Abstract
CD46 is a ubiquitously expressed type I transmembrane protein, first identified as a regulator of complement activation, and later as an entry receptor for a variety of pathogens. The last decade has also revealed the role of CD46 in regulating the adaptive immune response, acting as an additional costimulatory molecule for human T cells and inducing their differentiation into Tr1 cells, a subset of regulatory T cells. Interestingly, CD46 regulatory pathways are defective in T cells from patients with multiple sclerosis, asthma and rheumatoid arthritis, illustrating its importance in regulating T cell homeostasis. Indeed, CD46 expression at the cell surface is tightly regulated in many different cell types, highlighting its importance in several biological processes. Notably, CD46 is the target of enzymatic processing, being cleaved by metalloproteinases and by the presenilin/gamma secretase. This processing is required for its functions, at least in T cells. This review will summarize the latest updates on the regulation of CD46 expression and on its effects on T cell activation.
Keywords: CD46, enzymatic processing, gamma-secretase, Matrix metalloproteinases, T cells
Introduction
CD46 is a type I transmembrane protein expressed on all nucleated human cells (Liszewski and Atkinson, 1992; Seya, et al., 1999; Seya, et al., 1986). Its primary function is to bind to C3b/C4b to allow their cleavage by the serine protease Factor I, thereby protecting autologous cells from complement attack. However, it is a complex molecule as several isoforms, produced by splicing of various exons, can be co-expressed. Moreover, multiple ligands bind to CD46, and its ligation induces a variety of pleiotropic actions on different cell types. Labeled a “pathogen’s magnet” as several viruses and bacteria use CD46 as their receptor (Cattaneo, 2004), CD46 has also been shown to modulate autophagy which could control early pathogen infection (Joubert, et al., 2009). In addition, CD46 affects reproductive biology as it is involved in sperm-egg fusion during the fertilization process (Harris, et al., 2006; Riley, et al., 2002). Murine CD46 is only expressed in testes, underlining the important role of CD46 in this process. CD46 also has the ability to modulate acquired immunity. It affects the functions of T cells and antigen presenting cells, such as monocytes and dendritic cells (DCs) (Karp, 1999; Russell, 2004; Schneider-Schaulies and Schneider-Schaulies, 2008). The role of CD46 on T cells has been predominantly studied over the past decade. It was initially shown that CD46 could act as a costimulatory molecule for human T cells (Astier, et al., 2000). Most importantly, it was then shown that CD46 co-activation could in fact drive Tr1 differentiation (Kemper, et al., 2003). Tr1 cells are a subset of Treg characterized by their secretion of IL-10, a potent anti-inflammatory molecule. Indeed, CD46 has the ability to switch T cell phenotypes from Th1 to Tr1 depending notably on the cytokine milieu (Cardone, et al., 2010). The Tr1 differentiation pathway is defective in patients with multiple sclerosis (MS), as IL-10 production upon CD46 activation was impaired in ~50% of patients (Astier, et al., 2006; Ni Choileain and Astier, 2011). Defects in CD46-mediated Tr1 differentiation are not only observed in MS but also in patients with asthma (Xu, et al., 2010) and rheumatoid arthritis (Cardone, et al., 2010). This highlights the importance of CD46 in controlling T cell activation. CD46 expression at the surface of numerous cell types is controlled by different mechanisms. In epithelial cells (Weyand, et al., 2010) and T cells (Ni Choileain, et al., 2011b), the CD46 ectodomain is shed after cleavage by metalloproteinases (MMP), and its two intracytoplasmic tails cleaved by the presenilin-gamma secretase (P/γS) enzymatic complex. The importance of CD46 processing as a means of ensuring proper T cell activation and termination, is further discussed in this review.
CD46 structure
CD46 was initially identified as the membrane cofactor protein (MCP), a member of the regulators of complement activation (RCA) family gene cluster on chromosome 1, 1q32 (Lublin, et al., 1988). The CD46 ectodomain is composed of four conserved short consensus repeats (SCR) that are also called sushi domains or complement control protein modules (CCP). This is the binding site for either complement or pathogens, although Neisseria also requires the following domain, a region rich in serine, threonine and proline (STP region), which is heavily O-glycosylated (Kallstrom, et al., 2001; Persson, et al., 2011). A twelve amino-acid segment of unknown function then precedes a transmembrane segment and a short cytoplasmic tail (Liszewski and Atkinson, 1996). The CD46 gene consists of 14 exons and 14 transcripts have been reported due to extensive RNA splicing (Russell et al 1992). However, only four main isoforms are commonly expressed, and represented in Figure 1. They are generated by alternative splicing at two sites. One of them is within the STP region in the ectodomain. This region consists of 3 exons called A, B and C. The four major isoforms of CD46 utilize the C region, while the B exon is alternatively spliced, giving rise to either a BC or C STP region. Isoforms containing the A exon of this region have mainly been reported on malignant cells or intestine and salivary glands (Xing, et al., 1994). Only the C isoform was initially described in brain while an increased BC expression was observed in kidney and salivary gland (Johnstone, et al., 1993a). However, the BC isoform was also later identified in brain homogenate of patients who died of subacute sclerosing panencephalitis following measles infection (Buchholz, et al., 1996). The other main splicing event results in the generation of different short cytoplasmic tails, mainly Cyt1 and Cyt2. The exon coding for Cyt1 can be spliced, giving rise to Cyt2. Their sequences are different due to the presence of a stop codon in Cyt1. The transcripts coding for two other tails, called Cyt3 and Cyt4, have been reported but no proteins have been so far identified (Hara, et al., 1998; Purcell, et al., 1991). Again, most cell types in the human body express both Cyt1 and Cyt2 cytoplasmic tails. However, strikingly, a preferential expression of Cyt2 was detected in testes (Riley, et al., 2002) and brain (Johnstone, et al., 1993b). These data suggest that the expression of CD46 in specific organs such as brain, testis and salivary glands is differently controlled compared to other ones.
Figure 1. Representation of the four main isoforms of CD46.
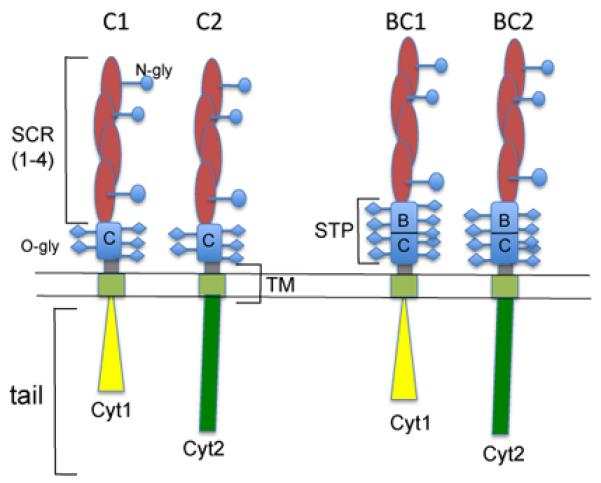
These isoforms arise from the alternative splicing of exon B, giving rise to a BC or C isoform. The exon coding for Cyt1 can also be spliced to produce either Cyt1 or Cyt2 tail. As Cyt1 contains a stop codon, Cyt1 and Cyt2 sequences are different. Hence, the four main isoforms are BC1, C1, BC2 and C2.
CD46 and its multitude of ligands
CD46 has cofactor activity for inactivation of C3b and C4b, as it allows their cleavage by the serine protease Factor I, protecting the cells from complement attack. The role of CD46 in the regulation of complement activity is evidenced in several pathologies. Heterozygous mutations in CD46 cause atypical hemolytic uremic syndrome (aHUS) (Fremeaux-Bacchi, et al., 2006; Noris, et al., 2003; Richards, et al., 2003). In contrast, many tumoral cells exhibit increased expression levels of CD46 and other complement regulators, rendering them resistant to complement attack (Geis, et al., 2010).
CD46 has also been labeled as a ‘pathogen’s magnet’ (Cattaneo, 2004), as at least seven pathogens, including several viruses and bacteria, use CD46 as their cellular receptor. This includes the Edmonston strain of measles virus, human herpesvirus-6 (HHV-6), adenoviruses A and B, type IV pili of Neisseria gonorrhoeae and Neisseria meningitidis as well as group A streptococcus (Cattaneo, 2004; Riley-Vargas, et al., 2004). As mentioned earlier, CD46 triggering induces autophagy (Joubert, et al., 2009). This is mediated by the Cyt1 tail, which interacts with the scaffold protein GOPC linked to autophagosome formation. The ligation of CD46 by measles virus as well as Streptococcus induces autophagy, which could provide a means to control infections. Interestingly, we have recently observed that CD46 ligation on primary T cells can lead to increased levels of LC3-II proteins which are indicative of autophagy (Mathieson, Astier, unpublished data). Finally, an indirect role for CD46 has been proposed for Escherichia coli-mediated urinary infections. Opsonized E. Coli are internalized via CD46 by epithelial cells which favours the uptake of the pathogenic bacteria (Li, et al., 2006). Moreover, the binding of pathogens to CD46 can modulate the immune response in their favor. For example, Streptococcus pyogenes can trigger Treg differentiation and IL-10 production to suppress ongoing effector responses (Price, et al., 2005). A decrease in IL-12 has been observed upon measles virus and HHV-6 infection of monocytes/macrophages (Karp, et al., 1996; Smith, et al., 2003). However, increased expressions of IL-12p40 and IL-23 have also been reported upon CD46 ligation in macrophages and dendritic cells, respectively, suggesting their regulation may also be dependant on the maturation stage on the cells (Kurita-Taniguchi, et al., 2000; Vaknin-Dembinsky, et al., 2008).
Intriguingly, different domains bind to the various ligands (Persson, et al., 2011). For example, SCR1 is not involved in binding to C3b and C4b, and the binding sites for C3b and C4b are different (Liszewski, et al., 2000). Moreover, glycosylation seems important and the N-glycosylation in SCR2 and SCR4 is necessary for CD46 complement regulatory functions (Liszewski, et al., 1998). However, there are some reports of effective recombinant bacteria-derived CD46 suggesting that glycosylation might be differently required depending on the ligand or the binding domain (Kallstrom, et al., 1997; Lovkvist, et al., 2008). In contrast, measles virus and adenovirus bind to SCR1 and SCR2 (Fleischli, et al., 2005; Gaggar, et al., 2005; Manchester, et al., 1997), and HHV-6 binds to SCR2 and SCR3 (Greenstone, et al., 2002). Streptococcus interacts with SCR4 (Blom, et al., 2000) while interaction of Neisseria with CD46 requires the SCR3 domain but also the STP region (Kallstrom, et al., 2001). The multitude of binding sites in CD46 raises the question as to whether its ligation at different sites differently modulates its functions.
CD46, an important regulator of T cell functions
Efficient T cell activation requires two signals, provided by TCR engagement and the concomitant stimulation of a costimulatory molecule (Rudd and Schneider, 2003; Sharpe and Freeman, 2002). The role of CD46 in the regulation of the adaptive immune response was demonstrated a decade ago. It was originally shown that CD46 triggering on PBMC transduced intracellular signals that led to enhanced T cell proliferation, hence acting as an additional costimulatory molecule for human T cells (Astier, et al., 2000; Marie, et al., 2002; Zaffran, et al., 2001). CD46-coactivated T cells also morphologically change, showing actin reorganization and a ‘spread’ in the well suggestive of a migratory profile (Zaffran, et al., 2001). Indeed, CD46-activated T cells express the integrin α4β7, LIGHT and the chemokine receptor CCR9 in presence of retinoic acid (Alford, et al., 2008). A similar costimulatory function for murine T cells was simultaneously reported for Crry, the murine analog of CD46 (Fernandez-Centeno, et al., 2000; Gaglia, et al., 2001; Jimenez-Perianez, et al., 2005). More recently, it was shown that activation of CD55, another complement regulator, could also promote T cell activation (Capasso, et al., 2006). These data highlight the importance of these complement regulatory proteins in regulating the adaptive immune response (reviewed in (Kemper and Atkinson, 2007; Le Friec and Kemper, 2009; Longhi, et al., 2006; Morgan, et al., 2005)).
The complexity of CD46 in the regulation of T cell activation was first revealed upon the investigation of the function of its two cytoplasmic tails. The two main intracellular tails of CD46 produced by alternative spicing (Cyt1 and Cyt2) are usually co-expressed in human cells. Hence, their role in T cell activation was first investigated in a CD46-transgenic mouse model (Marie, et al., 2002). Unexpectedly, it was found that these two tails exerted opposite effects on T cell-induced inflammation (Marie, et al., 2002). Each isoform differently controlled cell proliferation and the amounts of IL-2 and IL-10 secreted, and only the Cyt1 isoform induced T cell spreading. Overall, in vivo, the expression of Cyt1 resulted in a strong decrease in inflammation, while in contrast expression of Cyt2 promoted inflammation. Expression of the two tails showed the dominant anti-inflammatory effect of Cyt1. Further studies in human T cells demonstrated that CD46 could drive Tr1 differentiation in the presence of IL-2, characterized by secretion of large amount of IL-10 (Kemper, et al., 2003) and Granzyme B (Grossman, et al., 2004). Sanchez et al however reported that CD46 activation could lead towards a Th1 phenotype with high levels of IFNγ produced (Sanchez, et al., 2004). The paradox was somewhat elucidated in a recent report demonstrating that CD46 could in fact switch T cells from a Th1 to a Tr1 phenotype, depending notably on the concentration of IL-2 in the milieu (Cardone, et al., 2010). This report highlights the ability of CD46 to adapt its response depending on its environment, and this is described in depth in the review by Heeger and Kemper in this issue.
CD46 and multiple sclerosis
CD46 activation was first reported to be defective in T cells from patients with MS, as evidenced by a lack of IL-10 production by CD46-activated T cells in these patients. While CD46 activation of CD4+T cells from healthy donors led to IL-10 production, IL-10 production was strongly impaired in most patients with MS, in the relapsing remitting stage (Astier, 2008; Astier, et al., 2006). Of note, polymorphisms in the alpha chain of the IL-2 receptor (IL-2RA) have been identified as risk factors in MS patients (Hafler, et al., 2007). Considering the crucial role of IL-2 in differentiating Tr1 cells, it is possible that this contributes to the defects identified in the CD46 pathway. Moreover, CXCR3 expression levels are elevated in CD46-activated T cells from patients compared to the levels expressed in cells from healthy donors which may contribute to their recruitment to the brain (Ni Choileain and Astier, unpublished data and (Ni Choileain, et al., 2011a)). Importantly, the lack of Tr1 differentiation in MS was corroborated by another study (Martinez-Forero, et al., 2008), as well as in an in vivo monkey model of MS (Ma, et al., 2009). Moreover, in MS, CD46 activation might lead to the production of increased levels of IL-1beta and IL-17A (Yao, et al., 2010). Very interestingly, abnormal CD46 regulation of T cells has now also been demonstrated in asthma (Xu, et al., 2010) and rheumatoid arthritis (Cardone, et al., 2010). The role of CD46 in so many important diseases underlines its importance in maintaining immune homeostasis, and highlights the need to further understanding its function.
Defects of CD46 in MS are not only confined to T cells. CD46-activation of dendritic cells (DCs) was also skewed towards a pro-inflammatory profile. The pro-inflammatory cytokine IL-23 is essential for the survival of IL-17 producing effector cells (McGeachy, et al., 2009), and increased IL-23 production by DCs from patients was reported (Vaknin-Dembinsky, et al., 2006). Indeed, CD46 activation strongly increased IL-23 secretion levels by activated DCs of MS patients. In addition, CD46-activated DCs supernatant significantly increased IL-17 secretion by CD4+ T cells (Vaknin-Dembinsky, et al., 2008). Hence, it appears that, at least in MS but probably in other diseases, CD46 switches both T cells and DCs cells towards a pro-inflammatory phenotype. It would be interesting to test CD46 functions in other cell types.
Of note, a preliminary study has described the presence of inhibitory autoantibodies to CD46 and CD59 in the sera of MS patients that may contribute to CNS complement attack, although this small study has not been replicated yet (Pinter, et al., 2000).
Finally, it is intriguing that many infections have been associated with MS, mainly EBV (Maghzi, et al., 2010; Serafini, et al., 2010) and HHV-6 (Fogdell-Hahn, et al., 2005; Soldan, et al., 1997), albeit controversially (Ingram, et al., 2010; Lindsey and Hatfield, 2010; Tuke, et al., 2004). Relapses as well as increased Expanded Disability Status Scale (EDSS) scores have been correlated with active HHV-6 replication in remitting relapsing MS patients (Alvarez-Lafuente, et al., 2006). Increased levels of HHV-6 DNA have also been reported in MS plaques and serum that correlated with clinical exacerbations (Berti, et al., 2002; Cermelli, et al., 2003). Expression of CD46 on oligodendrocytes and astrocytes facilitates cell-cell fusion with infected T cells and may support transmission of HHV-6 from the periphery to the CNS (Tang, et al., 2008). Hence, it is possible that HHV-6 binding to CD46 in MS triggers CD46 co-stimulation and its defective pathway.
The timing of CD46 ligation controls T cell activation
Whilst CD46 indisputably promotes T cell activation (Astier, et al., 2000), regulates inflammation (Marie, et al., 2002) and Tr1 differentiation (Kemper, et al., 2003), as well as affects T cell morphology (Zaffran, et al., 2001) and polarity (Oliaro, et al., 2006), these actions require the co-engagement of the T cell receptor. However, the timing of CD46 ligation can dramatically alter T cell fate (Oliaro, et al., 2006). Ligation of CD46 prior to T cell activation leads an altered polarization, preventing the formation of an immunological synapse, and hence inhibiting T cell activation (Oliaro, et al., 2006). Indeed, it abrogated recruitment of the microtubule-organizing centre (MTOC) to the site of TCR triggering. A similar effect was also observed on NK cells as ligation of CD46 inhibited the recruitment of the MTOC to the interface with target cells and correlated with reduced killing (Oliaro, et al., 2006). This inhibitory effect on cell activation is notably due to the recruitment of CD46 to the lipid rafts upon its ligation, hence recruiting lipid rafts away from the site of TCR stimulation (Ludford-Menting, et al., 2011). Importantly, both cytoplasmic isoforms Cyt1 and Cyt2 were able to relocate to the rafts upon their ligation, which required the presence of the transmembrane palmitoylated cysteine. Surprisingly though, only recruitment of Cyt1 could inhibit the MTOC formation at the site of TCR triggering (Ludford-Menting, et al., 2011). As CD46-Cyt1 has been shown to interact with the protein DLG4 that is involved in cell polarization (Ludford-Menting, et al., 2002), it is likely that multiple events contribute to the inhibitory effect of CD46 ligation on MTOC formation. Hence, CD46 ligation controls cellular activation depending on how and when it occurs.
The expression of CD46 is tightly regulated
CD46 Surface Downregulation
Cell surface expression of CD46 on a variety of cell types is tightly regulated. Ligation of CD46 by several viruses and bacteria induced its downregulation from the cell surface (Gill, et al., 2003; Lovkvist, et al., 2008; Mahtout, et al., 2009; Naniche, et al., 1993; Sakurai, et al., 2007). The YXXL motif present in the juxtamembrane region is required for its downregulation (Yant, et al., 1997). Several modes of downregulation have been described, and depend in part on the strength of ligation. In DCs and other non-lymphoid cells, CD46 is constitutively internalized via clathrin-coated pits and recycled to the surface. However, multivalent cross-linking of CD46 induces a process similar to macropinocytosis, in both lymphoid and non-lymphoid cells (Crimeen-Irwin, et al., 2003). Shedding of the entire molecule in vesicles has been observed for cancer cells (Hakulinen, et al., 2004) as well as apoptotic cells (Hakulinen and Keski-Oja, 2006). Lastly, shedding of the ectodomain of CD46 by metalloproteinases and related proteins from the family of A Disintegrin And Metalloproteinase (ADAM) has been reported. Indeed, necrosis of cells and apoptosis of epithelial and neuronal cells induced CD46 shedding from the cell surface and from the apoptotic vesicles (Cole, et al., 2006; Elward, et al., 2005; Hakulinen and Keski-Oja, 2006). This contributes to the resolution of inflammation by inducing the clearance of apoptotic cells. Inhibition of caspases decreased the release of shed CD46 (Hakulinen and Keski-Oja, 2006). Similarly, infection of epithelial cells by Streptococcus pyogenes induced CD46 shedding from the cell surface, and this was associated with apoptotic cell death (Lovkvist, et al., 2008). The metalloproteinases MMP-3, MMP-8, MMP-9 as well as ADAM-10 have been involved in CD46 cleavage in apoptotic epithelial and neuronal cells (Cole, et al., 2006; Hakulinen and Keski-Oja, 2006). It is possible that altered protease levels involved in CD46 shedding affects CD46 processing and subsequent functions. Loxoceles spider venom provokes the MMP-dependent loss of CD46 from leukocytes, resulting in loss of protection against complement-mediated lysis (Van Den Berg, et al., 2002). Binding of pathogenic Neisseria to epithelial cells also induces the MMP-dependent shedding of CD46 ectodomain (Weyand, et al., 2010). Strikingly, CD46 ectodomain is also shed after co-activation of primary human T cells in a metalloproteinase-dependent manner (Ni Choileain, et al., 2011b). Addition of active Vitamin D to T cell culture modulates CD46 expression at the surface of activated T cells and promotes Tr1 differentiation (Kickler, Ni Choileain and Astier, unpublished data). This effect could be related to the regulation of MMPs by Vitamin D (Coussens, et al., 2009). Therefore, CD46 cell surface expression is tightly regulated in a variety of cell types, even upon cell activation, such as activation of T cells.
Shedding of CD46 results in production of soluble CD46 (sCD46) in the supernatants of CD46-activated T cells (Ni Choileain, et al., 2011b). Although the exact cleavage site has not been identified so far, it is likely close to or in the transmembrane domain as determined using CD59-CD46 fusion proteins (Van Den Berg, et al., 2002). Soluble CD46 has been identified in tears, plasma and seminal fluid (Hara, et al., 1992). Interestingly, increased levels of sCD46 have been documented in sera from cancer patients (Seya, et al., 1995), and from systematic lupus erythematosus (SLE) and MS patients (Kawano, et al., 1999; Soldan, et al., 2001). Moreover, sCD46 retains its ligand binding capacity, as sCD46 bound to HHV-6 was isolated from the serum of MS patients (Fogdell-Hahn, et al., 2005; Soldan, et al., 2001). It is intriguing that activated T cells secrete some C3b that, in turn, has been proposed to activate T cells (Cardone, et al., 2010). Alternatively, the C3b produced might bind to the shed sCD46, resulting in T cell inhibition in a negative feedback loop.
Cytoplasmic tail processing
Subsequent to their ectodomain cleavage, a number of proteins (such as amyloid precursor protein (APP), CD44, Notch and ERBB4) are subjected to further enzymatic processing. The C-terminal transmembrane fragment (CTF) generated by MMP-cleavage of the ectodomain is cleaved by the presenilin-γ-secretase (P/γS). This generates intracellular domains (ICDs) with signaling abilities (Parks and Curtis, 2007). Upon Neisseria gonorrheae or Neisseria meningitides infection of epithelial cell, CD46 tails are processed by the P/γS (Weyand, et al., 2010). This was inhibited by pre-incubation with a chemical inhibitor of MMPs, indicating that the MMP-dependent shedding of CD46 ectodomain was required for the subsequent tail processing. N. gonorrhoeae-induced CD46 tail processing required the presence of type IV pili (Tfp) and PilT, the Tfp retraction motor, suggesting that mechanotransduction plays a role in this event (Weyand, et al., 2010). Similarly, CD46 processing by the P/γS is tightly regulated in CD46-activated Tr1 cells, as further discussed below (Ni Choileain, et al., 2011b). It is intriguing that CD46 and Notch, both substrates of the P/γS, participate in IL-10 production. Indeed, Notch1 is upregulated by CD46 ligation in primary human T cells (Ni Choileain and Astier, unpublished data, and review from Heeger and Kemper), suggestive of a common path.
Processing of CD46 cytoplasmic isoforms – rheostat for T cell activation
The two main intracellular tails of CD46 produced by alternative spicing (Cyt1 and Cyt2) are usually co-expressed in human cells. Although short (Cyt1 contains 16 amino-acids and Cyt2 23), both tails can transmit signals within the cells (Lee, et al., 2002; Russell, 2004; Wong, et al., 1997; Yant, et al., 1997). As mentioned earlier, they exert opposite effects on T cell-induced inflammation in an in vivo CD46-transgenic mouse model (Marie, et al., 2002). Interestingly, we observed increased levels of Cyt2 mRNA in CD46-activated T cells from patients with MS, compared to T cells from healthy donors (Astier, et al., 2006). At the protein level, the expression of Cyt1 and Cyt2 in the cytoplasm fluctuates upon T cell activation in a timely fashion. Cyt1 levels first decreased concomitantly with an increase in Cyt2 expression, followed by a decrease in Cyt2 expression levels, likely due to their temporal cleavage by the P/γS (Ni Choileain, et al., 2011b). These data also reinforce the notion of specific roles of CD46 isoforms in T cell function. Indeed, it was shown that Cyt1 expression could drive IL-10 production by transfected Jurkat cells, which was dependent on its interaction with SKAP (Cardone, et al., 2010). We have observed that the expression and specific activation of Cyt1 in primary T cells promotes IL-10 secretion, while the expression and activation of Cyt2 isoform decreased IFNγ expression but increased CTLA-4 expression (Ni Choileain, et al., 2011b). Most importantly, the cleavage by the P/γS was required for the function of CD46 cytoplasmic isoforms. Cleavage of Cyt1 was indeed required to activate T cells, promote proliferation, and increase CD25 expression and IL-10 secretion. These parameters were largely impaired when an uncleavable Cyt1 was expressed in primary human T cells. In contrast, whilst little effect was observed when wild-type Cyt2 was expressed, expression of the uncleavable mutant led to increased proliferation, increased CD25 expression and increased IFNγ production. Therefore, CD46 acts as a sort of ‘yin and yang’ molecule for T cells, with the cleavage of Cyt1 promoting T cell activation, and the cleavage of Cyt2 turning T cells off (Ni Choileain, et al., 2011b), as represented in Figure 2. Hence, the balance in the expression of the two cytoplasmic isoforms of CD46 appears crucial to determine the outcome of the CD46-activated T cell. This also strongly supports the hypothesis that the abnormal Cyt1/Cyt2 ratio observed in MS participates in the inflammation. Moreover, in MS, the temporal downregulation of Cyt1 and Cyt2 was not detected (Ni Choileain, et al., 2009). Where do these tails go upon cleavage? Both tails have a nuclear localization motif, suggesting that similarly to Notch, they have the ability to translocate into the nucleus and probably regulate gene transcription. Indeed, our preliminary data suggest their nuclear localization in activated T cells (Ni Choileain and Astier, unpublished data). It is likely that an understanding of the behavior of the cleaved tails will give new insights into their biological functions, and highlight altered molecular pathways in human autoimmune diseases.
Figure 2. Proposed model of the role of CD46 processing on T cell activation.
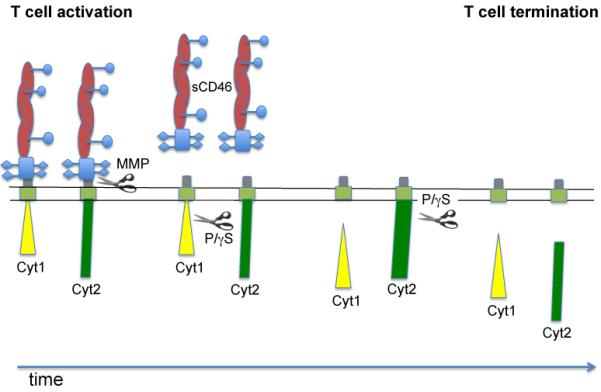
Upon CD46 co-activation, CD46 is cleaved by a metalloproteinase (MMP), resulting in the release of soluble CD46 in the milieu. sCD46 is likely to retain its binding function, suggesting a potential ability to further regulate T cell activation or neighboring cells. CD46 Cyt1 cytoplasmic tail is then cleaved by the presenilin/γ-secretase concomitantly with an increased in Cyt2 expression. This provides an “on” signal for T cells, resulting in proliferation, IL-10 secretion, and increased CD25 expression. Cleavage of Cyt2 by the P/γS terminates T cell activation by inhibiting T cell proliferation, decreasing IFNγ production and CD25 expression.
Conclusion
This past decade has brought a new focus for complement biologists as the interplay between complement regulators and adaptive immunity has become more apparent. Indeed, the impaired CD46-mediated Tr1 differentiation observed in patients exhibiting a C3 deficiency, along with defects in B cell memory and DC differentiation, underlines the importance of the complement receptors in the regulation of acquired immunity (Ghannam, et al., 2008). Moreover, it is now well established that this interplay, at least for CD46, is altered in MS and other human pathologies, resulting in abnormal immune responses and inflammation. While antigen presenting cells and T cells have mostly been studied, it is very likely that CD46 ligation modulates other cell types. Indeed, a recent paper describes its role in modulating B cell responses (Jabara, et al., 2011). It will now be interesting to see what CD46, and possibly the other complement regulators, do to other cell types in the context of human pathologies. The other main notion recently emerging is the complexity of CD46. Its biochemical structure is itself a source of complex regulation with many isoforms produced, and its expression is tightly regulated by different signals, such as apoptosis and cellular activation. Identifying new means to modulate CD46 expression, such as use of Vitamin D, could be a useful tool to modify immune response. Functionally, not only does CD46 fine-tune T cell responses depending on its environment, but it also has the ability to initiate and then terminate T cell responses through its tight regulation of expression and enzymatic processing. The recent data generated on CD46 indeed illustrate the plasticity of CD46 in regulating T cell activation. It will now be important to understand how to modulate this plasticity to potentially use it in future drug designs.
Acknowledgements
This work was supported by a research grant from the MS society (UK) to ALA. The authors thank Dr. Anna Richards for helpful discussion and critical reading of the manuscript.
Abbreviations
- MS
multiple sclerosis
- DCs
dendritic cells
- MMP
Matrix Metalloproteinases
- P/γS
presenilin/gamma secretase
- RA
Rheumatoid arthritis
- PBMC
peripheral blood mononuclear cells
References
- Alford SK, Longmore GD, Stenson WF, Kemper C. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J Immunol. 2008;181:2544–2555. doi: 10.4049/jimmunol.181.4.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, De Las Heras V, Bartolome M, Garcia-Montojo M, Arroyo R. Human herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathol. 2006;16:20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Astier AL. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology. 2008;124:149–154. doi: 10.1111/j.1365-2567.2008.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti R, Brennan MB, Soldan SS, Ohayon JM, Casareto L, McFarland HF, Jacobson S. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol. 2002;8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- Blom AM, Berggard K, Webb JH, Lindahl G, Villoutreix BO, Dahlback B. Human C4b-binding protein has overlapping, but not identical, binding sites for C4b and streptococcal M proteins. J Immunol. 2000;164:5328–5336. doi: 10.4049/jimmunol.164.10.5328. [DOI] [PubMed] [Google Scholar]
- Buchholz CJ, Gerlier D, Hu A, Cathomen T, Liszewski MK, Atkinson JP, Cattaneo R. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology. 1996;217:349–355. doi: 10.1006/viro.1996.0122. [DOI] [PubMed] [Google Scholar]
- Capasso M, Durrant LG, Stacey M, Gordon S, Ramage J, Spendlove I. Costimulation via CD55 on human CD4+ T cells mediated by CD97. J Immunol. 2006;177:1070–1077. doi: 10.4049/jimmunol.177.2.1070. [DOI] [PubMed] [Google Scholar]
- Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, Hayday A, Kemper C. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermelli C, Berti R, Soldan SS, Mayne M, D’Ambrosia J,M, Ludwin SK, Jacobson S. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- Cole DS, Hughes TR, Gasque P, Morgan BP. Complement regulator loss on apoptotic neuronal cells causes increased complement activation and promotes both phagocytosis and cell lysis. Mol Immunol. 2006;43:1953–1964. doi: 10.1016/j.molimm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, Wilkinson RJ, Martineau AR. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–548. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimeen-Irwin B, Ellis S, Christiansen D, Ludford-Menting MJ, Milland J, Lanteri M, Loveland BE, Gerlier D, Russell SM. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, Gasque P. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280:36342–36354. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Centeno E, de Ojeda G, Rojo JM, Portoles P. Crry/p65, a membrane complement regulatory protein, has costimulatory properties on mouse T cells. J Immunol. 2000;164:4533–4542. doi: 10.4049/jimmunol.164.9.4533. [DOI] [PubMed] [Google Scholar]
- Fleischli C, Verhaagh S, Havenga M, Sirena D, Schaffner W, Cattaneo R, Greber UF, Hemmi S. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J Virol. 2005;79:10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogdell-Hahn A, Soldan SS, Shue S, Akhyani N, Refai H, Ahlqvist J, Jacobson S. Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res. 2005;110:57–63. doi: 10.1016/j.virusres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V, Moulton EA, Kavanagh D, Dragon-Durey MA, Blouin J, Caudy A, Arzouk N, Cleper R, Francois M, Guest G, Pourrat J, Seligman R, Fridman WH, Loirat C, Atkinson JP. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17:2017–2025. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP, Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglia JL, Mattoo A, Greenfield EA, Freeman GJ, Kuchroo VK. Characterization of endogenous Chinese hamster ovary cell surface molecules that mediate T cell costimulation. Cell Immunol. 2001;213:83–93. doi: 10.1006/cimm.2001.1867. [DOI] [PubMed] [Google Scholar]
- Geis N, Zell S, Rutz R, Li W, Giese T, Mamidi S, Schultz S, Kirschfink M. Inhibition of membrane complement inhibitor expression (CD46, CD55, CD59) by siRNA sensitizes tumor cells to complement attack in vitro. Curr Cancer Drug Targets. 2010;10:922–931. doi: 10.2174/156800910793357952. [DOI] [PubMed] [Google Scholar]
- Ghannam A, Pernollet M, Fauquert JL, Monnier N, Ponard D, Villiers MB, Peguet-Navarro J, Tridon A, Lunardi J, Gerlier D, Drouet C. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008;181:5158–5166. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- Gill DB, Koomey M, Cannon JG, Atkinson JP. Down-regulation of CD46 by piliated Neisseria gonorrhoeae. J Exp Med. 2003;198:1313–1322. doi: 10.1084/jem.20031159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstone HL, Santoro F, Lusso P, Berger EA. Human Herpesvirus 6 and Measles Virus Employ Distinct CD46 Domains for Receptor Function. J Biol Chem. 2002;277:39112–39118. doi: 10.1074/jbc.M206488200. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Junnikkala S, Sorsa T, Meri S. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004;34:2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Keski-Oja J. ADAM10-mediated release of complement membrane cofactor protein during apoptosis of epithelial cells. J Biol Chem. 2006;281:21369–21376. doi: 10.1074/jbc.M602053200. [DOI] [PubMed] [Google Scholar]
- Hara T, Kuriyama S, Kiyohara H, Nagase Y, Matsumoto M, Seya T. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clin Exp Immunol. 1992;89:490–494. doi: 10.1111/j.1365-2249.1992.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Suzuki Y, Nakazawa T, Nishimura H, Nagasawa S, Nishiguchi M, Matsumoto M, Hatanaka M, Kitamura M, Seya T. Posttranslational modification and intracellular localization of a splice product of CD46 cloned from human testis: role of the intracellular domains in Oglycosylation. Immunology. 1998;93:546–555. doi: 10.1046/j.1365-2567.1998.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CL, Mizuno M, Morgan BP. Complement and complement regulators in the male reproductive system. Mol Immunol. 2006;43:57–67. doi: 10.1016/j.molimm.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Ingram G, Bugert JJ, Loveless S, Robertson NP. Anti-EBNA-1 IgG is not a reliable marker of multiple sclerosis clinical disease activity. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- Jabara HH, Angelini F, Brodeur SR, Geha RS. Ligation of CD46 to CD40 inhibits CD40 signaling in B cells. Int Immunol. 2011;23:215–221. doi: 10.1093/intimm/dxq474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Perianez A, Ojeda G, Criado G, Sanchez A, Pini E, Madrenas J, Rojo JM, Portoles P. Complement regulatory protein Crry/p65-mediated signaling in T lymphocytes: role of its cytoplasmic domain and partitioning into lipid rafts. J Leukoc Biol. 2005;78:1386–1396. doi: 10.1189/jlb.1104642. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Loveland BE, McKenzie IF. Identification and quantification of complement regulator CD46 on normal human tissues. Immunology. 1993a;79:341–347. [PMC free article] [PubMed] [Google Scholar]
- Johnstone RW, Russell SM, Loveland BE, McKenzie IF. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993b;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- Joubert PE, Meiffren G, Gregoire IP, Pontini G, Richetta C, Flacher M, Azocar O, Vidalain PO, Vidal M, Lotteau V, Codogno P, Rabourdin-Combe C, Faure M. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kallstrom H, Blackmer Gill D, Albiger B, Liszewski MK, Atkinson JP, Jonsson AB. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell Microbiol. 2001;3:133–143. doi: 10.1046/j.1462-5822.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- Kallstrom H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- Karp CL. Measles: immunosuppression, interleukin-12, and complement receptors. Immunol Rev. 1999;168:91–101. doi: 10.1111/j.1600-065x.1999.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus [published erratum appears in Science 1997 Feb 21;275(5303):1053] Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Kawano M, Seya T, Koni I, Mabuchi H. Elevated serum levels of soluble membrane cofactor protein (CD46, MCP) in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;116:542–546. doi: 10.1046/j.1365-2249.1999.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4(+) cells with CD3 and CD46 induces a Tregulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Kurita-Taniguchi M, Fukui A, Hazeki K, Hirano A, Tsuji S, Matsumoto M, Watanabe M, Ueda S, Seya T. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J Immunol. 2000;165:5143–5152. doi: 10.4049/jimmunol.165.9.5143. [DOI] [PubMed] [Google Scholar]
- Le Friec G, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp (Warsz) 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- Lee SW, Bonnah RA, Higashi DL, Atkinson JP, Milgram SL, So M. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. J Cell Biol. 2002;156:951–957. doi: 10.1083/jcb.200109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Feito MJ, Sacks SH, Sheerin NS. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J Immunol. 2006;177:2543–2551. doi: 10.4049/jimmunol.177.4.2543. [DOI] [PubMed] [Google Scholar]
- Lindsey JW, Hatfield LM. Epstein-Barr virus and multiple sclerosis: Cellular immune response and cross-reactivity. J Neuroimmunol. 2010;2010 doi: 10.1016/j.jneuroim.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Atkinson JP. Membrane cofactor protein. Curr Top Microbiol Immunol. 1992;178:45–60. doi: 10.1007/978-3-642-77014-2_4. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Atkinson JP. Membrane cofactor protein (MCP; CD46). Isoforms differ in protection against the classical pathway of complement. J Immunol. 1996;156:4415–4421. [PubMed] [Google Scholar]
- Liszewski MK, Leung M, Cui W, Subramanian VB, Parkinson J, Barlow PN, Manchester M, Atkinson JP. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46) J Biol Chem. 2000;275:37692–37701. doi: 10.1074/jbc.M004650200. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Leung MK, Atkinson JP. Membrane cofactor protein: importance of N- and O-glycosylation for complement regulatory function. J Immunol. 1998;161:3711–3718. [PubMed] [Google Scholar]
- Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lovkvist L, Sjolinder H, Wehelie R, Aro H, Norrby-Teglund A, Plant L, Jonsson AB. CD46 Contributes to the severity of group A streptococcal infection. Infect Immun. 2008;76:3951–3958. doi: 10.1128/IAI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin DM, Liszewski MK, Post TW, Arce MA, Le Beau MM, Rebentisch MB, Lemons LS, Seya T, Atkinson JP. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988;168:181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludford-Menting MJ, Crimeen-Irwin B, Oliaro J, Pasam A, Williamson D, Pedersen N, Guillaumot P, Christansen D, Manie S, Gaus K, Russell SM. The Reorientation of T-Cell Polarity and Inhibition of Immunological Synapse Formation by CD46 Involves Its Recruitment to Lipid Rafts. J Lipids. 2011;2011:521863. doi: 10.1155/2011/521863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludford-Menting MJ, Thomas SJ, Crimeen B, Harris LJ, Loveland BE, Bills M, Ellis S, Russell SM. A functional interaction between CD46 and DLG4: a role for DLG4 in epithelial polarization. J Biol Chem. 2002;277:4477–4484. doi: 10.1074/jbc.M108479200. [DOI] [PubMed] [Google Scholar]
- Ma A, Xiong Z, Hu Y, Qi S, Song L, Dun H, Zhang L, Lou D, Yang P, Zhao Z, Wang X, Zhang D, Daloze P, Chen H. Dysfunction of IL-10-producing type 1 regulatory T cells and CD4+CD25+ regulatory T cells in a mimic model of human multiple sclerosis in Cynomolgus monkeys. International Immunopharmacology. 2009;9:599–608. doi: 10.1016/j.intimp.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Maghzi AH, Marta M, Bosca I, Etemadifar M, Dobson R, Maggiore C, Giovannoni G, Meier UC. Viral pathophysiology of multiple sclerosis: A role for Epstein-Barr virus infection? Pathophysiology. 2010 doi: 10.1016/j.pathophys.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtout H, Chandad F, Rojo JM, Grenier D. Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiol Immunol. 2009;24:396–400. doi: 10.1111/j.1399-302X.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- Manchester M, Gairin JE, Patterson JB, Alvarez J, Liszewski MK, Eto DS, Atkinson JP, Oldstone MB. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR1-2. Virology. 1997;233:174–184. doi: 10.1006/viro.1997.8581. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, Lopez-Diaz de Cerio A, Palacios R, Sepulcre J, Moreno B, Gonzalez Z, Fernandez-Diez B, Melero I, Bendandi M, Villoslada P. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: central to innate immunity and bridging to adaptive responses. Immunol Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Astier AL. CD46 plasticity and its inflammatory bias in multiple sclerosis. Arch Immunol Ther Exp (Warsz) 2011;59:49–59. doi: 10.1007/s00005-010-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Stephen J, Weller B, Astier A. CD46, Chemotaxis and MS - Are These Linked? Nova Science Publishers Inc; New York: 2011a. pp. 85–108. [Google Scholar]
- Ni Choileain S, Weyand NJ, Neumann C, Thomas J, So M, Astier AL. The dynamic processing of CD46 intracellular domains provides a molecular rheostat for T cell activation. PLoS One. 2011b;6:e16287. doi: 10.1371/journal.pone.0016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Weyand NJ, So M, Weller B, Photiou D, Astier AL. Dysregulated control of expression of CD46 cytoplasmic isoforms in T cells from patients with multiple sclerosis. Eur J Immunol. 2009;39:S487. Abstract ECI 2009. [Google Scholar]
- Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, Porrati F, Gamba S, Remuzzi G. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362:1542–1547. doi: 10.1016/S0140-6736(03)14742-3. [DOI] [PubMed] [Google Scholar]
- Oliaro J, Pasam A, Waterhouse NJ, Browne KA, Ludford-Menting MJ, Trapani JA, Russell SM. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci U S A. 2006;103:18685–18690. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Persson BD, Schmitz NB, Santiago C, Zocher G, Larvie M, Scheu U, Casasnovas JM, Stehle T. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog. 2011;6 doi: 10.1371/journal.ppat.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter C, Beltrami S, Caputo D, Ferrante P, Clivio A. Presence of autoantibodies against complement regulatory proteins in relapsingremitting multiple sclerosis. J Neurovirol. 2000;6(Suppl 2):S42–46. [PubMed] [Google Scholar]
- Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- Purcell DF, Russell SM, Deacon NJ, Brown MA, Hooker DJ, McKenzie IF. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics. 1991;33:335–344. doi: 10.1007/BF00216692. [DOI] [PubMed] [Google Scholar]
- Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Muslumanoglu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003;100:12966–12971. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RC, Kemper C, Leung M, Atkinson JP. Characterization of human membrane cofactor protein (MCP; CD46) on spermatozoa. Mol Reprod Dev. 2002;62:534–546. doi: 10.1002/mrd.10144. [DOI] [PubMed] [Google Scholar]
- Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 coreceptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–118. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Sakurai F, Akitomo K, Kawabata K, Hayakawa T, Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Feito MJ, Rojo JM. CD46-mediated costimulation induces a Th1-biased response and enhances early TCR/CD3 signaling in human CD4+ T lymphocytes. Eur J Immunol. 2004;34:2439–2448. doi: 10.1002/eji.200324259. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Schneider-Schaulies S. Receptor interactions, tropism, and mechanisms involved in morbillivirus-induced immunomodulation. Adv Virus Res. 2008;71:173–205. doi: 10.1016/S0065-3527(08)00004-3. [DOI] [PubMed] [Google Scholar]
- Serafini B, Severa M, Columba-Cabezas S, Rosicarelli B, Veroni C, Chiappetta G, Magliozzi R, Reynolds R, Coccia EM, Aloisi F. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J Neuropathol Exp Neurol. 2010;69:677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- Seya T, Hara T, Iwata K, Kuriyama S, Hasegawa T, Nagase Y, Miyagawa S, Matsumoto M, Hatanaka M, Atkinson JP, et al. Purification and functional properties of soluble forms of membrane cofactor protein (CD46) of complement: identification of forms increased in cancer patients’ sera. Int Immunol. 1995;7:727–736. doi: 10.1093/intimm/7.5.727. [DOI] [PubMed] [Google Scholar]
- Seya T, Hirano A, Matsumoto M, Nomura M, Ueda S. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–1260. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–2884. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Fogdell-Hahn A, Brennan MB, Mittleman BB, Ballerini C, Massacesi L, Seya T, McFarland HF, Jacobson S. Elevated serum and cerebrospinal fluid levels of soluble human herpesvirus type 6 cellular receptor, membrane cofactor protein, in patients with multiple sclerosis. Ann Neurol. 2001;50:486–493. doi: 10.1002/ana.1135. [DOI] [PubMed] [Google Scholar]
- Tang H, Kawabata A, Takemoto M, Yamanishi K, Mori Y. Human herpesvirus-6 infection induces the reorganization of membrane microdomains in target cells, which are required for virus entry. Virology. 2008;378:265–271. doi: 10.1016/j.virol.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Tuke PW, Hawke S, Griffiths PD, Clark DA. Distribution and quantification of human herpesvirus 6 in multiple sclerosis and control brains. Mult Scler. 2004;10:355–359. doi: 10.1191/1352458504ms1057oa. [DOI] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Murugaiyan G, Hafler DA, Astier AL, Weiner HL. Increased IL-23 secretion and altered chemokine production by dendritic cells upon CD46 activation in patients with multiple sclerosis. J Neuroimmunol. 2008;195:140–145. doi: 10.1016/j.jneuroim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg CW, De Andrade RM, Magnoli FC, Marchbank KJ, Tambourgi DV. Loxosceles spider venom induces metalloproteinase mediated cleavage of MCP/CD46 and MHCI and induces protection against C-mediated lysis. Immunology. 2002;107:102–110. doi: 10.1046/j.1365-2567.2002.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand NJ, Calton CM, Higashi DL, Kanack KJ, So M. Presenilin/gamma-secretase cleaves CD46 in response to Neisseria infection. J Immunol. 2010;184:694–701. doi: 10.4049/jimmunol.0900522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TC, Yant S, Harder BJ, Korte-Sarfaty J, Hirano A. The cytoplasmic domains of complement regulatory protein CD46 interact with multiple kinases in macrophages. J Leukoc Biol. 1997;62:892–900. doi: 10.1002/jlb.62.6.892. [DOI] [PubMed] [Google Scholar]
- Xing PX, Russell S, Prenzoska J, McKenzie I. Discrimination between alternatively spliced STP-A and -B isoforms of CD46. Immunology. 1994;83:122–127. [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Gao YD, Yang J, Guo W. A defect of CD4+CD25+ regulatory T cells in inducing interleukin-10 production from CD4+ T cells under CD46 costimulation in asthma patients. J Asthma. 2010;47:367–373. doi: 10.3109/02770903.2010.481340. [DOI] [PubMed] [Google Scholar]
- Yant S, Hirano A, Wong TC. Identification of a cytoplasmic Tyr-X-XLeu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–770. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Graham J, Akahata Y, Oh U, Jacobson S. Mechanism of neuroinflammation: enhanced cytotoxicity and IL-17 production via CD46 binding. J Neuroimmune Pharmacol. 2010;5:469–478. doi: 10.1007/s11481-010-9232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signalregulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]


