Abstract
There are 33 human tetraspanin proteins, emerging as key players in malignancy, the immune system, fertilization, cellular signaling, adhesion, morphology, motility, proliferation, and tumor invasion. CD9, a member of the tetraspanin family, associates with and influences a variety of cell-surface molecules. Through these interactions, CD9 modifies multiple cellular events, including adhesion, migration, proliferation, and survival. CD9 is therefore considered to play a role in several stages during cancer development. Reduced CD9 expression is generally related to venous vessel invasion and metastasis as well as poor prognosis. We found that treatment of mice bearing human gastric cancer cells with anti-CD9 antibody successfully inhibited tumor progression via antiproliferative, proapoptotic, and antiangiogenic effects, strongly indicating that CD9 is a possible therapeutic target in patients with gastric cancer. Here, we describe the possibility of CD9 manipulation as a novel therapeutic strategy in gastric cancer, which still shows poor prognosis.
Keywords: CD9, Tetraspanin, Gastric cancer, Tumorigenicity, Therapeutic target
Core tip: Tetraspanin CD9 is a cell-surface protein with four transmembrane domains and is found in several organs. Although CD9 was primarily identified as a tumor suppressor, it exhibits diverse functions through its association with various partner proteins. CD9 relates to tumor proliferation, apoptosis, migration, adhesion, and angiogenesis, therefore involving several steps of tumor formation: communication with the environment, dissemination, and metastasis. In this review, we describe the possibility of CD9 manipulation as a novel therapeutic strategy to improve clinical outcome in gastric cancer.
INTRODUCTION
Gastric cancer is one of the most common malignancies, remaining a major public health issue as the fourth most common cancer and the second leading cause of cancer death worldwide[1], with a particularly high incidence in Japan, China, South Korea, Chile and Costa Rica. The large regional incidence variations possibly reflect different prevalences of Helicobacter pylori infection, which is responsible for > 60% of gastric cancer globally. Advanced gastric cancer is an aggressive disease, and the prognosis remains poor. The 5-year survival rate for locoregional disease is 25%-35%[2-4] and the median survival ranges from 10 to 14 mo in advanced disease[5,6]. Although various treatment modalities have been developed and the mortality rate of gastric cancer has gradually decreased over recent decades[7], many of them have failed to eliminate gastric cancer cells curatively[8]. Therefore, a novel therapeutic strategy is clinically desired.
CD9, a member of the tetraspanin family, has been reported to relate to growth and invasion of tumor cells. There are many reports of the relationship between CD9 expression and disease prognosis. In addition, molecular mechanisms of CD9 functions have been gradually clarified. In this field, we also reported apoptotic signals after CD9 ligation in gastric cancer cells, as well as the treatment of gastric-cancer-bearing mice with anti-CD9 antibody.
We review the characteristics of CD9 and discuss the possibility of CD9 as a novel therapeutic target in gastric cancer.
CD9 FUNCTIONS
Tetraspanins, which have four putative membrane-spanning domains, are integral membrane proteins including at least 33 distinct family members, such as CD9,CD37, CD53, CD63, CD81, CD82, and CD151[9-11]. Members of this family are involved in many physiological and pathological processes, such as fertilization, cellular adhesion, motility, and tumor invasion[9-12]. To date, tetraspanins are believed to act as molecular facilitators or adaptors, which form a network of interaction among the cell-surface molecules, known as the “tetraspanin web” or tetraspan-enriched microdomains[12,13]. Notably, some tetraspanin proteins have key roles in tumor initiation, promotion, metastasis, and angiogenesis.
CD9, which was identified as a suppressor of cancer spread[14], belongs to the tetraspanin family. Like other tetraspanins, CD9 has four putative transmembrane domains, which provide the short N- and C-terminal cytoplasmic domains, a small intracellular loop, and two extracellular loops[11,12] (Figure 1). CD9 is widely expressed on the surface of several types of cells, including many malignant tumor cells as well as normal hematopoietic, endothelial and epithelial cells[11,12].
Figure 1.
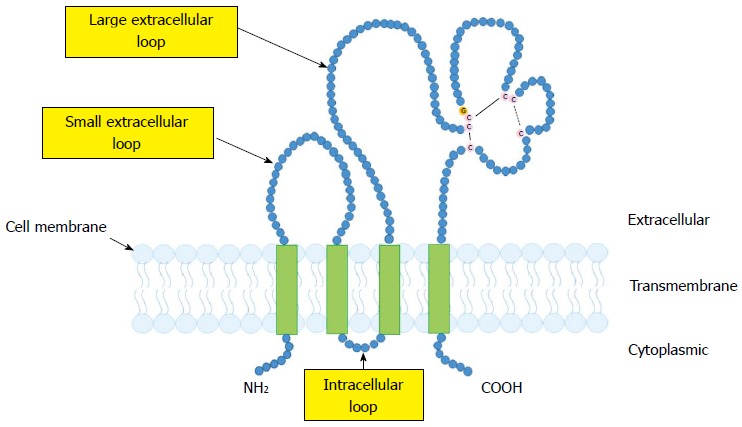
Structural features of CD9. CD9 has four putative transmembrane domains, which provide the short N- and C-terminal cytoplasmic domains, a small intracellular loop, and two extracellular loops. C: Cysteine; G: Glycine.
CD9 interacts with a number of transmembrane proteins, including integrins, immunoglobulin superfamily member EWI proteins (EWI-2 and EWI-F) and other tetraspanins (e.g., CD81 and CD151)[10-13], Claudin-1[15], epidermal growth factor receptor (EGFR)[16], and membrane-bound ligands for EGFR[17-19] (Table 1). These interactions form functional complexes, which facilitate cell adhesion, motility, and signaling[10,20-24]. For examples, antibody (Ab) ligation of CD9 induces homotypic aggregation of pre-B cells and augments their adhesion to bone marrow fibroblasts through the modification of integrins[10]. Treatment with anti-CD9 Ab can induce strong adhesion between stromal and hematopoietic cells[25,26] as well as inhibit the migration of malignant cells[27]. In addition, CD9 acts as a co-receptor for diphtheria toxin. CD9 does not bind directly to the toxin, but interacts with the diphtheria toxin receptor (transmembrane precursor of heparin-binding epidermal-growth-factor-like growth factor; HB-EGF), leading to the elevation of juxtacrine activity of HB-EGF[28,29]. Also, CD9 functionally associates with Fcγ receptors, and co-cross-linking of CD9-Fcγ receptors modifies signals for phagocytosis and inflammatory responses on macrophages[30].
Table 1.
CD9 associated with partner proteins
| Partner protein | Function | Ref. |
| EWI-2 | Modulates integrin-dependent cell motility, morphology and/or spreading | [5-6,8,44,45,50] |
| EWI-F | Functions unknown | [5-6,8,46,47] |
| Integrin β1 | CD9 modulates integrin-dependent cell morphology, cell migration, signaling and adhesion strengthening | [5,11] |
| Other tetraspanins (e.g., CD81, CD151) | Form TEMs | [7,8] |
| Claudin-1 | CD9 stabilizes expression of non-junctional Claudin-1 | [10] |
| EGFR | CD9 enhances the internalization of EGFR and reduces EGF-EGFR-induced signals | [11] |
| HB-EGF | CD9 upregulates both diphtheria toxin binding and mitogenic functions of HB-EGF | [23,24] |
| PKC isoforms | Contribute to signaling and tumor-suppressor functions | [29] |
| Type II PI4K | Contribute to signaling and tumor-suppressor functions | [30] |
EGFR: Epidermal growth factor receptor; HB-EGF: Heparin-binding epidermal-growth-factor-like growth factor; PKC: Protein kinase C; TEMS: Tetraspan-enriched microdomains.
CD9 affects physical processes, such as cell proliferation, apoptosis and tumor metastasis[31-33]. Treatment of cells with anti-CD9 Ab has revealed antiproliferative effects[16,18] via the suppression of extracellular signal-regulated kinase (ERK) 1/2 activity[31]. In addition, CD9 ligation concurrently induces apoptosis via the selective activation of the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and p38 mitogen-activated protein kinase (MAPK) pathway, as well as caspase-3 and the p46 Shc isoform[31]. Moreover, CD9 can associate with conventional protein kinase C (PKC) isoforms including PKCα and PKCβ[34], as well as type II phosphatidylinositol 4-kinase[35], which could contribute to tumor-suppressor functions. In addition, CD9 may affect the Wnt signaling pathway by downregulating Wnt genes[36]. Expression of CD9 also acts to protect transforming growth factor α from cleavage, thereby regulating cell proliferation and migration[19]. Therefore, CD9 expression has an ability to regulate a variety of intracellular signals.
CD9 AND CANCER
From experiments manipulating CD9 in tumor cell lines, CD9 has been demonstrated to be primarily a suppressor of metastasis[27,37-40]. Several clinical studies have also shown an important prognostic value of CD9. The reduced CD9 expression is associated with poor prognosis in melanoma[41], non-small-cell lung cancer[28], and breast[37,42], colon[43], pancreatic[44], ovarian[45] and prostate[46] cancer. Expression of CD9 is also related to metastasis of the gastrointestinal carcinoma[43,44,47,48]. For example, reduced CD9 expression is significantly associated with more venous vessel invasion and liver metastasis in patients with colon cancer[27,43]. Although diverse physiological functions (clinical data) of CD9 have been suggested[49,50], we and others have found that the amount of CD9 is inversely correlated with lymph node status in gastric cancer[48] and in esophageal squamous cell carcinoma[47]. Moreover, expression of CD9 protein in gastric cancer tissues was significantly stronger in patients without regional lymph node or distant metastasis than in those with metastasis[51]. Furthermore, the reduction of CD9 protein was associated with distant metastasis of gastric cancer. Thus, decreased levels of CD9 are strongly associated with an increased risk of recurrence, especially in patients with N0 nodal status and M0 metastatic status. Low levels of CD9 expression are related to poor prognosis. These findings are consistent with previous reports. Therefore, reduced CD9 expression is generally related to more venous vessel invasion and metastasis as well as poor prognosis in most common types of cancer.
As mentioned above, many investigators believe that CD9 is a suppressor of tumor development.
POSSIBILITY OF CD9-TARGETED THERAPY IN GASTRIC CANCER
Anti-CD9 monoclonal Abs (mAbs), ALB6 and PAINS-13 are ligand-mimic Abs, therefore, Ab ligation of CD9 with these antibodies enhances, but does not inhibit, CD9 functions (Figure 2). We first introduce some interesting data concerning mechanisms of CD9 functions obtained by using these Abs. We previously reported that treatment with anti-CD9 mAb (ALB6), which enhances CD9 functions, inhibited cell growth in CD9-positive tumor cell lines (MKN-28, MKN-45, SW480, HT-29, CaCO2, MIA-PaCa-2 and A459)[31]. In a gastric cancer line MKN-28, CD9 ligation induced apoptosis. ALB6 treatment activated JNK/SAPK and p38 MAPK as well as caspase-3[31]. Notably, ALB6 treatment selectively induced tyrosine phosphorylation of the p46 Shc isoform, and overexpression of its dominant-negative form completely cancelled the ALB6-induced activation of JNK/SAPK, p38 MAPK and caspase-3, leading to loss of apoptosis. Therefore, Ab ligation of CD9 induced apoptotic signals via restricted activation of the p46 Shc isoform. We also reported that CD9 ligation enhanced the internalization of EGFR[16]. ALB6 treatment induced a dotted or patch-like aggregation composed of CD9-EGFR and CD9-β1 integrin on the surface of MKN-28 cells. Furthermore, expression of CD9 specifically attenuated EGFR signaling in CD9-overexpressing CHO cells via the downregulation of surface expression of EGFR[16]. Therefore, CD9 expression negatively regulates cell surface EGFR expression levels. Finally, we examined in vivo effects of ALB6 Ab to treat patients with gastric cancer. MKN-28 cells were inoculated subcutaneously into SCID mice. After a tumor was visualized, the MKN-28-bearing mice were injected with ALB6 or control Ab three times per week. In the ALB6 treatment group, tumor volume was significantly suppressed, and the apoptotic indexes were increased. Therefore, administration of mice bearing human gastric cancer cells with anti-CD9 Ab successfully inhibited tumor progression[52]. Similar to our results, it has been reported that anti-CD9 mAb PAINS 13 inhibited in vivo tumor growth of colon cancer cells[53]. The inhibition of cell proliferation in colon carcinoma cells caused by anti-CD9 mAbs PAINS-13 was related to the enhanced integrin-dependent adhesion and the increased expression of membrane tumor necrosis factor (TNF)-α.
Figure 2.
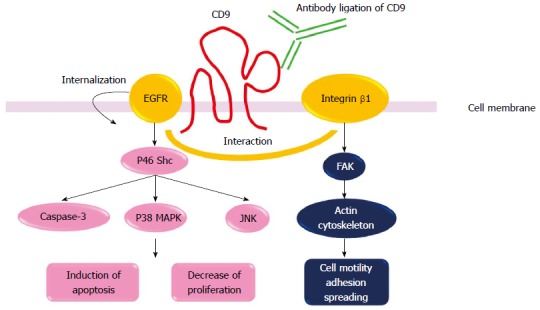
CD9 signaling. CD9-EGFR and CD9-β1 integrin co-localize on the cell surface. CD9 enhances the internalization of EGFR and reduces EGF-EGFR-induced signals[11]. CD9 ligation induced apoptosis via the selective activation of JNK and p38 MAPK pathway as well as caspase-3 and the p46 Shc isoform[26]. CD9 modulates integrin-dependent cell motility, cell migration, adhesion strengthening, and spreading[5,11]. EGFR: Epidermal growth factor receptor; p38 MAPK: p38 mitogen-activated-protein kinase; JNK: c-Jun NH2-terminal kinase; FAK: Focal adhesion kinase.
Therefore, TNF-α partly mediates the antiproliferative effects of CD9 in this case.
Overexpression of vascular endothelial growth factor (VEGF)-A is associated with tumor angiogenesis, nodal metastasis, and poor prognosis in cancer patients[54,55]. A report that CD9 gene transduction could downregulate VEGF-A expression is now available[36]. In this situation, CD9 is also likely to regulate tumor development negatively.
With regard to interactions between CD9 and integrins, CD9 seems to positively and/or negatively involve tumor development through functional modification of integrins. Indeed, the enhancement of integrin-mediated cell adhesion by CD9 inhibits metastasis and invasion of tumor cells and contributes to cell-adhesion-mediated drug resistance[56].
PRESENT TREATMENT FOR PATIENTS WITH GASTRIC CANCER
Improving molecular characterization has translated into better survival in select patients with advanced gastric and esophageal cancer. Trastuzumab, an antibody targeting the anti-human epidermal growth factor receptor 2 (HER2) extracellular domain, induces antibody-dependent cellular cytotoxicity and inhibits the HER2 downstream signals. In the ToGA study, standard chemotherapy regimens (capecitabine plus cisplatin or fluorouracil plus cisplatin) combined with trastuzumab resulted in a longer survival time than standard regimens without trastuzumab in patients with HER2-positive gastric cancer[57,58]. In addition, ramucirumab, an mAb targeting vascular endothelial growth factor receptor (VEGFR)-2, is the first biological treatment that showed survival benefits as a single-agent therapy for the second-line chemotherapy (REGARD trial) in patients with advanced gastric cancer who progressed after first-line chemotherapy[59]. An early report of the phase III RAINBOW trial, testing ramucirumab in combination with paclitaxel for the second-line therapy after platinum-fluoropyrimidine failure, also demonstrated an overall survival benefit of 9.6 mo vs 7.4 mo as compared with paclitaxel alone[60]. With recent success of ramucirumab, investigations with several other antiangiogenic agents have begun. These include the VEGFR-2 inhibitor, apatinib, and the multi-targeted tyrosine kinase receptor inhibitors, axitinib and pazopanib[60]. In addition to the HER family and VEGFRs, the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (mTOR) and the c-MET signaling pathways are promising candidates, and some molecular targeting agents are now in clinical investigation[61].
FUTURE PROSPECTS
A number of recent reports have suggested that tetraspanin targeting by Abs, soluble large-loop proteins, RNAi technology, or adenoviral transduction methods could be therapeutically beneficial[62]. In the case of CD9, we and others have proposed that CD9 ligation is likely to be useful to treat malignancies. Ectopic expression of CD9 in small-cell lung carcinoma cells inhibited their proliferation[63], and adenoviral transduction of CD9 inhibited lymph node metastasis in an orthotopic lung cancer model[40]. With cDNA expression microarray experiments, CD9 was reported to be one of the genes upregulated in gastric cancer[64]. Thus, CD9 expression in non-cancerous tissues is lower than that in gastric cancer tissues, indicating that adverse effects of anti-CD9 treatment on normal gastrointestinal tissues might be tolerable.
Tumor growth is dependent on angiogenesis, which forms new blood vessels[65]. Targeting tumor vessels provides several advantages over traditional anti-tumor approaches. CD9 enhancement contributes to tumor angiogenesis, presumably by affecting endothelial cell function, although their contributions to angiogenesis have not been shown using de novo tumor models. It was previously reported that CD9 gene transduction could downregulate VEGF-A expression, which is essential for angiogenesis[36]. Therefore, enhancement of CD9 functions may also be worthwhile in particular circumstances.
With regard to tumor metastasis, CD9 is involved in cell adhesion via enhancing integrin functions. In addition, associations of CD9 with EWI-2[10,11,13,66,67], EWI-F[68,69], EPCAM[70], Claudin-1[10] or HB-EGF[23,24] could have different effects on tumor cell invasion and metastasis. Indeed, the CD9 partners EWI-F[71] and EWI-2 can markedly affect cell migration[72], and EWI-2 influences the association of CD9 with membrane-type 1 matrix metalloproteinase (MT1-MMP; also known as MMP14) and MMP2[73], which could alter proteolysis during invasion. Thus, CD9 acts on multiple steps of tumorigenesis, and because CD9 function is dependent on its associating proteins, efficacy of the CD9-targeting therapy may be determined by expression of these associating molecules as well as CD9 itself.
CONCLUSION
Molecular mechanisms for CD9 functions have been understood through identification of CD9-associating proteins. Ab ligation of CD9 is a powerful tool to change CD9 functions, and we showed apoptotic signals after CD9 ligation in gastric cancer cells as well as successful treatment of gastric-cancer-bearing mice with anti-CD9 Ab. CD9 influences intracellular signals, cell adhesion, and cell proliferation, and is involved in several events during development of gastric cancer. Taken together with evidence from clinical data, the manipulation of CD9 is likely to have the potential to improve clinical results of therapy for gastric cancer. When implementing CD9-targeted therapy in gastric cancer, we should come up with various ideas to enhance CD9 functions.
A new therapy to target HER2, VEGFR-2, is responsible for a significant increase in survival of patients with advanced gastric cancer. Unfortunately, advanced gastric cancer continues to have a poor prognosis. In the future, new strategies to target CD9 will hopefully be developed and implemented for gastric cancer treatment.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 24, 2014
First decision: August 15, 2014
Article in press: December 16, 2014
P- Reviewer: Luo QF, Qi F S- Editor: Yu J L- Editor: Kerr C E- Editor: Liu XM
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 6.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 8.Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237–3242. doi: 10.3748/wjg.v12.i20.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 10.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 11.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 12.Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 14.Miyake M, Koyama M, Seno M, Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991;174:1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovalenko OV, Yang XH, Hemler ME. A novel cysteine cross-linking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol Cell Proteomics. 2007;6:1855–1867. doi: 10.1074/mcp.M700183-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Murayama Y, Shinomura Y, Oritani K, Miyagawa J, Yoshida H, Nishida M, Katsube F, Shiraga M, Miyazaki T, Nakamoto T, et al. The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol. 2008;216:135–143. doi: 10.1002/jcp.21384. [DOI] [PubMed] [Google Scholar]
- 17.Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inui S, Higashiyama S, Hashimoto K, Higashiyama M, Yoshikawa K, Taniguchi N. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J Cell Physiol. 1997;171:291–298. doi: 10.1002/(SICI)1097-4652(199706)171:3<291::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Fan H, Shum L, Derynck R. The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation. J Cell Biol. 2000;148:591–602. doi: 10.1083/jcb.148.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones PH, Bishop LA, Watt FM. Functional significance of CD9 association with beta 1 integrins in human epidermal keratinocytes. Cell Adhes Commun. 1996;4:297–305. doi: 10.3109/15419069609010773. [DOI] [PubMed] [Google Scholar]
- 21.Baudoux B, Castanares-Zapatero D, Leclercq-Smekens M, Berna N, Poumay Y. The tetraspanin CD9 associates with the integrin alpha6beta4 in cultured human epidermal keratinocytes and is involved in cell motility. Eur J Cell Biol. 2000;79:41–51. doi: 10.1078/s0171-9335(04)70006-0. [DOI] [PubMed] [Google Scholar]
- 22.Yáñez-Mó M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landázuri MO, Sánchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3 beta1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw AR, Domanska A, Mak A, Gilchrist A, Dobler K, Visser L, Poppema S, Fliegel L, Letarte M, Willett BJ. Ectopic expression of human and feline CD9 in a human B cell line confers beta 1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem. 1995;270:24092–24099. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- 25.Oritani K, Wu X, Medina K, Hudson J, Miyake K, Gimble JM, Burstein SA, Kincade PW. Antibody ligation of CD9 modifies production of myeloid cells in long-term cultures. Blood. 1996;87:2252–2261. [PubMed] [Google Scholar]
- 26.Aoyama K, Oritani K, Yokota T, Ishikawa J, Nishiura T, Miyake K, Kanakura Y, Tomiyama Y, Kincade PW, Matsuzawa Y. Stromal cell CD9 regulates differentiation of hematopoietic stem/progenitor cells. Blood. 1999;93:2586–2594. [PubMed] [Google Scholar]
- 27.Cajot JF, Sordat I, Silvestre T, Sordat B. Differential display cloning identifies motility-related protein (MRP1/CD9) as highly expressed in primary compared to metastatic human colon carcinoma cells. Cancer Res. 1997;57:2593–2597. [PubMed] [Google Scholar]
- 28.Higashiyama M, Taki T, Ieki Y, Adachi M, Huang CL, Koh T, Kodama K, Doi O, Miyake M. Reduced motility related protein-1 (MRP-1/CD9) gene expression as a factor of poor prognosis in non-small cell lung cancer. Cancer Res. 1995;55:6040–6044. [PubMed] [Google Scholar]
- 29.Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaji K, Takeshita S, Miyake K, Takai T, Kudo A. Functional association of CD9 with the Fc gamma receptors in macrophages. J Immunol. 2001;166:3256–3265. doi: 10.4049/jimmunol.166.5.3256. [DOI] [PubMed] [Google Scholar]
- 31.Murayama Y, Miyagawa J, Oritani K, Yoshida H, Yamamoto K, Kishida O, Miyazaki T, Tsutsui S, Kiyohara T, Miyazaki Y, et al. CD9-mediated activation of the p46 Shc isoform leads to apoptosis in cancer cells. J Cell Sci. 2004;117:3379–3388. doi: 10.1242/jcs.01201. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana I, Hemler ME. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol. 1999;146:893–904. doi: 10.1083/jcb.146.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono M, Handa K, Withers DA, Hakomori S. Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation. Cancer Res. 1999;59:2335–2339. [PubMed] [Google Scholar]
- 34.Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem. 2001;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- 35.Yauch RL, Hemler ME. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphoinositide 4-kinase. Biochem J. 2000;351 Pt 3:629–637. [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, Ueno M, Miyake M. MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene. 2004;23:7475–7483. doi: 10.1038/sj.onc.1208063. [DOI] [PubMed] [Google Scholar]
- 37.Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–1755. [PubMed] [Google Scholar]
- 38.Miyake M, Nakano K, Ieki Y, Adachi M, Huang CL, Itoi S, Koh T, Taki T. Motility related protein 1 (MRP-1/CD9) expression: inverse correlation with metastases in breast cancer. Cancer Res. 1995;55:4127–4131. [PubMed] [Google Scholar]
- 39.Kusukawa J, Ryu F, Kameyama T, Mekada E. Reduced expression of CD9 in oral squamous cell carcinoma: CD9 expression inversely related to high prevalence of lymph node metastasis. J Oral Pathol Med. 2001;30:73–79. doi: 10.1034/j.1600-0714.2001.300202.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeda T, Hattori N, Tokuhara T, Nishimura Y, Yokoyama M, Miyake M. Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res. 2007;67:1744–1749. doi: 10.1158/0008-5472.CAN-06-3090. [DOI] [PubMed] [Google Scholar]
- 41.Si Z, Hersey P. Expression of the neuroglandular antigen and analogues in melanoma. CD9 expression appears inversely related to metastatic potential of melanoma. Int J Cancer. 1993;54:37–43. doi: 10.1002/ijc.2910540107. [DOI] [PubMed] [Google Scholar]
- 42.Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998;153:973–983. doi: 10.1016/s0002-9440(10)65639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori M, Mimori K, Shiraishi T, Haraguchi M, Ueo H, Barnard GF, Akiyoshi T. Motility related protein 1 (MRP1/CD9) expression in colon cancer. Clin Cancer Res. 1998;4:1507–1510. [PubMed] [Google Scholar]
- 44.Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang CL, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M, et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer. 1998;79:509–516. doi: 10.1002/(sici)1097-0215(19981023)79:5<509::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Houle CD, Ding XY, Foley JF, Afshari CA, Barrett JC, Davis BJ. Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol Oncol. 2002;86:69–78. doi: 10.1006/gyno.2002.6729. [DOI] [PubMed] [Google Scholar]
- 46.Wang JC, Bégin LR, Bérubé NG, Chevalier S, Aprikian AG, Gourdeau H, Chevrette M. Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin Cancer Res. 2007;13:2354–2361. doi: 10.1158/1078-0432.CCR-06-1692. [DOI] [PubMed] [Google Scholar]
- 47.Uchida S, Shimada Y, Watanabe G, Li ZG, Hong T, Miyake M, Imamura M. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 1999;79:1168–1173. doi: 10.1038/sj.bjc.6690186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Isozaki K, Yamamori K, Mizuno H, Ishiguro S, Kiyohara T, Miyazaki Y, et al. Significance of the association between heparin-binding epidermal growth factor-like growth factor and CD9 in human gastric cancer. Int J Cancer. 2002;98:505–513. doi: 10.1002/ijc.10198. [DOI] [PubMed] [Google Scholar]
- 49.Fricker G, Drewe J, Vonderscher J, Kissel T, Beglinger C. Enteral absorption of octreotide. Br J Pharmacol. 1992;105:783–786. doi: 10.1111/j.1476-5381.1992.tb09057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz OG, Orhan O. Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol Res Pract. 2010;206:607–610. doi: 10.1016/j.prp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z, Gu S, Trojanowicz B, Liu N, Zhu G, Dralle H, Hoang-Vu C. Down-regulation of TM4SF is associated with the metastatic potential of gastric carcinoma TM4SF members in gastric carcinoma. World J Surg Oncol. 2011;9:43. doi: 10.1186/1477-7819-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamoto T, Murayama Y, Oritani K, Boucheix C, Rubinstein E, Nishida M, Katsube F, Watabe K, Kiso S, Tsutsui S, et al. A novel therapeutic strategy with anti-CD9 antibody in gastric cancers. J Gastroenterol. 2009;44:889–896. doi: 10.1007/s00535-009-0081-3. [DOI] [PubMed] [Google Scholar]
- 53.Ovalle S, Gutiérrez-López MD, Olmo N, Turnay J, Lizarbe MA, Majano P, Molina-Jiménez F, López-Cabrera M, Yáñez-Mó M, Sánchez-Madrid F, et al. The tetraspanin CD9 inhibits the proliferation and tumorigenicity of human colon carcinoma cells. Int J Cancer. 2007;121:2140–2152. doi: 10.1002/ijc.22902. [DOI] [PubMed] [Google Scholar]
- 54.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 55.Masuya D, Huang C, Liu D, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S, Haba R, Yokomise H. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer. 2001;92:2628–2638. doi: 10.1002/1097-0142(20011115)92:10<2628::aid-cncr1616>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 56.Carloni V, Mazzocca A, Mello T, Galli A, Capaccioli S. Cell fusion promotes chemoresistance in metastatic colon carcinoma. Oncogene. 2013;32:2649–2660. doi: 10.1038/onc.2012.268. [DOI] [PubMed] [Google Scholar]
- 57.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 58.de Mello RA, Marques AM, Araújo A. HER2 therapies and gastric cancer: a step forward. World J Gastroenterol. 2013;19:6165–6169. doi: 10.3748/wjg.v19.i37.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 60.Wilke H, Cutsem EV, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, et al. RAINBOW: A global, phage III, randomized, double-blind study of ramucirumab plus paclitaxel vs placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922(I4T-IE-JVBE) J Clin Oncol. 2014;32 Suppl 3:(abstr LBA7). [Google Scholar]
- 61.Yang W, Raufi A, Klempner SJ. Targeted therapy for gastric cancer: molecular pathways and ongoing investigations. Biochim Biophys Acta. 2014;1846:232–237. doi: 10.1016/j.bbcan.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Hemler ME. Targeting of tetraspanin proteins--potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng R, Yano S, Zhang H, Nakataki E, Tachibana I, Kawase I, Hayashi S, Sone S. CD9 overexpression suppressed the liver metastasis and malignant ascites via inhibition of proliferation and motility of small-cell lung cancer cells in NK cell-depleted SCID mice. Oncol Res. 2005;15:365–372. doi: 10.3727/096504005776449699. [DOI] [PubMed] [Google Scholar]
- 64.Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580–585. doi: 10.3748/wjg.v8.i4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J Biol Chem. 2001;276:40545–40554. doi: 10.1074/jbc.M107338200. [DOI] [PubMed] [Google Scholar]
- 67.Charrin S, Le Naour F, Labas V, Billard M, Le Caer JP, Emile JF, Petit MA, Boucheix C, Rubinstein E. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J. 2003;373:409–421. doi: 10.1042/BJ20030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charrin S, Le Naour F, Oualid M, Billard M, Faure G, Hanash SM, Boucheix C, Rubinstein E. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J Biol Chem. 2001;276:14329–14337. doi: 10.1074/jbc.M011297200. [DOI] [PubMed] [Google Scholar]
- 69.Stipp CS, Orlicky D, Hemler ME. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J Biol Chem. 2001;276:4853–4862. doi: 10.1074/jbc.M009859200. [DOI] [PubMed] [Google Scholar]
- 70.Le Naour F, André M, Greco C, Billard M, Sordat B, Emile JF, Lanza F, Boucheix C, Rubinstein E. Profiling of the tetraspanin web of human colon cancer cells. Mol Cell Proteomics. 2006;5:845–857. doi: 10.1074/mcp.M500330-MCP200. [DOI] [PubMed] [Google Scholar]
- 71.Chambrion C, Le Naour F. The tetraspanins CD9 and CD81 regulate CD9P1-induced effects on cell migration. PLoS One. 2010;5:e11219. doi: 10.1371/journal.pone.0011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 regulates alpha3beta1 integrin-dependent cell functions on laminin-5. J Cell Biol. 2003;163:1167–1177. doi: 10.1083/jcb.200309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolesnikova TV, Kazarov AR, Lemieux ME, Lafleur MA, Kesari S, Kung AL, Hemler ME. Glioblastoma inhibition by cell surface immunoglobulin protein EWI-2, in vitro and in vivo. Neoplasia. 2009;11:77–86, 4p following 86. doi: 10.1593/neo.81180. [DOI] [PMC free article] [PubMed] [Google Scholar]


