Abstract
It has been suggested that the accumulation of biomolecular damage caused by reactive oxygen species (ROS) contributes to aging. The antioxidant activity is related to the ability of certain compounds to protect against the potentially harmful effect of processes or reactions involving ROS. This ability is associated with the termination of free radical propagation in biological systems. From Heterotheca inuloides various compounds which have shown to possess antioxidant capacity and scavenging ROS. The aim of this study was to determine the antioxidant capacity of additional natural components isolated from H. inuloides and some semisynthetic derivatives, their anti-inflammatory activity and the effect on Caenorhabditis elegans nematode life span. Compounds showed ability to inhibit various biological processes such as lipid peroxidation, scavenge nonbiological important oxidants such as 1O2, OH∙, H2O2, and HOCl and scavenge non biological stable free radicals (DPPH). Some cadinane type compounds showed possess antioxidant, ROS scavenging capacity, anti-inflammatory activity, and effect on the C. elegans life span. Flavonoid type compounds increased the life of the nematode and quercetin was identified as the compound with the greatest activity. The modification of chemical structure led to a change in the antioxidant capacity, the anti-inflammatory activity, and the survival of the worm.
1. Introduction
Reactive oxygen species (ROS) exist as products of normal cellular physiology and play vital roles in the stimulation of signaling pathways in plant and animal cells [1]. Aerobic organisms produce ROS during the reduction of molecular oxygen by mitochondria [2]. ROS include free radicals such as superoxide anion (O2 −), hydroxyl radical (OH∙), and nonradical molecules like hydrogen peroxide (H2O2), singlet oxygen (1O2), and other species such as nitric oxide (NO), hypochlorous acid (HOCl), and peroxynitrite (ONOO−). When the level of ROS exceeds away cellular factors responsible for protecting cellular biomolecules against damage generated by oxidizing species is said to be in a state of “oxidative stress.” Under these conditions ROS can damage biomolecules like nucleic acids, proteins, lipids, carbohydrates, and enzymes. This condition has been implicated in the pathogenesis of a number of multiple pathologies such as senescence [3], ischemia/reperfusion injury [4], neurodegenerative diseases [5], infectious processes [6], rheumatoid arthritis [7], arterial diseases [8], obesity, diabetes, chronic kidney disease [9], and other ailments.
Heterotheca inuloides is commonly known as “Mexican arnica” and it is known by other names in different regions of Mexico [10, 11]. In Mexican traditional medicine the infusions of this plant are mainly used for treatment of contusions and bruises [12]. Phytochemical studies of this plant, allowed isolating different classes of compounds, mainly cadinane type sesquiterpenes [13], flavonoids [14], and phytosterols [15]. Previous studies have reported that metanolic extract and certain natural products isolated from H. inuloides dried flowers have antioxidant activity and ability to inhibit lipid peroxidation, scavenging ROS and act as hepatoprotective agents [16–18]. However, the antioxidant activity of natural products isolated from the acetone extract has not been reported. In the present study, we evaluated the antioxidant and ROS scavenging capacity of H. inuloides metabolites isolated from the acetonic extract and prepared semisynthetic derivatives and also evaluated their anti-inflammatory effects and effect on the C. elegans life span. Here we report the results considering the structure-activity relationships.
2. Materials and Methods
2.1. Reagents
All reagents used were of analytical grade. Sodium pyruvate, dimethyl thiourea (DMTU), nordihydroguaiaretic acid (NDGA), ascorbic acid, histidine, xylenol orange (FOX), 2,2-diphenyl-1-picrylhydrazyl (DPPH), dimethylsulfoxide (DMSO), N,N-dimethyl-4-nitrosoaniline (DMNA), catalase, xanthine, xanthine oxidase, nitroblue tetrazolium (NBT), dL-penicillamine 2-thiobarbituric acid (TBA), α-tocopherol, Folin and Ciocalteu's phenol reagent, 3,5-di-tert-4-butylhydroxytoluene (BHT), and 5-fluoro-2′-deoxyuridine were purchased from Sigma-Aldrich (Toluca, Mexico, or Sigma, St. Louis, MO). Absolute ethanol, hydrogen peroxide (H2O2), sulfuric acid (H2SO4), methanol, ethylenediaminetetraacetic acid disodium salt (EDTA), NaCl, KH2PO4, NaHPO4·7H2O, NaOH, and H2SO4 were purchased from Merck-Mexico. Sodium hypochlorite (NaOCl) was purchased from Hycel (Mexico City, Mexico).
2.2. Strains of C. elegans
Experimental C. elegans strain was wild type N2 (Bristol). The strain was obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA) and was maintained on nematode growth medium (NGM) at 20°C as described previously by Brenner [19]. Age-synchronized worms were generated in all experiments through the sodium hypochlorite method. Worms were allowed to hatch in Petri dishes in liquid S-medium with concentrated Escherichia coli OP50 as food [20].
2.3. Plant Material
Flowers of H. inuloides (provided by MIXIM Laboratories, Naucalpan, Mexico) were collected in 2010, in the town of Mesas Altas de San Juan Xoconusco, municipality of Donato Guerra (State of Mexico), and authenticated by MS Abigail Aguilar-Contreras. A voucher of plant material was deposited under code IMSSM-16064 in the Medicinal Plant Herbarium of the Instituto Mexicano del Seguro Social (IMSS, Mexico City).
2.4. Heterotheca inuloides Metabolites and Derivatives
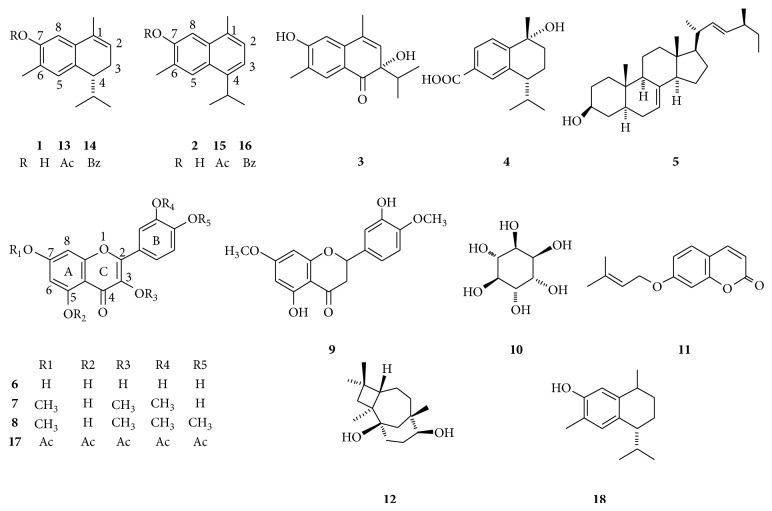
Compounds 1–12 were isolated from the acetone extract of dried flowers of H. inuloides in a previous study. Semisynthetic compounds 7-acetoxy-3,4-dihydrocadalene (13), 7-benzoxy-3,4-dihydrocadalene (14), 7-acetoxycadalene (15), 7-benzoxycadalene (16), quercetin pentaacetate (17), and 7-hydroxy calamenene (18) were obtained by conventional chemical procedures as was previously described [21] (Figure 1). Due to the paucity of material compounds 10 and 11 were not included in all the bioassays.
Figure 1.
Natural products isolated from H. inuloides flowers and derivatives.
2.5. Antioxidant Effects and ROS Scavenging of H. inuloides Metabolites
2.5.1. Estimation of Lipid Peroxidation
Lipid peroxidation was measured by TBARS assay using rat brain homogenates [22], with some modifications. Adult male Wistar rats (200–250 g) were provided by the Instituto de Fisiología Celular, UNAM, and their use was approved by the Animal Care and Use Committee [23]. The animals sacrifice was carried out avoiding unnecessary pain with CO2, cerebral tissue (whole brain) was rapidly dissected and homogenized. The homogenate was centrifuged for 10 min at 3400 rpm to yield a pellet that was discarded; protein content in the supernatant was measured using the Folin and Ciocalteu's phenol reagent [24] and adjusted to 2.66 mg protein/mL with PBS. The supernatant (375 μL) was incubated at 37°C for 30 min in presence of test sample (25 μL) dissolved in DMSO or ethanol and 50 μL of EDTA solution (20 μM). Lipid peroxidation was started adding 50 μL of a freshly prepared 100 μM FeSO4 solution (final concentration 10 μM) and incubated at 37°C for 60 min TBARS were determined as described by Ohkawa et al. [25] with some modifications. Concentration of TBARS was calculated by interpolation in a standard curve of tetrametoxipropane (TMP) [26]. Final results were expressed as mmoles of TBARS per mg of protein. The inhibition ratio (%) was calculated using the following formula:
| (1) |
where C was the absorbance of control and E was the absorbance of the test sample.
2.5.2. DPPH∙ Scavenging Capacity
DPPH assay was assessed in 96-well microtiter plates according to the method of Blois [27] with minor changes. Briefly, 0.05 mL of test compounds in DMSO at different concentrations was added to an ethanolic solution of 2,2-diphenylpicrylhydrazyl (DPPH∙) (133.33 μM, 0.150 mL). The control sample contained distilled water. Reaction mixtures were incubated at 37°C for 30 min in the dark. After incubation the absorbance was measured at 515 nm in a microplate reader ELx808 (BioTek Instruments, Inc., Vermont, USA). The % inhibition of each compound was determined by comparison with a DPPH ethanol blank solution [28]. The scavenging capacity is given as percent (%) DPPH scavenged, calculated as [(optical density of control − optical density of compound)/(optical density of control) × 100]; α-tocopherol was used as standard.
2.5.3. Superoxide Radical-Scavenging Activity
The superoxide radicals (O2 −) were generated through the system xanthine oxidase [29]. O2 − in the assay system and xanthine oxidase activity were measured as NBT reduction using a DU-640 series Beckman spectrophotometer. 800 μL of the following reaction mixtures: 90 μM xanthine, 16 mM Na2CO3, 22.8 μM NBT, and 18 mM phosphate buffer (pH 7.0) was mixed with 100 μL of different concentrations of H. inuloides metabolites. The reaction was started by the addition of 100 μL of xanthine oxidase (168 U/L). Optical density was registered both at 295 (for uric acid production) and at 560 nm (for O2 − in the assay system). The absorbance was determined using a Beckman DU640 Spectrophotometer (Beckman Coulter, Inc. California, USA).
2.5.4. Peroxide Scavenging Activity
Determination of scavenging capacity was made by ferrous ion oxidation-xylenol orange (FOX) assay [30]. Concentration of H2O2 was calculated from a standard curve prepared with increasing H2O2 concentrations. A solution of 75 μM H2O2 was mixed (1 : 1 v/v) with water (0% scavenging tube) or with different concentrations of H. inuloides metabolites and incubated for 30 min at room temperature. After this, H2O2 was measured by the following method. Briefly, 9 volumes of 4.4 mM BHT in HPLC-grade methanol were mixed with 1 volume of 1 mM xylenol orange and 2.56 mM ammonium ferrous sulphate in 0.25 M H2SO4 to give the working FOX reagent. 45 μL of the metabolite solutions and 45 μL of 75 μM H2O2 were dispensed in 1.5 mL Eppendorf tubes and mixed with 10 μL of HPLC-grade methanol immediately followed by the addition of 0.9 mL of FOX reagent, mixed on a vortex mixer for 5 s and then incubated at room temperature for 10 min. The tubes were centrifuged for 15,000 g for 10 min and absorbance at 560 nm was read against a methanol blank using a Beckman DU640 Spectrophotometer (Beckman Coulter, Inc. California, USA). The concentration of H2O2 was calculated from a standard curve prepared with increasing H2O2 concentrations. Sodium pyruvate was used as standard for H2O2 scavenging activity.
2.5.5. Hydroxyl Radical-Scavenging Activity
The malondialdehyde formed from the decay of deoxyribose was evaluated in reaction with thiobarbituric acid and measured at 532 nm [31]. The reaction mixture containing deoxyribose (0.056 mM), H2O2 (1 mM), potassium phosphate buffer (10 mM, pH 7.4), FeCl3 (0.2 mM), EDTA (0.2 mM), ascorbic acid (0.2 mM), and 100 μL of different concentrations of H. inuloides was incubated in a water bath at 37 ± 0.5°C for 1 h. The extent of the deoxyribose degradation by the OH∙ formed was measured by the thiobarbituric acid test at 532 nm using a Beckman DU640 Spectrophotometer (Beckman Coulter, Inc. California, USA). The ability of H. inuloides metabolites to scavenge OH∙ was compared with that of DMTU (0, 1, 2, 10, 20, and 31 μg/mL). The final results were expressed as inhibition percent in relation to a control test (without the sample).
2.5.6. Singlet Oxygen (1O2) Scavenging Activity
The ability of the compounds to scavenging 1O2 was detected spectrophotometrically at 440 nm using the bleaching of N,N-dimethyl p-nitrosoaniline (DMNA) as a specific detector [32]. The assay mixture contained 45 mM Na-phosphate buffer (pH 7.1), 10 mM histidine, 10 mM NaOCl, 10 mM H2O2, 50 μM DMNA, and 0.1 mL of different concentration of compounds. The total volume of reaction (2.0 mL) was incubated at 30°C for 40 min. The extent of 1O2 production was determined by measuring the decrease in the absorbance of DMNA at 440 nm using a Beckman DU640 Spectrophotometer (Beckman Coulter, Inc. California, USA). The relative scavenging efficiency (% inhibition production of 1O2) of H. inuloides metabolites was estimated from the difference in absorbance of DMNA with and without the addition of compounds being tested or reference compound. Glutathione (0, 0.92, 1.53, 2.15, 2.45, and 3.07 mg/mL) was used as standard for 1O2 scavenging.
2.5.7. HOCl Scavenging Assay
The HOCl scavenging activity was evaluated by measuring the decrease in absorbance of catalase at 404 nm and was carried out as described by Aruoma and Halliwell [33] with minor changes. Briefly, 150 μL of 49.8 μM bovine liver catalase solution (16.6 μM, final concentration) was mixed with 150 μL of 18 mM HOCl (6 mM, final concentration) and 150 μL of H. inuloides metabolite solutions in increasing concentrations or the reference compound ascorbic acid (0, 22, 44, 88 and 176 μg/mL). Spectra (370–450 nm) of catalase alone, catalase plus HOCl, or catalase plus HOCl and H. inuloides metabolites or the reference compound were registered and the optical densities (OD) at 404 nm were determined using a Beckman DU640 Spectrophotometer (Beckman Coulter, Inc., California, USA). The value of the OD of catalase alone minus the OD of catalase plus HOCl was considered as 100% of degradation of catalase (or 0% of scavenging activity), and the difference of the catalase alone minus the OD of the catalase plus HOCl in presence of either H. inuloides metabolites or reference compound was compared against this value. The ability of TPM to scavenge HOCl was compared with that of ascorbic acid.
2.6. Evaluation of Anti-Inflammatory Effect
The biological model employed was ear edema in mice induced by 13-ethyl-12-O-tetradecanoylphorbol (TPA), as described by Rao et al. [34] with slight modifications. CD1 male mice were used. Mice were administered with sodium pentobarbital (31.5 mg/kg, ip) and TPA solution (2.5 μg/ear) dissolved in ethanol (10 μL). This was done topically on the right ear, in both faces of the ear. The left ear received only ethanol, 10 min after the test substances (1 μmol/ear) or indomethacin (0.31 μmol/ear) as drug reference. The tested substances and indomethacin were dissolved in 20 μL of acetone and administered on both sides of the ears (10 μL/side). The control only received the vehicle (20 μL of acetone). Four hours later the mice were killed with CO2. A 7 mm diameter plug was removed from each ear. The swelling was assessed as the difference in weight between right and left ear plugs. The anti-inflammatory activity was expressed as inhibition of edema (IE) in percent relative to the edema formed in control animals according with the following formula: IE (%) = 100 − [B × 100/A], where A = edema induced by TPA alone, and B = edema induced by TPA plus sample.
2.7. Lifespan Assay
Lifespan assays were assessed in liquid medium at 20°C in 96-well plates (Corning, NY) and were carried out according to the established protocols [35]. Briefly, the nematodes were age-synchronized and distributed in wells as L1 larvae (10–20 animals per well) together with Escherichia coli OP50. To prevent self-fertilization, 5-fluoro-2′-deoxyuridine was added 36 h after seeding (0.12 mM final). Media were supplemented with different doses of compounds (100 or 200 μM). DMSO/ethanol (50/50% v/v) was included as a solvent control. The worms were monitored daily to observe the number of live worms in each treatment, the fraction of animals alive was scored on the basis of body movement. Observations and worm counts were performed using a microscope Eclipse TS 100 (Nikon Instruments Inc., Tokyo, Japan).
2.8. Statistical Analysis
For lipid peroxidation and DPPH scavenging data were represented as mean ± standard error of mean (SEM). Data were analyzed by one-way ANOVA followed by Dunnett's test for comparisons against control. Values of P ≤ 0.05 (∗) and P ≤ 0.01 (∗∗) were considered statistically significant. The inhibitory concentration 50 (IC50) was estimated by means of a linear regression equation. For scavenging capacity data were expressed as mean ± SEM. The data were compared against the blank tube without H. inuloides metabolites or the reference compounds, using one-way analysis of variance (ANOVA) followed by the Dunnett's Multiple Comparison test (GraphPad Prism 4.0 Software, San Diego, CA, USA). P < 0.05 was considered statistically significant. The scavenging capacity was expressed as the 50% inhibitory concentration (IC50) value, which denotes the concentration of H. inuloides metabolites or the reference compounds required to give a 50% reduction in oxidating effect relative to the blank tube. The data of the lifespan assays were processed using the GraphPad Prism 4.0 Software, (GraphPad Software, Inc., San Diego, CA, USA). Survival was plotted by the Kaplan-Meier method and the curves compared for significance using the log-rank test.
3. Results
3.1. Antioxidant Effects and ROS Scavenging
Our results showed that only compounds 2, 6, and 9 displayed the ability to reduce DPPH radical with a IC50 of 30.66 ± 8.14 μM, 13.11 ± 1.2 μM, and 6.97 ± 0.14 μM. Compounds 1 and 7 showed slight activity with IC50 higher than 100 μM. The other compounds tested showed a rate of antioxidant capacity less than 25% during the preliminary screening and therefore were not considered active (Table 1).
Table 1.
Capacity to inhibit lipid peroxidation and to reduce the DPPH radical.
| Compound | IC50 μM | |
|---|---|---|
| Lipid peroxidation | Reduction of DPPH | |
| 1 | 4.72 ± 0.11 | >100 |
| 2 | 3.09 ± 0.19 | 30.66 ± 8.14 |
| 3 | nd* | nd |
| 4 | nd | nd |
| 6 | 4.30 ± 0.27 | 13.11 ± 1.2 |
| 7 | 6.16 ± 0.06 | >100 |
| 8 | nd | nd |
| 9 | 14.46 ± 0.61 | 6.97 ± 0.14 |
| 11 | 17.35 ± 1.40 | nd |
| 12 | nd | — |
| 13 | 1.82 ± 0.12 | nd |
| 14 | >100 | nd |
| 15 | >100 | nd |
| 16 | >100 | nd |
| 17 | 3.67 ± 0.43 | — |
| 18 | 0.58 ± 0.008 | nd |
| α-Tocopherol | 6.78 ± 2.16 | 31.74 ± 1 |
*Not determined: at the concentrations tested the activity of compounds was low.
In peroxidation assay some compounds showed ability to inhibit this process. The inhibitory capacity of these compounds decreased in the following order: 18 > 13 > 2 > 17 > 6 > 1 > 7 > 9 > 11. Compound 18 had an IC50 of 0.58 ± 0.008 μM and showed to possess greater ability to inhibit lipid peroxidation than α-tocopherol used as reference substance whose IC50 was 6.78 ± 2.16 μM. The derivatives 14, 15, and 16 showed little ability to inhibit lipid peroxidation with IC50 higher than 100 μM.
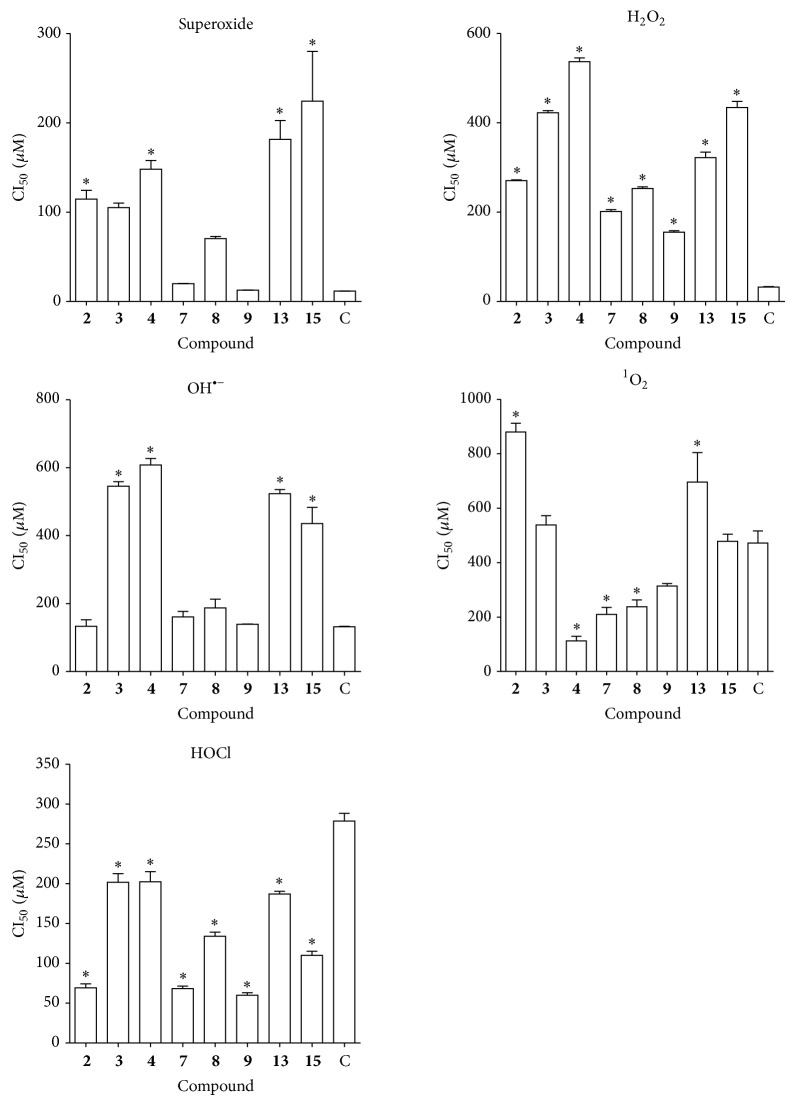
The H. inuloides natural products, derivatives as well as the reference compounds, displayed O2 −, HOCl, H2O2, 1O2, and OH∙ scavenging activity in a concentration-dependent way. The IC50 values were calculated from the dose-response curve (Table 2, Figure 2).
Table 2.
Scavenging capacity of H. inuloides metabolites on hypochlorous acid, hydroxyl radical, peroxide, superoxide anion, and singlet oxygen (IC50).
| Compound | Scavenging capacity IC50 μM | ||||
|---|---|---|---|---|---|
| HOCl | OH• | H2O2 | O2 − | 1O2 | |
| 2 | 69.1 ± 8.8* | 133.4 ± 32.6 | 270 ± 2.7* | 114.6 ± 13.9* | 880.4 ± 55.4* |
| 3 | 202.3 ± 21.9* | 545.4 ± 23.0* | 422 ± 8.0* | 105 ± 7.2* | 538.9 ± 58.6 |
| 4 | 203.5 ± 23.9* | 607.9 ± 33.5* | 536 ± 14* | 148 ± 13.0* | 112.4 ± 29.3* |
| 7 | 68.1 ± 5.77* | 161.2 ± 27.2 | 201 ± 6.7* | 20 ± 0.27* | 209.7 ± 44.5* |
| 8 | 133.9 ± 9.0* | 187.1 ± 44.6 | 252 ± 6.5* | 70.4 ± 3.3* | 237.8 ± 43.1* |
| 9 | 60.0 ± 5.2* | 139.5 ± 1.1 | 154 ± 6.3* | 12.2 ± 0.22 | 314.4 ± 15.8 |
| 13 | 106.1 ± 4.4* | 523.8 ± 20.4* | 322 ± 21.0* | 181.5 ± 29.8* | 697 ± 187.0* |
| 15 | 110.0 ± 15.8* | 435.78 ± 82.0* | 434.5 ± 23.3* | 244.1 ± 78.0* | 478.6 ± 44.7* |
|
| |||||
| Reference compound | Ascorbic acid | DMTU | Pyruvate | NDGA | GSH |
| 278.6 ± 16.7 | 131.5 ± 3.6 | 32.2 ± 1.7 | 11.6 ± 0.13 | 471 ± 77 | |
Data represent the mean ± SE of the three independent assays. The results were analyzed by ANOVA. The Dunnet's multiple comparison test was used to compare outcomes between experimental and control group. * P < 0.05 versus reference compound.
Figure 2.
ROS scavenging capacity of H. inuloides metabolites and semisynthetic derivatives. C=NDGA (superoxide); pyruvate (H2O2); DMTU (OH−); GSH (1O2); ascorbic Ac. acid (HOCl). The results were analyzed by ANOVA. The Dunnett's Multiple Comparison test was used to compare outcomes between experimental and control group. * P < 0.05.
The hypochlorous acid scavenging capability decreases in the following order 9 > 7 > 2 > 13 > 15 > 8 > 3 > 4. Compound 9 showed to possess the higher scavenging ability against HOCl. Compounds 13 and 15, acetylated derivatives of compounds 1 and 2, displayed a reduction in radical scavenging ability, with an increase in the IC50 value. The standard ascorbic acid showed an IC50 value of 278.6 ± 16.7 μM.
In the case of hydroxyl radical, the capacity to scavenging decreased in the following order: 2 > 9 > 7 > 8 > 15 > 13 > 3 > 4. The IC50 value for the dimethylthiourea used as reference substance was 131.5 ± 3.6 μM and was lower than those of the compounds tested.
H. inuloides metabolites were capable of scavenging hydrogen peroxide in an amount-dependent manner. Results showed that the scavenging activity values decreased in the order of 9 > 7 > 8 > 2 > 13 > 3 > 15 > 4. Compound 9 showed the best scavenging ability with an IC50 of 154 ± 6.3 μM; however, none of the compounds showed higher activity than the pyruvate used as reference substance whose IC50 was 32.2 ± 1.7 μM.
The superoxide scavenger capacity decreased in the following order: 9 > 7 > 8 > 3 > 2 > 4 > 13 > 15. Compound 9 showed to possess the best ability to trap superoxide radical with IC50 value of 12.2 ± 0.22 μM; however, it is not statistically different from NDGA reference compound whose IC50 was 11.6 ± 0.13 μM.
With respect to the singlet oxygen scavenger capacity the ability of the compounds to trap the singlet oxygen decreased in the following order 4 > 7 > 8 > 9 > 15 > 3 > 13 > 2. The calamenoic acid (4) showed comparatively better activity than the GHS. The IC50 values (Table 2) of calamenoic acid and GHS were 112.4 ± 29.3 μM y 471 ± 77 μM.
3.2. Evaluation of Anti-Inflammatory Effect
The results show that only the compounds 2, 4 and 16 displayed a significant anti-inflammatory activity, but this was less than that of indomethacin used as reference drug. The rest of the compounds showed a percentage of inhibition lower than 50%. (Table 3).
Table 3.
Anti-inflammatory activities of additional H. inuloides metabolites.
| Compound (1 μmol/ear) |
Edema | % |
|---|---|---|
| (mg, average SE) | Inhibition | |
| Negative control | 12.07 | — |
| 1 | 10.50 ± 0.99 | 5.41 |
| 2 | 1.85 ± 0.52 | 83.33** |
| 4 | 5.27 ± 0.70 | 65.73** |
| 5 | 9.80 ± 0.36 | 7.98 |
| 7 | 7.30 ± 1.15 | 38.86** |
| 9 | 7.57 ± 0.45 | 36.63** |
| 13 | 11.87 ± 0.66 | −11.42 |
| 14 | 11.07 ± 1.15 | 11.06 |
| 15 | 13.60 ± 0.70 | −13.90 |
| 16 | 3.73 ± 1.62 | 66.44** |
| 17 | 9.07 ± 0.70 | 19.46 |
| 18 | 13.17 ± 0.51 | 13.57 |
| Indomethacin (0.31 μ mol) | 61.90** |
The data represent the mean of three animals ± the standard error of the mean. All the compounds were tested at 1 μmol/ear doses. The results were analyzed by ANOVA. Student's t-test was used to compare outcomes between experimental and control groups. * P < 0.05 versus reference drug.
3.3. Life Span Assay
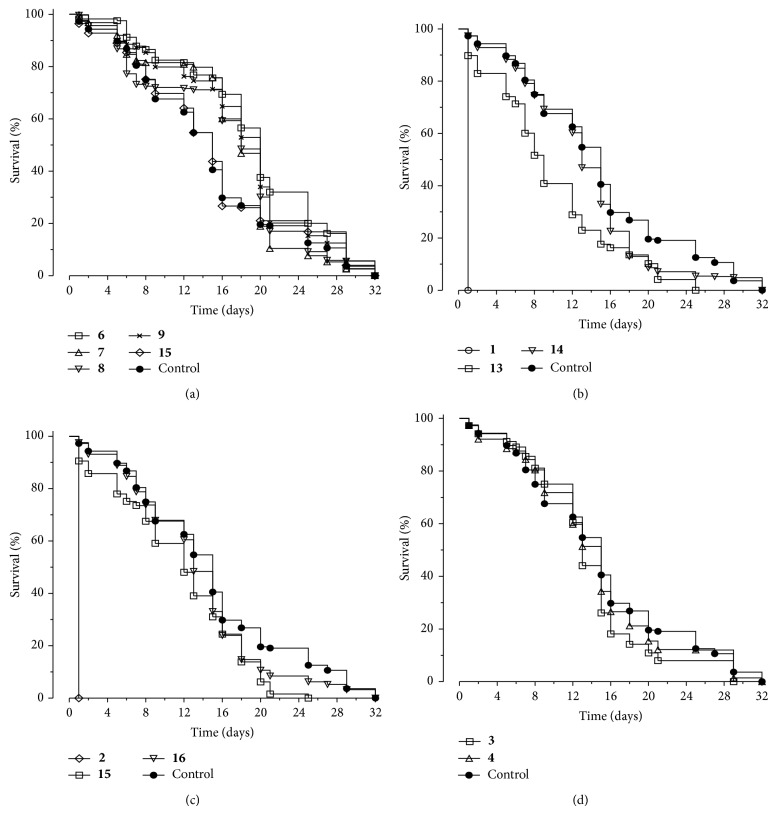
Exposure of C. elegans to compounds gave the following results: at 100 μM flavonoids type compounds extended half-life compared to the control (Table 4). Significant differences were observed for treatments compared with the vehicle-treated control (P < 0.001). The survival curve exhibited significant difference among worms fed with flavonoid compounds. There was not an increase in lifespan of the worm in the presence of cadinane compounds and some of them decreased survival as shown in Figure 3.
Table 4.
Summary of Caenorhabditis elegans life span.
| Treatment | Adult life span, 20°C | (n) | P versus control | |
|---|---|---|---|---|
| Mean | CI 95% | (Log-rank) | ||
| 1 | 1 | 0.171–1.962 | 449 | <0.0001 |
| 2 | 1 | 0.169–1.964 | 479 | <0.0001 |
| 3 | 13.661 | 13.13–14.209 | 513 | <0.0001 |
| 4 | 14.391 | 13.776–15.006 | 557 | 0.1175 |
| 6 | 19.2 | 18.41–19.82 | 444 | <0.0001 |
| 7 | 16.991 | 16.387–17.596 | 470 | <0.0001 |
| 8 | 16.583 | 15.926–17.239 | 575 | <0.0001 |
| 9 | 17.74 | 17.04–18.44 | 466 | <0.0001 |
| 13 | 11.012 | 10.414–11.609 | 434 | 0.0080 |
| 14 | 13.424 | 12.845–14.003 | 531 | 0.0001 |
| 15 | 11.659 | 11.103–12.215 | 499 | 0.0150 |
| 16 | 13.520 | 12.917–14.123 | 496 | 0.0001 |
| 17 | 15.216 | 14.478–15.955 | 499 | 0.1751 |
| Control | 14.943 | 14.471–15.414 | 1098 | |
Figure 3.
Lifespan survival curves in the presence H. inuloides metabolites. Labels denote the compound used (100 μM). Curve (a) flavonoids, curves (b), (c), and (d) natural and semisynthetic cadinane type compounds.
4. Discussion
H. inuloides metabolites showed to possess the ability to inhibit lipid peroxidation and scavenging free radicals and ROS. In lipid peroxidation assay we observed that the replacement of H at the hydroxyl in position 7 of sesquiterpenes 1 and 2 led to a decrease in the activity. It is further noted that the increase in the unsaturation of cadinanes led to a decrease in their activity, which is observed for compound 18 whose activity is greater than 1 that is greater than 2. In the case of flavonoid type compounds, 6 (quercetin) had the best ability to inhibit lipid peroxidation; natural methoxy derivatives 7 and 8 showed lower activity. These results are consistent with those reported that indicate that methoxy substituent perturbs the planarity due to steric hindrance imparted by the methyl group [36]. It is reported that the antioxidant activity of flavonoids is proportional to the number of hydroxyl groups present in the molecule [37]. Compound 6 (quercetin) having a 3,5,7,3′,4′-pentahydroxy group showed DPPH radical scavenging activity. Compounds 1 and 2 showed a statistically similar activity to 6 and higher than that of α-tocopherol. We noted that several tested compounds had the ability to inhibit lipid peroxidation; however, few of them reduced the DPPH radical. The above observations may be explained because the DPPH radical has a long life, and no resemblance to the highly reactive peroxyl radicals involved in lipid peroxidation, and some antioxidants that react quickly with peroxyl radicals react slowly or are inert to DPPH [38].
Hypochlorous acid radicals have the ability to inactivate the antioxidant enzyme catalase through degrade its heme group [33]. Catalase inactivation is inhibited in the presence of some H. inuloides compounds. The results showed that compounds are more efficient scavenger than the standard ascorbic acid. Among the flavonoid type compounds, 9 showed the highest ability to scavenge hypochlorous acid while 7 and 8 showed lower activity. In the last two compounds the hydroxyl at position 3, present in the quercetin molecule has been replaced by a methoxy group. It has been reported that the hydroxyl at position 3 of the C ring of quercetin plays an important role in the antioxidant activity including scavenging hypochlorite [39].
Hydroxyl free radicals cause damage to oxidative cells trough damage DNA, lipids, and proteins [31]. The flavonoids scavenge hydroxyl (OH∙) radicals. Hydroxyl radical (OH∙) generated by the Fenton reaction system was evaluated by TBARS assay. We observed that compound 9 was more active than 7, which in turn was more active than 8, with only one hydroxyl group. The results obtained are consistent with reports indicating that the scavenging activity of flavonoids increases with the number of free hydroxyl groups, and it is independent of the presence of double bond between C-2 and C-3 of ring C [40].
In biological systems H2O2 produces hydroxyl ions and hydroxyl radicals by reacting with Fe2+ and Cu2+ ions and they have been related to the initiation of many toxic effects [41]. It is therefore biologically advantageous for cells to control the accumulation of H2O2. H. inuloides metabolites showed ability to trap peroxide; however, they were no better than pyruvate.
The superoxide radical is a highly reactive species and not readily diffuses through the cell. Because the main production site of O2 − is the inner mitochondrial membrane, it has been proposed that mitochondrial DNA (mtDNA) is the main target of damage [42]. We noted that compounds 9 and 7 have the capacity to scavenging superoxide. It has been reported that the superoxide scavenging capacity of flavonoids is dependent on the number of hydroxyl groups in the B ring, on the presence of a free hydroxyl group at C3, on the presence of a saturated C2-C3 bond, and the absence of a C4 carbonyl group [43]. Also it has been reported that the number of conjugated double bonds and the presence of conjugated keto groups increase the quenching rate, while the presence of a hydroxyl, epoxy, and methoxy group has lesser effects [44]. Cadinane type compound 4 and flavonoid 9 showed the best ability to scavenge the singlet oxygen 1O2; other flavonoid type compounds showed lesser activity. The 1O2 scavenger activity of flavonoids is associated with the presence of a catechol moiety on ring B and with the presence of a hydroxyl group activating the double bond on C ring [45]. The cadinane type sesquiterpenes showed capacity to trap singlet oxygen greater than glutathione. In another study the ability of 7-hydroxy-3,4-dihydrocadalene to trap this species has been reported [46].
Preparations from H. inuloides are used as anti-inflammatory and analgesic agents in Mexican traditional medicine, and these properties have been investigated in several studies [13, 47, 48]. The results of anti-inflammatory assay activity showed that acetylation of compounds 1 and 2 to the corresponding derivatives 13 and 15, respectively, generated a change in biological response, since these derivatives exhibited proinflammatory activity. However, the benzoyl derivatives 14 and 16 (obtained from 1 and 2) resulted in opposite effects. Compound 14 increased the anti-inflammatory activity with respect to 1, while compound 16 displayed less activity in comparison with 2. It has previously been reported that quercetin (6) showed anti-inflammatory capacity in the mice ear edema test [13]; in this study it was observed that naturally methylated derivative 7 and semisynthetic acetylated derivative 17 displayed little activity in this assay (see Table 3).
We evaluated the effect of some natural products isolated from H. inuloides and derivatives in extension of life of C. elegans nematode (Figure 3). Treatment of C. elegans with some H. inuloides metabolites prolonged the lifespan of the worm and the best effects were obtained with flavonoid type compounds. The results are consistent with other studies conducted wherein it has been reported that certain compounds of flavonoid type increase lifespan of C. elegans, which protect against oxidative stress and cause an increase in the translocation of the transcription factor DAF-16 [49]. In another study it was observed that blueberry polyphenols increased lifespan and slowed aging related decline in C. elegans, but these benefits did not just reflect antioxidant activity [50]. Similarly, results of Wilson et al. [51] showed that the prolongevity effect of myricetin is dependent on DAF-16 and not on direct antioxidative effects of the flavonoid. Büchter et al. [52] observed that quercetin significantly increased reproductive capacity of C. elegans and enlarged the body size, whereas no modification of these characteristics was induced by their methylated derivatives, isorhamnetin, and tamarixetin. Certain cadinane type compounds isolated from H. inuloides have shown to possess antioxidant activity, but its antioxidant capacity was not reflected in an increase in the life span of C. elegans. Although several compounds isolated from H. inuloides have antiantigiardial activity [21] and it has been found that structurally similar compounds showed activity against Leishmania chagasi promastigotes [53].
5. Conclusions
In the present paper, we have shown that H. inuloides metabolites in vitro scavenged to O2 −, HOCl, H2O2, OH∙, and 1O2 in a concentration-dependent way. The IC50 in some cases were comparable to the reference compound. These observations suggest that metabolites of H. inuloides have the ability to capture free radicals. The results of the anti-inflammatory assay of the natural products and some derivatives showed both anti- and proinflammatory effects, depending on the functionalization of the OH− groups. We have found that antioxidant capacities are not predictive of C. elegans lifespan benefits. Even where a life span extension and antioxidant effect were observed, it appears that other factors are also likely to be involved in modulating lifespan.
Acknowledgments
José Luis Rodríguez-Chávez thanks the Consejo Nacional de Ciencia y Tecnología (México) for providing a graduate scholarship. The authors thank B. Quiroz, R. Patiño, M. I. Chávez, A. Peña, E. Huerta, R. Gaviño, L. Velasco, J. Pérez, Instituto de Química, UNAM, and D. Ortega Cuellar, Instituto Nacional de Pediatría, for technical assistance. The authors also acknowledge UNAM-DGAPA-PAPIIT (Project IG200514) for support. This paper is dedicated to Dr. Bernardino Huerta-Gertrudis (In memoriam), Instituto Nacional de Pediatría, México.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochemical Pharmacology. 1999;57(3):231–245. doi: 10.1016/S0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 2.Venditti P., Di Stefano L., Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion. 2013;13(2):71–82. doi: 10.1016/j.mito.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta J., Kar S., Liu R., et al. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. Journal of Cellular Physiology. 2010;225(1):52–62. doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama T., Tawa M., Yamagishi N., et al. Role of superoxide production in post-ischemic cardiac dysfunction and norepinephrine overflow in rat hearts. European Journal of Pharmacology. 2013;711(1–3):36–41. doi: 10.1016/j.ejphar.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Xie H., Hou S., Jiang J., Sekutowicz M., Kelly J., Bacskai B. J. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):7904–7909. doi: 10.1073/pnas.1217938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohanka M. Role of oxidative stress in infectious diseases. A review. Folia Microbiologica. 2013;58(6):503–513. doi: 10.1007/s12223-013-0239-5. [DOI] [PubMed] [Google Scholar]
- 7.Kundu S., Ghosh P., Datta S., Ghosh A., Chattopadhyay S., Chatterjee M. Oxidative stress as a potential biomarker for determining disease activity in patients with Rheumatoid Arthritis. Free Radical Research. 2012;46(12):1482–1489. doi: 10.3109/10715762.2012.727991. [DOI] [PubMed] [Google Scholar]
- 8.Marín C., Yubero-Serrano E. M., López-Miranda J., Pérez-Jiménez F. Endothelial aging associated with oxidative stress can be modulated by a healthy Mediterranean diet. International Journal of Molecular Sciences. 2013;14(5):8869–8889. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan S. R. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urological Research. 2012;40(2):95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz J. L. Índice y Sinonimia de las Plantas Medicinales de México. México City, Mexico: IMEPLAM; 1976. (Monografías Científicas I). [Google Scholar]
- 11.Argueta A., Cano L., Rodarte M. E. Atlas de las plantas medicinales de la medicina tradicional mexicana. 1–3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994. [Google Scholar]
- 12.Lozoya X., Aguilar A., Camacho R. Encuesta sobre el uso actual de plantas en la Medicina Tradicional Mexicana. Revista Médica del Instituto Mexicano del Seguro Social. 1987;25:283–291. [Google Scholar]
- 13.Delgado G., Del Socorro Olivares M., Chávez M. I., et al. Antiinflammatory constituents from Heterotheca inuloides . Journal of Natural Products. 2001;64(7):861–864. doi: 10.1021/np0005107. [DOI] [PubMed] [Google Scholar]
- 14.Jerga C., Merfort I., Willuhn G. Flavonoidaglyka aus den blüten von Heterotheca inuloides . Planta Medica. 1990;56(1):122–123. doi: 10.1055/s-2006-960903. [DOI] [PubMed] [Google Scholar]
- 15.Willuhn G., Schneider R. Sesquiterpene, Triterpene und Sterine aus Blüten von Heterotheca inuloides (‘Mexikanische Arnikablüten’) Archiv der Pharmazie. 1987;320(5):393–396. doi: 10.1002/ardp.19873200503. [DOI] [Google Scholar]
- 16.Kubo I., Chaudhuri S. K., Kubo Y., et al. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides . Planta Medica. 1996;62(5):427–430. doi: 10.1055/s-2006-957932. [DOI] [PubMed] [Google Scholar]
- 17.Haraguchi H., Saito T., Ishikawa H., Sanchez Y., Ogura T., Kubo I. Inhibition of lipid peroxidation by sesquiterpenoid in Heterotheca inuloides . Journal of Pharmacy and Pharmacology. 1996;48(4):441–443. doi: 10.1111/j.2042-7158.1996.tb05949.x. [DOI] [PubMed] [Google Scholar]
- 18.Coballase-Urrutia E., Pedraza-Chaverri J., Cárdenas-Rodríguez N., et al. Hepatoprotective effect of acetonic and methanolic extracts of Heterotheca inuloides against CCl4-induced toxicity in rats. Experimental and Toxicologic Pathology. 2011;63(4):363–370. doi: 10.1016/j.etp.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J. A., Fleming J. T. Basic culture methods. In: Epstein H. F., Shakes D. C., editors. Methods in Cell Biology. Vol. 48. San Diego, Calif, USA: Academic Press; 1995. pp. 3–29. [PubMed] [Google Scholar]
- 21.Rodríguez-Chávez J. L., Rufino-González Y., Ponce-Macotela M., Delgado G. In vitro activity of ‘Mexican Arnica’ Heterotheca inuloides Cass natural products and some derivatives against Giardia intestinalis . Parasitology. 2014 doi: 10.1017/s0031182014001619. [DOI] [PubMed] [Google Scholar]
- 22.Ng T. B., Liu F., Wang Z. T. Antioxidative activity of natural products from plants. Life Sciences. 2000;66(8):709–723. doi: 10.1016/S0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 23.Secretaría de Agricultura. Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio
- 24.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 25.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 26.Esterbauer H., Cheeseman K. H. Determination of aldehydic lipid peroxidation products. Methods in Enzymology. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 27.Blois M. S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 28.Cotelle N., Bernier J.-L., Catteau J.-P., Pommery J., Wallet J.-C., Gaydou E. M. Antioxidant properties of hydroxy-flavones. Free Radical Biology & Medicine. 1996;20(1):35–43. doi: 10.1016/0891-5849(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 29.Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. The Journal of Biological Chemistry. 1970;245(16):4053–4057. [PubMed] [Google Scholar]
- 30.Wolff S. P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods in Enzymology. 1994;233:182–189. doi: 10.1016/S0076-6879(94)33021-2. [DOI] [Google Scholar]
- 31.Halliwell B., Gutteridge J. M. C., Aruoma O. I. The deoxyribose method: a simple ‘test-tube’ assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry. 1987;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty N., Tripathy B. C. Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiology. 1992;98(1):7–11. doi: 10.1104/pp.98.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aruoma O. I., Halliwell B. Action of hypochlorous acid on the antioxidant protective enzymes superoxide dismutase, catalase and glutathione peroxidase. Biochemical Journal. 1987;248(3):973–976. doi: 10.1042/bj2480973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao Y. K., Fang S.-H., Tzeng Y.-M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima . Journal of Ethnopharmacology. 2005;100(3):249–253. doi: 10.1016/j.jep.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Solis G. M., Petrascheck M. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. Journal of Visualized Experiments: JoVE. 2011;(49) doi: 10.3791/2496.e2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dugas A. J., Jr., Castañeda-Acosta J., Bonin G. C., Price K. L., Fischer N. H., Winston G. W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. Journal of Natural Products. 2000;63(3):327–331. doi: 10.1021/np990352n. [DOI] [PubMed] [Google Scholar]
- 37.Cao G., Sofic E., Prior R. L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical Biology and Medicine. 1997;22(5):749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 38.Amorati R., Menichetti S., Viglianisi C., Foti M. C. Proton-electron transfer pathways in the reactions of peroxyl and dpph∙ radicals with hydrogen-bonded phenols. Chemical Communications. 2012;48(97):11904–11906. doi: 10.1039/c2cc36531f. [DOI] [PubMed] [Google Scholar]
- 39.Hirose Y., Kakita M., Washizu T., Matsugo S. Structural determination of oxidation products of flavonoids in alcoholic aqueous solution with reactive oxygen species. Journal of Photoscience. 2002;9(2):424–426. [Google Scholar]
- 40.Husain S. R., Cillard J., Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry. 1987;26(9):2489–2491. doi: 10.1016/s0031-9422(00)83860-1. [DOI] [Google Scholar]
- 41.Nappi A. J., Vass E. Hydroxyl radical production by ascorbate and hydrogen peroxide. Neurotoxicity Research. 2000;2(4):343–355. doi: 10.1007/bf03033342. [DOI] [Google Scholar]
- 42.Kirkinezos I. G., Moraes C. T. Reactive oxygen species and mitochondrial diseases. Seminars in Cell and Developmental Biology. 2001;12(6):449–457. doi: 10.1006/scdb.2001.0282. [DOI] [PubMed] [Google Scholar]
- 43.Hu J. P., Calomme M., Lasure A., et al. Structure-activity relationship of flavonoids with superoxide scavenging activity. Biological Trace Element Research. 1995;47(1–3):327–331. doi: 10.1007/bf02790134. [DOI] [PubMed] [Google Scholar]
- 44.Di Mascio P., Murphy M. E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. The American Journal of Clinical Nutrition. 1991;53(1, supplement):194S–200S. [PubMed] [Google Scholar]
- 45.Tournaire C., Croux S., Maurette M.-T., et al. Antioxidant activity of flavonoids: efficiency of singlet oxygen (1Δg) quenching. Journal of Photochemistry and Photobiology B: Biology. 1993;19(3):205–215. doi: 10.1016/1011-1344(93)87086-3. [DOI] [PubMed] [Google Scholar]
- 46.Coballase-Urrutia E., Pedraza-Chaverri J., Camacho-Carranza R., et al. Antioxidant activity of Heterotheca inuloides extracts and of some of its metabolites. Toxicology. 2010;276(1):41–48. doi: 10.1016/j.tox.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Segura L., Freixa B., Ringbom T., et al. Anti-inflammatory activity of dichloromethane extract of Heterotheca inuloides in vivo and in vitro. Planta Medica. 2000;66(6):553–555. doi: 10.1055/s-2000-8613. [DOI] [PubMed] [Google Scholar]
- 48.Rocha-González H. I., Blaisdell-López E., Granados-Soto V., Navarrete A. Antinociceptive effect of 7-hydroxy-3,4-dihydrocadalin isolated from Heterotheca inuloides: role of peripheral 5-HT1 serotonergic receptors. European Journal of Pharmacology. 2010;649(1–3):154–160. doi: 10.1016/j.ejphar.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Kampkötter A., Nkwonkam C. G., Zurawski R. F., et al. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans . Toxicology. 2007;234(1-2):113–123. doi: 10.1016/j.tox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Saul N., Pietsch K., Menzel R., Stürzenbaum S. R., Steinberg C. E. W. Catechin induced longevity in C. elegans: from key regulator genes to disposable soma. Mechanisms of Ageing and Development. 2009;130(8):477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Wilson M. A., Shukitt-Hale B., Kalt W., Ingram D. K., Joseph J. A., Wolkow C. A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans . Aging Cell. 2006;5(1):59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Büchter C., Ackermann D., Havermann S., et al. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. International Journal of Molecular Sciences. 2013;14(6):11895–11914. doi: 10.3390/ijms140611895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues I. A., Azevedo M. M. B., Chaves F. C. M., et al. In vitro cytocidal effects of the essential oil from Croton cajucara (red sacaca) and its major constituent 7-hydroxycalamenene against Leishmania chagasi . BMC Complementary and Alternative Medicine. 2013;13, article 249 doi: 10.1186/1472-6882-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]