Abstract
Cardiovascular disease, and the incidence of sudden cardiac death (SCD), will increase significantly in low- and middle-income countries (LMIC). Thus, SCD threatens to become a global public health problem. We present a summary of the current research that has investigated the epidemiology of SCD in LMIC. Few studies of SCD in LMIC exist, and they are of variable methodological quality. Risk factors for SCD are described, taking into account recent global burden of disease and risk factor statistics. We describe 1 proposal for a community-based, prospective, multiple-source methodology for SCD monitoring and surveillance that can be implemented in LMIC. Further research into the epidemiology of SCD in LMIC, using standardized methodology, would allow investigators and policy makers to determine the regions, communities, and individuals most at need for SCD prevention. Focusing on SCD and its prevention in LMIC should be a priority for the global health community.
Sudden cardiac death (SCD) is defined as death from an unexpected circulatory arrest, usually due to a cardiac arrhythmia, occurring within an hour of symptom onset [1]. Given the current and projected burden of cardiovascular disease (CVD) in low- and middle-income countries (LMIC) [2,3], it is likely that SCD will also increase in LMIC in the future. Therefore, SCD threatens to become a global public health problem, affecting populations in LMIC as well as those in high-income countries (HIC). Most reports of the epidemiology of SCD have been confined to HIC [4,5]. Unfortunately, SCD data from LMIC are generally lacking, of variable quality, and derived from different methodologies. This limits the applicability of a systematic review of SCD epidemiology in LMIC and precludes the possibility of performing a meta-analysis. Instead, we present a summary of the research that has investigated the epidemiology of SCD in LMIC, highlighting the methodological variation among the different studies, gaps in knowledge, and future research opportunities. Further, we describe a proposal for a community-based, prospective, multiple-source methodology for SCD monitoring and surveillance that can be implemented in LMIC.
EPIDEMIOLOGY OF SCD IN LMIC
Determining true occurrences of SCD is challenging. First, SCD occurs in the general population in an unpredictable manner. Second, it is critical to exclude subjects that are likely to have died of a noncardiac cause. Third, even though there is a universal definition of SCD, the practical application of this definition is challenging, particularly with respect to assignment of the appropriate International Classification of Diseases (ICD)-10 code on death certificates. Thus, death certificate records may inaccurately record SCD as the cause of death and retrospective analyses of death certificates reflect that same inaccuracy [6,7]. An accurate estimate of SCD incidence requires prospective ascertainment of cases rather than retrospective review of death certificates. Even in the United States, the true incidence of SCD is unclear [5].
The ORE-SUDS (Oregon Sudden Unexpected Death Study) used prospective surveillance involving multiple sources of information, including the emergency medical response system, the county medical examiner’s office, and emergency rooms of local hospitals [8]. Cases were identified by physicians from the emergency medical services or by the county medical examiner; these potential cases were then screened to determine which ones met SCD criteria. The ORE-SUDS investigators reported an annual SCD incidence of 53 per 100,000, using this methodology. Studies in other HIC using similar methodology have reported equivalent incidence rates for SCD, such as Ireland (51 per 100,000) [9]. Using these incidence rates, it has been estimated that the annual global incidence of SCD would be approximately 4 to 5 million cases [10]. However, this number may be inaccurate, as the SCD incidence rates in LMIC may not be equivalent to those in HIC.
A recent analysis from China used a prospective, multiple-source surveillance methodology to ascertain incident SCD events [11]. Three levels of case reporting and ascertainment were used, and the investigators worked intimately within the already established administrative and bureaucratic structure in China. This multilevel system of reporting and ascertainment allowed for rigorous, prospective verification and confirmation of SCD cases within the community. The first level of reporting included the household administrative office and health station within neighborhoods. Case reporting at this level involved examination of death certificates, review of medical records, autopsy data, interviews with family members, and review of data collected by the household administrative officer. The second level consisted of data verification and diagnosis ascertainment at local hospitals and health centers specific to each geographic region. The third level involved final case adjudication at the research coordinating center. Using this multiple-source, multi-level surveillance methodology, the China investigators found an overall annual incidence of 41.8 per 100,000. The specific data sources used in this study differed from ORE-SUDS due to differences in healthcare infrastructure and government record-keeping practices between the United States and China. However, the methodology as described by the investigators appears to be quite robust. The investigators also reported significant variability in SCD incidence rates by geographic region, with age-standardized incidence rates higher in rural provinces. Given the multiple levels of case reporting and ascertainment used in this study, it is unlikely that these results reflect information bias.
Other studies from LMIC have used diverse methodologies of variable quality. An earlier study from China, using multiple sources, but with a retrospective methodology, reported SCD incidence rates between 7.3 and 29.5 per 100,000. A report from South Africa investigated out-of-hospital cardiac arrest based only on data from first responders and yielded a SCD incidence of 6.4 per 100,000 [13]. This value is likely an underestimate, because a multiple-source methodology was not used. A recent study from southern India used an innovative, but retrospective, methodology involving surveys of medical student respondents and their relatives and found that SCD contributed to 10.3% of recallable deaths [14]. The data provided by the investigators, however, do not allow for determination of SCD incidence.
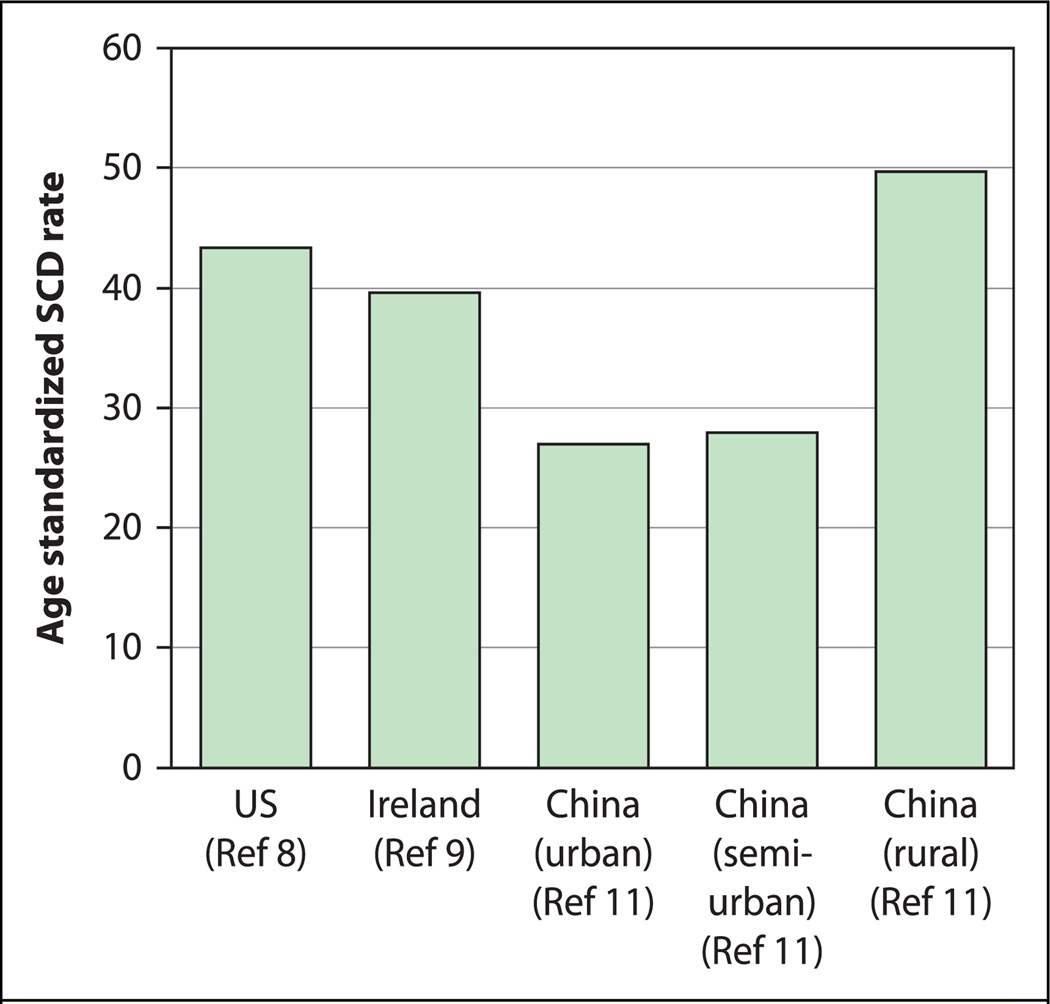
These studies, despite the disparate methodologies and quality of study design, highlight that SCD incidence rates are likely to differ significantly between HIC and LMIC, as well as between different geographical regions [15] (Fig. 1). The near totality of other studies of SCD in LMIC are autopsy studies [16–23], hospital-based studies [24,25], or are summary statistics of number of implantable cardioverter-defibrillators [26–28]. Such studies are unable to provide estimates of SCD incidence in the community. Further research into SCD epidemiology in LMIC—using a uniform, robust, community-based, prospective, multiple-source methodology—would help to estimate the true burden of SCD in LMIC.
Fig. 1.
Sudden cardiac death (SCD) incidence rates (per 100,000) from different countries and regions, standardized to the World Health Organization standard population [15]. US, United States.
RISK FACTORS FOR SCD—IMPLICATIONS OF GLOBAL DISEASE BURDEN
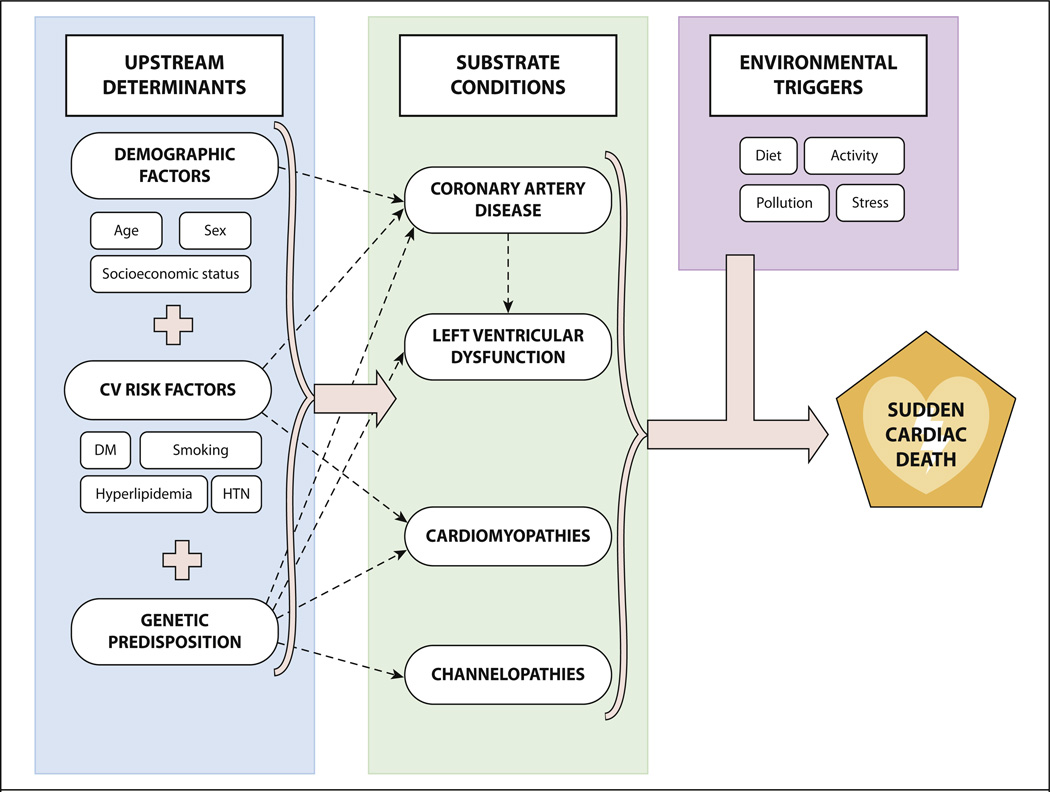
SCD is the consequence of complex interaction between upstream determinants, a variety of cardiac-substrate conditions, and environmental triggers (Fig. 2).
Fig. 2.
Schematic demonstrating the interaction among the risk factors for sudden cardiac death. CV, cardiovascular; DM, diabetes mellitus; HTN, hypertension.
Upstream determinants: age and sex
Men have a higher risk of SCD than women do, although this difference decreases with age, likely due to the increased risk of coronary artery disease (CAD) among post-menopausal women [29]. Over time, SCD prevalence has increased among women relative to men; whereas earlier studies had reported a 1:3 ratio of women to men, more recent data suggest that women now comprise approximately 40% of SCD cases in the United States [8].
Average life expectancy is increasing in LMIC at a more rapid pace than occurred in HIC in the 20th century [30,31]. The disease burden of this increasingly elderly population will consist of diseases such as CAD and its complications, including SCD. At the same time, CVD is affecting younger individuals in LMIC; age-adjusted mortality from CVD is greater in LMIC than in HIC [32]. SCD is a devastating event, and it may threaten the economically productive members of LMIC societies.
Upstream determinants: socioeconomic status
In ORE-SUDS, the incidence of SCD was 30% to 80% higher among individuals living in neighborhoods in the lowest socioeconomic quartile than it was in those in the highest quartile [33], a pattern similarly seen in other HIC settings [34,35]. Likewise, SCD incidence in China was found to be higher in the rural, less-developed regions than it was in urban, more-developed cities [11]. The cause of this socioeconomic gradient of SCD burden is not fully understood, but it may be related to a higher burden of CAD risk factors combined with poorer access to health care [29]. In many LMIC, there is an inverse relationship between socioeconomic status and burden of CVD and CVD risk factors [32,36]. Thus, SCD may disproportionately affect the poor in these regions.
Upstream determinants: cardiovascular risk factors
CAD risk factors such as hypertension, dyslipidemia, tobacco, and diabetes mellitus (DM) all contribute to SCD risk by increasing the risk of CAD. In addition, DM has consistently been identified as a strong independent predictor of SCD risk [37–39]. DM-specific accelerated forms of atherosclerosis may exist with enhanced thrombogenicity [40]. Additionally, there is a high prevalence of abnormal QT interval prolongation among diabetic individuals [41,42]. QT interval prolongation, regardless of the primary cause, has been associated with increased overall cardiac mortality [43].
Elevated blood glucose currently accounts for over 20% of ischemic heart disease deaths and over 15% of stroke deaths worldwide [44], and the global burden of DM is increasing rapidly, especially in LMIC [45]. In China, the prevalence of DM increased nearly 3-fold from 1980 to 1994, and current projections predict that this will continue to increase [46]. Similar trends have been reported in India [47] and South Africa [48]. This trend foreshadows an even greater and very concerning global burden of DM and its associated complications, including SCD, in the upcoming decades in LMIC.
Upstream determinants: genetic predisposition
The association between genetics and SCD is clearly seen in hereditary abnormalities in ion channel function such as the long QT and Brugada syndromes [29]. Other associations are not as clear, but emerging data have provided evidence that genetic factors may contribute to the risk of SCD in patients without an overt channelopathy [49,50]. In addition, even monogenic syndromes, such as the long QT syndrome, may involve modifier genes and varying degrees of penetrance [51]. Certain conditions are more common in certain ethnic populations, such as Brugada syndrome in Asians [52]. In addition, there are an increasing number of genetic studies being conducted in LMIC for various arrhythmic syndromes that lead to SCD [53–55].
Substrate conditions: coronary artery disease
Approximately 80% of SCD are attributed to CAD [8,56,57]. The 2 major mechanisms of fatal ventricular arrhythmias in patients with CAD include: 1) acute coronary ischemia, usually as a result of plaque rupture and occlusion of an epicardial coronary artery; and 2) re-entry associated with areas of slow conduction and previous myocardial scarring [58]. In LMIC, the prevalence of CAD is already substantial in nearly every geographic region of the world [59]. It is expected that the burden of CAD and CAD risk factors in LMIC will increase significantly over the next 2 decades [3,44]. Autopsy studies from several LMIC already indicate that CAD accounts for a substantial proportion of SCD cases [12,16,22]. SCD from CAD in LMIC will inevitably increase in the future as the burden of CAD rises.
Substrate conditions: left ventricular dysfunction
Severe left ventricular dysfunction is the most widely used predictor of SCD risk, independent of the presence or absence of CAD. An autopsy report from Nigeria concluded that the majority of SCD cases were due to left ventricular failure secondary to hypertensive heart disease [17]. In Latin America, Chagas disease is a significant risk factor for SCD and is responsible for a substantial proportion of SCD cases [60]. Congestive heart failure as a result of CAD, rheumatic heart disease, hypertensive heart disease, and inflammatory heart disease is estimated to affect nearly 6 million people per year worldwide [3]. The global human immunodeficiency virus (HIV) epidemic is of particular concern in this regard, as both HIV infection [61] and antiretroviral medications [62] have been associated with SCD. The dual and potentially overlapping epidemics of HIV and CVD in LMIC may have profound implications for SCD in these regions.
Substrate conditions: cardiomyopathies and channelopathies
Cardiomyopathies (e.g., hypertrophic cardiomyopathy, dilated cardiomyopathies, arrhythmogenic right ventricular dysplasia, sarcoidosis, and amyloidosis), congenital cardiac conditions (e.g., coronary anomalies, cyanotic/noncyanotic diseases), and genetic channelopathies (e.g., long QT syndromes, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia) account for the remainder of SCD cases. As has already been described, awareness is increasing about specific cardiomyopathies and channelopathies in LMIC that increase SCD risk [53–55].
Environmental triggers
A variety of environmental triggers, in combination with the described substrate conditions, has the potential to incite an episode of unstable arrhythmia that can lead to SCD. These triggers include activity (both vigorous activity [63,64] and sleep [65]), stress [66], diet [67], and pollution [68]. There remains a lack of consensus regarding the relative importance of these different triggers. In addition, the exact mechanisms by which each trigger is related to the occurrence of SCD remain unknown. However, some triggers, such as air pollution, already contribute substantially to the global burden of disease and are expected to increase over time [44].
GLOBAL EPIDEMIOLOGY OF SCD: A WAY FORWARD
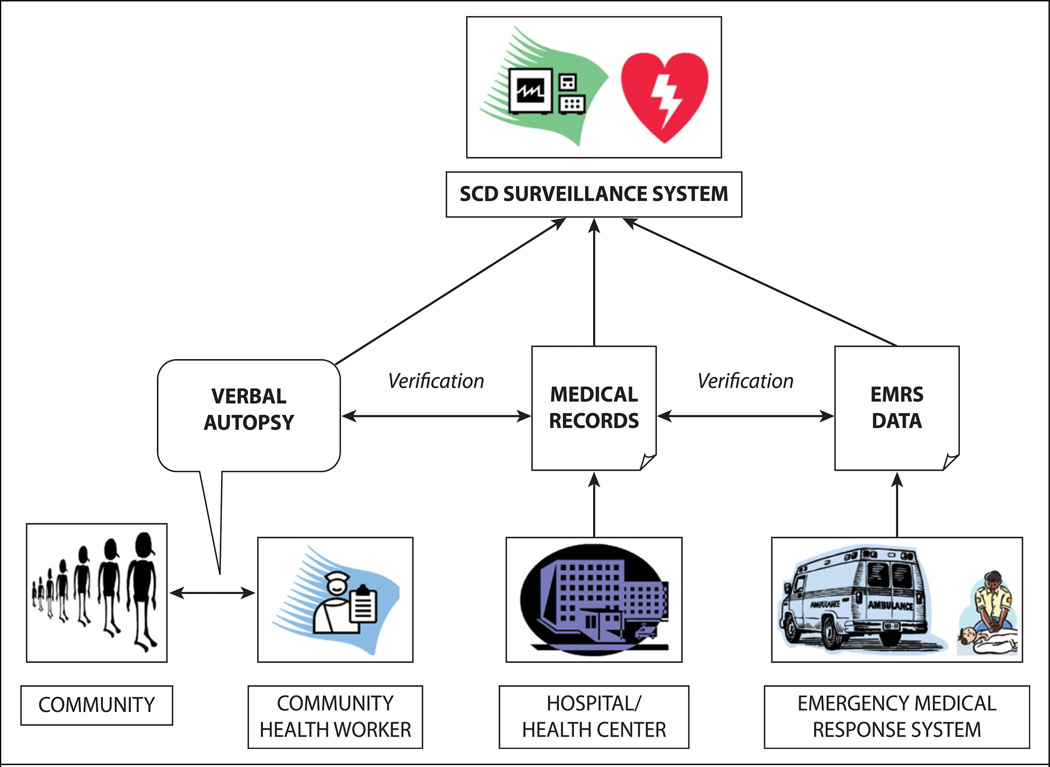
To improve the understanding of SCD epidemiology in LMIC, the global community should consider developing and implementing more robust monitoring and surveillance systems that are prospective and multiple-source with reliable case ascertainment. In the absence of widespread and mature emergency medical response systems, alternative community-based strategies will be required. One potential solution is to use community health workers (CHW), who are community-based workers who have received basic training to support individuals and groups in their own communities to access health and social services, to educate community members about various health issues, and to support overall community development [69–72]. These individuals are able to conduct verbal autopsies of individuals who die in their respective jurisdictions. A verbal autopsy is an interview carried out with family members and/or caregivers of the deceased using a structured questionnaire to elicit signs and symptoms and other pertinent information that can be used to assign a probable underlying cause of death. Standardized protocols for verbal autopsy—including age group-specific questionnaires, cause-of-death certification, ICD-10 coding, and data management procedures—have been developed by the World Health Organization [73]. Whereas verbal autopsy is subject to several limitations—including reliability, accuracy, standardization, comparability, and validity—it has the potential to improve the current situation of extremely limited data with respect to SCD in LMIC. Where possible, this information can be supplemented by hospital or health center records and medical response system data (Fig. 3).
Fig. 3.
Prospective, multiple-source methodology for sudden cardiac death (SCD) surveillance. EMRS, emergency medical response system.
Given the World Health Organization’s support for integrating CHW into national health workforce plans worldwide, this type of community-based strategy for SCD monitoring and surveillance can be considered for implementation in LMIC. However, given the important competing demands and obligations for CHW—including infant and child health, immunizations, nutrition, pre-natal care, control of sexually transmitted diseases, and general preventive care in adults—it is quite possible that CHW in LMIC will not have the time or resources to monitor and document suspected cases of SCD.
Therefore, we propose that this type of community-based strategy for SCD monitoring and surveillance be studied and evaluated in a few sentinel surveillance and research settings in both LMIC and HIC such as the United States. In addition to assessing the accuracy of verbal autopsy for SCD diagnosis, it would be possible to ascertain the costs associated with this type of surveillance, spillover effects (both positive and negative) on other duties of CHW, as well as impact on mortality surveillance in general, which may have important relevance for national vital statistics. These data can then inform policy makers regarding the usefulness and value of this proposal as compared to alternatives.
CONCLUSIONS
In light of the burden of CVD and CVD risk factors in LMIC [3,44], it is likely that SCD will become increasingly prevalent worldwide. However, there are currently insufficient data—and those that exist are of variable quality—to arrive at robust epidemiologic conclusions regarding the true burden of SCD in LMIC and the regions, communities, and individuals most at need for SCD and risk factor prevention. This will require continued, focused investigations using standardized methodology. We recommend that SCD surveillance systems, based on a prospective multiple-source methodology, be studied and evaluated to determine whether they would be appropriate, feasible, and affordable in LMIC. The results of this research will allow investigators and policy makers to determine whether to implement community-based SCD surveillance on a widespread basis. In addition, in light of the complex interaction among genetic predisposition, behavioral risk factors, biological substrates, and environmental triggers that lead to SCD, ongoing investigation to identify high-risk markers and predictors of SCD may ultimately allow for development of risk scores that can be tested in a wide spectrum of patient populations. As we witness the epidemiologic transition worldwide, focus on global SCD and its prevention should be a priority for the global cardiovascular community.
ACKNOWLEDGMENT
The authors would like to thank Vishal Marwah for his assistance in developing the figures for this paper.
REFERENCES
- 1.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva, Switzerland: World Health Organization; 2008. The Global Burden of Disease: 2004 Update. [Google Scholar]
- 4.Kuisma M, Repo J, Alaspaa A. The incidence of out-of-hospital ventricular fibrillation in Helsinki, Finland, from 1994 to 1999. Lancet. 2001;358:473–474. doi: 10.1016/S0140-6736(01)05634-3. [DOI] [PubMed] [Google Scholar]
- 5.Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Every NR, Parsons L, Hlatky MA, et al. Use and accuracy of state death certificates for classification of sudden cardiac deaths in high-risk populations. Am Heart J. 1997;134:1129–1132. doi: 10.1016/s0002-8703(97)70035-8. [DOI] [PubMed] [Google Scholar]
- 7.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Jr, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82:50–53. doi: 10.1016/s0002-9149(98)00240-9. [DOI] [PubMed] [Google Scholar]
- 8.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Byrne R, Constant O, Smyth Y, et al. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the West of Ireland. Eur Heart J. 2008;29:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 10.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua W, Zhang LF, Wu YF, et al. Incidence of sudden cardiac death in China: analysis of 4 regional populations. J Am Coll Cardiol. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZJ. Sudden cardiac death in the People’s Republic of China. Cor Vasa. 1986;28:90–95. [PubMed] [Google Scholar]
- 13.Stein C. Out-of-hospital cardiac arrest cases in Johannesburg, South Africa: a first glimpse of short-term outcomes from a paramedic clinical learning database. Emerg Med J. 2009;26:670–674. doi: 10.1136/emj.2008.066084. [DOI] [PubMed] [Google Scholar]
- 14.Rao BH, Sastry BK, Chugh SS, et al. Contribution of sudden cardiac death to total mortality in India: a population based study. Int J Cardiol. 2012;154:163–167. doi: 10.1016/j.ijcard.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. GPE Discussion Paper Series. Geneva, Switzerland: World Health Organization; 2001. Age Standardization of Rates: a New WHO Standard. [Google Scholar]
- 16.Sudha ML, Sundaram S, Purushothaman KR, Kumar PS, Prathiba D. Coronary atherosclerosis in sudden cardiac death: an autopsy study. Indian. J Pathol Microbiol. 2009;52:486–489. doi: 10.4103/0377-4929.56130. [DOI] [PubMed] [Google Scholar]
- 17.Rotimi O, Fatusi AO, Odesanmi WO. Sudden cardiac death in Nigerians—the Ile-Ife experience. West Afr J Med. 2004;23:27–31. doi: 10.4314/wajm.v23i1.28076. [DOI] [PubMed] [Google Scholar]
- 18.Rotimi O, Ajayi AA, Odesanmi WO. Sudden unexpected death from cardiac causes in Nigerians: a review of 50 autopsied cases. Int J Cardiol. 1998;63:111–115. doi: 10.1016/s0167-5273(97)00274-x. [DOI] [PubMed] [Google Scholar]
- 19.Schneider J, Bezabih K. Causes of sudden death in Addis Ababa, Ethiopia. Ethiop Med J. 2001;39:323–340. [PubMed] [Google Scholar]
- 20.Park HY, Weinstein SR. Sudden unexpected nocturnal death syndrome in the Mariana Islands. Am J Forensic Med Pathol. 1990;11:205–207. doi: 10.1097/00000433-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Voevoda MI, Kulikov IV, Maksimov VN, et al. Sudden cardiac death and polymorphism of genes-candidates of cardiovascular diseases. Kardiologiia. 2009;49:52–57. [PubMed] [Google Scholar]
- 22.Onciu M, Baz R, Onciu C, Leonte T, Sapte E. Epidemiologic aspects of sudden death in the Constanţa District. Rev Med Chir Soc Med Nat Iasi. 2005;109:373–376. [PubMed] [Google Scholar]
- 23.Kopytina RA, Cherkesov VV, Kobets GP, et al. Incidence and pathomorphological characteristics of the development of sudden coronary death in coal miners. Ter Arkh. 1993;65:41–43. [PubMed] [Google Scholar]
- 24.Gupta RR, Mishra N, Jain S, et al. Epidemiology and profile of sudden cardiac deaths (SCD) in hospitalized patients (abstr) Indian Heart J. 2008;60 [abstract no. 355]. [Google Scholar]
- 25.Lokhandwala Y, Panicker GK, Deshpande S. Sudden cardiac death-an Indian perspective. CVD Prev Control. 2009;4:103–108. [Google Scholar]
- 26.Mond HG. The World Survey of Cardiac Pacing and Cardioverter Defibrillators: calendar year 1997–Asian Pacific, Middle East, South America, and Canada. Pacing Clin Electrophysiol. 2001;24:856–862. doi: 10.1046/j.1460-9592.2001.00856.x. [DOI] [PubMed] [Google Scholar]
- 27.Mond HG, Irwin M, Ector H, Proclemer A. The World Survey of Cardiac Pacing and Cardioverter-Defibrillators: calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202–1212. doi: 10.1111/j.1540-8159.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 28.Muratore CA, Batista Sa LA, Chiale PA, et al. Implantable cardioverter defibrillators and Chagas’ disease: results of the ICD Registry Latin America. Europace. 2009;11:164–168. doi: 10.1093/europace/eun325. [DOI] [PubMed] [Google Scholar]
- 29.Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seale C. Changing patterns of death and dying. Soc Sci Med. 2000;51:917–930. doi: 10.1016/s0277-9536(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 31.Population Reference Bureau. Graphics Bank: Aging. [Accessed October 30, 2012];PowerPoint presentation database. Available at: http://www.prb.org/Home/Publications/GraphicsBank/Aging.aspx. [Google Scholar]
- 32.Fuster V, Kelly BB, editors. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 33.Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70:186–192. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Hemingway H, Malik M, Marmot M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur Heart J. 2001;22:1082–1101. doi: 10.1053/euhj.2000.2534. [DOI] [PubMed] [Google Scholar]
- 35.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 36.Rastogi T, Reddy KS, Vaz M, et al. Diet and risk of ischemic heart disease in India. Am J Clin Nutr. 2004;79:582–592. doi: 10.1093/ajcn/79.4.582. [DOI] [PubMed] [Google Scholar]
- 37.Balkau B, Jouven X, Ducimetière P, Eschwège E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 38.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 39.Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 40.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 41.Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC. The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia. 1999;42:68–75. doi: 10.1007/s001250051115. [DOI] [PubMed] [Google Scholar]
- 42.Pourmoghaddas A, Hekmatnia A. The relationship between QTc interval and cardiac autonomic neuropathy in diabetes mellitus. Mol Cell Biochem. 2003;249:125–128. [PubMed] [Google Scholar]
- 43.Rana BS, Lim PO, Naas AA, et al. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91:44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Geneva, Switzerland: World Health Organization; 2009. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. [Google Scholar]
- 45.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Bennett GL, Xiaoren P. China. In: Ekoe PZ, Williams R, editors. The epidemiology of diabetes mellitus: an international perspective. Chichester, England: J Wiley; 2001. pp. 247–251. [Google Scholar]
- 47.Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M. Rising prevalence of NIDDM in an urban population in India. Diabetologia. 1997;40:232–237. doi: 10.1007/s001250050668. [DOI] [PubMed] [Google Scholar]
- 48.Motala AA. Diabetes trends in Africa. Diabetes Metab Res Rev. 2002;18(Suppl 3):S14–S20. doi: 10.1002/dmrr.284. [DOI] [PubMed] [Google Scholar]
- 49.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 50.Friedlander Y, Siscovick DS, Weinmann S, et al. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 51.Benhorin J, Moss AJ, Bak M, et al. Variable expression of long QT syndrome among gene carriers from families with five different HERG mutations. Ann Noninvasive Electrocardiol. 2002;7:40–46. doi: 10.1111/j.1542-474X.2001.tb00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. doi: 10.1161/01.cir.99.5.666. [DOI] [PubMed] [Google Scholar]
- 53.Watkins DA, Hendricks N, Shaboodien G, et al. for the CASSA Investigators. Clinical features, survival experience, and profile of plakophylin-2 gene mutations in participants of the arrhythmogenic right ventricular cardiomyopathy registry of South Africa. Heart Rhythm. 2009;6:S10–S17. doi: 10.1016/j.hrthm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Shi R, Zhang Y, Yang C, et al. The cardiac sodium channel mutation delQKP 1507–1509 is associated with the expanding phenotypic spectrum of LQT3, conduction disorder, dilated cardiomyopathy, and high incidence of youth sudden death. Europace. 2008;10:1329–1335. doi: 10.1093/europace/eun202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouali S, Boughzela E, Haggui A, et al. Clinical and electrophysiological profile of Brugada syndrome in the Tunisian population. Pacing Clin Electrophysiol. 2011;34:47–53. doi: 10.1111/j.1540-8159.2010.02890.x. [DOI] [PubMed] [Google Scholar]
- 56.Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 57.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 58.Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007;115:2451–2457. doi: 10.1161/CIRCULATIONAHA.106.683235. [DOI] [PubMed] [Google Scholar]
- 59.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rassi A, Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115:1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 61.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh M, Arora R, Jawad E. HIV protease inhibitors induced prolongation of the QT interval: electrophysiology and clinical implications. Am J Ther. 2010;17:e193–e201. doi: 10.1097/MJT.0b013e3181ad3437. [DOI] [PubMed] [Google Scholar]
- 63.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 64.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305:1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy PR, Reinier K, Singh T, et al. Physical activity as a trigger of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Int J Cardiol. 2009;131:345–349. doi: 10.1016/j.ijcard.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eliot RS, Buell JC. Role of emotions and stress in the genesis of sudden death. J Am Coll Cardiol. 1985;5(Suppl 6):95B–98B. doi: 10.1016/s0735-1097(85)80535-0. [DOI] [PubMed] [Google Scholar]
- 67.Varró A, Baczkó I. Possible mechanisms of sudden cardiac death in top athletes: a basic cardiac electrophysiological point of view. Pflugers Arch. 2010;460:31–40. doi: 10.1007/s00424-010-0798-0. [DOI] [PubMed] [Google Scholar]
- 68.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhutta ZA, Lassi ZS, Pariyo G, Huicho L. Geneva, Switzerland: World Health Organization; 2010. Global Experience of Community Health Workers for Delivery of Health Related Millennium Development Goals: A Systematic Review, Country Case Studies, and Recommendations for Integration into National Health Systems. [Google Scholar]
- 70.The Earth Institute. One Million Community Health Workers: Technical Task Force Report. New York, NY: The Earth Institute, Columbia University; 2011. [Google Scholar]
- 71.Lehman U, Sanders D. The State of the Evidence on Programmes, Activities, Costs and Impact on Health Outcomes of Using Community Health Workers. Geneva, Switzerland: World Health Organization; 2007. Community Health Workers: What Do We Know About Them? [Google Scholar]
- 72.Richter RW, Bengen B, Alsup PA, Bruun B, Kilcoyne MM, Challenor BD. The community health worker: a resource for improved health care delivery. Am J Public Health. 1974;64:1056–1061. doi: 10.2105/ajph.64.11.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Geneva, Switzerland: World Health Organization; 2007. Verbal Autopsy Standards: Ascertaining and Attributing Cause of Death. [Google Scholar]