Abstract
Background and Objectives
Economic evaluations provide information to aid the optimal utilization of limited healthcare resources. Costs of biologics for Rheumatoid arthritis (RA) are remarkably high, which makes these agents an important target for economic evaluations. This systematic review aims to identify existing studies examining the cost-effectiveness of biologics for RA, assess their quality and report their results systematically.
Methods
A literature search covering Medline, Scopus, Cochrane library, ACP Journal club and Web of Science was performed in March 2013. The cost-utility analyses (CUAs) of one or more available biological drugs for the treatment of RA in adults were included. Two independent investigators systematically collected information and assessed the quality of the studies. To enable the comparison of the results, all costs were converted to 2013 euro.
Results
Of the 4890 references found in the literature search, 41 CUAs were included in the current systematic review. While considering only direct costs, the incremental cost-effectiveness ratio (ICER) of the tumor necrosis factor inhibitors (TNFi) ranged from 39,000 to 1 273,000 €/quality adjusted life year (QALY) gained in comparison to conventional disease-modifying antirheumatic drugs (cDMARDs) in cDMARD naïve patients. Among patients with an insufficient response to cDMARDs, biologics were associated with ICERs ranging from 12,000 to 708,000 €/QALY. Rituximab was found to be the most cost-effective alternative compared to other biologics among the patients with an insufficient response to TNFi.
Conclusions
When 35,000 €/QALY is considered as a threshold for the ICER, TNFis do not seem to be cost-effective among cDMARD naïve patients and patients with an insufficient response to cDMARDs. With thresholds of 50,000 to 100,000 €/QALY biologics might be cost-effective among patients with an inadequate response to cDMARDs. Standardization of multiattribute utility instruments and a validated standard conversion method for missing utility measures would enable better comparison between CUAs.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease with the prevalence of 0.2–1% among adult population in Europe and North-America [1]. RA affects physical health causing pain, stiffness, progressive joint destruction and physical disability. Medical treatment, joint replacement surgery and productivity losses due to sick leave and early retirements lead to significant expenses for society [2]. The treatment target of RA is remission or low disease activity and the medication initially comprises conventional disease-modifying antirheumatic drugs (cDMARDs) such as methotrexate (MTX), sulphasalazine (SSZ), hydroxychloroquine (HCQ) and leflunomide (LEF), low-dose prednisolone and their combinations [3]. However, not all patients achieve remission or low disease activity with cDMARDs due to intolerance or lack of effectiveness. Biologic disease-modifying antirheumatic drugs (bDMARDs), also known as biologics, cover TNF inhibitors (TNFi) (adalimumab (ADA) (Humira, AbbVie Ltd.), certolizumab pegol (CER) (Cimzia, UCB Pharma SA), etanercept (ETN) (Enbrel, Pfizer Ltd.), golimumab (GOL) (Simponi, Janssen Biologics B.V), infliximab (IFX) (Remicade, Janssen Biologics B.V.)) and agents based on other mechanisms of action (abatacept (ABT) (Orencia, Bristol-Myers Squibb Pharma EEIG), anakinra (ANA) (Kineret, Biovitrum AB), rituximab (RTX) (MabThera, Roche Registration Ltd) and tocilizumab (TOC) (RoActemra, Roche Registration Ltd.)). Biologics have proven to be an effective treatment for RA, but because of the high price, they are recommended only for patients with insufficient response or intolerance to cDMARDs [3–6].
Economic evaluations provide information on the benefits and costs of these expensive treatments to aid the optimal utilization of limited healthcare resources [7]. Cost-effectiveness analysis (CEA) is the most typical form of economic evaluation for health care interventions. In CEA, costs and effectiveness of two or more treatments are compared. The costs are measured in monetary units and effectiveness in natural units, for example in life years or pain free days. Cost-utility analysis (CUA) is a subtype of CEA, applying quality adjusted life years (QALY) as a measure of effectiveness. The primary outcome measure in CUAs is incremental cost-effectiveness ratio ICER, which describes the ratio of the additional costs of a treatment (compared to an alternative) to QALYs gained. An ICER is not reported if one treatment is both cheaper and more effective than another, e.g. if it is dominant.
Biologics for RA are an important target for economic evaluations because of the associated high costs. Previous systematic reviews suggest that biologics might be cost-effective at the willingness to pay (WTP) threshold of 50,000–100,000 $/QALY among patients with insufficient treatment response to cDMARD but not in cDMARD naïve patients [8–10]. However, these reviews involve some weaknesses such as lack of quality assessment [9], insufficient reporting of study characteristics [8] or omission of between-biologics comparison [10]. The aim of our systematic review is to identify all existing studies examining the cost-utility of one or more biologics for RA in adults, assess their quality and report their results systematically.
Methods
Literature search
We performed a literature search aiming to identify existing CUAs assessing the cost-effectiveness of biologics for treatment of RA. The search covering Medline, SCOPUS (including EMBase), Cochrane library (Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, Cochrane Database of Systematic Reviews, NHS Economic Evaluation Database, Cochrane Central Register of Controlled Trials and Cochrane Methodology Register), ACP Journal club and Web of science was executed in March 2013 using a search strategy developed with a librarian. The search strategy included terms describing study design (CUA), intervention (Biologics) and patients (RA) in different spellings. The complete search strategy for PubMed is presented in S1 File.
No time or language restrictions were made to the literature search. The number of non-English publications was used to investigate the existence of a language bias and publication bias was assessed based on the number of conference abstract published as full-text.
Study selection
All references identified by the literature search were imported to reference management software (Refworks), where duplicate records were removed. Of the remaining references, the CUAs of one or more currently available biologics for the treatment of RA in adults were selected using a pre-defined inclusion and exclusion criteria (S1 Table). The evaluation for inclusion was conducted independently by two persons (JJ and KA) at first by titles and afterwards by full-text. In case of disagreement, a third opinion (MB) was requested. Studies without active comparison treatment (cDMARDs or other biologics) or QALYs as measure of effectiveness were excluded from this systematic review. Reporting of ICER was required, if applicable. Studies published only as conference abstracts and articles without English full-text were excluded.
Data collection
The Data on patients, interventions, controls, study design (country, perspective, time horizon, the year of resource utilization, included costs, discount rate, the source of effectiveness, the instrument for utility measures, study funding) and outcomes were extracted using a Microsoft Excel—based collection form. Two assessors (JJ and SH) independently extracted the data and discrepancies were resolved by consulting the third investigator (MB). Due to limited time and resources, authors were not contacted for complementary information.
Quality assessment
As currently recommended, the quality of economic evaluations included was assessed using the British Medical Journal (BMJ) checklist and in addition, the Philips`checklist for modelling studies [11–13]. Two investigators (JJ and SH) assessed the quality of the studies independently and the third investigator (MB) was consulted when necessary. BMJ checklist involves 35 items and Philips’ checklist 57 items. Quality scores based on fulfilment of items and average percentages of the applicable criteria met were calculated. To assess the relative quality of the studies we divided studies in three categories (good, adequate and poor quality) ranking them by using the average percentages.
Representation of results
The quantitative synthesis of the results of the studies included is not possible owing to heterogeneous study designs. Results of the CUAs included were stratified into five subgroups by type of drug used, previous treatments and response to them, and the comparator treatment as follows: 1) Biologics for cDMARDs naive patients, 2) Biologics compared with cDMARD in patients with an inadequate response to one or several cDMARDs, 3) Biologics compared with other biologics among patients with an inadequate response to cDMARDs, 4) Biologics compared with cDMARDs among patients with an inadequate response to TNFi(s) and 5) Biologics compared with other biologics among patients with an inadequate response to TNFi(s). Further, CUAs were stratified according to adequateness of the comparator treatment. Adequate comparator was defined as a cDMARD not used before [3].
To enable a comparison of the results, all of the reported costs were converted to euro using the European Central Bank exchange rates (http://sdw.ecb.europa.eu) and adjusted to the price level of the year 2013 using the price index of Health care expenditure in Finland (Statistics Finland). ICERs including only direct costs were considered primary results due to differences in the ways indirect costs (e.g. productivity losses) were calculated in studies. In addition, ICERs including both direct and indirect costs were presented as secondary outcomes, if reported in the original studies.
Results
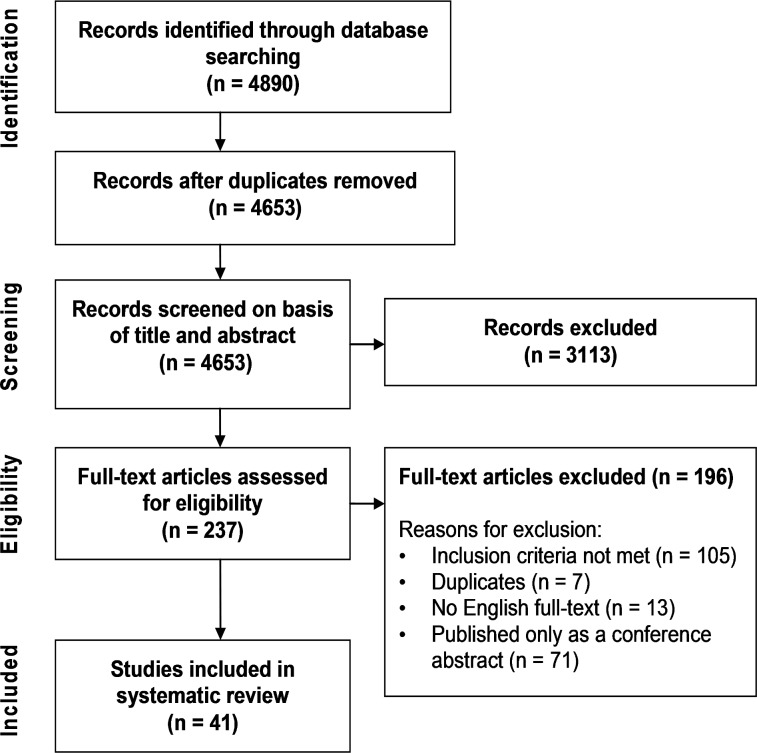
Altogether, 4653 non-duplicate references were identified with the literature search, of which 3113 were excluded during title and abstract screening (Fig. 1). After the assessment of 237 full-text articles, 41 were included in the current review. A majority of the studies excluded by full-text assessment did not meet the inclusion criteria (105 studies) or were published only as conference abstracts (71 studies). The list of the articles excluded after full-text assessment is displayed in S2 File.
Fig 1. Flow chart of the study selection process.
Characteristics of studies included in the current review
The 41 CUAs included were published 2002–2013 [14–54]. One study was based on empiric cost and effectiveness data from a randomised controlled trial (RCT) [19], two on observational data [16,37] while the remaining 38 studies used a modelling approach with multiple data sources [14,15,17,18,20–36,38–54]. In 33 of the 38 modelling studies effectiveness estimates were derived from one or more RCTs, while five modelling studies applied effectiveness obtained from national registers. A summary of the characteristics of the CUAs included is shown in Table 1.
Table 1. Characteristics of the studies included in the current review.
| Study, Year of publication, Country | Patients | Biologic treatment(s) | Comparator | Perspective | Time horizon | Study type | Source of effectiveness | Instrument for utility measures | Discount rate* |
|---|---|---|---|---|---|---|---|---|---|
| Bansback et al. 2005, Sweden [41] | Moderate to severe RA, inadequate response to 2 cDMARDs | ADA+MTX or ADA or ETN+MTX or ETN or IFX+MTX | cDMARD | Policy maker | Lifetime | Patient-level transition state model | RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | 3% |
| Barbieri et al. 2005, UK [51] | Severe RA, inadequate response to MTX | IFX+MTX | MTX | Payer (UK NHS) | Lifetime (model), 1 and 2 years and lifetime (treatment) | Markov model | RCT | VAS | Costs 6%, benefits 1.5% |
| Barton et al. 2004, UK [50] | RA, inadequate response to SSZ or MTX | ETN / IFX➔ cDMARDs | cDMARDs: GST ➔ AZA ➔ D-PEN ➔HCQ➔ LEF ➔ CSA ➔ MTX/CSA➔ Palliation | Payer (UK NHS) | Lifetime | Individual sampling model | RCTs | EQ-5D converted from HAQ, QoL = 0.862–0.327 x HAQ | Costs 6%, benefits 1.5% |
| Brennan et al. 2004, UK [49] | RA, inadequate response to at least 2 cDMARDs (MTX, SSZ) | ETN➔ cDMARDs | cDMARDs: GST➔LEF➔CSA | Payer | Lifetime | Individual patient-level simulation model | RCT | EQ-5D converted from HAQ, QoL = 0.86–0.20 x HAQ | Costs 6%, benefits 1.5% |
| Brennan et al. 2007, UK [15] | RA, inadequate response to at least 2 cDMARDs | TNFi (ETN, IFX, ADA) | cDMARDs | Payer (UK NHS) | Lifetime | Individual sampling model | British Registry (BSRBR) | EQ-5D converted from HAQ | Costs 6%, benefits 1.5% |
| Brodszky et al. 2010, Hungary [34] | Moderate to severe RA, inadequate response to cDMARDs and at least1 TNFi | RTX | 1.) MTX, 2.) Another TNFi | Health care provider | Lifetime (model), 2 infusions and 3 years (treatment) | Markov model | RCTs | EQ-5D converted from HAQ | 5% |
| CADTH 2010, Canada [48] | RA, inadequate response to at least 2 cDMARDs | ADA or ETN or IFX or GOL or ABT or Optimal sequence of biologics | MTX | Health care provider | 5 years | Markov model | MTC | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | Not stated |
| Chen et al. 2006, UK [28] | 1.) Early RA, no previous cDMARDs and 2.) RA, inadequate response to at least 2 cDMARDs (SSZ, MTX) | IFX+MTX / ADA+MTX / ETN+MTX / ETN / ADA➔cDMARDs or cDMARDs➔ IFX+MTX / ETN+MTX / ADA+MTX / ETN /ADA | cDMARDs:(MTX)➔ MTX+SSZ ➔ MTX+SSZ+HCQ ➔ LEF ➔ GST ➔ AZA (CSA ➔ CSA+MTX ➔ D-PEN or cDMARDs: MTX+SSZ+HCQ ➔ LEF ➔ GST ➔ AZA ➔ CSA ➔ CSA+MTX ➔ D-PEN | Payer (UK NHS) | Lifetime | Individual sampling model | Meta-analysis in same report | EQ-5D converted from HAQ, QoL = 0.862–0.327 x HAQ | Costs 6%, benefits 1.5% |
| Chiou et al. 2004 [47] | Moderate to severe RA | ETN+MTX or ETN or ADA+MTX or ADA or ANA+MTX or ANA or IFX+MTX | Comparison of biologics | Payer | 1 year | Decision analytic model | RCTs | VAS converted from ACR20, ACR50, ACR70 and no ACR responses | - |
| Clark et al. 2004, UK [22] | RA, inadequate response to cDMARDs and TNFi (SSZ, MTX, HCQ, (GST), LEF, ETN, IFX) | ANA➔cDMARDs or cDMARDs➔ANA | cDMARDs: (GST)➔ AZA➔CSA➔ MTX+CSA | Payer (UK NHS) | Lifetime | Individual sampling model | Meta-analysis in same report | EQ-5D converted from HAQ, QoL = 0.862–0.327 x HAQ | Costs 6%, benefits 1.5% |
| Coyle et al. 2006, Canada [46] | RA, no response to cDMARDs (MTX, MTX+SSZ, MTX+SSZ+HCQ) | IFX+MTX / ETN➔GST or GST➔IFX+MTX / ETN | GST | Third party payer (Ministry of Health) | 5 years | Markov model | Systematic review in same report | EQ-5D converted from HAQ | 5% |
| Davies et al. 2009, USA [21] | Early RA (< 3 years), no previous MTX | ADA+MTX / ETN / IFX+MTX ➔ cDMARDs or ADA+MTX➔ ETN➔ cDMARDs | cDMARDs: MTX ➔ MTX+HCQ ➔ LEF ➔ GST ➔ Palliation | Payer | Lifetime | Individual patient-level simulation model | Several RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ | 3% |
| Diamantopoulos et al. 2012, Italy [33] | RA, inadequate response to cDMARDs | TOC+MTX ➔ biologics: (ADA+MTX ➔ RTX+MTX ➔ ABA+MTX ➔ Palliation) | ETN+MTX ➔ biologics | Payer | Lifetime | Individual patient-level simulation model | MTC | EQ-5D converted from HAQ, QoL = 0.82–0.11 x HAQ—0.07 x HAQ² | 3% |
| Farahani et al. 2006, Canada [37] | RA | ETN + cDMARD | cDMARD (MTX, SSZ, HCQ etc.) | Societal | 1 year | Observational analysis, no modelling used | RCT and observational study (efficacy vs. effectiveness data) | EQ-5D converted from HAQ, QoL = 0.862–0.327 x HAQ | - |
| Finckh et al. 2009, USA [27] | Early RA (< 3 months), no previous cDMARDs | 1.) cDMARDs ➔ 1.TNFi+MTX ➔ 2.TNFi+MTX➔ 3.TNFi 2.)1.TNFi+MTX ➔ 2.TNFi+MTX➔ 3.TNFi ➔ cDMARDs 3.)NSAID ➔ cDMARDs ➔ 1.TNFi+MTX ➔ 2.TNFi+MTX ➔ 3.TNFi | Comparison of treatment 3 strategies containing TNFi | Health care provider, societal | Lifetime | Individual sampling model | Meta-analysis | EQ-5D converted from HAQ | 3% |
| Hallinen et al. 2010, Finland [54] | Severe RA, no response to TNFi | RTX+MTX / ADA+MTX / ETN+MTX / IFX+MTX/ ABT+MTX➔ cDMARDs or Optimal sequence of biologics | cDMARDs: GST ➔ CSA+MTX | Societal | Lifetime (up to the age of 100 years) | Patient-level Markov model | RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | 3% |
| Jobanputra et al. 2002, UK [32] | RA, no response at least 2 cDMARDs (SSZ, MTX) | ETN / IFX+MTX ➔ cDMARDs or cDMARDs➔ ETN / IFX+MTX | cDMARDs: GST ➔ AZA ➔ D-PEN ➔ HCQ ➔ LEF ➔ CSA ➔ CSA+MTX | Payer (UK NHS) | Lifetime | Individual sampling model | Meta-analysis in same report | EQ-5D converted from HAQ | Costs 6%, benefits 1.5% |
| Kielhorn et al. 2008, UK [31] | RA, inadequate response to 2 cDMARDs and a TNFi | RTX ➔ MTX ➔ cDMARDs or RTX+MTX ➔ ADA+MTX ➔ IFX+MTX ➔ cDMARDs | cDMARDs: LEF ➔ GST ➔ CSA ➔ MTX or ADA+MTX ➔ IFX+MTX ➔ cDMARDs | Payer (UK NHS) | Lifetime | Patient-level Markov model | RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | 3,5% |
| Kobelt et al. 2003, UK & Sweden [52] | Advanced RA, no response to MTX | IFX+MTX | MTX | Not stated | 10 years (model), 1 and 2 years (treatment) | Markov model | RCT | EQ-5D converted from HAQ | UK: Costs 6%, benefits 1.5%; Sweden: 3% |
| Kobelt et al. 2004, Sweden [16] | RA, inadequate response to at least 2 cDMARDs, including MTX | TNF (IFX, ETN) | Baseline (DMARD) | Societal | 1 year | Observational analysis, no modelling used | Observational study | EQ-5D | - |
| Kobelt et al. 2005, Sweden [36] | RA, inadequate response to cDMARD (excluding MTX) | ETN+MTX or ETN | MTX | Societal | 5 and 10 years (model); 2, 5 and 10 years (treatment) | Patient-level Markov model | RCT | EQ-5D converted from HAQ | 3% |
| Kobelt et al. 2011, Sweden [38] | Early RA, no previous MTX | ETN+MTX ➔ Half-dose ETN+MTX ➔ cDMARD / 2. biologic | MTX ➔ cDMARD / biologic | Societal | 10 years | Patient-level Markov model | RCT | EQ-5D converted from HAQ | 3% |
| Lekander et al. 2010, Sweden [26] | RA, inadequate response to at least 2 cDMARDs | IFX + cDMARD | cDMARD | Societal | 20 years | Markov cohort model | Registry (STURE) | EQ-5D converted from HAQ | 3% |
| Lekander et al. 2013, Sweden [25] | 1.) RA, inadequate response to at least 2 cDMARDs or 2.) RA, inadequate response to a TNFi | TNFi (ADA, IFX, ETN) + cDMARD or TNFi or ETN+cDMARD or ETN | cDMARD | Societal | 20 years | Markov cohort model | Registry (Swedish Rheumatology Register) | EQ-5D converted from HAQ | 3% |
| Lindgren et al. 2009, Sweden [45] | RA, inadequate response to a TNFi | RTX ➔ 2.TNFi (ADA, ETN, IFX) | 2. TNFi ➔ 3. TNFi | Societal | Lifetime | Discrete event simulation model | RCT and Registry (SSTAG) | EQ-5D converted from HAQ and DAS 28 | 3% |
| Malottki et al. 2011, UK [53] | RA, inadequate response to a TNFi | ADA / ETN / IFX / RTX / ABT ➔ cDMARDs | cDMARDs: LEF ➔ GST ➔ CSA ➔ AZA | Payer (UK HNS) | Lifetime | Individual sampling model | Meta-analysis | EQ-5D converted from HAQ, QoL = 0.804–0.203 x HAQ—0.045 x HAQ² | 3,5% |
| Marra et al. 2007, Canada [44] | RA, refractory to standard therapy | IFX+MTX | MTX | Societal | 10 years | Patient-level Markov model | RCT | HUI-2, HUI-3, EQ-5D and SF6D converted from HAQ | 3% |
| Merkesdal et al. 2010, Germany [18] | RA, inadequate response to ETN | RTX+MTX ➔ ADA+MTX ➔ IFX+MTX ➔ GST ➔ CSA ➔ MTX | ADA+MTX ➔ IFX+MTX ➔ GST ➔ CSA ➔ MTX | Payer | Lifetime | Patient-level Markov model | RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | 3,5% |
| Nguyen et al. 2012, USA [24] | Moderate to severe RA, moderate or no response to MTX | ADA+MTX / IFX+MTX / CER+MTX / GOL+MTX ➔ TOC | ETN+MTX ➔ TOC / MTX ➔ TOC | Payer | 5 years | Markov cohort model | RCTs (Systematic review) | VAS converted from ACR20, ACR50, ACR70 and no ACR responses | 3% |
| Schipper et al. 2011, the Netherland [30] | Early RA, no previous cDMARDs | 1.)1.TNFi+MTX ➔ 2.TNFi+MTX➔ RTX+MTX 2.)MTX+LEF➔ 1.TNFi+MTX ➔ 2.TNFi+MTX➔ RTX+MTX 3.)MTX(MTX+LEF ➔ 1.TNFi+MTX ➔ 2.TNFi+MTX➔ RTX+MTX | Comparison of treatment 3 strategies containing TNFi | Payer, societal | 5 years | Patient-level Markov model | Registries (Nijmegen and DREAM) | EQ-5D | 4% |
| Soini et al. 2012, Finland [20] | Moderate to severe RA, inadequate response to at least 1 cDMARD | TOC+MTX / ADA+MTX / ETN+MTX ➔ RTX+MTX ➔ IFX+MTX ➔ LEF ➔ CSA ➔ MTX | MTX➔ RTX+MTX ➔ IFX+MTX ➔ LEF ➔ CSA ➔ MTX | Payer, societal | Lifetime | Individual sampling model | MTC | EQ-5D converted from HAQ, QoL = 0.82–0.11 x HAQ—0.07 x HAQ² | 3% |
| Spalding & Hay 2006, USA [14] | Early RA (< 3 months), no previous cDMARDs | ADA+MTX or ADA or IFX+MTX or ETN | MTX | Payer, societal | Lifetime | Markov model | Several RCTs | HUI3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE + 0,001 x AGE | 3% |
| Tanno et al. 2006, Japan [35] | RA, inadequate response to busillamine (cDMARD) | ETN ➔ cDMARDs | cDMARDs: MTX ➔ SSZ ➔ MTX+SSZ ➔ no cDMARD | Societal | Lifetime | Markov model | RCT | EQ-5D converted from HAQ, QoL = 0.74–0.17 x HAQ | Costs 6%, benefits 1.5% |
| Wailoo et al. 2008, USA [40] | Established RA | ADA / IFX / ETN / ANA ➔ cDMARD | Comparison of biologics | Payer (Medicare) | Lifetime | Model, unspecified | Meta-analysis | EQ-5D converted from HAQ | 3% |
| van den Hout et al. 2009, the Netherlands [19] | Early RA (≤ 2 years), no previous cDMARDs | 1.)MTX ➔ MTX+SSZ ➔ MTX+SSZ+HCQ➔ MTX+SSZ+HCQ+CS ➔ IFX+MTX ➔ MTX+CSA+CS ➔ LEF ➔ AZA+CS 2.)IFX+MTX➔ SSZ ➔ LEF ➔MTX+CSA+ CS ➔ GST+CS ➔ AZA+CS 3.)MTX(SSZ➔ LEF ➔ IFX+MTX ➔ GST+ CS ➔ MTX+CSA+CS ➔ AZA+ CS 4.)MTX+SSZ+CS➔ MTX+CSA+ CS ➔ IFX+MTX ➔ LEF ➔ GST+ CS ➔ AZA+CS | Comparison of treatment 4 strategies containing TNFi | Societal | 2 years | Empiric CUA, no modelling used10 | RCT | EQ-5D (British and Dutch valuations), SF6D, TTO | 3% |
| Welsing et al. 2004, the Netherland [23] | Active RA, inadequate response to at least 2 cDMARDs (SSZ, MTX) | ETN➔Usual care or LEF➔ETN(Usual care or ETN➔LEF(Usual care | Usual care or LEF(Usual care | Societal, Payer (Third party payer) | 5 years | Markov model | RCTs | EQ-5D converted from DAS28 responses | 4% |
| Vera-Llonch et al. 2008a, USA [17] | Moderate to severe RA, inadequate response to MTX | ABT+MTX | MTX | Third party payer | 10 years, lifetime | Patient-level simulation model | RCT | EQ-5D converted from HAQ | 3% |
| Vera-Llonch et al. 2008b, USA [43] | Moderate to severe RA, inadequate response to TNFi | ABT+MTX | MTX | Third party payer | 10 years, lifetime | Patient-level simulation model | RCT | EQ-5D converted from HAQ | 3% |
| Wong et al. 2002 [39] | Active refractory RA | IFX+MTX | MTX | Payer, societal | Lifetime (model), 54 weeks (treatment) | Markov cohort model | RCT | VAS | 3% |
| Wu et al. 2012, China [29] | Moderate to severe RA, inadequate response to at least 2 cDMARDs (including MTX) | ETN / IFX / ADA➔ cDMARDs or ETN(RTX➔ cDMARDs or IFX(RTX➔ cDMARDs or ADA(RTX➔ cDMARDs | cDMARDs: GST ➔ LEF ➔ CSA ➔ MTX | Payer, societal | Lifetime | Markov cohort model | RCTs | HUI-3 converted from HAQ, QoL = 0.76–0.28 x HAQ + 0.05 x FEMALE | 3% |
| Yuan et al. 2010, USA [42] | Active RA, inadequate response to a TNFi | ABA+MTX / RTX+MTX ➔ MTX | MTX | Payer | Lifetime | Patient-level simulation model | RCTs | EQ-5D converted from HAQ | 3% |
➔ = switch to next treatment in case of an inadequate response, ABT = abatacept, ADA = adalimumab, ANA = anakinra, AZA = azathioprine, bDMARD = biologic disease-modifying antirheumatic drugs, CADTH = Canadian Agency for Drugs and Technologies in Health, cDMARD = conventional disease-modifying antirheumatic drugs, CER = certolizumab pegol, CS = corticosteroids, CSA = cyclosporin A, DAS28 = Disease Activity Score 28, D-PEN = D-Penicillin, EQ-5D = EuroQol-5D, ETN = etanercept, GOL = golimumab, GST = Gold, HAQ = Health Assessment Questionnaire, HCQ = hydroxychloroquine, HUI-2 = Health Utility Index 2, HUI-3 = Health utility Index 3, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, LEF = leflunomide, MTC = mixed-treatment comparison, MTX = methotrexate, NSAID = non-steroidal anti-inflammatory drug, QALY = quality-adjusted life year, QoL = quality of life, RA = rheumatoid arthritis, RCT = randomized controlled trial, RTX = rituximab, SF-6D = Short Form 6D, SSZ = sulfasalazine, TNFi = TNF inhibitor, TOC = tocilizumab, TTO = Time Trade-off, UK NHS = The National Health Service of the United Kingdom, VAS = the Visual Analogue Scale,
*People and society tend to value present costs and benefits more than future ones. This is taken into account by discounting future costs and benefits with a predefined rate.
Cost-effectiveness of biologics in patients with early RA and naïve to cDMARDs
The cost-effectiveness of biologics for patients with early RA and naïve to cDMARDs were analysed in seven studies (Table 2). Four studies performed a comparison between biologics and cDMARDs [14,21,28,38]. The ICERs of TNFi in comparison to cDMARDs ranged from 39,000 to 1 273,000 €/QALY when only direct costs were considered (Table 2). IFX was associated with the highest ICERs ranging from 422,000 to 1 273,000 €/QALY while ICERs for ETN and ADA as a monotherapy were below 100,000 €/QALY. As a combination therapy with MTX, ICERs for ETN and ADA were substantially higher. If both direct and indirect costs were considered, ICERs for biologics were slightly more favourable.
Table 2. Cost-effectiveness of biologics in cDMARD naïve patients.
| Treatments | Study | ICER €/QALY (only direct costs) | ICER €/QALY (direct and indirect costs) | Results of deterministic sensitivity analysis €/QALY | Source of research funding |
|---|---|---|---|---|---|
| TNFi vs. cDMARDs | |||||
| IFX | Chen et al. 2006 [28] | 1 273,007 | - | 40,876—dominated | NICE (UK) |
| Davies et al. 2009 [21] | Extended dominance by ADA | Extended dominance by ADA | - | Abbott | |
| Spalding & Hay 2006 [14] | 422,215 | - | 422,114–573,650 | University of Southern California | |
| ADA | Chen et al. 2006 [28] | 152,021 (ADA+MTX) | - | 40,876—dominated (ADA+MTX) | NICE (UK) |
| Chen et al. 2006 [28] | 58,672 (ADA) | - | 36,983—dominated (ADA) | NICE (UK) | |
| Davies et al. 2009 [21] | 41,178 (ADA+MTX) | 20,413 | 31,435–61,124 | Abbott | |
| Davies et al. 2009 [21] | 37,309 (ADA+MTX ➔ ETN) | - | - | Abbott | |
| Spalding & Hay 2006 [14] | 200,620 (ADA+MTX) | - | 200,570 (ADA+MTX) | University of Southern California | |
| Spalding & Hay 2006 [14] | 65,745 (ADA) | - | 67,962 (ADA) | University of Southern California | |
| ETN | Spalding & Hay 2006 [14] | 92,503 | 81,408 | 80,027–108,051 | University of Southern California |
| Davies et al. 2009 [21] | Extended dominance by ADA | Extended dominance by ADA | - | Abbott | |
| Kobelt et al. 2011 [38] | 38,639 | 15,315 | 2,473–38,639 | Wyeth (now Pfizer) | |
| Chen et al. 2006 [28] | 332,850 (ETN+MTX) | - | 35,037—dominated (ETN+MTX) | NICE (UK) | |
| Chen et al. 2006 [28] | 96,157 (ETN) | - | 35,037–231,633 (ETN) | NICE (UK) | |
| Comparison of treatment strategies containing TNFi | |||||
| 1.)MTX➔MTX+SSZ➔ MTX+SSZ+HCQ➔ MTX+SSZ+HCQ+CS ➔IFX(MTX+CSA+ CS ➔ LEF➔AZA+CS 2.)IFX(SSZ➔LEF➔ MTX+CSA+CS➔GST+CS➔AZA+CS | Van den Hout et al. 2009 [19] | 2 vs.1: 215,256 | 2 vs.1: 147,280 | 24,924–362,537 | Dutch Health Care Insurance Board, Schering-Plough and Centocor (now Janssen Biologics B.V) |
| 1.)1.TNFi➔2.TNFi➔RTX 2.)MTX+LEF➔1.TNFi➔ 2.TNFi➔RTX 3.)MTX➔MTX+LEF ➔1.TNFi ➔2.TNFi➔RTX | Schipper et al. 2011 [30] | 2 vs.3: 462,576 | 2 vs.3: 461,476 | 2 vs.1: 456,946–791,788 | Wyeth (now Pfizer) |
| Schipper et al. 2011 [30] | 1 vs.3: 145,784 | 1 vs.3: 143,831 | 1 vs.3: 120,136–545,603 | Wyeth (now Pfizer) | |
| Schipper et al. 2011 [30] | 2 vs.1: 1 dominates | 2 vs.1: 1 dominates | - | Wyeth (now Pfizer) | |
| 1.)cDMARDs ➔ 1.TNFi ➔ 2.TNFi ➔ 3.TNFi 2.➔1.TNFi ➔ 2.TNFi ➔ 3.TNFi ➔ cDMARDs 3.)NSAID ➔ cDMARDs➔ 1.TNFi ➔2.TNFi➔3.TNFi | Finckh et al. 2009 [27] | 1 vs.3: 4,234 | 1 vs.3: 1 is cost-saving | 1 vs.3: 1 is cost saving—14,738 | Arthritis research foundation and an anonymous donor |
| Finckh et al. 2009 [27] | 2 vs.3: 635,597 | 2 vs.3: 471,575 | 2 vs.3: 30,624–3 dominates | Arthritis research foundation and an anonymous donor | |
| Finckh et al. 2009 [27] | 2 vs.1: 1 dominates | 2 vs.1: 1 dominates | 2 vs.1: 40,956–1 dominates. | Arthritis research foundation and an anonymous donor | |
➔ = switch to next treatment in case of an inadequate response, ADA = adalimumab, AZA = azathioprine, cDMARD = conventional disease-modifying antirheumatic drugs, CS = corticosteroids, CSA = cyclosporin A, ETN = etanercept, GST = Gold, HCQ = hydroxychloroquine, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, LEF = leflunomide, MTX = methotrexate, NICE = National Institute for Health and Care Excellence, NSAID = non-steroidal anti-inflammatory drug, QALY = quality-adjusted life year, SSZ = sulfasalazine, TNFi = TNF inhibitor
Three out of the seven studies examined the cost-effectiveness of different treatment strategies for early RA including TNFi in all treatment options, with only its time of usage in a treatment sequence being altered [19,27,30]. Two studies found a late introduction of TNFi to be a dominant strategy compared to initiation of the treatment with TNFi. Meanwhile van den Hout and colleagues found the ICER for TNFi as a first-line treatment option to be 215,000 €/QALY compared to its later introduction (Table 2).
Cost-effectiveness of biologics among patients with an inadequate response to cDMARD
There were 21 studies comparing the biologics and cDMARDs in patients with an insufficient response to cDMARDs (Table 3). When only direct costs were considered ICERs for IFX, ADA and ETN were 12,000–282,000; 44,000–274,000 and 40,000–708,000, respectively. ABT and TOC were associated with narrower ranges of ICERs (42,000 to 47,000 and 19,000 to 21,000, respectively). ICERs below 35,000 €/QALY were found in three studies [20,29,51] and below 50,000 €/QALY in ten studies [15,17,28,39,41,49,52]. The quality scores of the studies were not associated with the magnitude of ICER values. Adequate comparator was applied in nine of 21 CUAs [15,23,28,29,32,35,36,46,50]. These studies provided higher ICERs compared to other studies: only one CUAs with an adequate comparison treatment provided ICERs below 35,000 €/QALY for biologics when considering only direct costs [29].
Table 3. Cost-effectiveness of biologics in comparison with cDMARD among patients with an insufficient response to cDMARD.
| Biologic | Study | ICER €/QALY (only direct costs) | ICER €/QALY (direct and indirect costs) | Results of deterministic sensitivity analysis €/QALY | Source of research funding |
|---|---|---|---|---|---|
| IFX | Bansback et al. 2005 [41] | 69,717–93,665 | - | - | Abbott |
| Barbieri et al. 2005 [51] | 12,438–89,108 | - | 9,325–103,753 | Schering-Plough | |
| Barton et al. 2004 [50] | 166,921 | - | 96,287–213,008 | NICE (UK) | |
| CADTH 2010 [48] | Extended dominance by ADA | - | - | Health Canada and the governments of provinces and territories | |
| Chen et al. 2006 [28] | 59,173–270,563 (IFX➔cDMARDs) | - | 37,957—dominated (IFX➔cDMARDs) | NICE (UK) | |
| Chen et al. 2006 [28] | 73,772 (cDMARDs➔IFX) | - | 50,027–117,763 (cDMARDs➔IFX) | NICE (UK) | |
| Coyle et al. 2006 [46] | 98,132 (IFX➔GST) | - | 85,279–138,948 (IFX➔GST) | Health Canada and the governments of provinces and territories | |
| Coyle et al. 2006 [46] | 84,931 (GST➔IFX) | - | 71,298–101,084 (GST➔IFX) | Health Canada and the governments of provinces and territories | |
| Jobanputra et al. 2002 [32] | 282,151 (IFX➔cDMARDs) | - | 128,590–641,955 (IFX➔cDMARDs) | NICE (UK) | |
| Jobanputra et al. 2002 [32] | 230,698 (cDMARDs➔IFX) | - | 68,157–413,593 (cDMARDs➔IFX) | NICE (UK) | |
| Kobelt et al. 2003 [52] | 38,945–76,392 | 4,684–65,635 | IFX is cost saving—60,597 | Schering-Plough | |
| Lekander et al. 2010 [26] | - | 27,321 | 10,005–56,246 | Schering-Plough | |
| Marra et al. 2007 [44] | - | 30,267–66,008 | IFX dominates—139,343 | Canadian Arthritis Network | |
| Wu et al. 2012 [29] | 20,254 (IFX) | 20,150 (IFX) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| Wu et al. 2012 [29] | 21,946 (IFX➔RTX) | 21,833 (IFX➔RTX) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| Wong et al. 2002 [39] | 44,737 | 13,348 | IFX is cost saving—137,292 | Schering-Plough and National Institutes of Health | |
| ADA | Bansback et al. 2005 [41] | 49,284–63,493 (ADA+MTX) | - | - | Abbott |
| Bansback et al. 2005 [41] | 59,949–94,478 (ADA) | - | - | Abbott | |
| CADTH 2010 [48] | 92,326 | - | - | Health Canada and the governments of provinces and territories | |
| Chen et al. 2006 [28] | 58,784–125,354 (ADA+MTX➔ cDMARDs) | - | 37,178–291,974 (ADA+MTX➔ cDMARDs) | NICE (UK) | |
| Chen et al. 2006 [28] | 67,349–274,456 (ADA➔ cDMARDs) | - | 41,266- dominated (ADA➔ cDMARDs) | NICE (UK) | |
| Chen et al. 2006 [28] | 57,811 (cDMARDs➔ ADA+MTX) | - | 43,018–83,699 (cDMARDs➔ ADA+MTX) | NICE (UK) | |
| Chen et al. 2006 [28] | 78,054 (cDMARDs➔ADA) | - | 52,750–124,770 (cDMARDs➔ADA) | NICE (UK) | |
| Wu et al. 2012 [29] | 43,943 (ADA) | 43,876 (ADA) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| Wu et al. 2012 [29] | 38,689 (ADA➔ RTX) | 38,641 (ADA➔ RTX) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| ETN | Bansback et al. 2005 [41] | 51,581–74,972 (ETN+MTX) | - | - | Abbott |
| Bansback et al. 2005 [41] | 53,265–61,274 (ETN) | - | - | Abbott | |
| Barton et al. 2004 [50] | 122,754 | - | 73,350–157,370 | NICE (UK) | |
| Brennan et al. 2004 [49] | 39,740 | 18,950 | 18 950–103 145 | Not stated, two of authors are employees of Wyeth (now Pfizer) | |
| CADTH 2010 [48] | Dominated by ADA | - | - | Health Canada and the governments of provinces and territories | |
| Chen et al. 2006 [28] | 55,475–96,935 (ETN+MTX➔ cDMARDs) | - | 34,648–187,058 (ETN+MTX➔ cDMARDs) | NICE (UK) | |
| Chen et al. 2006 [28] | 59,173–92,264 (ETN➔cDMARDs) | - | 36,399–185,695 (ETN➔ cDMARDs) | NICE (UK) | |
| Chen et al. 2006 [28] | 46,327 (cDMARDs➔ ETN+MTX) | - | 35,037–66,181 (cDMARDs➔ ETN+MTX) | NICE (UK) | |
| Chen et al. 2006 [28] | 46,132 (cDMARDs➔ ETN) | - | 35,232–65,013 (cDMARDs➔ ETN) | NICE (UK) | |
| Coyle et al. 2006 [46] | 125,661 (ETN➔ GST) | - | 109,335–173,251 (ETN➔ GST) | Health Canada and the governments of provinces and territories | |
| Coyle et al. 2006 [46] | 109,161 (GST➔ETN) | - | 94 919–129,916 (GST(ETN) | Health Canada and the governments of provinces and territories | |
| Jobanputra et al. 2002 [32] | 202,218 (ETN➔ cDMARDs) | - | 93,643–448,885 (ETN➔ cDMARDs) | NICE (UK) | |
| Jobanputra et al. 2002 [32] | 174,388 (cDMARDs➔ ETN) | - | 51,662–312,186 (cDMARDs➔ ETN) | NICE (UK) | |
| Kobelt et al. 2005 [36] | 69,550 (ETN+MTX) | 49,314–72,058 (ETN+MTX) | 33,704–69,550 | Wyeth (now Pfizer) | |
| Kobelt et al. 2005 [36] | - | Dominated by ETN+MTX (ETN) | - | Wyeth (now Pfizer) | |
| Lekander et al. 2013 [25] | - | 52,671 (ETN+cDMARD) | 33,922–78,770 (ETN+cDMARD) | Wyeth (now Pfizer) | |
| Lekander et al. 2013 [25] | - | 68,535 (ETN) | 40,818–127,988 (ETN) | Wyeth (now Pfizer) | |
| Soini et al. 2012 [20] | 22,745 | - | 9,437–57,025 | Roche | |
| Tanno et al. 2006 [35] | - | 25,993 | 19,547–32,439 | Ministry of Education, Science, Sports and Culture and the Ministry of Health, Japan | |
| Welsing et al. 2004 [23] | 233,867 (LEF➔ ETN➔ Usual care vs. Usual care) | 216,059 (LEF➔ ETN➔ Usual care vs. Usual care) | - | Not stated | |
| Welsing et al. 2004 [23] | 413,169 (ETN➔ LEF➔ Usual care vs. Usual care) | 392,539 (ETN➔ LEF➔Usual care vs. Usual care) | - | Not stated | |
| Welsing et al. 2004 [23] | 440,322 (LEF➔ ETN(Usual care vs. LEF➔ Usual care) | 419,588 (LEF➔ ETN➔ Usual care vs. LEF➔ Usual care) | - | Not stated | |
| Welsing et al. 2004 [23] | 708,060 (ETN➔ LEF➔ Usual care vs. LEF➔ Usual care) | 683,041 (ETN➔ LEF➔ Usual care vs. LEF➔ Usual care) | - | Not stated | |
| Wu et al. 2012 [29] | 58,711 (ETN) | 58,684 (ETN) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| Wu et al. 2012 [29] | 50,409 (ETN➔ RTX) | 50,389 (ETN➔ RTX) | - | Shanghai Hospital Association, National Natural Science Foundation of China and Shanghai Natural Science Foundation | |
| ABT | CADTH 2010 [48] | Extended dominance by ADA | - | - | Health Canada and the governments of provinces and territories |
| Vera-Llonch et al. 2008a [17] | 42,382–47,177 | - | 36,976–69,134 | Bristol-Myers Squibb | |
| GOL | CADTH 2010 [48] | Extended dominance by ADA | - | - | Health Canada and the governments of provinces and territories |
| TOC | Soini et al. 2012 [20] | 18,693–20,776 | 18,731–20,813 | 7,629–53,17 | Roche |
| TNFi | Brennan et al. 2007 [15] | 46,486 (TNFi as a group) | - | 24,378–93,833 | The British Society for Rheumatology (BSR) |
| Kobelt et al. 2004 [16] | 62,419 | 61,016 | 51,759–180,244 | Österlund and Kock Foundations, The King Gustav V 80 year fund and The Reumatikerförbundet | |
| Lekander et al. 2013 [25] | 75,799 (TNFi+cDMARD) | 57,092 (TNFi+cDMARD) | 34,472–88,294 (TNFi+cDMARD) | Wyeth (now Pfizer) | |
| Lekander et al. 2013 [25] | 106,062 (TNFi) | 88,146 (TNFi) | 50 315–169 383 (TNFi) | Wyeth (now Pfizer) |
➔ = switch to next treatment in case of an inadequate response, ABT = abatacept, ADA = adalimumab, CADTH = Canadian Agency for Drugs and Technologies in Health, cDMARD = conventional disease-modifying antirheumatic drugs, CER = certolizumab pegol, ETN = etanercept, GOL = golimumab, GST = Gold, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, LEF = leflunomide, MTX = methotrexate, NICE = National Institute for Health and Care Excellence, QALY = quality-adjusted life year, SSZ = sulfasalazine, TNFi = TNF inhibitor, TOC = tocilizumab
Six studies performed comparisons between different biologics used in patients with an inadequate response to cDMARDs [20,24,28,32,33,50]. The results of these studies were contradictory. Two studies [20,24] found ETN to be dominant over IFX and ADA, while three of the other studies[28,32,50] reported ICERs ranging from 23,000 to 109,000 €/QALY for ETN when only direct costs were included (Table 4). Two studies comparing TOC and ETN found TOC to be the dominant strategy. None of these CUAs included indirect costs.
Table 4. Comparison of biologics in patients with an insufficient response to cDMARD.
| Biologic | Comparator | Study | ICER €/QALY (only direct costs) | Results of deterministic sensitivity analysis €/QALY | Source of research funding |
|---|---|---|---|---|---|
| IFX | ETN | Nguyen et al. 2012 [24] | ETN dominates | - | One of the authors was funded by UCB Pharma |
| CER | Nguyen et al. 2012 [24] | CER dominates | - | One of the authors was funded by UCB Pharma | |
| ADA | GOL | Nguyen et al. 2012 [24] | ADA dominates | - | One of the authors was funded by UCB Pharma |
| ETN | Nguyen et al. 2012 [24] | ETN dominates | - | One of the authors was funded by UCB Pharma | |
| IFX | Chen et al. 2006 [28] | 4,983—IFX is cost saving (ADA➔ cDMARDs) | - | NICE (UK) | |
| IFX | Chen et al. 2006 [28] | ADA dominates (cDMARDs➔ ADA) | - | NICE (UK) | |
| ETN | IFX | Barton et al. 2004 [50] | 68,373 | 42,760–88,266 | NICE (UK) |
| IFX | Jobanputra et al. 2002 [32] | 109,297 (ETN➔ cDMARDs) | 51,908–231,484 (ETN➔ cDMARDs) | NICE (UK) | |
| IFX | Jobanputra et al. 2002 [32] | 101,714 (cDMARDs➔ETN) | 30,597–180,270 (cDMARDs➔ ETN) | NICE (UK) | |
| IFX | Chen et al. 2006 [28] | 38,541–47,884 (ETN➔ cDMARDs) | - | NICE (UK) | |
| IFX | Chen et al. 2006 [28] | 23,553 (cDMARDs➔ ETN) | - | NICE (UK) | |
| ADA | Soini et al. 2012 [20] | ETN dominates | - | Roche | |
| ADA | Chen et al. 2006 [28] | 35,621–61,315 (ETN➔ cDMARDs) | - | NICE (UK) | |
| ADA | Chen et al. 2006 [28] | 22,579–30,755 (cDMARDs➔ ETN) | - | NICE (UK) | |
| GOL | ETN | Nguyen et al. 2012 [24] | ETN dominates | - | One of the authors was funded by UCB Pharma |
| CER | Nguyen et al. 2012 [24] | CER dominates | - | One of the authors was funded by UCB Pharma | |
| CER | ETN | Nguyen et al. 2012 [24] | 1 756,213 | - | One of the authors was funded by UCB Pharma |
| TOC | ETN | Diamantopoulos et al. 2012 [33] | TOC dominates | TOC dominates—19,187 | Roche |
| ETN | Soini et al. 2012 [20] | TOC dominates—6,673 | - | Roche |
➔ = switch to next treatment in case of an inadequate response, ADA = adalimumab, CER = certolizumab pegol, ETN = etanercept, GOL = golimumab, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, NICE = National Institute for Health and Care Excellence, QALY = quality-adjusted life year, TOC = tocilizumab
Cost-effectiveness of biologics among patients with an inadequate response to at least one TNF inhibitor
Eight CUAs compared biologics and cDMARDs in patients who had had an insufficient response to at least one TNFi [22,25,31,34,42,43,53,54]. RTX was associated with the lowest ICERs ranging from 26,000 to 48,000 €/QALY (Table 5). Three of four studies evaluating RTX provided ICERs below 35,000 €/QALY and none of the studies reported ICERs more than 50,000 €/QALY. ANA was associated with the highest ICERs with a range of 234,000–1 347,000 €/QALY. ICERs for the other agents ranged from 41,000 to 143,000 €/QALY. Inadequate comparator (MTX) was applied in three studies [34,42,43], and one study [25] did not specify the comparator cDMARDs. However, the ICERs of these studies did not differ from those of the other studies. Results of the four studies comparing one biologic to another [18,31,34,53] indicated RTX as the most cost-effective biologic among patients with an insufficient response to a TNFi (Table 6).
Table 5. Cost-effectiveness of biologics in comparison with cDMARD among patients with an insufficient response to at least one TNF inhibitor.
| Biologic | Study | ICER €/QALY (only direct costs) | ICER €/QALY (direct and indirect costs) | Results of deterministic sensitivity analysis €/QALY | Source of research funding |
|---|---|---|---|---|---|
| RTX | Yuan et al. 2010 [42] | 47,931 | - | 57,370–96,012 | BMS |
| Kielhorn et al. 2008 [31] | 28,594 | - | 9,758–67,321 | Roche | |
| Brodszky et al. 2010 [34] | 26,304–46,389 | 31,382–37,266 | - | Center for Public Affairs Studies Foundation and Roche | |
| Hallinen et al. 2010 [54] | 34,269 | - | 24,929–52,929 | Roche | |
| Malottki et al. 2011 [53] | 30,021 | - | 16,220–65,448 | NICE (UK) | |
| IFX | Hallinen et al. 2010 [54] | 40,923 | - | 36,174–48,483 | Roche |
| Malottki et al. 2011 [53] | 51,362 | - | 40,976–98,029 | NICE (UK) | |
| ADA | Hallinen et al. 2010 [54] | 57,713 | - | 48,963–68,930 | Roche |
| Malottki et al. 2011 [53] | 48,801 | - | 39,980–87,216 | NICE (UK) | |
| ETN | Hallinen et al. 2010 [54] | 57,068 | - | 48,294–68,285 | Roche |
| Malottki et al. 2011 [53] | 55,346 | - | 44,248–108,558 | NICE (UK) | |
| Lekander et al. 2013 [25] | - | 74,743 (ETN+cDMARD) | 47,164–113,453 (ETN+DMARD) | Wyeth (now Pfizer) | |
| Lekander et al. 2013 [25] | - | 88,861 (ETN) | 53,769–175,126 (ETN) | Wyeth (now Pfizer) | |
| ABT | Hallinen et al. 2010 [54] | 75,910 | - | 65,232–90,234 | Roche |
| Malottki et al. 2011 [53] | 54,635 | - | 45,671–90,062 | NICE (UK) | |
| Vera-Llonch et al. 2008b [43] | 45,275–49,802 | - | 40,211–79,438 | Not stated, One of authors was an employee of BMS | |
| Yuan et al. 2010 [42] | 41,207 | - | 49,912–81,509 | BMS | |
| ANA | Clark et al. 2004 [22] | 620,109–1 347,287 (ANA➔cDMARDs) | - | 100,378–671,413 | NICE (UK) |
| Clark et al. 2004 [22] | 234,214–292,210 (cDMARDs➔ANA) | - | 82,533–216,370 | NICE (UK) | |
| TNFi | Lekander et al. 2013 [25] | 101,618 (TNFi+cDMARD) | 84,363 (TNFi+cDMARD) | 50,316–134,016 (TNFi+cDMARD) | Wyeth (now Pfizer) |
| Lekander et al. 2013 [25] | 143,745 (TNFi) | 126,813 (TNFi) | 71,022–328,903 (TNFi) | Wyeth (now Pfizer) |
➔ = switch to next treatment in case of an inadequate response, ABT = abatacept, ADA = adalimumab, ANA = Anakinra, BMS = Bristol-Myers Squibb, cDMARD = conventional disease-modifying antirheumatic drugs, ETN = etanercept, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, NICE = National Institute for Health and Care Excellence, QALY = quality-adjusted life year, RTX = rituximab, TNFi = TNF inhibitor
Table 6. Comparison of biologics among patients with an insufficient response to at least one TNF inhibitor.
| Biologic | Comparator | Study | ICER €/QALY (only direct costs) | ICER €/QALY (direct and indirect costs) | Results of deterministic sensitivity analysis €/QALY | Source of research funding |
|---|---|---|---|---|---|---|
| RTX | Another TNFi | Brodszky et al. 2010 [34] | RTX dominates | RTX dominates | - | Center for Public Affairs Studies Foundation and Roche |
| 2.TNFi➔ 3.TNFi | Lindgren et al. 2009 [45] | RTX dominant | RTX dominant | RTX dominates—41,044 | Roche | |
| ADA ➔ IFX ➔ cDMARDs | Merkesdal et al. 2010 [18] | 27,776 | 17,634 | 8,050–54,441 | Roche | |
| ADA ➔ IFX ➔ cDMARDs | Kielhorn et al. 2008 [31] | 22,581 | - | - | Roche | |
| IFX | RTX | Malottki et al. 2011 [53] | RTX dominates | - | 5,833—RTX dominates | NICE (UK) |
| ADA | RTX | Malottki et al. 2011 [53] | RTX dominates | - | 612—RTX dominates | NICE (UK) |
| ETN | Malottki et al. 2011 [53] | ADA dominates | - | ADA dominates-103,578 | NICE (UK) | |
| IFX | Malottki et al. 2011 [53] | ADA dominates | - | 27,033–40,834 | NICE (UK) | |
| ETN | RTX | Malottki et al. 2011 [53] | RTX dominates | - | RTX dominates | NICE (UK) |
| IFX | Malottki et al. 2011 [53] | 649,782 | - | 55,915—IFX dominates | NICE (UK) | |
| ABT | RTX | Malottki et al. 2011 [53] | 185,815 | - | 73,273–1 225,153 | NICE (UK) |
| ADA | Malottki et al. 2011 [53] | 66,017 | - | 57,053–119,656 | NICE (UK) | |
| ETN | Malottki et al. 2011 [53] | 53,781 | - | 47,663–71,992 | NICE (UK) | |
| IFX | Malottki et al. 2011 [53] | 59,329 | - | 52,500–81,952 | NICE (UK) |
➔ = switch to next treatment in case of an inadequate response, ABT = abatacept, ADA = adalimumab, ETN = etanercept, ICER = Incremental cost-effectiveness ratio, IFX = infliximab, NICE = National Institute for Health and Care Excellence, QALY = quality-adjusted life year, RTX = rituximab, TNFi = TNF inhibitor
Other studies
Three studies did not specify patients’ previous treatments, and therefore were not included in the subgroups described above [37,40,47]. Farahani et al. estimated ICER for ETN in comparison to cDMARDs to be 71,000 €/QALY while applying the efficacy estimates based on a RCT and 150,000 €/QALY when effectiveness estimates from an observational study were used [37]. Chiou et al. and Wailoo et al. performed comparisons of different biologics [40,47]. Both studies reported ETN to be dominant over IFX. Chiou et al. also found ETN to dominate ADA while Wailoo et al. estimated ICER of 95,000 €/QALY for ETN in comparison to ADA.
Quality of the included studies
The average quality scores of the 41 studies included in the present review were 25.7 out of 35 (range 17 to 31) and 32.3 out of 57 (range 16 to 46) when evaluated using BMJ checklist and Philips’ list, respectively (Table 7). The corresponding average percentages of the applicable items fulfilled were 81 (range 57 to 100) and 62 (range 31 to 90) for BMJ check list and Philips’ list, respectively. The most frequent quality issues were the incomplete reporting of the data sources, inappropriate comparator treatments, defects in the sensitivity analysis and the lack of quality assessment of data used.
Table 7. Results of quality assessment.
| Study | BMJ quality scores, max = 35 (items applicable in each study) | Applicable items % | Philip’s quality scores, max = 57 (items applicable in each study) | Applicable items % | Quality category |
|---|---|---|---|---|---|
| Bansback et al. 2005, Sweden [41] | 23 (31) | 74 | 38 (52) | 73 | Adequate |
| Barbieri et al. 2005, UK [51] | 25 (31) | 81 | 23 (53) | 43 | Poor |
| Barton et al. 2004, UK [50] | 29 (31) | 94 | 40 (49) | 82 | Good |
| Brennan et al. 2004, UK [49] | 29 (33) | 88 | 30 (54) | 56 | Adequate |
| Brennan et al. 2007, UK [15] | 26 (32) | 81 | 37 (49) | 76 | Good |
| Brodszky et al. 2010, Hungary [34] | 19 (30) | 63 | 16 (52) | 31 | Poor |
| CADTH 2010, Canada [48] | 17 (30) | 57 | 18 (53) | 34 | Poor |
| Chen et al. 2006, UK [28] | 31 (31) | 100 | 46 (51) | 90 | Good |
| Chiou et al. 2004, [47] | 23 (31) | 74 | 20 (53) | 38 | Poor |
| Clark et al. 2004, UK [22] | 30 (31) | 97 | 40 (50) | 80 | Good |
| Coyle et al. 2006, Canada [46] | 29 (31) | 94 | 28 (52) | 54 | Adequate |
| Davies et al. 2009 USA [21] | 29 (32) | 91 | 38 (55) | 69 | Good |
| Diamantopoulos et al. 2012, Italy [33] | 25 (32) | 78 | 32 (55) | 58 | Adequate |
| Farahani et al. 2006, Canada [37] | 19 (27) | 70 | No modelling used | - | Poor |
| Finckh et al. 2009, USA [27] | 28 (32) | 88 | 36 (54) | 67 | Good |
| Hallinen et al. 2010, Finland [54] | 29 (31) | 94 | 28 (52) | 54 | Adequate |
| Jobanputra et al. 2002, UK [32] | 28 (31) | 90 | 34 (49) | 69 | Good |
| Kielhorn et al. 2008, UK [31] | 25 (31) | 81 | 37 (53) | 70 | Adequate |
| Kobelt et al. 2003, UK & Sweden [52] | 23 (32) | 72 | 22 (49) | 45 | Poor |
| Kobelt et al. 2004, Sweden [16] | 25 (30) | 83 | No modelling used | - | Adequate |
| Kobelt et al. 2005, Sweden [36] | 22 (33) | 67 | 28 (53) | 53 | Poor |
| Kobelt et al. 2011, Sweden [38] | 26 (33) | 79 | 28 (54) | 52 | Poor |
| Lekander et al. 2010, Sweden [26] | 23 (33) | 70 | 31 (51) | 61 | Poor |
| Lekander et al. 2013, Sweden [25] | 24 (33) | 73 | 37 (53) | 70 | Adequate |
| Lindgren et al. 2009, Sweden [45] | 22 (33) | 67 | 34 (48) | 71 | Adequate |
| Malottki et al. 2011, UK [53] | 29 (31) | 94 | 46 (52) | 88 | Good |
| Marra et al. 2007, Canada [44] | 27 (33) | 82 | 31 (54) | 57 | Adequate |
| Merkesdal et al. 2010, Germany [18] | 27 (32) | 84 | 34 (52) | 65 | Adequate |
| Nguyen et al. 2012, USA [24] | 25 (31) | 81 | 28 (55) | 51 | Poor |
| Schipper et al. 2011, the Netherlands [30] | 25 (33) | 76 | 34 (52) | 65 | Adequate |
| Soini et al. 2012, Finland [20] | 31 (33) | 94 | 42 (54) | 78 | Good |
| Spalding & Hay 2006, USA [14] | 23 (32) | 72 | 30 (52) | 58 | Poor |
| Tanno et al. 2006, Japan [35] | 29 (32) | 91 | 23 (51) | 45 | Adequate |
| Wailoo et al. 2008, USA [40] | 25 (31) | 81 | 36 (55) | 65 | Adequate |
| van den Hout et al. 2009, the Netherlands [19] | 29 (31) | 94 | No modelling used | - | Good |
| Welsing et al. 2004, the Netherlands [23] | 22 (32) | 69 | 27 (55) | 49 | Poor |
| Vera-Llonch et al. 2008a, USA [17] | 26 (32) | 81 | 37 (50) | 74 | Good |
| Vera-Llonch et al. 2008b, USA [43] | 27 (32) | 84 | 37 (50) | 74 | Good |
| Wong et al. 2002 [39] | 23 (33) | 70 | 24 (51) | 47 | Poor |
| Wu et al. 2012, China [29] | 30 (32) | 94 | 41 (54) | 76 | Good |
| Yuan et al. 2010, USA [42] | 23(32) | 72 | 36 (53) | 68 | Adequate |
Discussion
We performed a systematic literature review of cost-effectiveness of biologics used for the treatment of RA. After the literature search and the selection process of the initially identified reports, 41 original articles were included in the current review. While considering only direct costs, the ICERs of the TNFis ranged from 39,000 to 1 273,000 €/ QALY in comparison to cDMARD in patients naïve to cDMARDs. Among patients with an inadequate response to cDMARDs, biologics were associated with ICERs ranging from 12,000 to 708,000 €/QALY. In this setting, none of the biologics appeared to be more cost-effective than any of the others. ICERs for the second line biologics ranged from 26,000 to 1 347,000 €/QALY in comparison to cDMARDs among patients with an inadequate response to TNFi. In this patient subgroup RTX was the most and ANA the least cost-effective biologic. The quality assessment revealed several problems, namely insufficient reporting of data sources and problematic methodological details, which possibly reduce the validity of the results.
When assessing whether biologics are cost-effective or not, it should be known what the willingness to pay for an additional QALY is. There is no widely accepted WTP threshold value for ICER although the National Institute for Health and Care Excellence (NICE) has published a threshold of 20,000–30,000 £/QALY (~24,000–35,000 €/QALY) in United Kingdom [55]. Based on this statement by NICE we used the WTP threshold of 35,000 €/QALY. With this threshold biologics are not cost-effective in cDMARD naïve patients. However, also much higher WTP thresholds have been proposed and applied in the literature, but even with the 100,000 €/QALY threshold biologics do not seem to be cost-effective in this patient subgroup. Slightly more preferable ICERs for ADA and ETN monotherapies do not count either: TNFi monotherapy has later been found less effective than its combination with MTX and therefore, biologics as monotherapies are not currently recommended [3,5]. In patients who have an insufficient response to cDMARDs, biologics are not cost-effective with the 35,000 €/QALY threshold, and with the higher thresholds of 50,000–100,000 €/QALY the evidence of their cost-effective is conflicting. It should be noted that ADA, ETN and IFX, which have been for the longest time on the market, have been assessed in several studies and are consequently associated with a wide range of different ICERs. Meanwhile the narrower ranges of ICER values for ABT and TOC probably reflect the lower number of studies rather than more consistent performance of these agents. Health technology assessment reports provided by independent organisations such as NICE tend to provide higher ICERs than CUAs funded by pharmaceutical companies, due to different premises of the studies. Such publicly funded and in this respect independent reports are not yet available for the newer agents such as TOC, which also may at least in part explain more favourable ICERs. Among the patients with an inadequate response to one TNFi, RTX appears cost-effective with the threshold of 35,000 €/QALY. With the higher thresholds also other TNFis and ABT might be cost effective. These findings are consistent with previous systematic reviews on the current topic [8–10].
We performed this review following current recommendations for systematic literature review of economic evaluations [11]. Standardized methodology is a certain guarantee for the quality and reliability of the current work. Source studies were restricted to CUAs, instead of all CEAs, because QALY as a single measure of the effectiveness enables more accurate comparison of the results. A further aim was to enhance the comparability of the studies by classifying them by previous treatments and comparator treatments. Such a classification seems almost to be necessary because the patient history is a key factor while assessing the external validity and trying to generalize the results and because the comparator treatment has a great impact on ICERs.
The importance of adequate comparator has been previously raised by Tsao and colleagues in their systematic review examining the cost-effectiveness of biologics in comparison to cDMARDs [9]. MTX was the most frequent comparator in the studies included in the current systematic review. MTX is the drug of choice in cDMARD naïve patient population [3]. On the other hand, in patients with MTX monotherapy treatment failure this drug does not represent an adequate treatment option. Instead patients should be treated with other cDMARDs or a combination of cDMARDs they have not received before. In the current study ICERs were assessed using comparator treatments and it seems that CUAs applying adequate comparators may provide rather high ICERs. However, in spite of the general acceptance of MTX as an anchor drug in RA, there is a lack of consensus on the optimal cDMARDs sequence, which poses a problem for CUAs.
It should be noticed that in spite of stratification of patients to subgroups, methodological differences make a comparison of different CUAs difficult. Heterogeneity in time horizons, discount rates, and perspectives were observed, all possibly inducing differences between the studies. For example, it is likely that a CUA with a longer time horizon produces more favourable ICERs compared to ones with shorter time horizons [17,36,43]. While biologics are expensive, they might induce future savings through decreased productivity losses and the lesser need for surgery and inpatient care. A discount rate depreciates the future costs and benefits of the treatment consequently reducing their impact on ICER.
Analyses counting only direct costs give an incomplete view of the pros and cons of different treatments, while various methods used to estimate indirect costs remain controversial. In the current study ICERs based only on direct costs and ICERs based on the inclusion of both direct and indirect costs are provided if they were reported in the original source publication. It is likely that biologics decrease productivity costs because they improve the health status of the patients [5,6]. However, the age and employment status of treated population and the overall labour costs have a major impact on indirect costs, introducing heterogeneity in the ICERs. For example, in China where labour costs are low, Wu et al. reported only small differences between ICERs including direct or both direct and indirect costs, while in Sweden much larger differences were observed [25,29,36,38,52]. The method used for the evaluation of productivity costs generate further variation in ICERs when also indirect costs are considered: Van den Hout et al. reported ICERs of 147,000€/QALY and 25,000€/QALY for early IFX treatment using friction cost and human capital methods, respectively [19]. For these reasons it is more transparent not to use ICERs with indirect costs when results of different studies are to be compared. Accordingly, conclusions in the current review are based on ICERs including only direct costs. Health service and other costs are always also related to national economy, health policy and price level and thus ICERs cannot directly be generalized when analysing results from different countries.
Different methodologies used for the QALY measures have effect on ICERs. In most studies, the utility scores of the multiattribute utility (MAU) instruments (e.g. EQ-5D) were derived from the Health assessment questionnaire (HAQ) or some other disease specific measures. This is necessary due to the fact that the MAU instruments have been applied in few RCTs, while disease specific measures such as HAQ have been commonly used in RCTs. Application of different formulas for conversions introduce a further source of heterogeneity in ICERs estimates [44]. Different MAU instruments without any conversions produce different utility scores and hence, different ICERs [19]. Standardization of MAU instruments and a validated standard conversion method for missing utility measures would enable better comparison between different CUAs.
In most studies the effectiveness estimates were based on one or several RCTs, representing rather estimates for efficacy. While RCTs are the key source for the efficacy evidence in medicines, they have some weaknesses if applied as source of effectiveness estimates in economic evaluations. Firstly, the results of RCTs are generally better than in the clinical practice because patients are carefully selected and adherence is usually better to RCTs than to regular clinical practices. Consequently, ICERs based on efficacy estimates from RCTs tend to be much lower than those based on observational data as shown by Farahani et al. [37]. Secondly, an objective of RCTs is usually to explore an efficacy of a single treatment in comparisons to placebo (or MTX in case of several RCTs studying biologics for RA), rather than compare complex treatment strategies. In contrast, CUAs aim to compare active treatments reflecting real life practices, and therefore indirect comparisons of RCTs are often necessary. However, some CUAs which used effectiveness estimates obtained from several RCTs reported indirect comparisons inadequately. This, restricted clinical evidence and therefore somewhat inconsistent results from CUAs explain that the ranking of biologics remains unclear among patients having inadequate response for cDMARDs [6]. To advance CUAs even further, indirect comparisons could in the future be performed and reported according to current guidelines [56].
The quality of economic evaluations was assessed using two different checklists, and was found to be suboptimal. The quality scores according the BMJ checklist were rather high while Philips’ checklist provided less favorable estimates of the study qualities. The reason for this discrepancy is probably the extensiveness of the Philips’ checklist, which covers several topics not considered in the BMJ checklist. An interesting finding was that quality scores of the studies were not associated with the magnitude of ICER. This is perhaps based on the nature of checklists: a single and simple modeling assumption may have a great impact on ICERs even if its effect on quality scores remains minor. In addition to the quality assessment of the individual studies, we assessed the bias across the CUAs. Only a few of the older conference abstracts identified through the literature search have been published later as a full article, indicating a reporting bias. However, conference abstracts were not included in the current systematic literature review due to incomplete information and problems with quality assessment that may bias their results. The risk of a language bias seems minor based on the small number of non-English papers excluded.
Conclusions
With the WTP threshold of 35,000 €/QALY, biologics do not seem to be cost-effective among cDMARD naïve patients or cDMARD resistant patients. Among patients with an inadequate response to TNFi(s), RTX seems to be cost-effective. With thresholds of 50,000–100,000 €/QALY biologics might be cost-effective among cDMARD resistant patients.
Supporting Information
(DOCX)
(DOCX)
Duplicate references (n = 7) are excluded from a list.
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JTJ has received a 22,000€ grant from the Research Foundation of the University of Helsinki (http://www.helsinki.fi/tiedesaatio/grants/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36: 182–188. [DOI] [PubMed] [Google Scholar]
- 2. Lundkvist J, Kastäng F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ. 2008;8: S49–60. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73: 492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordström DC, Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One. 2012;7: e30275 10.1371/journal.pone.0030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM a, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69: 976–986. 10.1136/ard.2009.126573 [DOI] [PubMed] [Google Scholar]
- 6. Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73: 516–528. 10.1136/annrheumdis-2013-204577 [DOI] [PubMed] [Google Scholar]
- 7. Drummond M, Sculpher M, Torrance G, Bernie O, Stoddart G. Methods for Economic Evaluation of Health Care Programmes. 3rd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 8. Schoels M, Wong J, Scott DL, Zink A, Richards P, Landewé R, et al. Economic aspects of treatment options in rheumatoid arthritis: A systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69: 995–1003. 10.1136/ard.2009.126714 [DOI] [PubMed] [Google Scholar]
- 9. Tsao NW, Bansback NJ, Shojania K, Marra C a. The issue of comparators in economic evaluations of biologic response modifiers in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2012;26: 659–676. 10.1016/j.berh.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 10. Van der Velde G, Pham B, Machado M, Ieraci L, Witteman W, Bombardier C, et al. Cost-effectiveness of biologic response modifiers compared to disease-modifying antirheumatic drugs for rheumatoid arthritis: a systematic review. Arthritis Care Res(Hoboken). 2011;63: 65–78. 10.1002/acr.20338 [DOI] [PubMed] [Google Scholar]
- 11. Shemilt I, Mugford M, Byford S, Drummond M, Eisenstein E, Knapp M, et al. Incorporating economics evidence In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration; 2008. pp. 449–479. 10.1002/14651858.CD004741.pub2 [DOI] [Google Scholar]
- 12. Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Philips Z, Ginnelly L, Sculpher M, Claxton K. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8: 1–172. [DOI] [PubMed] [Google Scholar]
- 14. Spalding JR, Hay J. Cost effectiveness of tumour necrosis factor-α inhibitors as first-line agents in rheumatoid arthritis. Pharmacoeconomics. 2006;24: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 15. Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost effectiveness of TNF-α antagonists in the management of rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Registry. Rheumatology(Oxford). 2007;46: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 16. Kobelt G, Eberhardt K, Geborek P. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: Costs and outcomes in a follow up study of patients with Ra treated with etanercept or infliximab in southern Sweden. Ann Rheum Dis. 2004;63: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to methotrexate. Rheumatology (Oxford). 2008;47: 535–541. 10.1093/rheumatology/ken007 [DOI] [PubMed] [Google Scholar]
- 18. Merkesdal S, Kirchhoff T, Wolka D, Ladinek G, Kielhorn A, Rubbert-Roth A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur J Health Econ. 2010;11: 95–104. 10.1007/s10198-009-0205-y [DOI] [PubMed] [Google Scholar]
- 19. Van Den Hout WB, Goekoop-Ruiterman YPM, Allaart CF, Vries-Bouwstra JKD, Hazes JMM, Kerstens PJSM, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Care Res(Hoboken). 2009;61: 291–299. [DOI] [PubMed] [Google Scholar]
- 20. Soini EJ, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15: 340–51. 10.3111/13696998.2011.649327 [DOI] [PubMed] [Google Scholar]
- 21. Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol. 2009;36: 16–25. 10.3899/jrheum.080257 [DOI] [PubMed] [Google Scholar]
- 22. Clark W, Jobanputra P, Barton P, Burls A. The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis. Health Technol Assess. 2004;8: 1–117. [DOI] [PubMed] [Google Scholar]
- 23. Welsing PMJ, Severens JL, Hartman M, van Riel PLCM, Laan RFJM. Modeling the 5-year cost effectiveness of treatment strategies including tumor necrosis factor-blocking agents and leflunomide for treating rheumatoid arthritis in the Netherlands. Arthritis Care Res(Hoboken). 2004;51: 964–973. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen CM, Bounthavong M, Mendes MAS, Christopher MLD, Tran JN, Kazerooni R, et al. Cost Utility of Tumour Necrosis Factor- a Inhibitors for Rheumatoid Arthritis. Pharmacoeconomics. 2012;30: 575–593. 10.2165/11594990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 25. Lekander I, Borgström F, Lysholm J, van Vollenhoven RF, Lindblad S, Geborek P, et al. The cost-effectiveness of TNF-inhibitors for the treatment of rheumatoid arthritis in Swedish clinical practice. Eur J Health Econ. 2013;14: 863–873. 10.1007/s10198-012-0431-6 [DOI] [PubMed] [Google Scholar]
- 26. Lekander I, Borgstrm F, Svarvar P, Ljung T, Carli C, Van Vollenhoven RF. Cost-effectiveness of real-world infliximab use in patients with rheumatoid arthritis in Sweden. Int J Technol Assess Health Care. 2010;26: 54–61. 10.1017/S0266462309990596 [DOI] [PubMed] [Google Scholar]
- 27. Finckh A, Bansback N, Marra CA, Anis AH, Michaud K, Lubin S, et al. Treatment of Very Early Rheumatoid Arthritis With Symptomatic Therapy, Disease-Modifying Antirheumatic Drugs, or Biologic Agents: A Cost-Effectiveness Analysis. Ann Intern Med. 2009;151: 612–621. 10.7326/0003-4819-151-9-200911030-00006 [DOI] [PubMed] [Google Scholar]
- 28. Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10: 1–248. [DOI] [PubMed] [Google Scholar]
- 29. Wu B, Wilson A, Wang F-f., Wang S-l., Wallace DJ, Weisman MH, et al. Cost Effectiveness of Different Treatment Strategies in the Treatment of Patients with Moderate to Severe Rheumatoid Arthritis in China. PLoS One. 2012;7: e47373 10.1371/journal.pone.0047373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schipper LG, Kievit W, den Broeder AA, van der Laar MA, Adang EMM, Fransen J, et al. Treatment strategies aiming at remission in early rheumatoid arthritis patients: Starting with methotrexate monotherapy is cost-effective. Rheumatology (Oxford). 2011;50: 1320–1330. 10.1093/rheumatology/ker084 [DOI] [PubMed] [Google Scholar]
- 31. Kielhorn A, Porter D, Diamantopoulos A, Lewis G. UK cost-utility analysis of rituximab in patients with rheumatoid arthritis that failed to respond adequately to a biologic disease-modifying antirheumatic drug. Curr Med Res Opin. 2008;24: 2639–50. 10.1185/03007990802321683 [DOI] [PubMed] [Google Scholar]
- 32. Jobanputra P, Barton P, Bryan S, Burls A. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: A systematic review and economic evaluation. Health Technol Assess. 2002;6: 1–110. [DOI] [PubMed] [Google Scholar]
- 33. Diamantopoulos A, Benucci M, Capri S, Berger W, Wintfeld N, Giuliani G, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ. 2012;15: 576–585. 10.3111/13696998.2012.665110 [DOI] [PubMed] [Google Scholar]
- 34. Brodszky V, Orlewska E, Péntek M, Kárpáti K, Skoupá J, Gulacsi L. Challenges in economic evaluation of new drugs: Experience with rituximab in Hungary. Med Sci Monit. 2010;16: 1–5. [PubMed] [Google Scholar]
- 35. Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M, et al. Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: A preliminary analysis. Mod Rheumatol. 2006;16: 77–84. [DOI] [PubMed] [Google Scholar]
- 36. Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept(Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis. 2005;64: 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farahani P, Levine M, Goeree R. A comparison between integrating clinical practice setting and randomized controlled trial setting into economic evaluation models of therapeutics. J Eval Clin Pract. 2006;12: 463–470. [DOI] [PubMed] [Google Scholar]
- 38. Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost-effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. Int J Technol Assess Health Care. 2011;27: 193–200. 10.1017/S0266462311000195 [DOI] [PubMed] [Google Scholar]
- 39. Wong JB, Singh G, Kavanaugh A. Estimating the cost-effectiveness of 54 weeks of infliximab for rheumatoid arthritis. Am J Med. 2002;113: 400–408. [DOI] [PubMed] [Google Scholar]
- 40. Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum. 2008;58: 939–46. 10.1002/art.23374 [DOI] [PubMed] [Google Scholar]
- 41. Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis. 2005;64: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan Y, Trivedi D, Maclean R, Rosenblatt L. Indirect cost-effectiveness analyses of abatacept and rituximab in patients with moderate-to-severe rheumatoid arthritis in the United States. J Med Econ. 2010;13: 33–41. 10.3111/13696990903508021 [DOI] [PubMed] [Google Scholar]
- 43. Vera-Llonch M, Massarotti E, Wolfe F, Westhovens R, Sofrygin O, Maclean R, et al. Cost-Effectiveness of Abatacept in Patients with Moderately to Severely Active Rheumatoid Arthritis and Inadequate Response to Tumor Necrosis Factor- α Antagonists. J Rheumatol. 2008;35: 1745–1753. [PubMed] [Google Scholar]
- 44. Marra CA, Marion SA, Guh DP, Najafzadeh M, Wolfe F, Esdaile JM, et al. Not all “quality-adjusted life years” are equal. J Clin Epidemiol. 2007;60: 616–624. [DOI] [PubMed] [Google Scholar]
- 45. Lindgren P, Geborek P, Kobelt G. Modeling the cost-effectiveness of treatment of rheumatoid arthritis with rituximab using registry data from Southern Sweden. Int J Technol Assess Health Care. 2009;25: 181–189. 10.1017/S0266462309090230 [DOI] [PubMed] [Google Scholar]
- 46.Coyle D, Judd M, Blumenauer B, Cranney A, Tugwell P, Well GA. Infliximab and Etanercept in Patients with Rheumatoid Arthritis: A Systematic Review and Economic Evaluation [Technology report no 64]. Ottawa: Canadian Coordinating Office for Health Technology Assessment. 2006. Available: http://www.cadth.ca/en/products/health-technology-assessment/publication/610. Accessed 4 February 2015.
- 47. Chiou C-F, Choi J, Reyes CM. Cost-effectiveness analysis of biological treatments for rheumatoid arthritis. Expert Rev Pharmacoecon Outcomes Res. 2004;4: 307–315. 10.1586/14737167.4.3.307 [DOI] [PubMed] [Google Scholar]
- 48.Canadian Agency for Drugs and Technologies in Health. Clinical and Economic Overview: Biological Response Modifier Agents for Adults with Rheumatoid Arthritis. CADTH Therapeutic Review. 2010. Available: http://www.cadth.ca/media/pdf/TR_RA_Clinical_and_Economic_Overview_e.pdf. Accessed 4 February 2015.
- 49. Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology(Oxford). 2004;43: 62–72. [DOI] [PubMed] [Google Scholar]
- 50. Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess. 2004;8: 1–104. [DOI] [PubMed] [Google Scholar]
- 51. Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics. 2005;23: 607–618. [DOI] [PubMed] [Google Scholar]
- 52. Kobelt G, Jönsson L, Young A, Eberhardt K. The cost-effectiveness of infliximab (Remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology(Oxford). 2003;42: 326–335. [DOI] [PubMed] [Google Scholar]
- 53. Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infiximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: A systematic review and economic evaluation. Health Technol Assess. 2011;15: 1–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hallinen TA, Soini EJ, Eklund K, Puolakka K. Cost-utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology(Oxford). 2010;49: 767–777. 10.1093/rheumatology/kep425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Institution for Health and Clinical Excellence. Guide to the methods of technology appraisal 2013. 2013. Available: http://publications.nice.org.uk/pmg9. Accessed 4 February 2015.
- 56. Hoaglin DC, Hawkins N, Jansen JP, Scott D a, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14: 429–437. 10.1016/j.jval.2011.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Duplicate references (n = 7) are excluded from a list.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.