenic protein (Mfp) and outer membrane protein 19 (Omp19) in bacterial pathogenesis. In this study, we showed that B. abortus Δmfp::kan and Δomp19::kan deletion mutant strains have reduced persistence in vivo in C57BL/6 and interferon regulatory factor 1 (IRF-1) knockout (KO) mice. Additionally, 24 h after macrophage infection with a Δmfp::kan or Δomp19::kan strain expressing green fluorescent protein (GFP) approximately 80% or 65% of Brucella-containing vacuoles (BCVs) retained the late endosomal/lysosomal marker LAMP-1, respectively, whereas around 60% of BCVs containing wild-type S2308 were found in LAMP-1-negative compartments. B. abortus Δomp19::kan was attenuated in vivo but had a residual virulence in C57BL/6 and IRF-1 KO mice, whereas the Δmfp::kan strain had a lower virulence in these same mouse models. Furthermore, Δmfp::kan and Δomp19::kan strains were used as live vaccines. Challenge experiments revealed that in C57BL/6 and IRF-1 KO mice, the Δmfp::kan strain induced greater protection than the vaccine RB51 and protection similar that of vaccine S19. However, a Δomp19::kan strain induced protection similar to that of RB51. Thus, these results demonstrate that Brucella Mfp and Omp19 are critical for full bacterial virulence and that the Δmfp::kan mutant may serve as a potential vaccine candidate in future studies.
INTRODUCTION
Brucellosis is a chronic infectious disease caused by bacteria of the genus Brucella. This disease affects many species of animals, resulting in great economic losses, and is therefore an important bacterial zoonotic disease worldwide (1). The genus Brucella replicates inside trophoblasts, macrophages, and dendritic cells and colonizes the reticuloendothelial system and reproductive organs (2). Additionally, brucellosis is not only the major cause of abortion and infertility in animals but also a debilitating disease in humans (2–7).
To overcome the immune system and establish a chronic infection, B. abortus utilizes diverse evasion mechanisms. This pathogen can penetrate host cells through lipid rafts (8). Once inside cells, the establishment of a persistent infection relies on the ability of the bacterium to form a Brucella-containing vacuole (BCV), which traffics from the endocytic compartment to the endoplasmic reticulum (ER), forming a replicative BCV. It is in this replicative BCV that the bacteria begin to multiply (8, 9).
Extensive vaccination programs have been undertaken to prevent brucellosis in animals. Despite their availability, live vaccine strains have critical disadvantages (10). The main vaccines currently available for brucellosis are S19 and RB51 (derived from B. abortus) and Rev-1 (derived from Brucella melitensis). However, these vaccine strains are virulent for humans and can induce abortion in animals. The strains S19 and Rev-1 do not enable a differential diagnosis between infected and vaccinated animals in serological diagnostic tests. Additionally, the strains RB51 and Rev-1 have variable efficacies, and RB51 is resistant to rifampin, the antibiotic of choice for the treatment of brucellosis in humans (11, 12). Numerous attempts to develop safer and more effective vaccines, including the use of DNA vaccines or recombinant protein vaccines, have had limited success (13–15). Considering the mechanism of infection of Brucella, the use of attenuated strains capable of efficiently inducing cellular immunity offers the best approach.
To better understand the pathogenesis associated with this disease, significant efforts have focused on defining the relevant Brucella virulence genes. Carrica et al. have characterized a highly conserved protein in Brucella, termed Brucella membrane fusogenic protein (Mfp) (16). Although the function of Brucella Mfp is unknown, this protein adopts an amphipathic alpha-helical structure in the presence of phospholipid vesicles, high ionic strength, or acidic pH, and the interaction of Brucella Mfp with phospholipid vesicles promotes in vitro membrane fusion (16). The structural similarity between Brucella Mfp and fusogenic proteins commonly observed in viruses, bacteria, and eukaryotes is a strong indicator of their biological relevance (16–19). The distinctive properties of the Brucella outer membrane are critical to Brucella virulence. Omp19 is an immunoreactive outer membrane lipoprotein that is one of many proteins considered critical for Brucella virulence (20). In the present study, the gene replacement Δmfp::kan and Δomp19::kan mutant strains were tested in comparison to the wild-type strain for intracellular localization in vitro and persistence in vivo. Additionally, the potential use of these mutant strains as vaccines was tested in immunocompromised and immunocompetent mice.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. All B. abortus strains were grown on brucella broth (BB) medium (Becton Dickinson) or on plates of BB medium containing 1.5% agar (BA). Escherichia coli strains were grown on Luria-Bertani (LB) medium (Invitrogen). When necessary, the medium was supplemented with ampicillin (100 μg/ml) and/or kanamycin (50 μg/ml) and/or chloramphenicol (5 μg/ml). The bacteria were grown at 37°C with stirring at 180 rpm. After growth, the bacteria were aliquoted, frozen, and quantified (CFU/ml).
TABLE 1.
Bacterial strains and plasmid used in this study
| Bacterial strain or plasmid | Characteristicsa | Source or referenceb |
|---|---|---|
| B. abortus strains | ||
| B. abortus S2308 | Wild type, smooth | LIDI |
| B. abortus S2308(pBBR4-gfp) | Wild type expressing GFP, smooth | LIDI |
| B. abortus S19 | Vaccine strain, smooth | LIDI |
| B. abortus RB51 | Vaccine strain, rough | LIDI |
| B. abortus Δmfp::kan | Kanr, mutant of S2308, smooth | CICVyA-INTA |
| B. abortus Δmfp(pBBR4-gfp) | Kanr Ampr, mutant of S2308, smooth | This study |
| B. abortus Δmfp(pBBR1-mfp) | Kanr Cmr, mutant of S2308, smooth | CICVyA-INTA |
| B. abortus Δmfp(pBBR1-mfp, pBBR4-gfp) | Kanr Cmr Ampr, mutant of S2308, smooth | This study |
| B. abortus Δomp19::kan | Kanr, mutant of S2308, smooth | CICVyA-INTA |
| B. abortus Δomp19(pBBR4-gfp) | Kanr Ampr, mutant of S2308, smooth | This study |
| B. abortus Δomp19(pBBR1-omp19) | Kanr Cmr, mutant of S2308, smooth | CICVyA-INTA |
| B. abortus Δomp19 (pBBR1-omp19, pBBR4-gfp) | Kanr Cmr Ampr, mutant of S2308, smooth | This study |
| E. coli strains | ||
| E. coli BL21 | Competent strain for protein expression | LIDI |
| E. coli S17 | Competent conjugative donor strain | LIDI |
| Plasmid | ||
| GFP | pBBR4-gfp Ampr | DNA 2.0 |
Kanr, kanamycin resistance; Ampr, ampicillin resistance; Cmr, chloramphenicol resistence.
LIDI, Laboratório de Imunologia de Doenças Infecciosas, UFMG, Belo Horizonte, Brazil; CICVyA-INTA, Instituto de Biotecnología, CICVyA-INTA, Buenos Aires, Argentina (Silvio L. Cravero).
Mice.
C57BL/6 mice and interferon regulatory factor 1 (IRF-1) knockout (KO) mice were obtained from the Federal University of Minas Gerais (UFMG) animal facility. They were used at 6 to 8 weeks of age and in groups of six to eight animals.
BMDM culture.
Bone marrow-derived macrophages (BMDM) were obtained from C57BL/6 mice as follows. Each femur and tibia was flushed with 5 ml of Hanks' balanced salt solution (HBSS). The resulting cell suspension was centrifuged, and the cells were resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS; Gibco), penicillin-streptomycin (10 μg/ml), and 10% L929 cell-conditioned medium (LCCM) as a source of macrophage colony-stimulating factor (M-CSF). Cells were distributed into 24-well plates and incubated at 37°C in a 5% CO2 atmosphere. Three days after seeding, another 0.1 ml of LCCM was added. On the 7th day, the medium was renewed, and on the 10th day, the cells were completely differentiated into macrophages (21).
Generation of GFP-expressing B. abortus mutant strains.
The B. abortus Δmfp::kan and Δomp19::kan defined deletion mutants were constructed by gene replacement as described previously (21). The mfp or omp19 wild-type gene was replaced by double homologous recombination with the mfp or omp19 allele containing the kanamycin resistance gene, resulting in the Δmfp::kan or Δomp19::kan mutant strain (Table 1). To generate B. abortus wild-type (S2308) or mutant strains expressing green fluorescent protein (GFP), a plasmid containing the GFP sequence (pBBR4-gfp) was transformed into chemically competent E. coli S17, a conjugative donor strain (22). E. coli S17-GFP cells were then cultured with a B. abortus Δmfp::kan or Δomp19::kan strain in brucella agar without antibiotics to allow conjugation. After 48 h at 37°C, the colonies were seeded in brucella agar containing kanamycin (50 μg/ml) and ampicillin (100 μg/ml) (BAkan/amp) to select the Brucella bacteria that expressed GFP, resulting in a Δmfp(pBBR4-gfp) or Δomp19(pBBR4-gfp) strain. The growing CFU were seeded again on BAkan/amp to ensure purification, and the typical B. abortus morphology was assessed by Gram staining. The strain B. abortus S2308(pBBR4-gfp) generated by conjugation was obtained from our laboratory stock. The complementation experiments were performed using the entire mfp or omp19 gene inserted into the plasmid pBBR1MCS (with chloramphenicol resistance) that replicates in Brucella, resulting in the complemented Δmfp(pBBR1-mfp) or Δomp19(pBBR1-omp19) strain as previously described by our group (21). GFP was also expressed in complemented mutant strains by conjugation using the plasmid pBBR4-gfp as described above. The complemented strains expressing GFP were termed the Δmfp(pBBR1-mfp, pBBR4-gfp) and Δomp19(pBBR1-omp19, pBBR4-gfp) strains.
Confocal microscopy.
BMDM were differentiated on 12-mm glass coverslips in 24-well plates. Infection with the S2308(pBBR4-gfp), Δmfp(pBBR4-gfp), Δomp19(pBBR4-gfp), Δmfp(pBBR1-mfp, pBBR4-gfp), or Δomp19(pBBR1-omp19, pBBR4-gfp) strain was performed at a multiplicity of infection (MOI) of 100:1. Bacteria were centrifuged with the plated macrophages at 600 × g for 10 min at 4°C. After 30 min of incubation at 37°C with 5% CO2, each well was washed four times with HBSS (500 μl) and incubated for 90 min in DMEM supplemented with 10% FBS, streptomycin (100 μg/ml), and gentamicin (100 μg/ml) to kill extracellular bacteria. Thereafter, the antibiotic concentration was decreased to 10 μg/ml. At 2 and 24 h postinfection, the BMDM were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde, pH 7.4, at room temperature for 15 min. Once the cells were fixed, the coverslips were washed again and maintained in PBS at 4°C overnight. The cells were incubated with the primary antibody rabbit anti-LAMP-1 (Sigma) diluted in 10% FBS and 0.1% saponin in PBS for 1 h at room temperature. After samples were washed three times with PBS, the coverslips were incubated for 30 min at room temperature with an anti-rabbit secondary antibody conjugated to Alexa 546 (Jackson ImmunoResearch). The cells were washed once with 0.1% saponin in PBS, once in PBS, and once in water and then mounted in the mounting medium Prolong Gold with 4′,6′-diamidino-2-phenylindole (DAPI; Invitrogen). The samples were examined by confocal microscopy (Nikon C2 confocal microscope).
Persistence of B. abortus Δmfp and Δomp19 strains in C57BL/6 mice.
C57BL/6 mice were intraperitoneally (i.p.) injected with 106 CFU of a B. abortus Δmfp::kan or Δomp19::kan strain or with B. abortus S2308 as a control. Eight mice from each group were sacrificed at 1, 3, 6, and 12 weeks postinfection, and spleens were removed, macerated, and plated on BA medium after serial dilutions. CFU were counted after 3 days of incubation at 37°C with 5% CO2.
Virulence of B. abortus Δmfp and Δomp19 strains in IRF-1 KO mice.
IRF-1 KO mice were i.p. injected with 106 CFU of the wild-type strain S2308, the Δmfp::kan or Δomp19::kan mutant strain, and the Δmfp(pBBR1-mfp) or Δomp19(pBBR1-omp19) complemented strain. Mouse survival was monitored daily for 90 days postinfection.
Effectiveness of immunization with Δmfp and Δomp19 strains in C57BL/6 and IRF-1 KO mice.
C57BL/6 and IRF-1 KO mice were i.p. injected with 105 CFU of the Δmfp::kan or Δomp19::kan strain or with the vaccine strain S19 (105 CFU) or RB51 (107 CFU) as a control. Control unimmunized mice were injected with 0.1 ml of PBS. At 6 weeks postimmunization, all C57BL/6 and IRF-1 KO mice were challenged i.p. with B. abortus S2308 at 106 CFU. Two weeks later, the CFU counts from the spleens of C57BL/6 mice were taken as described above. For IRF-1 KO mice, survival was monitored for 30 days after infection with B. abortus S2308. We used a higher dose for the rough vaccine strain B. abortus RB51 to optimize protection levels, as demonstrated by Ko et al. (23).
IFN-γ detection in splenocytes from mice vaccinated with B. abortus Δmfp and Δomp19 strains.
C57BL/6 mice were i.p. injected with 105 CFU of a Δmfp::kan or Δomp19::kan strain or with the S19 (105 CFU) or RB51 (107 CFU) vaccine strain as a vaccine control. Control unimmunized mice were injected with 0.1 ml of PBS. Six weeks after immunization, C57BL/6 mice were sacrificed, and their spleens were removed and macerated. Cells were suspended in RPMI 1640 medium (Gibco) supplemented with 2 mM l-glutamine, 25 mM HEPES, 10% FBS, penicillin G (100 μg/ml), and streptomycin (100 μg/ml). Erythrocytes were eliminated with ACK lysis solution (150 mM NH4Cl, 1 mM KHCO3, 1 mM Na2-EDTA [pH 7.3]). Splenocytes were cultured in 96-well microtiter plates (106 cells/well/200 μl) to assess cytokine production. The culture was stimulated with B. abortus S2308 (MOI of 100:1) or 5 μg/ml of concanavalin A (ConA). Negative-control experiments were performed using supplemented RPMI medium alone. Cells were incubated at 37°C with 5% CO2. Supernatant aliquots were collected after 72 h of culture for gamma interferon (IFN-γ) measurement by enzyme-linked immunosorbent assay (ELISA) using a commercially available Duoset ELISA Development System kit (R&D Systems, Minneapolis, MN).
Statistical analysis.
Posttests were performed using the Bonferroni method after analysis of variance (ANOVA) with the software GraphPad Prism, version 6 (GraphPad, Inc.).
Ethics statement.
All procedures described in this study had prior approval from the animal ethics committee of the Universidade Federal de Minas Gerais, CEUA no. 128/2014.
RESULTS
BCVs containing Δmfp and Δomp19 mutants retain lysosomal markers in BMDM.
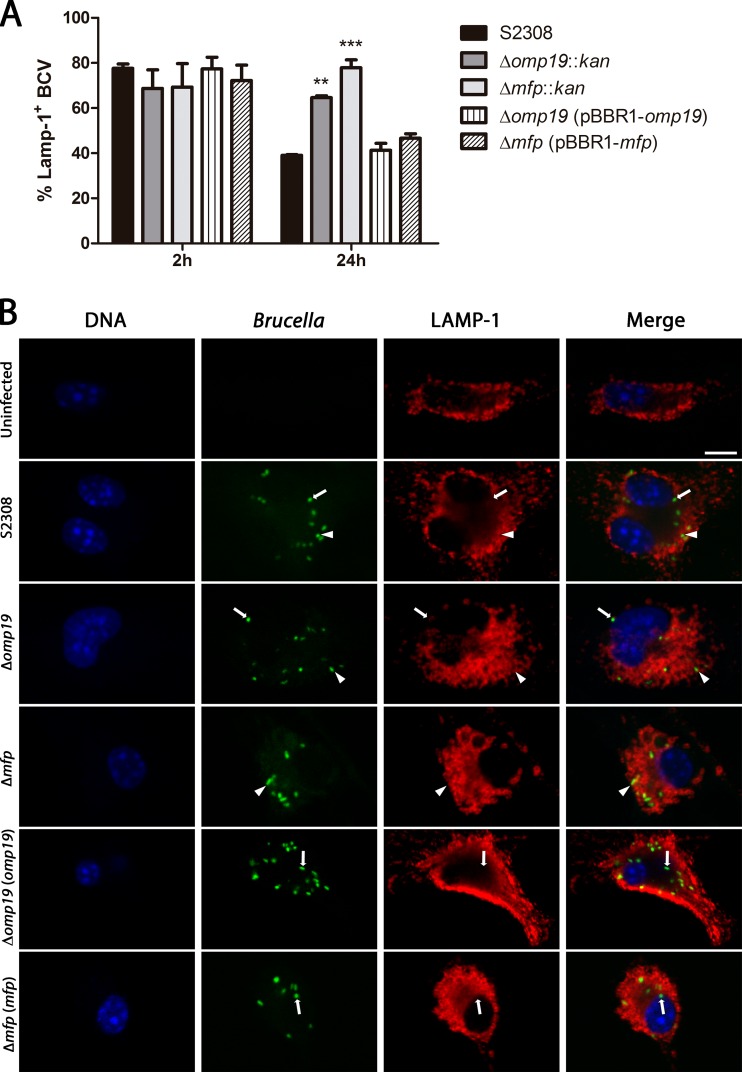
Inside the cell, the maturation process of the Brucella-containing vacuoles (BCVs) is characterized by transient interactions and partial fusions with organelles of the endocytic pathway. Such fusion events are required for bacteria to redirect their trafficking to the ER and for further maturation of BCVs into ER-derived replicative organelles. The formation of the replicative BCVs is characterized by the loss of lysosomal markers, such as lysosomal-associated membrane protein 1 (LAMP-1) (8, 9). Therefore, we analyzed whether the BCVs containing a Δmfp(pBBR4-gfp) or Δomp19(pBBR4-gfp) mutant strain lose or retain the LAMP-1 marker after BMDM infection. At 2 h postinfection, approximately 70% of the BCVs of the wild-type strain B. abortus S2308, the mutant Δmfp(pBBR4-gfp) or Δomp19(pBBR4-gfp) strain, and the complemented Δmfp(pBBR1-mfp, pBBR4-gfp) or Δomp19(pBBR1-omp19, pBBR4-gfp) strain were found in LAMP-1-positive compartments. However, at 24 h postinfection, approximately 60% of the BCVs containing the B. abortus S2308 and the complemented Δmfp(pBBR1-mfp, pBBR4-gfp) or Δomp19(pBBR1-omp19, pBBR4-gfp) strain were found in LAMP-1-negative compartments, while around 80% or 65% of the BCVs containing the Δmfp(pBBR4-gfp) or Δomp19(pBBR4-gfp) strain retained the LAMP-1 marker, respectively. Therefore, we conclude that Δmfp(pBBR4-gfp) and Δomp19(pBBR4-gfp) strains remain in LAMP-1-containing compartments 24 h after infection (Fig. 1). Additionally, we determined whether bacterial growth in Brucella broth was modified by the absence of the proteins Mfp or Omp19. Both the Δmfp::kan (193 ± 10 min) and Δomp19::kan (196 ± 6.4 min) mutant strains showed a generation time (TG) slightly greater than that of the wild-type strain B. abortus S2308 (180 ± 2.8 min); however, these differences were not statistically significant (P < 0.05), as determined by posttests using the Bonferroni method after ANOVA (data not shown).
FIG 1.
Multiplication and intracellular localization of B. abortus Δmfp::kan, Δomp19::kan, Δmfp(pBBR1-mfp), Δomp19(pBBR1-omp19), and wild-type strains in BMDM. BMDM were infected (MOI of 100:1) with the B. abortus S2308(pBBR4-gfp), Δmfp(pBBR4-gfp), Δomp19(pBBR4-gfp), Δmfp(pBBR1-mfp, pBBR4-gfp), or Δomp19(pBBR1-omp19, pBBR4-gfp) strain. At 2 and 24 h postinfection, BCVs marked with LAMP-1 were directly enumerated by optical visualization using a confocal microscope. At least 50 BMDM were counted for each triplicate view. (A) Quantification of the percentage of wild-type or Brucella mutant BCVs containing LAMP-1 by confocal microscopy. Statistically significant differences in relation to the S2308(pBBR4-gfp) strain are indicated as follows: **, P < 0.01; ***, P < 0.001. (B) Representative confocal images of BMDM at 24 h postinfection with wild-type B. abortus or mutants. Brucella strains are labeled in green (GFP), LAMP-1 is shown in red (Alexa 546), and DNA is shown in blue (DAPI). Arrows point to Brucella bacteria outside LAMP-positive vesicles, and arrowheads indicate Brucella bacteria inside the LAMP-positive compartments. Scale bar, 10 μm.
B. abortus Δmfp and Δomp19 strains have reduced persistence and confer immunoprotection to C57BL/6 mice.
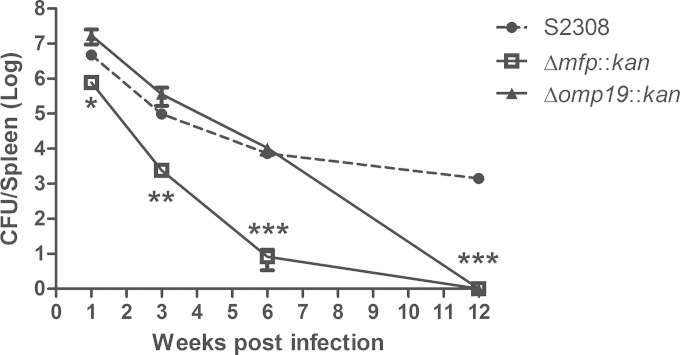
To determine whether Brucella Mfp and Omp19 are involved in the persistence of B. abortus, we compared the bacterial load in spleens of C57BL/6 mice inoculated with B. abortus wild-type strain S2308 or the Δmfp::kan and Δomp19::kan mutant strains. The Δmfp::kan strain demonstrated reduced persistence during all time periods analyzed compared to that of the wild-type strain S2308 (Fig. 2). The persistence of the Δomp19::kan strain was significantly reduced at 6 weeks postinfection. At 12 weeks postinoculation, both mutant strains were completely eliminated from the mouse spleens.
FIG 2.
Persistence of B. abortus S2308, Δmfp, and Δomp19 strains in C57BL/6 mice. Mice were intraperitoneally injected with 106 CFU of B. abortus S2308 and the Δmfp::kan or Δomp19::kan strain. Spleens were removed at 1, 3, 6, and 12 weeks after infection, and the CFU were enumerated. The data points are presented as the log10 CFU per spleen. Statistically significant differences in relation to B. abortus wild-type S2308 are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. This result is representative of two independent experiments.
To determine whether the Δmfp::kan and Δomp19::kan strains could induce protective immunity against infection, C57BL/6 mice immunized with these mutant strains were challenged with B. abortus S2308. Bacterial CFU numbers in the spleens were determined 2 weeks after challenge. The degree of vaccine efficacy in C57BL/6 mice was determined by subtracting the mean number of CFU/spleen recovered from mice after immunization and challenged with B. abortus S2308 from the mean number of CFU/spleen recovered from unimmunized but challenged control mice (PBS). All immunized mice had significant reductions in the numbers of B. abortus S2308 bacteria in the spleen compared with the amounts in the spleen of unimmunized mice (Table 2). No significant differences were observed between mice immunized with the S19 and Δmfp::kan strains as well as with the RB51 and Δomp19::kan strains. However, the S19 and Δmfp::kan strains (1.73 and 1.47 log units, respectively) induced greater protection than the RB51 and Δomp19::kan strains (0.77 and 0.73 log units, respectively).
TABLE 2.
Protective immunity induced by immunization with a B. abortus S19, RB51, Δmfp, or Δomp19 strain in C57BL/6 mice
| Vaccine | Log10 CFU of B. abortus S2308 in spleen (mean ± SD)a | Protection (log10 U) |
|---|---|---|
| PBS control | 6.67 ± 0.13 | |
| B. abortus S19 | 4.94 ± 0.41 | 1.73b,c |
| B. abortus RB51 | 5.90 ± 0.42 | 0.77b |
| B. abortus Δmfp::kan | 5.20 ± 0.42 | 1.47b,c |
| B abortus Δomp19::kan | 5.94 ± 0.35 | 0.73b |
Mice were inoculated with PBS, 107 CFU of RB51, or 105 CFU of the other strains. Six weeks later, mice were challenged i.p. with 106 CFU of S2308, and spleen CFU were enumerated 2 weeks after challenge. Results are representative of two independent experiments.
Significantly different compared to the PBS control group (P ≤ 0.0004).
Significantly different compared to the Δomp19::kan strain and RB51 (P ≤ 0.003).
B. abortus Δmfp induces IFN-γ production similar to that of the vaccine strains S19 and RB51.
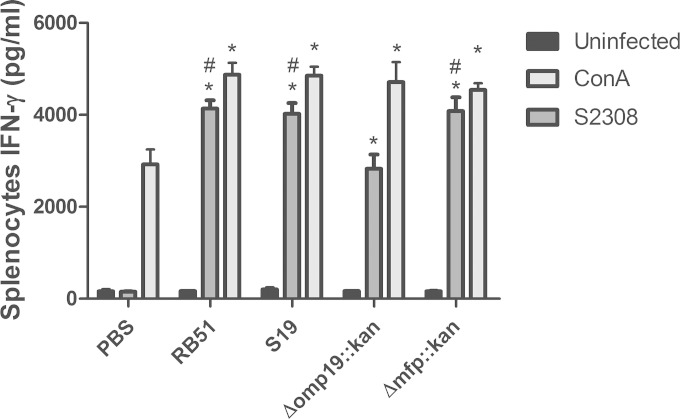
IFN-γ is required for the bactericidal activity of macrophages and is a key cytokine in immunity against Brucella (24, 25). Therefore, we investigated the IFN-γ production of splenocytes in mice previously immunized with the Δmfp::kan or Δomp19::kan strain in response to challenge with the B. abortus wild-type strain. The Δmfp::kan mutant strain induced levels of IFN-γ similar to the level induced in response to the vaccine strains RB51 and S19 (Fig. 3). In contrast, the Δomp19::kan strain induced less IFN-γ in spleen cells of vaccinated mice than the Δmfp::kan, S19, and RB51 strains in spleen cells of immunized animals.
FIG 3.
Production of IFN-γ in splenocytes of C57BL/6 mice vaccinated with a B. abortus Δmfp or Δomp19 mutant strain. C57BL/6 mice were intraperitoneally injected with the wild-type S2308 (105 CFU), with vaccine strain S19 (105 CFU) or RB51 (CFU 107), or with the Δmfp::kan (105 CFU) or Δomp19::kan (105 CFU) strain. Unimmunized control mice were inoculated with PBS. After 6 weeks, a splenectomy was performed, and spleen cells were activated in vitro with B. abortus S2308 or ConA. After 72 h, the supernatant was collected, and the amount of IFN-γ was measured by ELISA. Statistically significant differences are indicated as follows: *, P < 0.001, compared to the PBS control group; #, P < 0.001, compared to the Δomp19::kan group. This result is representative of two independent experiments.
B. abortus Δmfp and Δomp19 strains are attenuated and confer immunoprotection in IRF-1 KO mice.
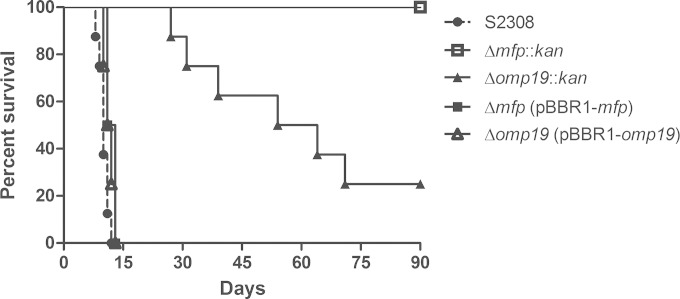
Immunocompromised IRF-1 KO mice can detect different levels of Brucella virulence, providing a useful tool for evaluating attenuation of Brucella mutants (23). To test the attenuation of the mutants, IRF-1 KO mice were infected with the S2308, Δmfp::kan, and Δomp19::kan strains and with the Δmfp(pBBR1-mfp) or Δomp19(pBBR1-omp19) complemented strain at 106 CFU, and survival was monitored. All mice inoculated with the wild-type strain S2308 died between the 5th and 11th day after infection. After inoculation with the Δomp19::kan strain, 87.5% (7/8) of mice survived the first 4 weeks, and 25% (2/8) survived the entire period of observation (90 days). All mice inoculated with the Δmfp::kan strain survived the entire period of observation. These results indicate an attenuation of virulence of both mutant strains and especially of the Δmfp::kan strain, which showed complete attenuation (Fig. 4). Additionally, complementation of the Δmfp and Δomp19 mutants restored the wild-type strain 2308 phenotype since all mice inoculated with the Δmfp(pBBR1-mfp) or Δomp19(pBBR1-omp19) strain died within 15 days of infection. Our research group has previously demonstrated the virulence of the vaccine strains S19 and RB51 in IRF-1 KO mice. Only 70 to 80% of IRF-1 KO mice inoculated with S19 at 106 CFU survived the first 4 weeks after infection, while all mice inoculated with RB51 at 106 or 108 CFU survived the first 4 weeks (21, 26). Our data suggest the following order of virulence in IRF-1 KO mice: the S2308 strain > Δomp19::kan strain > Δmfp::kan strain.
FIG 4.
Virulence of the B. abortus S2308, Δmfp, Δomp19, Δmfp(pBBR1-mfp), or Δomp19(pBBR1-omp19) strain in IRF-1 KO mice. Mice were intraperitoneally injected with 106 CFU of each strain, and mouse survival was monitored for 90 days after infection. This result is representative of three independent experiments.
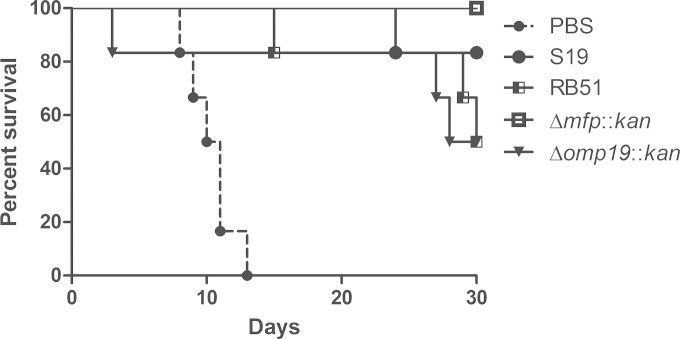
To determine the protection efficacy of immunizations with the Δmfp::kan and Δomp19::kan strains, IRF-1 KO mice were vaccinated with the B. abortus mutants or with the vaccine strain S19 or RB51 as a control. Eight weeks after vaccination, all mice were challenged with wild-type S2308, and mouse survival was monitored for 30 days. After the challenge with B. abortus S2308, all mice that received saline died between the 8th and 14th days. All vaccinated mice presented increased survival compared to that of control animals inoculated with PBS, demonstrating that all strains activated the immune system and induced some degree of protection. However, the Δmfp::kan strain was the only one with 100% protection efficacy. At the end of the observation period, the percent survival of immunized mice was as follows: for the Δmfp::kan strain, 100%; S19, 83.3%; RB51, 50%; and the Δomp19::kan strain, 50% (Fig. 5).
FIG 5.
Effectiveness of immunization with the B. abortus S19, RB51, Δmfp, or Δomp19 strain in IRF-1 KO mice. Mice were immunized with S19 (105 CFU) or RB51 (107 CFU) or with the Δmfp::kan (105 CFU) or Δomp19::kan (105 CFU) strain. Control unimmunized mice were injected with PBS. After 8 weeks, all mice were challenged with 106 CFU of B. abortus S2308. Mouse survival was monitored for 30 days. This result is representative of three independent experiments.
DISCUSSION
B. abortus is the main etiological agent of brucellosis, a zoonotic disease that affects numerous animal species, causing serious economic loss and posing a public health threat (2–7, 27). Therefore, many research efforts have focused on characterizing new virulence factors of B. abortus with the aim of developing live vaccine strains that combine both safety and efficacy (11). Commonly, B. abortus S19 and RB51 have been used, but these vaccine strains have several drawbacks, and the development of improved vaccines to control brucellosis has been challenging (11, 28, 29).
Previous studies from our laboratory have identified genes related to survival and virulence in B. abortus (13, 14, 21, 26). Here, we investigated the importance of the Brucella proteins Mfp and Omp19 in microbial pathogenesis by evaluating the persistence of the B. abortus gene replacement Δomp19::kan and Δmfp::kan mutant strains both intracellularly and in vivo. In this study, we showed that the absence of Omp19 and Mfp in B. abortus is associated with reduced virulence in mice. However, attenuation was predominantly associated with the Δmfp::kan strain. Brucella virulence is dependent on survival and replication within host cells, including phagocytic cells. Once internalized, Brucella is found within BCVs that transiently interact with early and late endosomes, acquiring lysosomal markers. However, after fusion with lysosomes, the bacterium redirects its trafficking to the ER and establishes a replicative BCV. Consequently, the loss of lysosomal markers on BCVs is a hallmark of virulent Brucella infection. We therefore analyzed the endosomal/lysosomal marker LAMP-1 (8, 9) (Fig. 1). Most of the BCVs containing wild-type B. abortus S2308 lost LAMP-1, which suggests the formation of replicative BCVs. In contrast, most BCVs containing the Δmfp::kan or Δomp19::kan strain retained LAMP-1. Considering the in vitro membrane fusion activity of Brucella Mfp (16) and its similarity to fusogenic proteins commonly observed in viruses, bacteria, and eukaryotes (17–19), Brucella Mfp might be involved in fusion between the BCV and the ER and subsequently in the formation of the replicative BCV. However, this hypothesis has yet to be proven. Furthermore, in vivo persistence was observed in C57BL/6 and IRF-1 KO mice. B. abortus Δmfp::kan and Δomp19::kan mutant strains also had reduced persistence in C57BL/6 mice (Fig. 2) and were attenuated in IRF-1 KO mice (Fig. 4). However, while the Δmfp::kan strain already had reduced persistence in C57BL/6 spleens 1 week after infection, the Δomp19::kan strain did not display diminished persistence compared to that of the wild-type strain until 6 weeks postinfection (Fig. 3). Therefore, the Δomp19::kan strain had residual virulence in C57BL/6 and IRF-1 KO mice, whereas the Δmfp::kan strain presented greater attenuation in these mouse models. Additionally, the Δmfp(pBBR1-mfp) or Δomp19(pBBR1-omp19) complemented strain recovered full virulence and killed all IRF-1 KO mice within 15 days of infection.
A Th1 cell-dominant response is required for protection against Brucella infection (25, 30). Therefore, the induction of a Th1 profile can be used as an indicator of potential Brucella vaccine efficacy in the preliminary screenings of potential candidates (12). Both the Δmfp::kan and Δomp19::kan strains induced IFN-γ production in spleen cells of vaccinated mice; however, the Δmfp::kan strain induced greater amounts than the Δomp19::kan strain (Fig. 3). To evaluate the immunoprotection conferred by the Δmfp::kan and Δomp19::kan strains, in vivo assays were performed in C57BL/6 and IRF-1 KO mice (Table 2 and Fig. 5). Both mutant strains conferred significant immunoprotection in C57BL/6 and IRF-1 KO mice. However, the Δmfp::kan strain was more efficient in both animal models. Over 30 days of observation, the Δmfp::kan strain conferred 100% protection to the IRF-1 KO mice when they were challenged with the B. abortus virulent strain.
Previous studies with Brucella mutants suggest that some level of persistence is required for protection (12). This residual virulence cannot be so great as to cause disease or so low as to fail to successfully stimulate protective immunity. Our results suggest that the Δmfp::kan strain has a level of persistence that allows it to elicit potent protective immunity without causing disease. Moreover, although it was more attenuated, the Δmfp::kan strain induced greater protection than the Δomp19::kan strain. The low protection induced by the Δomp19::kan strain highlights the antigenic importance of Omp19, which corroborates the fact that immunization with Omp19 is enough to confer partial protection against wild-type B. abortus 2308 infection (31). Additionally, mutation in the mfp and omp19 genes does not grossly alter lipopolysaccharide (LPS) formation since both the Brucella Δmfp::kan and Δomp19::kan strains showed a smooth LPS phenotype, similar to that of the wild-type 2308 strain (data not shown). Further, Brucella Δmfp::kan presented levels of Omp19 expression similar to the level of wild-type bacteria, demonstrating that lack of Mfp does not influence the production of important antigens such as Omp19 (data not shown).
In conclusion, Omp19 and Mfp are critical for the full virulence of B. abortus. Additionally, the Δmfp::kan and Δomp19::kan mutant strains are able to induce protective immunity in C57BL/6 and IRF-1 KO mice at levels similar to those of the commercial strains S19 and RB51, respectively. Due to its protection efficacy in the mouse models studied here, the Δmfp::kan strain shows great potential as a vaccine for brucellosis, and this result justifies further investigation in large animals.
ACKNOWLEDGMENTS
This work was supported by grants from CNPq, CNPq/CONICET, FAPEMIG, FAPEMIG/CNPq (PRONEX), CAPES/PNPD, CNPq/CT-Biotec, CNPq/REPENSA, and INCT-Vacinas.
REFERENCES
- 1.Pappas G. 2010. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents 36(Suppl 1):S8–S11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Martirosyan A, Moreno E, Gorvel J-P. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev 240:211–234. doi: 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 3.Corbel MJ. 1997. Brucellosis: an overview. Emerg Infect Dis 3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernués A, Manrique E, Maza MT. 1997. Economic evaluation of bovine brucellosis and tuberculosis eradication programmes in a mountain area of Spain. Prev Vet Med 30:137–149. doi: 10.1016/S0167-5877(96)01103-8. [DOI] [PubMed] [Google Scholar]
- 5.Grillo M-J, Blasco J-M, Gorvel J-P, Moriyon I, Moreno E. 2012. What have we learned from brucellosis in the mouse model? Vet Res 43:29–29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Review article: brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 7.Peniche-Cardena A, Martinez-Herrera D, Franco-Zamora JL, Canudas-Lara E, Barradas-Pina F, Molina-Sanchez B, Gutierrez-Ruiz EJ, Williams JDJ, Morales-Alvarez JF, Flores-Castro R. 2012. Economic analysis of efficiency of RB51 strain vaccine of Brucella abortus applied in herds naturally infected with brucellosis in tropical climate. J Anim Vet Adv 11:1784–1789. doi: 10.3923/javaa.2012.1784.1789. [DOI] [Google Scholar]
- 8.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. 2008. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 9.von Bargen K, Gorvel JP, Salcedo SP. 2012. Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol Rev 36:533–562. doi: 10.1111/j.1574-6976.2012.00334.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira SC, Giambartolomei GH, Cassataro J. 2011. Confronting the barriers to develop novel vaccines against brucellosis. Expert Rev Vaccines 10:1291–1305. doi: 10.1586/erv.11.110. [DOI] [PubMed] [Google Scholar]
- 11.Schurig GG, Sriranganathan N, Corbel MJ. 2002. Brucellosis vaccines: past, present and future. Vet Microbiol 90:479–496. doi: 10.1016/S0378-1135(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. 2013. Progress in vaccine development. Front Biol (Beijing) 8:60–77. doi: 10.1007/s11515-012-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerq S, Harms JS, Rosinha GMS, Azevedo V, Oliveira SC. 2002. Induction of a Th1-type of immune response but not protective immunity by intramuscular DNA immunisation with Brucella abortus GroEL heat-shock gene. J Med Microbiol 51:20–26. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira SC, Splitter GA. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959–962. doi: 10.1016/0264-410X(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 15.Toth TE, Cobb JA, Boyle SM, Roop RM, Schurig GG. 1995. Selective humoral immune response of Balb/C mice to Brucella abortus proteins expressed by vaccinia virus recombinants. Vet Microbiol 45:171–183. doi: 10.1016/0378-1135(95)00047-E. [DOI] [PubMed] [Google Scholar]
- 16.Carrica MDC, Craig PO, Alonso SDV, Goldbaum FA, Cravero SL. 2008. Brucella abortus MFP: a trimeric coiled-coil protein with membrane fusogenic activity. Biochemistry 47:8165–8175. doi: 10.1021/bi800462y. [DOI] [PubMed] [Google Scholar]
- 17.Hayward RD, McGhie EJ, Koronakis V. 2000. Membrane fusion activity of purified SipB, a Salmonella surface protein essential for mammalian cell invasion. Mol Microbiol 37:727–739. doi: 10.1046/j.1365-2958.2000.02027.x. [DOI] [PubMed] [Google Scholar]
- 18.Fasshauer D. 2003. Structural insights into the SNARE mechanism. Biochim Biophys Acta 1641:87–97. doi: 10.1016/S0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 19.Dutch RE, Jardetzky TS, Lamb RA. 2000. Virus membrane fusion proteins: biological machines that undergo a metamorphosis. Biosci Rep 20:597–612. doi: 10.1023/A:1010467106305. [DOI] [PubMed] [Google Scholar]
- 20.Tibor A, Wansard V, Bielartz V, Delrue R-M, Danese I, Michel P, Walravens K, Godfroid J, Letesson J-J. 2002. Effect of omp10 or omp19 deletion on Brucella abortus outer membrane properties and virulence in mice. Infect Immun 70:5540–5546. doi: 10.1128/IAI.70.10.5540-5546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trant CG, Lacerda TL, Carvalho NB, Azevedo V, Rosinha GM, Salcedo SP, Gorvel J-P, Oliveira SC. 2010. The Brucella abortus phosphoglycerate kinase mutant is highly attenuated and induces protection superior to that of vaccine strain 19 in immunocompromised and immunocompetent mice. Infect Immun 78:2283–2291. doi: 10.1128/IAI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 23.Ko J, Gendron-Fitzpatrick A, Ficht TA, Splitter GA. 2002. Virulence criteria for Brucella abortus strains as determined by interferon regulatory factor 1-deficient mice. Infect Immun 70:7004–7012. doi: 10.1128/IAI.70.12.7004-7012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandao AP, Oliveira FS, Carvalho NB, Vieira LQ, Azevedo V, Macedo GC, Oliveira SC. 2012. Host susceptibility to Brucella abortus infection is more pronounced in IFN-γ knockout than IL-12/β2-microglobulin double-deficient mice. Clin Dev Immunol 2012:589494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacerda TL, Cardoso PG, Augusto de Almeida L, Camargo IL, Afonso DA, Trant CC, Macedo GC, Campos E, Cravero SL, Salcedo SP, Gorvel J-P, Oliveira SC. 2010. Inactivation of formyltransferase (wbkC) gene generates a Brucella abortus rough strain that is attenuated in macrophages and in mice. Vaccine 28:5627–5634. doi: 10.1016/j.vaccine.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Boschiroli ML, Foulongne V, O'Callaghan D. 2001. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol 4:58–64. doi: 10.1016/S1369-5274(00)00165-X. [DOI] [PubMed] [Google Scholar]
- 28.Cheville NF, Olsen SC, Jensen AE, Stevens MG, Palmer MV, Florance AM. 1996. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am J Vet Res 57:1153–1156. [PubMed] [Google Scholar]
- 29.Smith LD, Ficht TA. 1990. Pathogenesis of Brucella. Crit Rev Microbiol 17:209–230. doi: 10.3109/10408419009105726. [DOI] [PubMed] [Google Scholar]
- 30.Rafiei A, Ardestani S, Kariminia A, Keyhani A, Mohraz M, Amirkhani A. 2006. Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J Infect 53:315–324. doi: 10.1016/j.jinf.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Pasquevich KA, Ibanez AE, Coria LM, Garcia Samartino C, Estein SM, Zwerdling A, Barrionuevo P, Oliveira FS, Seither C, Warzecha H, Oliveira SC, Giambartolomei GH, Cassataro J. 2011. An oral vaccine based on U-Omp19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PLoS One 6:e16203. doi: 10.1371/journal.pone.0016203. [DOI] [PMC free article] [PubMed] [Google Scholar]