Abstract
The maintenance of gastrointestinal mucosal integrity depends on the rapid alarm of protective mechanisms in the face of pending injury. To this end, the gastric mucosa is innervated by intrinsic sensory neurons and two populations of extrinsic sensory neurons: vagal and spinal afferents. Extrinsic afferent neurons constitute an emergency system that is called into operation when the gastrointestinal mucosa is endangered by noxious chemicals. The function of these chemoceptive afferents can selectively be manipulated and explored with the use of capsaicin which acts via a cation channel termed TRPV1. Many of the homeostatic actions of spinal afferents are brought about by transmitter release from their peripheral endings. When stimulated by noxious chemicals, these afferents enhance gastrointestinal blood flow and activate hyperaemia-dependent and hyperaemia-independent mechanisms of protection and repair. In the rodent foregut these local regulatory roles of sensory neurons are mediated by calcitonin gene-related peptide and nitric oxide. The pathophysiological potential of the neural emergency system is best portrayed by the gastric hyperaemic response to acid back-diffusion, which is governed by spinal afferent nerve fibres. This mechanism limits damage to the surface of the mucosa and creates favourable conditions for rapid restitution and healing of the wounded mucosa. Other extrinsic afferent neurons, particularly in the vagus nerve, subserve gastrointestinal homeostasis by signalling noxious events in the foregut to the central nervous system and eliciting autonomic, emotional-affective and neuroendocrine reactions. Under conditions of inflammation and injury, chemoceptive afferents are sensitized to peripheral stimuli and in this functional state contribute to the hyperalgesia associated with functional dyspepsia and irritable bowel syndrome. Thus, if GI pain is to be treated by sensory neuron-directed drugs it needs to be considered that these drugs do not inhibit nociception at the expense of GI mucosal vulnerability.
Keywords: Afferent neurons, Brainstem, Calcitonin gene-related peptide, Capsaicin, c-Fos, Chemonociception, Cytokines, Efferent-like function of afferent neurons, Gastric mucosal protection, Gut-brain axis, Hypersensitivity, Mucosal blood flow, Neurogenic inflammation, TRPV1, Vagus nerve
1. Introduction
The two major functions of the gastrointestinal (GI) tract are, on the one hand, to take up and digest food, absorb nutrients and water, eliminate useless material and, on the other hand, to recognize harmful food constituents, antigens and pathogens as well as neutralize or expel them via emesis and diarrhoea. These seemingly conflicting tasks of the alimentary canal require a careful analysis of the luminal contents and the functional status of the GI tract so that the appropriate effector programmes can be selected (Grundy, 2002; Holzer, 2002a). It is therefore not surprising that the digestive system is endowed with an elaborate network of surveillance systems among which sensory neurons play a particular role. This review focuses on a further role of sensory neurons that has been characterized in my laboratory: a group of nociceptive afferent nerve fibres not only monitors noxious events in the GI tract but through the peripheral release of neuropeptides also initiates local mechansims of defence.
At an occasion celebrating the fundamental achievements of Ivan Petrovich Pavlov in St. Petersburg one hundred years after their recognition by the award of a Nobel Prize it is appropriate to remember that Ivan Pavlov was the first to recognize that the gut and its innervation have the ability to adapt to their ever changing environment. We now know that part of the adaptive capacity of the nervous system relates to the plasticity of neural transmission. This concept was also influenced by Ivan Pavlov, as we can learn from Otto Loewi who in 1921 discovered the chemical nature of neurotransmission in Graz and in 1936 shared the Nobel Prize for Medicine or Physiology with Sir Henry Dale. In an unpublished lecture manuscript entitled “Adaptation and Regeneration” (circa 1940) Otto Loewi mentions adaptational phenomena in the central nervous system which “recently have been termed plasticity”. He goes on to say that “we owe to the experiments of one of the greatest physiologists of all times, the Russian Ivan Pavlov, the knowledge that such an adaptation can indeed occur” (Lembeck and Giere, 1968).
2. Sensory innervation of the gut
In keeping with its need of surveillance systems, the alimentary canal receives a complex network of sensory neurons. Unlike somatic structures which are supplied by one population of afferent neurons only, the alimentary canal is innervated by four classes of sensory neurons that can be differentiated on the basis of their origins and projections. Intrinsic primary afferent neurons (IPANs) of the enteric nervous system (ENS) have their cell bodies within the GI tract and originate either in the myenteric plexus or in the submucosal plexus (Holzer, 2002a). These two groups of IPANs are complemented by two groups of extrinsic afferent neurons whose cell bodies lie either in the jugular and nodose ganglia or in the dorsal root ganglia (Grundy, 2002; Holzer, 2002a). Importantly, 80 - 90 % of the axons in the vagus nerves are afferent nerve fibres that project to the nucleus tractus solitarii and area postrema of the brainstem (Grundy, 2002). Within the GI tract, intrinsic and extrinsic afferent neurons supply mucosa, submucosa (particularly arterioles), muscle, enteric nerve plexuses and serosa. These projections enable sensory nerve fibres to respond to changes of the chemical environment in the lumen, interstitial space and vasculature and to mechanical stimuli such as distortion of the villi, distension of the gut wall and contraction or relaxation of the muscle (Cervero, 1994; Berthoud and Neuhuber, 2000; Gebhart, 2000; Grundy, 2002).
3. Efferent-like roles of spinal afferent neurons
While IPANs supply the ENS with information that this brain of the gut requires for its independent control of digestion, afferent fibres of the vagal and spinal nerves convey information from the gut to the brain. In addition, a subgroup of spinal afferent neurons subserves a local protective function in the mucosa (Holzer, 1998a) inasmuch as these neurons can signal for vascular, secretory and motor reactions if mucosal integrity is challenged by chemical noxae (Figure 1). This efferent-like function is mediated by neuropeptide transmitters such as calcitonin gene-related peptide (CGRP) and tachykinins (substance P, neurokinin A) which upon noxious stimulation of sensory neurons are released from their peripheral endings in the tissue. Through this process, stimulation of sensory nerve endings increases blood flow and vascular permeability in somatic tissues including the skin, a process that is embodied in the term “neurogenic inflammation” (Jancsó, 1960; Holzer, 1988; Maggi, 1995). Neurogenic inflammation can be elicited by a large variety of noxious stimuli and is thought to assist repair of the injured tissue (Holzer, 1998b).
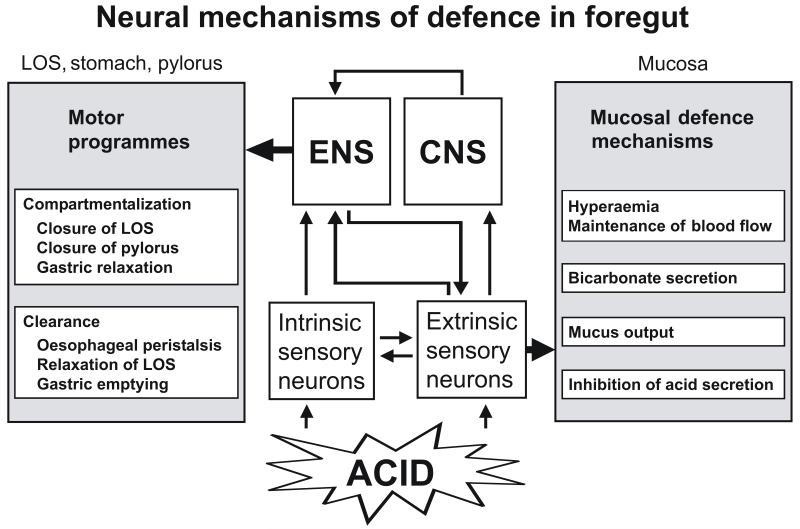
Figure 1.
Two neural mechanisms of gastric acid defence in the oesophagus, stomach and duodenum. Both mechanisms are alarmed by acid-sensitive afferent neurons. CNS, central nervous system; ENS, enteric nervous system; LOS, lower oesophageal sphincter.
Following up preliminary observations made by Szolcsányi and Barthó (1981), the study of neurogenic inflammation in the skin prompted me to address the possibility that similar protective mechanisms operate in the GI mucosa (Holzer and Sametz, 1986; Holzer and Lippe, 1988). This conjecture was tested with capsaicin, a unique neuropharmacological tool which acutely excites nociceptive afferent neurons and, at high doses, causes a long-lasting defunctionalization (functional ablation) of these neurons (Holzer, 1991). It was already N. Jancsó (1960) who had discovered that acute administration of capsaicin to the skin elicits neurogenic inflammation, whereas pretreatment of rats with a high dose of capsaicin abolishes this response. It was, however, not understood at that time how capsaicin acts so specifically on nociceptive afferent neurons and whether this action of capsaicin has any physiological or pathophysiological bearing. We now know that capsaicin acts specifically on TRPV1, the transient receptor potential cation channel of vanilloid type 1 (Caterina et al., 1997), which is expressed both by vagal and spinal afferents innervating the rat stomach (Schicho et al., 2004).
4. Spinal afferent nerve fibres as local emergency system in the oesophago-gastro-duodenal mucosa
With the help of capsaicin it was soon discovered that stimulation of afferent neurons protects the rat gastric mucosa against experimental injury (Holzer and Lippe, 1988), whereas functional ablation of capsaicin-sensitive afferent neurons makes the gastric mucosa highly vulnerable to damage by various ulcerogens (Holzer and Sametz, 1986; Holzer, 1998a). The gastroprotective effect of capsaicin-induced stimulation of sensory neurons is associated with a marked rise of gastric mucosal blood flow (Holzer et al., 1990), which is mediated by CGRP released from spinal afferent nerve endings in the gastric submucosa and mucosa (Chen et al., 1992). It was subsequently discovered that the same alarm system operates in the human gastroduodenal mucosa (Yeoh et al., 1995) and throughout the small and large intestine of experimental animals (Holzer and Barthó, 1996; Holzer, 1998a; Akiba et al., 2002).
The pathophysiological potential of this neural defence system is portrayed by the protective response to gastric acid backdiffusion. Hydrochloric acid and pepsin are highly aggressive secretions of the stomach which attack the mucosal tissue if they can overwhelm the epithelial barrier. This is thought to take place when the mucosal barrier is focally disrupted by the mechanical forces of digestion, by ingested alcohol, nonsteroidal anti-inflammatory drugs or irritant food, or by reflux of bile. The surge of acid intruding the lamina propria stimulates spinal afferents which via a peripheral mechanism of action increase blood flow through the gastroduodenal mucosa and initiate other mechanisms of defence (Holzer et al., 1991; Raybould et al., 1992; Holzer et al., 1994; Akiba et al., 1999). Acid-sensitive extrinsic afferents thus represent a neural emergency system that is called into operation in the face of pending injury (Holzer, 1998a). The hyperaemia elicited by sensory neurons in response to acid challenge is mediated by CGRP and nitric oxide (Li et al., 1992; Lippe and Holzer, 1992; Holzer et al., 1994). Further analysis of the underlying mechanisms indicates that acid-induced stimulation of nociceptive nerve endings in the gastroduodenal mucosa initiates an axon reflex-like circuitry that strengthens mucosal resistance by a multiplicity of actions: an increase in blood flow, bicarbonate and mucus secretion and an inhibition of acid secretion (Figure 1). The hyperaemia-independent gastroprotection brought about by sensory neurons involves tachykinins which themselves do not increase, but decrease gastric mucosal blood flow in the rat (Heinemann et al., 1996; Stroff et al., 1996).
Gastric acid challenge not only activates extrinsic afferent neurons but also intrinsic neurons of the rat gastric myenteric plexus as visualized by induction of c-Fos, a marker of neuronal excitation (Schicho et al., 2001; Schicho et al., 2003). Thus, about 12 % of all myenteric neurons in the rat stomach express c-Fos after luminal exposure to a noxious acid concentration; all of these neurons stain for nitric oxide synthase (NOS), vasoactive intestinal polypeptide (VIP) and neuropeptide Y (NPY), but not for choline acetyltransferase (Schicho et al., 2001; Schicho et al., 2003). As this chemical code is also found in nerve fibres supplying the external muscle and muscularis mucosae of the stomach, it would appear that gastric acid challenge stimulates inhibitory motor neurons (Schicho et al., 2003). This is consistent with observations that capsaicin-induced stimulation of gastric sensory nerve fibres causes relaxation of the stomach (Holzer, 1998a). The acid-evoked activation of NOS/VIP/NPY-positive neurons of the ENS seems to be brought about by a neural reflex, given that the expression of c-Fos in the ENS is blunted by functional ablation of capsaicin-sensitive extrinsic afferent neurons (Schicho et al., 2003). In view of these findings it has been proposed that acid-sensitive afferent neurons protect the oesophago-gastro-duodenal mucosa from acid by two principal mechanisms (Holzer, 2002b), by strengthening the epithelial barrier and by adapting foregut motility to this goal (Figure 1). Given that the gastric mucosa is most resistant to acid, the stomach is relaxed while the lower oesophageal and pyloric sphincters are closed in order to prevent excess acid from entering the oesophagus and duodenum (Holzer, 2002b).
5. Vagal afferent neuron-mediated systemic reactions to gastric acid challenge
Nociceptive afferent neurons promote tissue protection from injury not only via their local efferent-like role but also through their afferent function whereby they elicit behavioural, autonomic and neuroendocrine responses and elicit the sensation of pain. The physiological task of these systemic reactions is also to maintain or regain tissue homeostasis following a noxious attack. While the local protective reactions of the rat gastric mucosa to luminal acid challenge are initiated by spinal afferents (Raybould et al., 1992), the systemic reactions to excess gastric acid exposure are brought about by vagal afferents that signal an acute acid attack of the rat gastric mucosa to the brainstem (Schuligoi et al., 1998; Michl et al., 2001). Thus, mapping of the afferent input from the acid-threatened stomach to the spinal cord and brainstem has revealed that gastric acid challenge induces c-Fos only in the nucleus tractus solitarii and area postrema of the brainstem but not in the spinal cord (Schuligoi et al., 1998; Michl et al., 2001; Danzer et al., 2004). In contrast, gastric distension causes expression of c-Fos both in the spinal cord and brainstem (Traub et al., 1996). It follows that vagal and spinal afferents are specialized to mediate different homeostatic reactions to a noxious acid insult of the gastric mucosa (Holzer and Maggi, 1998). This functional dissociation of vagal and spinal afferent neurons in their response to gastric noxae has been confirmed by the observation that the visceromotor pain response to intragastric acid stimulation is likewise mediated by vagal afferents, whereas the pain reaction to gastric distension is signaled by spinal afferents (Lamb et al., 2003).
The involvement of vagal afferents in the central signalling of a gastric mucosal acid insult (Schuligoi et al., 1998; Michl et al., 2001; Lamb et al., 2003) and peripheral immune challenge (Goehler et al., 2000; Konsman et al., 2002) is of obvious relevance to understanding visceral sensation in health and disease. After it has long been held that vagal sensory neurons do not play any role in visceral pain, there is now growing awareness that these neurons make a distinct contribution to the emotional-affective, neuroendocrine and behavioural aspects of GI nociception (Traub et al., 1996; Berthoud and Neuhuber, 2000; Gebhart, 2000). This view is in keeping with the processing of afferent input from the acid-threatened stomach in the central nervous system. Following its relay to the brainstem, the information is passed on to subcortical brain nuclei including the paraventricular nucleus of the hypothalamus, the central amygdala and other limbic areas, nuclei involved in the emotional, behavioural, autonomic and neuroendocrine reactions to noxious stimuli (Michl et al., 2001). There is, however, no activation of the insular cortex, the major cerebral representation area of afferent input from the stomach, which suggests that vagal afferent signalling of an acute mucosal acid insult does not give rise to the discriminative perception of pain.
6. Sensitization of gastric acid-sensitive vagal afferent pathways by proinflammatory cytokines
If vagal afferent neurons are to play a role in gastric chemonociception, the question arises as to whether they can be sensitized under conditions of infection, inflammation and immune challenge. There is increasing evidence that this is indeed the case (Lamb et al., 2003). Thus, acetic acid-induced ulceration of the rat stomach increases the excitability of nodose and dorsal root ganglion neurons, an effect that is related to an enhancement of tetrodotoxin-resistant sodium currents (Bielefeldt et al., 2002). Accordingly, the visceromotor pain response to gastric acid challenge is enhanced in rats with acetic acid-induced ulcers or iodoacetamide-induced gastritis (Lamb et al., 2003). My group has obtained experimental evidence that proinflammatory cytokines can sensitize vagal afferent pathways to gastric acid challenge. Thus, systemic administration of interleukin-1beta and tumour necrosis factor-alpha leads to an increase in the gastric acid-evoked expression of c-Fos in the brainstem (Holzer et al., 2004). This state of hypersensitivity is maintained for a period of more than 2 days and thought to be due to a peripheral cytokine action, given that intracisternal cytokine administration fails to amplify the acid-evoked expression of c-Fos in the brainstem and the sensitizing effect of systemic interleukin-1beta is prevented by systemic pretreatment with recombinant interleukin-1 receptor antagonist (Holzer et al., 2004). The ability of cytokines to sensitize vagal afferent pathways to gastric acid (Holzer et al., 2004) is in keeping with the implication of vagal afferents in the communication between the peripheral immune system and the brain (Goehler et al., 2000; Konsman et al., 2002).
7. Conclusions
The data reviewed here indicate that the foregut possesses structural and functional properties whereby its mucosa is protected from the deleterious actions of acid and other injurious chemicals. Two major mechanisms of acid defence can be differentiated (Figure 1). On the one hand, mucosal mechanisms constituting the oesophago-gastro-duodenal epithelial barrier ensure that gastric acid is prevented from intruding the mucosal tissue at cytotoxic concentrations and that any lesions are rapidly repaired (Holzer, 1998a). On the other hand, the foregut is highly compartmentalized in order to impede the movement of gastric acid to the oesophagus and duodenum and to control its transcompartmental passage through the lower oesophageal and pyloric sphincters. Both mechanisms of gastric acid defence need be quickly triggered and hence depend on neural communication pathways. Essential in these pathways are acid-sensitive spinal afferents that alarm the appropriate protective mechanisms in the face of pending injury. There is increasing awareness that mucosal disease in the upper gut can in some way be related to malfunction of sensory neurons and their warning function (Holzer, 1998a). Consequently, the role of sensory neurons as a signalling system in GI mucosal homeostasis has important implications for the diagnosis, management and pharmacotherapy of GI mucosal disease.
In addition to local tissue protection, sensory neurons also initiate systemic reactions that help maintaining or regaining tissue homeostasis. In the case of a gastric acid insult these systemic reactions appear to be mediated by vagal afferent neurons. Consistent with the overall homeostatic role of afferent neurons is their ability to adapt to changes in the GI environment. Unfortunately, sensory neurons also can fall victim to their adaptability if alterations in their sensitivity do not revert to normal once the triggering stimulus is over. Thus, patients with functional bowel disorders such as functional dyspepsia or irritable bowel syndrome suffer from GI pain and discomfort, and there is ample evidence that hypersensitivity of the extrinsic afferent nervous system is one factor in the complaints of these patients (Drossman et al., 2000). This concept identifies afferent neurons as a target at which novel therapies may be aimed (Holzer, 2004). Ideally, sensory neuron-directed drugs should block the exaggerated signalling of hypersensitive afferents, which implies that they hit molecular targets whose number or structure is modified in visceral hyperalgesia. However, the double role of sensory neurons in local tissue protection and visceral hyperalgesia necessitates these drugs to be designed such that they do not depress nociception at the expense of GI mucosal vulnerability.
Acknowledgements
Thanks are due to Evelin Painsipp for preparing the figure. Work in the author’s laboratory is currently supported by the Zukunftsfonds Steiermark (grant 262), the Jubilee Funds of the Austrian National Bank (grant 9858) and the Austrian Scientific Research Funds (FWF grant L25-B05).
References
- Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am. J. Physiol. 1999;277:G268–G274. doi: 10.1152/ajpgi.1999.277.2.G268. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J. Gastroenterol. 2002;37(Suppl. 14):133–138. doi: 10.1007/BF03326432. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Ozaki N, Gebhart GF. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology. 2002;122:394–405. doi: 10.1053/gast.2002.31026. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol. Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Chen RYZ, Li DS, Guth PH. Role of calcitonin gene-related peptide in capsaicin-induced gastric submucosal arteriolar dilation. Am. J. Physiol. 1992;262:H1350–H1355. doi: 10.1152/ajpheart.1992.262.5.H1350. [DOI] [PubMed] [Google Scholar]
- Danzer M, Jocic M, Samberger C, Painsipp E, Bock E, Pabst M-A, Crailsheim K, Schicho R, Lippe IT, Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am. J. Physiol. 2004;286:G403–G411. doi: 10.1152/ajpgi.00308.2003. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, editors. Rome II. The Functional Gastrointestinal Disorders. Second Edition Degnon Associates; McLean: 2000. [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. IV. Visceral afferent contributions to the pathobiology of visceral pain. Am. J. Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl. 1):i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann A, Jocic M, Herzeg G, Holzer P. Tachykinin inhibition of acid-induced gastric hyperaemia in the rat. Br. J. Pharmacol. 1996;119:1525–1532. doi: 10.1111/j.1476-5381.1996.tb16068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998a;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998b;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Holzer P. Sensory neurone responses to mucosal noxae in the upper gut: relevance to mucosal integrity and gastrointestinal pain. Neurogastroenterol. Motil. 2002a;14:459–475. doi: 10.1046/j.1365-2982.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Role of sensory neurons in mucosal protection from acid-induced lesions in the foregut. In: Dal Negro RW, Geppetti P, Morice AH, editors. Experimental and Clinical Pharmacology of Gastroesophageal Reflux-Induced Asthma. Pacini; Pisa: 2002b. pp. 25–33. [Google Scholar]
- Holzer P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin. Ther. Targets. 2004;8:107–123. doi: 10.1517/14728222.8.2.107. [DOI] [PubMed] [Google Scholar]
- Holzer P, Barthó L. Sensory neurons in the intestine. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. CRC Press; Boca Raton: 1996. pp. 153–167. [Google Scholar]
- Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience. 1988;27:981–987. doi: 10.1016/0306-4522(88)90201-1. [DOI] [PubMed] [Google Scholar]
- Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Holzer P, Sametz W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology. 1986;91:975–981. doi: 10.1016/0016-5085(86)90702-x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Danzer M, Schicho R, Samberger C, Painsipp E, Lippe IT. Vagal afferent input from the acid-challenged rat stomach to the brainstem: enhancement by interleukin-1beta. Neuroscience. 2004;129:439–445. doi: 10.1016/j.neuroscience.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Holzer P, Livingston EH, Guth PH. Sensory neurons signal for an increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology. 1991;101:416–423. doi: 10.1016/0016-5085(91)90020-l. [DOI] [PubMed] [Google Scholar]
- Holzer P, Pabst M-A, Lippe IT, Peskar BM, Peskar BA, Livingston EH, Guth PH. Afferent nerve-mediated protection against deep mucosal damage in the rat stomach. Gastroenterology. 1990;98:838–848. doi: 10.1016/0016-5085(90)90005-l. [DOI] [PubMed] [Google Scholar]
- Holzer P, Wachter C, Jocic M, Heinemann A. Vascular bed-dependent roles of the peptide CGRP and nitric oxide in acid-evoked hyperaemia of the rat stomach. J. Physiol. 1994;480:575–585. doi: 10.1113/jphysiol.1994.sp020385. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó N. Role of the nerve terminals in the mechanism of inflammatory reactions. Bull. Millard Fillmore Hosp. 1960;7:53–77. [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Lembeck F, Giere W, Loewi Otto. Springer; Berlin: 1968. [Google Scholar]
- Li DS, Raybould HE, Quintero E, Guth PH. Calcitonin gene-related peptide mediates the gastric hyperemic response to acid back-diffusion. Gastroenterology. 1992;102:1124–1128. [PubMed] [Google Scholar]
- Lippe IT, Holzer P. Participation of endothelium-derived nitric oxide but not prostacyclin in the gastric mucosal hyperaemia due to acid back-diffusion. Br. J. Pharmacol. 1992;105:708–714. doi: 10.1111/j.1476-5381.1992.tb09043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- Michl T, Jocic M, Heinemann A, Schuligoi R, Holzer P. Vagal afferent signaling of a gastric mucosal acid insult to medullary, pontine, thalamic, hypothalamic and limbic, but not cortical, nuclei of the rat brain. Pain. 2001;92:19–27. doi: 10.1016/s0304-3959(00)00467-x. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Sternini C, Eysselein VE, Yoneda M, Holzer P. Selective ablation of spinal afferent neurons containing CGRP attenuates gastric hyperemic response to acid. Peptides. 1992;13:249–254. doi: 10.1016/0196-9781(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur. J. Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Holzer P, Lippe IT. Mucosal acid challenge activates nitrergic neurons in the myenteric plexus of the rat stomach. Am. J. Physiol. 2001;281:G1316–G1321. doi: 10.1152/ajpgi.2001.281.5.G1316. [DOI] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Pabst M-A, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol. Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Jocic M, Heinemann A, Schöninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Stroff T, Plate S, Seyed Ebrahim J, Ehrlich K-H, Respondek M, Peskar BM. Tachykinin-induced increase in gastric mucosal resistance: role of primary afferent neurons, CGRP, and NO. Am J Physiol. 1996;271:G1017–G1027. doi: 10.1152/ajpgi.1996.271.6.G1017. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Barthó L. Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat. In: Mózsik G, Hänninen O, Jávor T, editors. Gastrointestinal Defense Mechanisms. Akadémiai Kiadó; Budapest: 1981. pp. 39–51. [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Yeoh KG, Kang JY, Yap I, Guan R, Tan CC, Wee A, Teng CH. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig. Dis. Sci. 1995;40:580–583. doi: 10.1007/BF02064374. [DOI] [PubMed] [Google Scholar]