Abstract
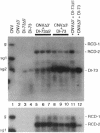
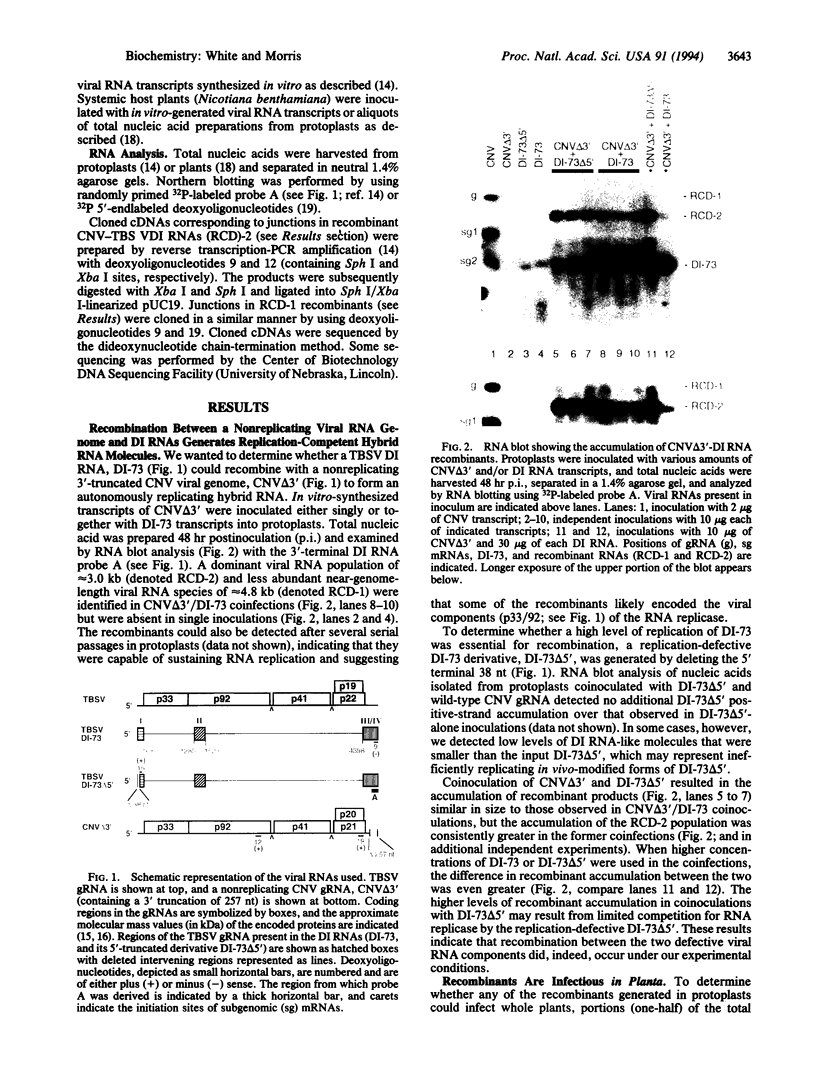
The tombusviruses represent a group of small icosahedral plant viruses that contain monopartite positive-sense RNA genomes. Tombusviruses are able to generate small replicating deletion mutants of their genomes (i.e., defective interfering RNAs) during infections via RNA recombination and/or rearrangement. To further study the process of RNA recombination and to determine whether tombusviruses were capable of trans-recombination, protoplasts were coinoculated with in vitro-generated transcripts of a nonreplicating 3'-truncated genomic RNA of cucumber necrosis tombusvirus and either replicative or replication-defective DI RNAs of tomato bushy stunt tombusvirus. After a 48-hr incubation, two dominant replicative chimeric recombinant viral RNA populations were detected that contained various large contiguous 5' segments of the cucumber necrosis tombusvirus genomic RNA fused to 3'-terminal regions of the tomato bushy stunt tombusvirus defective interfering RNA. Some of the larger chimeric recombinants formed in protoplasts were able to systemically infect plants and induce wild-type symptoms. In addition, a functional chimeric genome was generated in planta after direct coinoculation of whole plants with the defective RNA components. These results indicate that (i) RNA recombination can occur relatively efficiently in single-cell infections, (ii) trans-recombination can occur with nonreplicating viral RNA components, and (iii) functional chimeric genomes can be generated via recombination. Possible mechanisms for the formation of the recombinants are proposed, and evolutionary implications are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dzianott A. M. Generation and analysis of nonhomologous RNA-RNA recombinants in brome mosaic virus: sequence complementarities at crossover sites. J Virol. 1991 Aug;65(8):4153–4159. doi: 10.1128/jvi.65.8.4153-4159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone P. J., Carpenter C. D., Li X. H., Simon A. E. Recombination between satellite RNAs of turnip crinkle virus. EMBO J. 1990 Jun;9(6):1709–1715. doi: 10.1002/j.1460-2075.1990.tb08294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone P. J., Haydar T. F., Simon A. E. Sequences and structures required for recombination between virus-associated RNAs. Science. 1993 May 7;260(5109):801–805. doi: 10.1126/science.8484119. [DOI] [PubMed] [Google Scholar]
- Hearne P. Q., Knorr D. A., Hillman B. I., Morris T. J. The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology. 1990 Jul;177(1):141–151. doi: 10.1016/0042-6822(90)90468-7. [DOI] [PubMed] [Google Scholar]
- King A. M. Preferred sites of recombination in poliovirus RNA: an analysis of 40 intertypic cross-over sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11705–11723. doi: 10.1093/nar/16.24.11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr D. A., Mullin R. H., Hearne P. Q., Morris T. J. De novo generation of defective interfering RNAs of tomato bushy stunt virus by high multiplicity passage. Virology. 1991 Mar;181(1):193–202. doi: 10.1016/0042-6822(91)90484-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Lai M. M. RNA recombination in animal and plant viruses. Microbiol Rev. 1992 Mar;56(1):61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. D., Morris T. J. De novo generation and accumulation of tomato bushy stunt virus defective interfering RNAs without serial host passage. Virology. 1994 Jan;198(1):377–380. doi: 10.1006/viro.1994.1045. [DOI] [PubMed] [Google Scholar]
- Li Y., Ball L. A. Nonhomologous RNA recombination during negative-strand synthesis of flock house virus RNA. J Virol. 1993 Jul;67(7):3854–3860. doi: 10.1128/jvi.67.7.3854-3860.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P. D., Bujarski J. J. Genetic recombination in brome mosaic virus: effect of sequence and replication of RNA on accumulation of recombinants. J Virol. 1992 Nov;66(11):6824–6828. doi: 10.1128/jvi.66.11.6824-6828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P. D., Bujarski J. J. Targeting the site of RNA-RNA recombination in brome mosaic virus with antisense sequences. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6390–6394. doi: 10.1073/pnas.90.14.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Recombination and polymerase error facilitate restoration of infectivity in brome mosaic virus. J Virol. 1993 Feb;67(2):969–979. doi: 10.1128/jvi.67.2.969-979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Requirement for a viral trans-acting factor encoded by brome mosaic virus RNA-2 provides strong selection in vivo for functional recombinants. J Virol. 1990 May;64(5):2437–2441. doi: 10.1128/jvi.64.5.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon D. M., Johnston J. C. Infectious transcripts from cloned cucumber necrosis virus cDNA: evidence for a bifunctional subgenomic mRNA. Virology. 1991 Apr;181(2):656–665. doi: 10.1016/0042-6822(91)90899-m. [DOI] [PubMed] [Google Scholar]
- Rochon D. M., Tremaine J. H. Complete nucleotide sequence of the cucumber necrosis virus genome. Virology. 1989 Apr;169(2):251–259. doi: 10.1016/0042-6822(89)90150-5. [DOI] [PubMed] [Google Scholar]
- White K. A., Bancroft J. B., Mackie G. A. Coding capacity determines in vivo accumulation of a defective RNA of clover yellow mosaic virus. J Virol. 1992 May;66(5):3069–3076. doi: 10.1128/jvi.66.5.3069-3076.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Morris T. J. Nonhomologous RNA recombination in tombusviruses: generation and evolution of defective interfering RNAs by stepwise deletions. J Virol. 1994 Jan;68(1):14–24. doi: 10.1128/jvi.68.1.14-24.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Cascone P. J., Simon A. E. Recombination between satellite and genomic RNAs of turnip crinkle virus. Virology. 1991 Oct;184(2):791–794. doi: 10.1016/0042-6822(91)90454-j. [DOI] [PubMed] [Google Scholar]