Abstract
Peptide Nucleic Acids (PNAs) are single-stranded synthetic nucleic acids with a pseudopeptide backbone in lieu of the phosphodiester linked sugar and phosphate found in traditional oligos. PNA designed complementary to the bacterial Shine-Dalgarno or start codon regions of mRNA disrupts translation resulting in the transient reduction in protein expression. This study examines the use of PNA technology to interrupt protein expression in obligate intracellular Rickettsia sp. Their historically intractable genetic system limits characterization of protein function. We designed PNA targeting mRNA for rOmpB from Rickettsia typhi and rickA from Rickettsia montanensis, ubiquitous factors important for infection. Using an in vitro translation system and competitive binding assays, we determined that our PNAs bind target regions. Electroporation of R. typhi and R. montanensis with PNA specific to rOmpB and rickA, respectively, reduced the bacteria’s ability to infect host cells. These studies open the possibility of using PNA to suppress protein synthesis in obligate intracellular bacteria.
Introduction
Organisms in the genus Rickettsia are vector-borne Gram-negative α-proteobacteria that cause severe febrile disease in humans. The genus is grouped into Typhus Group (TG) and Spotted Fever Group (SFG) based on antigenicity of outer membrane proteins and the different diseases caused. The obligate intracellular lifestyle of Rickettsia species substantially limits the ability of researchers to genetically manipulate them in the laboratory, thus impeding progress in understanding their unique lifestyles and virulence. The use of homologous recombination in R. typhi [1] and transposon mutagenesis in R. monacensis and R. prowazekii [2–4] to express GFP indicate that the barriers to genetic manipulation are not insurmountable. Likewise, the use of the mariner-based Himar1 transposon system enabled researchers to inhibit actin-based motility of R. rickettsii through disruption of the autotransporter Sca2 and restore a nonlytic plaque phenotype to R. rickettsii spoT mutants [5,6]. However, these tools are underdeveloped when compared to those available to free-living bacterial systems and present challenges of their own. For instance, screening rickettsial transformants, is constrained by the number of resistance markers available due to the clinical importance of many antibiotics, and by the potential for spontaneous mutants arising following numerous passages [7]. In addition, the process of selecting mutants can take weeks due to poor transformation efficiencies and slow bacterial growth [5,6]. The need for better developed strategies for suppressing protein synthesis in obligate intracellular systems, such as Rickettsia, remains in order for improved molecular dissection of host-microbe interactions.
Antisense technologies offer temporary reduction of protein expression through sequence-specific recognition of mRNA. Peptide Nucleic Acids (PNAs) are DNA mimics possessing a pseudopeptide backbone with conventional purine and pyrimidine bases [8]. Antisense PNA molecules complementary to the Shine-Dalgarno or start codon region of mRNA effectively reduce translation [9,10]. PNAs designed complementary to essential components such as ribosomal RNA demonstrate their use as effective antimicrobials in a concentration dependent manner [9], while offering the possbility of using PNA to study essential protein function where a true knockout would preclude recovery of viable mutants. The ability of PNA to limit growth of facultative intracellular bacterium Brucella suis [11], implies the feasibility of using this strategy for reducing protein expression in obligate intracellular Rickettsia sp.
With this report we aim to provide a proof of principle for the utility of PNA-antisense technology to study Rickettsia spp. Based on the efficiency of protein inhibition demonstrated for bacteria from other genera, we hypothesize that PNA will reduce protein expression of RickA and rOmpB resulting in decreased infection of host cells. We targeted rickA from non-pathogenic R. montanensis (SFG) and rOmpB from endemic typhus-causing R. typhi (TG) because of the defined phenotypes associated with their functions. Studies targeting the R. rickettsii Arp2/3 activator RickA demonstrate its essential role during actin tail polymerization and intercellular spread [12]. Meanwhile, surface expressed rOmpB of R. conorii is sufficient for bacterial invasion of non-phagocytic mammalian cells [13,14]. Hence, disruption of either RickA or rOmpB expression should limit bacterial load due to their respective roles in establishing infection. Using in vitro translation and PNA-RNA hybridization assays, we show that the PNAs hybridize to the complementary upstream region for each target gene. When targeting PNA to either rickA in R. montanensis or rOmpB in R. typhi prior to in vitro infection, subsequent burden of infected L929 or Vero cells decreased 88% and 56%, respectively when compared to non-targeting controls at 24 hours. An 80% drop in R. montanensis burden persisted at 48 hours post-infection. Despite this, we measured no change in adherence to host cells following either rOmpB or rickA PNA treatments. We detected a 90% reduction in burden in vivo following injection of Dermacenter variabilis ticks with rickA PNA-treated R. montanensis. Taken together, these data point toward PNA as a viable methodology for studying genes of obligate intracellular species by successfully decreasing protein expression to elicit a phenotype.
Materials and Methods
Rickettsiae, host cell culture, and ticks
L929 (Mouse fibroblast, ATCC CCL-1) or Vero76 (African green monkey kidney ATCC CRL-1587) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% FBS at 34°C and 5% CO2. Host cells were inoculated with either R. montanensis strain M5/6 or R. typhi strain Wilmington (ATCC VR-144) at 80% confluency. Infected host cells were grown for 5 to 6 days. Rickettsiae were harvested by scraping infected cells into the media and sonicating on ice using a Sonic Dismembranator (Thermo Fisher Scientific Inc., Waltham, MA) for 5 cycles of 7 seconds on with 10 second rests in between. The lysates were centrifuged at 1000x g for 10 minutes to remove large host cell material. The rickettsial suspension was placed over an equal volume of 20% OptiPrep Density Gradient medium (Sigma-Aldrich, St. Louis, MO) in water or SPG buffer (218 mM sucrose, 3.76 mM KH2PO4, 7.1 mM K2HPO4, 4.9 mM potassium glutamate) and centrifuged at 10,000x g for 10 minutes. The pellets were washed with 250 mM sucrose, centrifuged at 14,000x g; and MOI 10 determined using the BacLight Live/Dead assay (Life Technologies, Grand Island, NY) or previous estimates. Unfed male and female D. variabilis ticks were provided by Daniel Sonenshine (Department of Biological Sciences, Old Dominion University). Maintenance of the tick colony was carried out according to protocols approved by the Institutional Animal Care and Use Committee at Old Dominion University.
PNA Synthesis, labelling, and electroporation
Custom PNA oligomers (Table 1) were synthesized by Bio Synthesis (Lewisville, TX). Oligomers were designed complementary to the predicted Shine Dalgarno region or inclusive of the start codon. A BLASTn analysis was performed for each PNA sequence against the full genomes of each respective bacterium to confirm specificity for the sequence of interest. PNA oligomers were biotinylated using EZ-Link Sulfo-NHS-Biotin, (Thermo Fisher Scientific Inc., Waltham, MA). Labeled PNA was purified using Pierce C18 Spin Columns (Thermo Fisher Scientific Inc.) Biotin-labeled PNA was used to confirm rOmpB PNA binds to the targeted region. Dot blots were used to confirm the successful biotinylation of oligomers. To deliver, 2 μM PNA was mixed with 1 x 107 purified rickettsia in a total volume of 100 μl of 250 mM sucrose in a 0.2 cm gap sterile cuvette on ice. Rickettsiae were then electroporated using a Gene Pulser Xcell Microbial System (Bio-Rad, Hercules, CA) by applying a 5 ms pulse (2500 V, 25 μF, 200 Ω). The PNA-rickettsiae suspension were transferred to a microcentrifuge tube and 400 μl of complete DMEM (5% FBS) medium was added to the electroporated rickettsia for recovery at room temperature for one hour.
Table 1. PNA Sequences.
| Target Gene | Description | PNA Sequence |
|---|---|---|
| rOmpB | Encodes an outer membrane protein sufficient for adherence and invasion of non-phagocytic cells by rickettsiae | H-(KFF) 3 K-TTT TTG AGC CAT AAT TT-NH 2 |
| rickA | Encodes a human WASP-like protein with the capacity to activate Arp 2/3 of host cells for intercellular spread | H-(KFF) 3 K-ACC TAC TAT AAA T-NH 2 |
| Non-target-1 | Scrambled control | H-(KFF) 3 K-TCT TAG TAT GTA TCT TA-NH 2 |
| Non-target-2 | Off-target control, encodes rifampin resistance | H-(KFF) 3 K-TAC CAT ATG AAA-NH 2 |
In vitro translation
The region starting 100 base pairs upstream of the start codon through 600 base pairs downstream of the rickA gene was amplifed using rickA forward primer 5’- ATTCGTTCATCTATCTTTTTTTTATTTATC-3’ and reverse primer 5’-TTTTTGTATTTCTTTAAGTTCTTTGACATTAG-3’ and cloned into pEXP5-CT (Life Technologies, Grand Island, NY). Translation was performed using the Expressway Cell-Free E. coli Expression System (Life Technologies). PNA targeted to rickA was added to the reaction to demonstrate PNA specificity for its target sequence as measured by impaired protein production. The CALML3 control vector was included in each reaction to serve as a loading control and to demonstrate PNA specificity. The total volume of each sample was resuspended in LDS loading buffer (Life Technologies) with reducing agent, heated for 10 minutes at 70°C and run on NuPAGE 4–12% Bis-Tris gels. Gels were transferred to PVDF membranes using an iBlot gel transfer device (Life Technologies). Membranes were probed using an anti-His antibody (1:3000) (Thermo Fisher Scientific, Waltham, MA) and developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Labeled PNA hybridization
Following biotinylation, PNA labeling was confirmed via dot blot. Briefly, 1 volume 20X SSC buffer was added to biotinylated PNA. Hybond-N+ nylon membrane (GE Healthcare Life Sciences, Pittsburgh, PA) was wet with 10X SSC buffer and spotted with 2 μl of sample and dried. Membranes were wet with denaturing solution (1.5 M NaCl, 0.5 M NaOH) for 5 minutes and transferred to filter paper soaked in neutralizing solution (1.5 M NaCl, 0.5 M Tris HCl, 0.001 M EDTA) for 1 minute. The membrane was dried, UV fixed and developed using Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific Inc., Waltham, MA) The target rOmpB ssRNA molecule 5’-GAAAAAAUUAUGGCUCAAAAACCAAA-3’ was synthesized by Integrated DNA Technologies, Inc (Coralville, IA). Next, 2.0 μl (120 ng) of ssRNA was mixed with either 235 ng of specific or non-targeting control-2 biotinylated PNA, or ratios of unlabeled to labeled rOmpB specific PNA. One microliter of RNase Inhibitor (Clontech Laboratories, Inc. Mountain View, CA) and 250 mM Tris (pH 7.2) were added and the total volume was brought to 15 μl using molecular biology grade water. The samples were incubated at 95° C for 10 minutes and left at room temperature to anneal for 30 minutes to 1 hour. The samples were diluted with loading dye supplemented with 0.1% SDS to give the hybridized PNA-RNA an overall negative charge allowing it to run toward the cathode. The samples were run n a 2% TBE agarose gel and transferred to a nylon membrane using an iBlot semi-dry transfer apparatus (Life Technologies, Grand Island, NY). Membranes were crosslinked with UV light and developed using Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific Inc., Waltham, MA).
RickA and OmpB protein reduction by titered Western blot
Rickettsiae were purified from L929 cells over an Optiprep cushion as described above. Approximately 5 x 106 of purified R. montanensis or equal volumes of purified R. typhi were resuspended in 100 μl of 250 mM sucrose containing 2 μM of PNA targeting rickA or rOmpB, or non-targeting control-2. The rickettsia-PNA suspension was placed into a pre-chilled 0.2 cm cuvette and electroporated as described above. The rickettsiae were transferred to a microcentrifuge tube and 400 μl of complete DMEM (5% FBS) medium was added to the electroporated rickettsia for recovery at room temperature for one hour. Rickettsia were centrifuged at 17,000x g for 10 minutes, the pellet resuspended in 39.5 μl of water, and two-fold serial dilutions were performed using water as the diluent. LDS sample buffer containing reducing agent was added to each tube containing a dilution for a total volume of 60 μl. The total volume of each sample was boiled for 10 minutes at 90°C, allowed to cool, and separated on a 4–12% Bis-Tris Bolt polyacrylamide gel (Life Technologies). The proteins were transferred to PVDF as described above and probed with anti-Ef-Ts (1:1000, load normalizer) and probed with either anti-RickA (1:2000) or anti-rOmpB (1:250) antibodies overnight at 4° C. Primary antibodies were detected with HRP-labeled donkey anti-rabbit IgG (BioLegend, San Diego, CA) at 1:3000 for 30–45 minutes. Blots were developed with WestPico Chemiluminescent detection substrate (Thermo Fisher Scientific Inc., Waltham, MA). Band densitometry was quantified using GeneTools image analysis software (Syngene USA, Frederick, MD).
Host cell infection with PNA treated rickettsiae
Following purification, PNA treatment and recovery (described above), R. typhi and R. montanensis were added to 1 x 104 L929 or Vero cells in 8-well chamber (Lab-Tek) slides at an MOI of 10. Bacteria were incubated on cells for 24 or 48 hours then washed with 1X PBS to remove nonadherent bacteria and fixed with freshly prepared 3.5% PFA. Extra- and intracellular rickettsiae were stained as previously described [15]. Briefly, extracellular bacteria were probed with either a mouse-anti-R. montanensis (1:200) or rat-anti-R. typhi serum (1:500), followed by either a goat-anti-mouse Alexa Fluor 594 or goat-anti-rat Alexa Fluor 594 secondary antibody (1:1000) (Life Technologies, Grand Island, NY). Host cells were permeabilized with 0.1% Triton-X 100 in PBS for 5 minutes at room temperature. Intracellular bacteria were probed as described above using the same primary antibodies but Alexa Fluor 488 secondary antibodies. Samples were mounted under Vectashield with DAPI (Vector Labs, Burlingame, CA) and visualized under oil at a total magnification of 1000x on a Nikon Eclipse E600 microscope. The slides were analyzed using QCapture Pro 5.1 imaging and analysis software. Intracellular rickettsia fluoresced as only green. Percent infection was determined by dividing the number of infected host cells by the total number rickettsiae-associated of cells. Percent association was determined by dividing the number of rickettsiae-associated cells by the total number of counted host cells. Host cells with one or more attached extracellular rickettsiae were considered “rickettsiae-associated.”
Tick infection with PNA-treated R. montanensis
Unfed male and female D. variabilis ticks were injected with 5000–15,000 rickA PNA-treated R. montanensis using pulled glass capillaries attached to a Nanoject II pump (Drummond Scientific, Broomall, PA). Ticks injected with non-targeting control-2 PNA-treated R. montanensis served as a control. Ticks were injected through the emargination cavity and incubated for 18 hours at 23°C with 95% humidity. Ticks were washed with 3% hydrogen peroxide, sterile water, 70% ethanol in succession, dried and placed in sterile 5 ml conical tubes on ice. Each tick was placed in a drop of AL buffer (DNeasy Blood and Tissue Kit, Qiagen, Valencia, CA) on a specimen slide and cut in two halves using a sterile razor blade. Each tick half and the dissecting buffer was removed to 180 μl of AL buffer. Tick samples were homogenized using a Tissuelyzer (Thermo Fisher Scientific Inc., Waltham, MA) and gDNA isolated according to the manufacturer’s instructions. Equal amounts of gDNA were placed into a qPCR reaction and the rickettsial housekeeping gene, gltA, and tick actin amplified using Quanta BioSciences SYBR Green I qPCR kit (Gaithersburg, MD) or Stratagene Brilliant SYBR Green QPCR Mastermix (Cedar Creek, TX). Amplification was done using glta forward primer 5’-CAGTCCGAATTGCCAGCTCA-3’ and reverse primer 5’- CGGGCCAAAGTGAGGCAATACCCG-3’ and actin forward primer 5’- GGAAGGACCTGTACGCCAACAC-3’ and reverse primer 5’- CGCCGATCTTTCATGGTGGAAGG-3’. Rickettsial burden was estimated by normalizing copies of gltA to copies of actin. Genomic copies were estimated using standard curves for each gene. Briefly, each gene was amplified with gene specific primers, the amplicon extracted from an agarose gel, and serial dilutions of the DNA performed to cover 103–1010 copies. Each dilution was amplified using qPCR. A standard curve plotting cycle thresholds versus Log10 gene copies was generated for the purpose of estimating bacterial load per tick.
Statistical analyses
Experiments were repeated at least twice, with individual ticks considered biological replicates. Each treatment was represented by at least ten individual biological replicates collected from all experiments performed. To exclude outlier data points from statistical analyses, interquatrile range outlier tests were performed. Data from each treatment group were subjected to the Shapiro—Wilk test and log transformed if lacking normality. An F-test was used to assess equality of variances between groups. Comparison of each treatment with a control was done via a one-tailed t-test. All statistical analyses were performed using Microsoft Excel.
Results
rickA PNA binds the complementary target in vitro
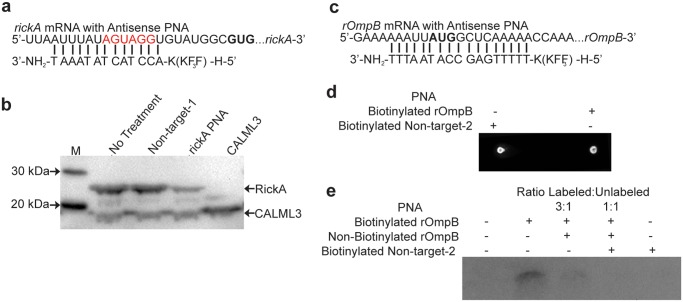
PNA complementary to the putative Shine-Dalgarno region of the well-characterized rickettsial gene, rickA, was tested for its ability to bind its target and suppress protein production using an in vitro translation system. We cloned the gene region starting 100 base pairs upstream of the rickA start codon through 600 base pairs downstream to include the sequence complementary to our PNA (Fig. 1A). An E. coli derived in vitro transcription/translation assay was then performed with the reactions treated with either no PNA, non-targeting control-1 PNA, or rickA PNA. The control vector coding for human calmodulin-like 3 (CALML3) was added to each reaction to serve as an internal loading control. A separate reaction containing the control vector alone was also performed. The entire volumes of each reaction were processed for Western Blotting to ensure equal loading. Reduced rickA production was observed when the in vitro translation reaction was supplemented with PNA specific to the gene, as compared with no PNA or non-targeting treatments (Fig. 1B). No change in CALML3 protein level was seen between the treatment groups demonstrating the specifity of the PNA for the rickA target sequence and equal loading. However, slightly increased CALML3 expression was seen in the CALML3-only reaction compared with those also including the rickA containing vector. This difference is likely due to equal concentrations of reagents being used for the translation of a single protein from a single vector versus the translation of two proteins from two vectors. A ghosted band appears at 25 kDa in all reactions, which may be an artifact of sample preparation, as its presence in the CALML3-only reaction indicates it is not associated with RickA expression. Thus, decreased expression of RickA following treatment with target PNA demonstrates its complementarity to and ability to bind the appropriate nucleotide sequence.
Fig 1. PNA is specific for designed target.
A) rickA PNA target with putative Shine Dalgarno region in red and start codon in bold B) Western blot of in vitro translation assay supplemented with PNA complementary to the cloned region of rickA, no PNA, or non-targeting control-1 PNA. In vitro translation reactions contained vectors coding both truncated RickA and the control CALML3, or CALML3 alone. C) rOmpB PNA target with start codon in bold D) Dot blot confirming biotinylation of rOmpB and non-targeting-2 PNA. E) Nylon membrane probed to detect biotinylated PNA-ssRNA pairs following rOmpB PNA target RNA denaturation and hybridization to biotinylated PNA, incubated with increasing ratios of unlabeled PNA for competitive binding assay. Incubations with either no PNA or biotinylated non-targeting-2 PNA serve as a control.
rOmpB PNA is complementary to its target sequence
Attempts to perform PNA treated in vitro translation of rOmpB were unsuccessful due to difficulty in cloning the appropriate rOmpB region. We chose instead to perform a hybridization assay to demonstrate the complementary binding ability of rOmpB PNA (Table 1) to the desired nucleotide sequence (Fig. 1C). To establish rOmpB-PNA pairs with the desired sequence, we biotinylated the PNA and hybridized it to a target rOmpB ssRNA molecule inclusive of the start codon. Biotinylation of PNA was confirmed via dot blot (Fig. 1D). Equal concentrations of rOmpB amplicon were mixed with biotinylated rOmpB PNA or non-targeting control-2 PNA, or different ratios of labeled to unlabeled rOmpB PNA. This would enable competitive binding to demonstrate specificity. Samples were heated to allow for denaturation then incubated at room temperature to anneal. No PNA hybridization occured when the reaction was supplemented with biotinylated non-targeting control-2 PNA or when the reaction contained no PNA (Fig. 1E). However, hybridization occured when the amplicon was mixed with biotinylated rOmpB PNA complementary to the target (Fig. 1E). We saw decreased hybridization of biotinylated PNA following competitive inhibition with increasing concentrations of unlabeled rOmpB PNA. This formation of a ssRNA-PNA pairing establishes the ability of our rOmpB PNA to bind the desired nucleotide target sequence.
rOmpB and RickA expression decrease in rickettsiae following PNA treatment
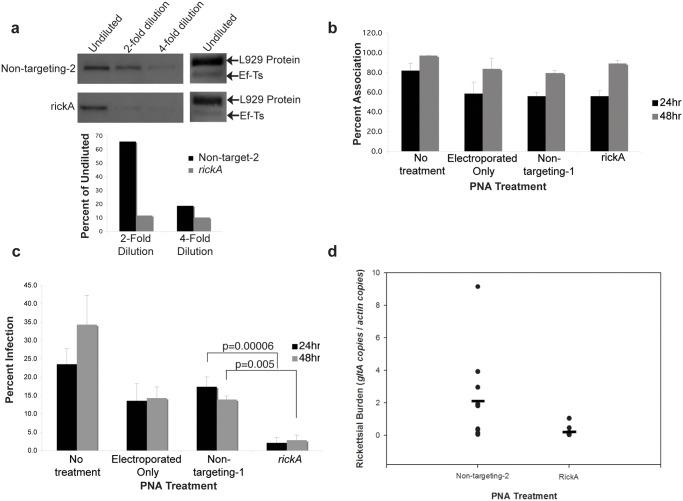
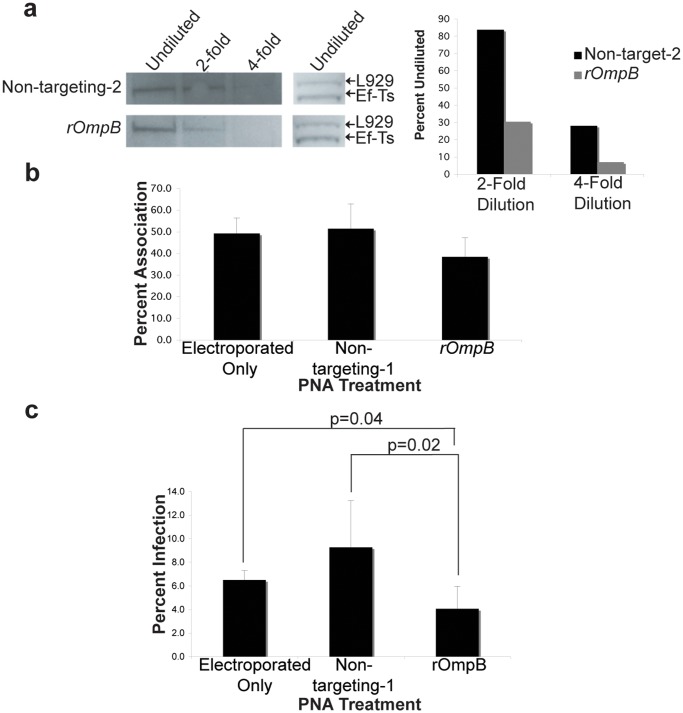
Knowing that PNA is specific for its target gene, we evaluated its ability to suppress protein expression in rickettsiae. R. typhi and R. montanensis were purified and electroporated with non-targeting control-2 and either rOmpB or rickA PNA. Following recovery, bacteria were serially diluted, lysed, and protein extracts run on a denaturing gel then transferred to PVDF. The goal of probing a dilution series was to overcome the saturation effect of highly expressed proteins like rOmpB and RickA. Given that PNA-mediated reduction likely affects only a percentage of the whole target protein population, we reasoned that a loss of protein could be measured by a loss of signal at higher dilutions as compared to non-targeting controls. Membranes were probed with antiserum for either rOmpB or RickA and translation elongation factor Ef-Ts, to demonstrate equal loading. We observed a loss of signal earlier in the dilution series in those bacteria treated with PNA specific for rickA or rOmpB compared with those treated with a non-targeting control-2 (Fig. 2A, Fig. 3A). Meanwhile, undiluted Ef-Ts bands were equivalent. The higher band seen when probing with anti-Ef-Ts serum is a previously documented host protein that cross reacts with our anti-Ef-Ts serum [16,17]. We performed quantitative densitometry graphing the signal of each dilution as a percent of the signal from the undiluted sample. The loss of signal earlier in the dilution series indicates the presence of less rOmpB or RickA, which we interpret as successful PNA knockdown of each protein’s expression respectively.
Fig 2. PNA decreases protein production and in vitro and in vivo infection by SFG R. montanensis.
A) Western blot of Opti-prep purified, serially diluted R. montanensis treated with rickA PNA and quantitative densitometry of the RickA expression as a percent of undiluted RickA. B) Adherence percentages and C) Infection percentages of L929 cells by R. montanensis treated with PNA designed to rickA at 24 and 48 hours. Infection by rickA PNA-treated R. montanensis is reduced 88% (p = 0.00006) at 24 hours and 80% (p = 0.005) at 48-hours post-infection. There is no statistically significant change in adherence with PNA treatment. In vitro experiments were repeated twice and performed in duplicate. Error bars represent standard deviation. D) Unfed D. variabilis adult ticks were injected with rickA PNA-treated (n = 11) or non-targeting control PNA-2-treated (n = 10) rickettsia. Rickettsial burden was measured using qPCR. Genomic copies for gltA were normalized to genomic copies for actin. Closed circles represent individual ticks and the closed horizontal bars represent the mean. Rickettsial burden in ticks infected with rickA PNA-treated R. montanensis is reduced 90% compared to the control (p = 0.004). In vivo experiments were repeated twice with 10 biological replicates per treatment.
Fig 3. PNA decreases rOmpB protein production and in vitro infection by TG R. typhi.
A) Western blot of Opti-prep purified, serially diluted rOmpB PNA-treated R. typhi and quantitative densitometry of the rOmpB expression as a percent of undiluted rOmpB. Adherence percentages of Vero cells by rOmpB or non-targetng control PNA-treated R. typhi. No statistical significance found between treatment groups. C) Infection percentages of Vero cells by rOmpB or non-targeting control PNA-treated R. typhi. Infection by rOmpB PNA treated R. typhi is reduced 56% (p = 0.02) compared to non-targeting control PNA. In vitro experiments were repeated twice and performed in duplicate. Error bars represent standard deviation.
Inhibition of Rickettsial entry but not association in vitro
RickA and rOmpB are well characterized for their role in intercelluar spread and host cell invasion respectively [12,18–20]. Hence, interruption of these proteins’ expression should reduce rickettsial infection. Purified R. montanensis were electroporated with either rickA or non-targeting-1 PNA, electroporated in the absence PNA, or left untreated. Following recovery, they were incubated with L929 mouse fibroblast cells for 24 or 48 hours. At the respective time points, cells were washed, fixed and stained differentially for internal and external bacteria to allow visual determination of infected or bacterially associated host cells by microscopy as described previously [15]. Bacterial electroporation alone caused a loss of infection compared to no treatment, indicating a loss of viability as a result of the procedure. However, an 88% and 80% drop in infection for rickA PNA treated bacteria occurred at 24 and 48 hours respectively, when compared with those electroporated only or treated with non-targeting control-1 PNA (Fig. 2C). Likewise, when we infected Vero cells with R. typhi treated with either rOmpB or non-targeting-1 PNA, we observed a 56% decrease in infection at 24 hours when the bacteria were treated with rOmpB PNA versus the non-targeting control (Fig. 3C). Taken together, these data illustrate the capability of using PNA to reduce a particular protein’s expression to in turn impair rickettsiae infection of host cells.
To verify that the loss of host cell infection by R. typhi and R. montanensis seen following rOmpB and rickA PNA treatments was not due to an inability to associate with host cells following PNA treatment, we next measured the ability of bacteria to associate with host cells. We used the aforementioned strategy of differentially staining rickettsiae. Host cells with associated (extracellular) bacteria were counted as a percentage of 100 total host cells. No significant difference in host cell association was seen when R. montanensis was treated with rickA PNA (Fig. 2B) or when R. typhi was treated with rOmpB PNA (Fig. 3B).
R. montanensis burden decreases in vivo following rickA PNA treatment
To evaluate the feasibility of PNA as a strategy for inhibiting protein synthesis in vivo, we injected D. variabilis ticks with R. montanensis treated with either rickA or non-targeting control-2 PNA. Following purification, PNA treatment, and recovery, R. montanensis were injected into the hoemocel via the emargination cavity of unfed male and female ticks. Ticks were incubated overnight, dissected, and burden assessed using qPCR comparing relative numbers of rickettsial and tick housekeeping genes. Rickettsial burden in the ticks decreased 90% following infection by rickA PNA-treated R. montanensis as compared to non-targeting control-2-treated rickettsia (Fig. 2D). This decrease in bacterial load complements the in vitro infection data and establishes PNA as an approach for successful inhibition of rickettsial protein expression leading to an in vivo phenotype.
Discussion
The use of PNA to reduce protein expression and subsequently impair bacterial growth has been investigated for both Gram-negative and Gram-positive bacteria [9,21]. PNA designed complementary to the ribsomal binding sites or inclusive of the start codon of rickA and rOmpB demonstrated the ability of this anti-sense technology to inhibit gene function essential to infection by rickettsiae. Subsequently, an overall decrease in the amount of RickA or rOmpB, as determined by immunoblotting serially diluted bacteria, is seen in PNA-treated R. montanensis or R. typhi, respectively. When treated with PNA specific to rOmpB, R. typhi infection of Vero cells decreased, and when treated with PNA complementary to rickA, R. montanensis infection of L929 cells decreases. Yet, no decrease in the percentage of bacterially associated cells took place with either rickA or rOmpB PNA treatments compared to controls, suggesting a role for other adhesin factors in the rickettsial adherence to host cells. Reduction in bacterial burden also occured in the tick vector D. variabilis following infection with rickA PNA-treated R. montanensis.
Traditionally, delivery of PNA occurs by linking the oligo to a basic stretch of amino acids, which increases uptake by free-living bacteria, and adding it to broth culture. While all our PNAs, experimental and non-targeting controls, were conjugated to the cell penetrating peptide (CPP), KFFKFFKFFK, [22] concern regarding the viability of rickettsiae if left extracellular for an extended period of time and the efficacy of getting PNA through both host and bacterial membranes led us to examine electroporation of purified Rickettsia as a delivery method. Electroporation is a tested method of delivering nucleic acids to rickettsial species [2–4]. Additionally, electroporation of Salmonella typhimurium infected macrophages with CPP-PNA targeting rpoD reduced the bacterial load more than those infected macrophages receiving no electroporation, implying it is a feasible method of PNA delivery [23]. While electroporation of purified Rickettsia to deliver PNA did cause a loss of bacterial viability (Fig. 2C), the remaining bacteria established productive infection in our control samples, demonstrating the utility of electroporation as a PNA delivery method. We argue that the limitation of infection in vitro and in vivo is due specifically to the PNA and not the CPP as both our non-targeting controls and experimental PNAs contain the CPP with no deleterious effects. Futhermore, previous work reports that adding CPP-PNA to host cell macrophages causes no detectable toxicity of the cell [11], suggesting that any drop in bacterial load is not due to PNA-induced host cell toxicity.
The reduction in infection seen following PNA treatment echos previous studies investigating the function of rickA and rOmpB from other rickettsial species. R. rickettsii Iowa stain, which is defective at cleaving rOmpB, displayed reduced virulence and plaque formation [19]. Likewise, inhibiting Ku70, the mammalian receptor of rOmpB, by antibody blocking or siRNA treatment reduced invasion by R. conorii, with no effect on bacterial adherence to host cells [20]. Though well characterized for its polymerization of host actin [12,18], recent reports further implicate RickA in invasion. Fewer infected A549 cells per infectious focus were calculated for rickA:tn mutant when compared with wild type R. parkeri 24-hours post-infection [24]. Additionally, R. bellii overexpressing rickA from R. monacensis, adhere to Vero cells in greater numbers at early time points (2–4 hours-post-infection), implying that rickA may have a role in the establishment of early infection, as adherence is required for invasion by the bacteria [25]. At 24-hours post-infection we saw less infection but not adherence by R. montanensis treated with rickA PNA, an identical phenotype to what we see when R. typhi is treated with rOmpB PNA. The trend continued with rickA PNA treatments at 48-hours post infection. We opted to not take rOmpB PNA-treated R. typhi samples at 48-hours because of the characterization of rOmpB as an invasion factor, presuming that a phenotype associated with reduced rOmpB would be best measured at earlier time points. Conversely, RickA is primarily known as an actin nucleator which could be important at later time points for intercellular spread. Yet, when D. variabilis tissues are treated with an Arp2/3 inhibitor prior to R. montanensis infection, burden is reduced, implying a role for actin polymerization in host cell invasion [26]. In addition, our observation that PNA-mediated suppression of RickA expression reduced the ability of R. montanensis to infect host cells and ticks argues that RickA plays a role during invasion. Consistent with this, our comparable infection and adherence data between R. typhi treated with rOmpB PNA and R. montanensis treated with rickA PNA further suggests that RickA, like rOmpB, may be involved in the host cell invasion process in addition to its well documented role in actin polymerization.
Off target effects are a concern when working with antisense oligos to interrupt gene function. In E. coli, two base mutations of the target site reduced PNA-mediated inhibition, while six base mutations virtually eliminated it [9]. Six base pair mismatches in either PNA or its target sequence ablated PNA inhibition in Staphylococcus aureus [21], further demonstrating the high specificity of PNA for its target sequence. When designing PNA oligos, we blasted the PNA sequences to ensure they aligned with only our target sequences, with our rickA PNA designed complementary to the putative Shine-Dalgarno region and the rOmpB PNA designed to include the start codon (Fig. 1A, 1C). Our rickA PNA inhibits RickA production but not CALML3 in an in vitro translation reaction. Additionally, we use two different non-targeting controls (Table 1) in our experiments. Non-targeting-1 was used for in vitro translation as well as cell culture infections, while Non-targeting-2 was used for ssRNA hybridization, in vivo protein suppression and tick infections. Non-targeting-1 is a scrambled control while Non-targeting-2 is designed for a rifampin resistance gene that our bacteria do not possess. Experimental PNAs, rOmpB and rickA, produced equivalent expression interruption results regardless of the control being used for comparison. Taken together these data point to the specificity of the technology for a target.
Our treatment of rickettsiae with 2 μM PNA to inhibit protein production is comparable to concentrations used in other systems. GFP expression from GFP-expressing S. aureus is reduced 30% following treatment with 2 μM PNA [21]. Interestingly, reduction in LacZ expression by E. coli is recorded following treatment with PNA at nanomolar concentrations [22]. Moreover, a positive correlation between PNA treatment and protein reduction exists in each system [21,22]. These observations suggest that further optimization is needed to establish the PNA dose requirements for the suppression of less ubiquitous targets and to determine the growth inhibitory concentrations for each system studied.
Our ability to recapitulate phenotypes reported by previous studies using PNA, illustrates its potential as a genetic tool in rickettsiae. While advances in the use of transposon delivery [5,6,27] and shuttle vectors [28] have increased the breadth of questions that can be asked about rickettsiae, tools to study obligate intracellular bacteria lag behind those available to study free-living bacteria. The field needs additional tools of genetic manipulation to better scrutinize the lifestyle and virulence of obligate intracellular bacteria, and allow molecular Koch’s postulates to be fulfilled. Our ability to inhibit protein expression in non-pathogenic Spotted Fever Group (SFG) R. montanensis and pathogenic Typhus Group (TG) R. typhi indicates the broad spectrum of uses for this technology. We propose that PNA could be utilized by a multitude of intracellular systems as a method for determining essential functions of genes to be targeted in the development of drugs and vaccines. PNA offers an advantage in that protein expression reduction is achieved in one day, no antibiotic selection is required, and partial reduction can be achieved allowing study of essential genes, where investigations of a phenotype imposed by a true knockout would be impossible due to low bacterial viability. For instance, PNA-mediated inhibition of essential genes involved with DNA replication, fatty acid synthesis, and RNA synthesis was successful in facultative intracellular B. suis[11]. These data also open the possibility of developing other anti-sense methods, such as transforming bacteria with vectors coding for an anti-sense transcript or supplementing host cell culture media with PNA molecules for delivery.
Current methods of delivery to free-living bacteria involve suplementation of culture media with PNA. Electroporation allows synchronization of PNA delivery to the entire bacterial population, increasing the effectiveness of PNA. Furthermore, studies that target proteins with transient expression can be conducted with greater accuracy and precision due to the synchronized method of PNA delivery. It was recently reported that electroporation enhances the availibility of CPP-PNA to S. typhimurium, following electroporation of infected macrophages compared to CPP-PNA added without electroporation [23]. Given these findings, it is possible that electroporation can reduce the costs of PNA synthesis for use in antisense studies by requiring no CPP and allowing lower concentrations to be used for experiments, but further optimization is required.
Many obligate intracellular bacteria cause serious morbidity and mortality in humans and lack effective vaccines. By targeting rickettsial genes of interest for the suppression of protein synthesis resulting in a gene specific phenotype, PNA offers promise as an additional tool for studying virulence and the intracellular lifestyle of these bacteria. Expanded use of this methodology to temporarily knock down protein expression for genes of interest may also allow the study of essential gene function in obligate intracellular bacteria.
Acknowledgments
The authors would like to thank Dr. Daniel Sonenshine (ODU, Norfolk, VA) for the ticks used in this project and Dr. Matthew Welch (UC Berkeley, Berkeley, CA) for providing the RickA antibody. We would also like to thank Dr. Kristen Rennoll-Bankert (UMB, Baltimore, MD) and Dr. Kelly Brayton (WSU, Pullman, OR) for critical review of this manuscript. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, Irish Cattle Breeders Federation Society Ltd. or The University of Maryland, College Park.
Data Availability
All relevant data are within the paper.
Funding Statement
Work for this manuscript was supported by funds from the National Institutes of Health/National Institute of Allergy and Infectious Diseases 1K22AI08040-01A1 to Shane M. Ceraul and R01AI043006 and R01AI017828 to Abdu F. Azad. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. http://www.niaid.nih.gov. National Institutes of Health/National Institute of Allergy and Infectious Diseases R01AI043006 and R01AI017828 to Abdu F. Azad provided partial support to personnel who authored this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Troyer JM, Radulovic S, Azad AF. Green fluorescent protein as a marker in Rickettsia typhi transformation. Infect Immun. 1999;67: 3308–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldridge GD, Burkhardt N, Herron MJ, Kurtti TJ, Munderloh UG. Analysis of fluorescent protein expression in transformants of Rickettsia monacensis, an obligate intracellular tick symbiont. Appl Environ Microbiol. 2005;71: 2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldridge GD, Kurtti TJ, Burkhardt N, Baldridge AS, Nelson CM, Oliva AS, et al. Infection of Ixodes scapularis ticks with Rickettsia monacensis expressing green fluorescent protein: a model system. J Invertebr Pathol. 2007;94: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu ZM, Tucker AM, Driskell LO, Wood DO. Mariner-based transposon mutagenesis of Rickettsia prowazekii . Appl Environ Microbiol. 2007;73: 6644–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun. 2010;78: 2240–2247. 10.1128/IAI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark TR, Ellison DW, Kleba B, Hackstadt T. Complementation of Rickettsia rickettsii RelA/SpoT restores a nonlytic plaque phenotype. Infect Immun. 2011;79: 1631–1637. 10.1128/IAI.00048-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rachek LI, Tucker AM, Winkler HH, Wood DO. Transformation of Rickettsia prowazekii to rifampin resistance. J Bacteriol. 1998;180: 2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 9. Good L, Nielsen PE. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16: 355–358. [DOI] [PubMed] [Google Scholar]
- 10. Good L, Nielsen PE. Peptide nucleic acid (PNA) antisense effects in Escherichia coli . Curr Issues Mol Biol. 1999;1: 111–116. [PubMed] [Google Scholar]
- 11. Rajasekaran P, Alexander JC, Seleem MN, Jain N, Sriranganathan N, Wattam AR, et al. Peptide nucleic acids inhibit growth of Brucella suis in pure culture and in infected murine macrophages. Int J Antimicrob Agents. 2013;41: 358–362. 10.1016/j.ijantimicag.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeng RL, Goley ED, D’Alessio JA, Chaga OY, Svitkina TM, Borisy GG, et al. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell Microbiol. 2004;6: 761–769. [DOI] [PubMed] [Google Scholar]
- 13. Martinez JJ, Cossart P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J Cell Sci. 2004;117: 5097–5106. [DOI] [PubMed] [Google Scholar]
- 14. Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 2009;11: 629–644. 10.1111/j.1462-5822.2008.01279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ceraul SM, Chung A, Sears KT, Popov VL, Beier-Sexton M, Rahman MS, et al. A Kunitz protease inhibitor from Dermacentor variabilis, a vector for spotted fever group rickettsiae, limits Rickettsia montanensis invasion. Infect Immun. 2011;79: 321–329. 10.1128/IAI.00362-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaur SJ, Rahman MS, Ammerman NC, Beier-Sexton M, Ceraul SM, Gillespie JJ, et al. TolC-dependent secretion of an ankyrin repeat-containing protein of Rickettsia typhi . J Bacteriol. 2012;194: 4920–4932. 10.1128/JB.00793-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pelc RS, McClure JC, Sears KT, Chung A, Rahman MS, Ceraul SM. Defending the fort: a role for defensin-2 in limiting Rickettsia montanensis infection of Dermacentor variabilis . Insect Mol Biol. 2014;23: 457–465. 10.1111/imb.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gouin E, Egile C, Dehoux P, Villiers V, Adams J, Gertler F, et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature. 2004;427: 457–461. [DOI] [PubMed] [Google Scholar]
- 19. Hackstadt T, Messer R, Cieplak W, Peacock MG. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect Immun. 1992;60: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii . Cell. 2005;123: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 21. Nekhotiaeva N, Awasthi SK, Nielsen PE, Good L. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol Ther. 2004;10: 652–659. [DOI] [PubMed] [Google Scholar]
- 22. Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE. Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol. 2001;19: 360–364. [DOI] [PubMed] [Google Scholar]
- 23. Ma S, Schroeder B, Sun C, Loufakis DN, Cao Z, Sriranganathan N, et al. Electroporation-based delivery of cell-penetrating peptide conjugates of peptide nucleic acids for antisense inhibition of intracellular bacteria. Integr Biol. 2014;6: 973–978. 10.1039/c4ib00172a [DOI] [PubMed] [Google Scholar]
- 24. Reed SC, Lamason RL, Risca VI, Abernathy E, Welch MD. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr Biol. 2014;24: 98–103. 10.1016/j.cub.2013.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliver JD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG. Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl Environ Microbiol. 2014;80: 1170–1176. 10.1128/AEM.03352-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petchampai N, Sunyakumthorn P, Guillotte ML, Verhoeve VI, Banajee KH, Kearney MT, et al. Novel identification of Dermacentor variabilis Arp2/3 complex and its role in rickettsial infection of the arthropod vector. PLoS One. 2014;9: e93768 10.1371/journal.pone.0093768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clark TR, Lackey AM, Kleba B, Driskell LO, Lutter EI, Martens C, et al. Transformation frequency of a mariner-based transposon in Rickettsia rickettsii . J Bacteriol. 2011;193: 4993–4995. 10.1128/JB.05279-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burkhardt NY, Baldridge GD, Williamson PC, Billingsley PM, Heu CC, Felsheim RF, et al. Development of shuttle vectors for transformation of diverse Rickettsia species. PLoS One. 2011;6: e29511 10.1371/journal.pone.0029511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.