Abstract
AIM: To evaluate the predictive value of the lymph node (LN) ratio (LNR, number of metastatic LNs/ examined LNs) for recurrence in patients with rectal cancer and to compare its applicability according to preoperative chemoradiotherapy (PCRT).
METHODS: From 2000 to 2009, 967 patients with metastatic LNs after curative resection for locally advanced rectal cancer were identified. Patients were categorized according to PCRT (PCRT vs No PCRT). The cut-off LNR was determined based on the pN1 vs pN2 when the recommended number of LNs was harvested. The 5-year recurrence-free survival (RFS) rates using the Kaplan-Meier method were compared according to p/yp N stage and the LNR in each group.
RESULTS: Among patients with the same p/ypN stage, the 5-year RFS rate differed according to the LNR. In addition, the 5-year RFS rate was significantly different between pN and LNR groups in patients with No PCRT. In PCRT group, however, only LNR was associated with prognosis. On multivariate analysis, both pN and LNR were significant independent prognostic factors for 5-year RFS in the No PCRT group. In the PCRT group, only LNR category was found to be associated with RFS (HR = 2.36, 95%CI: 1.31-3.84, and P = 0.001).
CONCLUSION: The LNR is an important prognostic predictor of RFS in rectal cancer patients especially treated with PCRT. Current pN categories could not discriminate between prognostic groups of RFS after PCRT.
Keywords: Rectal cancer, Preoperative chemoradiotherapy, Lymph node ratio, Prognosis, pN
Core tip: The number of metastatic lymph node might show different prognosis according to the number of examined lymph node. Retrieved number of lymph node after preoperative chemoradiotherapy (PCRT) has been known fewer than those without PCRT. However, number of metastatic lymph nodes used in pathologic staging was same between patients treated with PCRT and those without PCRT. The present study suggests the metastatic lymph node ratio would be useful prognostic indicator and it is more prominent in patients treated with PCRT.
INTRODUCTION
The current staging system for colorectal cancer is based solely on the number of metastatic lymph nodes (LNs)[1]. To accurately stage patients using this system, a sufficient number of LNs-greater than 12 for colorectal cancer-must be examined to avoid underestimation of nodal stage[2]. The number of identified metastatic LNs can be influenced by the total number of LNs examined, and this can affect staging[3-5]. However, it remains unclear whether the prognostic significance of the number of metastatic LNs differs between patients who have only a small number of LNs retrieved compared to patients who have several LNs retrieved.
To overcome this limitation of the TNM staging system, a complementary LN metastasis stratification method is needed. The LNR, defined as the ratio of metastatic to examined LNs, has been shown to be useful in identifying prognostic subgroups within gastric and esophageal cancer patients[6,7]. The prognostic value of the LNR has also been demonstrated in colon and rectal cancer[8-10]. These previous studies have shown that the LNR can be used not only as a prognostic indicator, but also as a parameter for a more accurate stratification system than the metastatic LN absolute number-based staging system in colon and rectal cancer.
Preoperative chemoradiotherapy (PCRT) has been shown to induce shrinkage of tumors and provide lymphatic drainage, and is associated with improved local control[11-13]. However, the applicability of postoperative pathologic results in patients treated by PCRT has not been fully assessed. Furthermore, while a correlation between lymph node metastasis and poor oncologic outcome in patients treated with PCRT and radical resection has been suggested, the value of the LNR after PCRT remains controversial[14,15]. PCRT has been shown to result in a significant decrease in both the size and number of LNs available for examination after resection[16-20]. Consequently, the number of LNs examined could be below the recommended number in patients with rectal cancer. Therefore, for patients with rectal cancer treated with PCRT, a complementary LN metastasis stratification method may be needed than for those treated with upfront surgery.
It is unclear whether the impact of the LNR on prognosis differs between rectal cancer patients treated with PCRT and those treated with upfront surgical resection. The aim of this study was to evaluate the prognostic impact of the LNR in rectal cancer patients with metastatic LNs after radical resection.
MATERIALS AND METHODS
Patients, clinical staging, and treatment
We performed a retrospective consecutive cohort study of patients with biopsy-proven, locally advanced mid and low rectal cancer who were treated at Asan Medical Center between 2000 and 2009. Patients were identified from our institutional colorectal cancer patient database and tumor registry. Among the identified patients, 967 patients proved to have metastatic LNs on final pathologic examination. Patients with concurrent distant metastasis, concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, emergent surgery, a prior history of radiotherapy to the pelvis, or a prior history of malignancy were excluded. Study approval was obtained from the Asan Medical Center Institutional Review Board.
Pretreatment clinical stage was assessed based on transrectal ultrasound (TUS), magnetic resonance imaging (MRI), or computed tomography (CT) findings. All patients also underwent full colonoscopic evaluation to exclude synchronous tumors, as well as digital rectal examination and proctoscopy to identify the tumor distance from the anal verge. Some patients were treated with PCRT, with a median radiotherapy dose of 50.4 Gy and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent infusional 5-fluorouracil or capecitabine). For patients treated with PCRT, operations were generally performed 6 to 8 wk following the completion of PCRT using total mesorectal excision principles.
Standard pathologic tumor staging of the resected specimen was then performed. Postoperative follow-up consisted of routine physical examination with carcinoembryonic antigen (CEA) measurement every 3 to 6 mo, as well as colonoscopy every 2 to 3 years and cross-sectional imaging every 6 to 12 mo for 5 years.
Statistical analysis
To investigate the association of the metastatic LNR with oncologic outcome, categorization of LNRs was performed. A cut-off value of 0.25 was chosen to facilitate patient assignment to subgroups because 0.25 represents the number of metastatic lymph node of pN1 category based on 12 LNs harvested, which are recommend by the current TNM staging system for proper staging.
Patients were then assigned to two groups based on their LNR: LNR1, less than or equal to 0.25; LNR2, greater than 0.25. Pathologic N category of TNM staging system was chosen for comparison of function as a prognostic predictor with the two LNR subgroups of patients.
Categorical data were summarized by frequency within each cohort, and comparisons were performed using the χ2 test for proportions. A test for binary correlation was used to assess associations between selected polynomial categorical variables. For recurrence-free survival (RFS) analysis, cases were identified as failures at the time of disease recurrence. RFS rates were determined for each LNR-based group using pT category and current TNM stage-based group. Cox proportional hazards regression analysis was performed for multivariate comparisons. P values less than 0.05 were considered statistically significant.
RESULTS
Patient population and tumor characteristics
A total of 256 patients who were treated with PCRT and 724 who were treated with upfront surgery for rectal cancer during the study period and had pathologically proven cancer with metastatic LNs (ypN+) were included. The median age was 55 years [interquartile range (IQR): 48-62 years)]. The median distance of the tumor from the anal verge was 5 cm (IQR: 4-7 cm). All patients underwent total mesorectal excision. A sphincter-saving procedure was performed in 777 patients (80.3%). Age, gender, and sphincter preservation rates did not differ between patients who underwent PCRT and those who did not. The number of harvested and metastatic LNs was significantly lower among patients treated with PCRT (Table 1).
Table 1.
Patient and tumor characteristics n (%)
| Non-PCRT, n = 724 | PCRT, n = 243 | P value | |
| Age (mean ± SD) (yr) | 54 ± 10.3 | 59.2 ± 11.3 | < 0.001 |
| < 50 | 154 (21.3) | 80 (32.9) | |
| 50-65 | 346 (47.8) | 130 (53.5) | < 0.001 |
| > 65 | 224 (30.9) | 33 (13.6) | |
| Gender | 0.005 | ||
| Male | 434 (59.9) | 148 (60.9) | |
| Female | 290 (40.1) | 95 (39.1) | |
| Location1 | < 0.001 | ||
| 6-10 cm | 474 (65.5) | 100 (41.2) | |
| ≤ 5 cm | 250 (34.5) | 143 (58.8) | |
| Sphincter preservation | 600 (82.9) | 177 (72.8) | < 0.001 |
| Among patients with low rectum | 133 (53.2) | 81 (56.6) | 0.370 |
| LNR2 | 0.25 ± 0.24 | 0.252 ± 0.19 | 0.170 |
| Number of harvested LNs | 18.2 ± 8.5 | 14.8 ± 7.1 | < 0.001 |
| < 12 LNs harvested | 153 (21.1) | 96 (39.5) | < 0.001 |
| Number of positive LNs | 3.9 ± 3.7 | 2.8 ± 2.8 | < 0.001 |
| p/yp N category | < 0.001 | ||
| p/yp N1 | 445 (61.5) | 181 (74.5) | |
| p/yp N2 | 279 (38.5) | 62 (25.5) | |
| Follow-up duration, (Interquartile range) (mo) | 60 (39-80) | 56 (43-68) | 0.540 |
From the anal verge;
Lymph node ratio. PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio; LNs: Lymph nodes.
There were 96 patients (39.5%) in the PCRT group and 153 patients (21.1%) in the -No PCRT group who had less than 12 LNs resected. Of the 724 patients in the No PCRT group, 445 (61.5%) were N1 and 279 (38.5%) were N2. In the PCRT group, 181 (74.5%) were N1 and 62 (25.5%) were N2 (Table 1). The mean LNR was not different between the two groups.
Recurrence-free survival and prognostic factors for recurrence-free survival
The median follow-up duration was 40 mo (IQR: 32-58 mo) for the entire cohort. Within the same ypN category, the 5-year RFS rate differed significantly according to the LNR group. By contrast, significant differences in ypN were not found within LNR groups (Table 2). RFS for each group according to the pN category and the LNR category was analyzed. Both pN category and LNR category showed stratification of RFS in the No PCRT group (Figure 1). In the PCRT group, however, RFS did not differ by the pN category. Only the LNR category showed stratification of RFS in the PCRT group (Figure 1).
Table 2.
Five-year recurrence-free survival for T-stage subgroups stratified by lymph node ratio and pN category
|
No PCRT |
P value2 |
PCRT |
P value2 | |||||
| pN1 | pN2 | Overall | pN1 | pN2 | Overall | |||
| LNR1 | 78.6% | 60.1% | 76.2% | < 0.001 | 58.7% | 42.0% | 59.2% | < 0.001 |
| LNR2 | 59.5% | 51.2% | 52.9% | 27.6% | 38.1% | 33.8% | ||
| Overall | 76.3% | 53.4% | 54.3% | 44.3% | ||||
| P value1 | < 0.001 | < 0.262 | ||||||
RFS according to the LNR;
RFS according to pN category. PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio; RFS: Recurrence-free survival.
Figure 1.
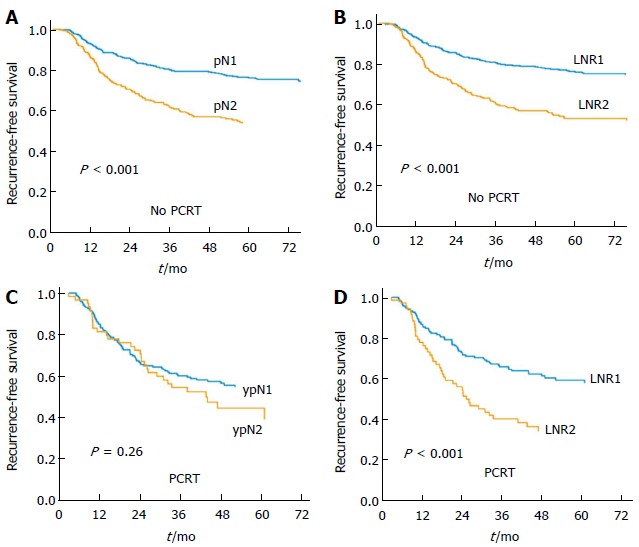
Recurrence-free survival. A: pN category in the no PCRT group; B: LNR category in the No PCRT group; C: ypN category in the PCRT group; D: LNR category in the PCRT group. LNR represents prognostic groups in both the No PCRT and the PCRT group. Current ypN status failed to show stratification with advancement of ypN status. PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio.
Influence of the pN and the LNR category on RFS was evaluated according to the number of harvested LNs. In the No PCRT group, RFS differed according to both the pN and the LNR category regardless of whether 12 LNs were examined. For the PCRT group, RFS differed according to LNR when < 12 and ≥ 12 LNs were harvested; in contrast, the pN category did not statistically significantly impact RFS irrespective of the number of harvested lymph node (Table 3).
Table 3.
Five-year recurrence-free survival stratified by lymph node ratio and pN-category according to the number of harvested lymph nodes
|
No PCRT |
PCRT |
|||||||
| < 12 | P value | ≥ 12 | P value | < 12 | P value | ≥ 12 | P value | |
| p/yp N1 | 67.8% | 0.01 | 79.2% | < 0.001 | 43.8% | 0.90 | 62.4% | 0.080 |
| p/yp N2 | 43.3% | 55.0% | 36.5% | 47.2% | ||||
| LNR1 | 74.2% | 0.01 | 76.6% | < 0.001 | 50.2% | 0.05 | 63.0% | 0.007 |
| LNR2 | 51.3% | 53.6% | 31.9% | 38.1% | ||||
PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio.
Risk factors of recurrence-free survival: Prognostic implication of pN and LNR category
In univariate analysis, LNR category was associated with RFS in both the No PCRT and the PCRT group. pN category, however, was not associated with RFS in the PCRT group. Other factors related with RFS in the No PCRT group were location of tumor, lymphovascular invasion, perineural invasion, and increased preoperative serum CEA (sCEA). In the PCRT group, perineural invasion was the only factor associated with RFS. In multivariate analysis, both the pN and the LNR category were confirmed as independent prognostic factors of RFS in the No PCRT group. However, in the PCRT group, only the LNR category was an independent prognostic factor showing stratification for RFS (Table 4).
Table 4.
Factors associated with recurrence-free survival: Multivariate analysis
| Factor |
No PCRT |
PCRT |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| p/yp N category | < 0.001 | 0.380 | ||||
| N1 | 1.00 | 1.00 | ||||
| N2 | 1.90 | 1.43-2.53 | 1.21 | 0.79-1.85 | ||
| LNR category | < 0.001 | 0.001 | ||||
| LNR1 | 1.00 | 1.00 | ||||
| LNR2 | 1.97 | 1.48-2.63 | 1.94 | 1.31-2.88 | ||
| Lymphovascular invasion | 0.34 | |||||
| None | 1.00 | 1.00 | 0.110 | |||
| Present | 1.24 | 0.93-1.67 | 1.42 | 0.89-2.27 | ||
| Perineural invasion | 0.04 | 0.030 | ||||
| None | 1.00 | 1.00 | ||||
| Undetermined | 1.65 | 1.12-2.43 | 1.86 | 1.18-2.93 | ||
| Location | 0.02 | 0.040 | ||||
| 6-10 cm | 1.00 | 1.00 | ||||
| ≤ 5 cm | 1.34 | 1.05-1.85 | 0.62 | 0.40-0.98 | ||
| Preoperative CEA | 0.02 | |||||
| Normal | 1.00 | |||||
| Increased | 1.46 | 1.07-1.98 | ||||
PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio; CEA: Carcinoembryoinc antigen.
Prognostic groups combined with p/ypT category and LNR
We compared the 5-year RFS according to the current 7th TNM stage (Figure 2). The current TNM stage could not effectively represent prognostic groups among patients of the PCRT group. We further analyzed the 5-year RFS considering the ypT and the LNR category and divided patients into three groups that showed statistical differences in RFS, R1 as ypT0-2 LNR1, R2 as ypT3-4 LNR1 and ypT0-2 LNR2, and R3 as ypT3-4 LNR2. These groups, which combined ypT and the LNR showed significant differences in 5-year RFS (Figure 2) and effectively separated patients into prognostic groups. For the No PCRT group, the R group, which is based on LNR and pT category, also showed stratification of RFS. In contrast, in the PCRT group, the R groups, but not the current TNM subgroups, showed significant differences in RFS.
Figure 2.
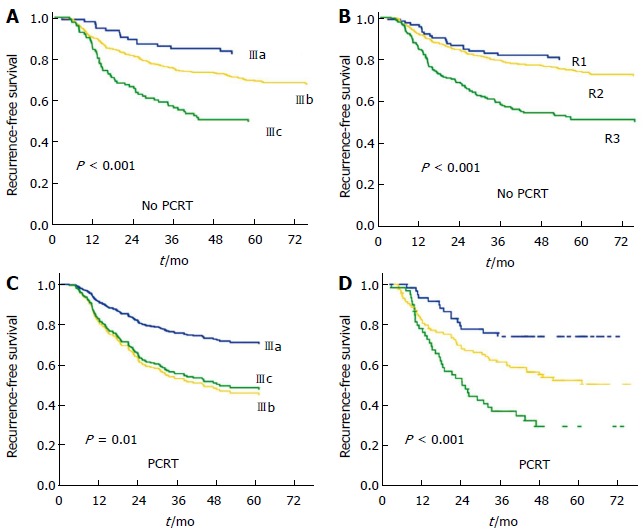
Recurrence-free survival. A: TNM stage by the 7th AJCC cancer staging system in the No PCRT group; B: R stage combined with pT/LNR in the No PCRT group; C: TNM stage in the PCRT group; D: R stage using yp T/LNR in the PCRT group. ypT/LNR combined groups showed significant categorization of prognostic groups. PCRT: Preoperative chemoradiotherapy; LNR: Lymph node ratio.
DISCUSSION
In the PCRT group, LNR was found to be the most significant prognostic factor for RFS in the present study. However, pN category could not discriminate patients into prognostic groups. Indeed, the LNR was significant both in patients with more than 12 LNs as well as in patients with less than 12 LNs examined. Although the harvesting of more than 12 LNs is recommended for proper staging, the number of LNs that can be harvested decreases in rectal cancer patients who have undergone PCRT[16,18]. Therefore, it is questionable whether the same absolute number-based staging system should be applied to all patients, including those with less than 12 retrieved LNs or patients who have been treated with PCRT. Based on the results of this study, the LNR could be applied to such patients as a prognostic predictor.
Patients within the same ypN category had a diverse distribution of the LNR. When patients within the same ypN category were stratified according to the LNR, there were significant differences in the 5-year RFS between LNR groups. In contrast, patients within the same LNR group did not show significant differences in RFS according to ypN category, except for the LNR1 group. This suggests that patients within the same ypN category could be further divided into different prognostic groups according to the LNR which might be a more proper discriminating category.
The LNR was confirmed as the only independent prognostic factor for RFS in the PCRT group using multivariate analysis. These findings suggest that a ratio-based approach is a better predictor of RFS than absolute number-based LN staging in patients with stage III rectal cancer treated with PCRT.
Invasion through the bowel wall is also an independent high risk factor for recurrence and survival. We compared the 5-year RFS according to the current TNM stage and R group combined with the pT/ypT and the LNR category. The 5-year RFS significantly differed according to both TNM stage and the ypT/LNR-based R group in the No PCRT group. However, the current TNM stage could not show corresponding poor outcome according to advanced stage in the PCRT group. R groups, in comparison, showed better stratification for RFS in the PCRT group. The inherent value of any cancer staging system lies in its reproducibility and applicability to current methods of pathological assessment. As the stage III patient group is defined by the identification and quantification of mesenteric nodes, accuracy of staging is directly proportional to nodal identification. Whereas examination of at least 12 LNs has been recommended for adequate determination of stage III colorectal cancer, the finding of any nodal involvement, regardless of the number of nodes examined, is defined as stage III disease. Therefore, a LNR was introduced to complement lymph node retrieval. In addition, the depth of invasion of tumor to bowel wall has to be considered alongside nodal status because nodal status was not the only determinant of pathologic stage. The present study compared RFS based on stage including the p/ypT category as well as the p/ypN category, and the LNR.
The results showed that the LNR-based category may be a useful prognostic factor accompanying p/yp T category. For patients in the PCRT group, LNR-based stage, but not the current TNM stage, was able to stratify patient for RFS.
This study has several limitations. Although the data were collected prospectively, the study was designed retrospectively, which may have introduced a selection bias. In addition, the prognostic significance of the LNR has been previously evaluated using different methodologies yielding varying results, particularly due to differences in the cut-off values used for grouping patients and heterogeneity of collected data[8-10,21-23] for colorectal cancer. For the practical use of the LNR as a prognostic variable, the most effective LNR cut-off values need to be determined. Although many studies, including our study, demonstrated that the LNR was a significant prognostic factor, further larger-scale comprehensive studies are warranted to determine the LNR cut-off values for rectal cancer. However, in the present study, the ratio between the number of positive LNs(4) which is generated by dividing pN2 from pN1 and the number of retrieved LNs(12) recommended for proper staging using the current staging system was used as a cut-off LNR value (0.25). Therefore, this LNR value is likely reasonable to compare the prognostic implication of p/yp N categories of the current TNM staging system with LNR-based categories.
Furthermore, studies regarding the LNR for rectal cancer patients treated with PCRT should be performed independently because PCRT influences LN status and significantly reduces the number of harvested LNs. Persistence of LN metastasis after PCRT may serve as a marker for a more aggressive biologic behavior of a tumor and the consequent need for more intensive postoperative treatment.
In conclusion, we found that the LNR was a more important prognostic factor for RFS in patients with lymph node metastasis after PCRT than those who did not undergo PCRT. Furthermore, absolute number-based nodal staging could not adequately predict prognosis for patients treated with PCRT. In addition, the predictive ability of the LNR was maintained when less than 12 LNs were harvested. Combined with ypT status, the LNR could be used to assign patients to a prognostic group as an alternative to the current TNM staging system. A large-scale comparative study to confirm the prognostic impact of the LNR and to determine the optimal LNR cut-off value is required.
COMMENTS
Background
The number of metastatic lymph node could be various according how many lymph nodes (LNs) were examined. The current staging system using number of metastatic LNs as N category for colorectal cancer, therefore, has a limitation in terms of influence by number of harvested LNs. The metastatic LN ratio (LNR), defined as the ratio of metastatic to examined LNs, has been shown to be useful in identifying prognostic subgroups for non-irradiated rectal cancer, colon cancer and other type of cancers. Preoperative chemoradiotherapy (PCRT) which is one of the standard treatment for locally advanced rectal cancer has been shown to influence on the number of retrieved lymph node. Therefore, the LNR may more useful for patients treated with PCRT. To evaluate the efficacy of LNR as a prognostic indicator in patient who receive PCRT, the present study compare oncologic outcomes according to current TNM stage and new classification using LNR among patients treated with PCRT and those without PCRT.
Research frontiers
Risk of recurrence was well stratified based on LNR in PCRT patients. In case of patients treated with PCRT, categories based on LNR had stronger association with recurrence-free survival. Future investigation is required to make staging system based on LNR and decide on clinical suitability of new staging system based on LNR in PCRT patients.
Innovations and breakthroughs
The current study shows the effectiveness of LNR to predict prognosis in patients who did not receive PCRT as well as those treated with PCRT. The present study gives importance of LNR on prognostication in PCRT than no-PCRT setting and suggests new staging system based on LNR and ypT category to apply practically.
Applications
The LNR-based category of patients with advanced rectal cancer treated by PCRT can be used to predict oncologic outcome. It is more accurate than LN number based staging system for prognostication. Adjuvant treatment and surveillance need to be given based on prognostic implication based on pathologic stage and LNR may have a role in case under-staging was suspicious based on current pathologic staging system.
Peer-review
This article is very important because it underlines the impact of neoadjuvant radiochemotherapy of rectal cancer and moreover can predict recurrence free survival. It would be useful to check these patients data according to tumor regression grade as well.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 8, 2014
First decision: August 27, 2014
Article in press: November 19, 2014
P- Reviewer: Elpek GO, Furka A, Steele SR S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (UICC International Union Against Cancer). 7th ed. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 2.Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol. 1996;106:209–216. doi: 10.1093/ajcp/106.2.209. [DOI] [PubMed] [Google Scholar]
- 3.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 4.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, Catalano PJ, Haller DG. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol. 2003;10:213–218. doi: 10.1245/aso.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–371. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, Grebner A, Ulm K, Höfler H, Nekarda H, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–978. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD, Lee SW, Kim JC, Yu CS, Kim HC, Kim TW, et al. lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:796–802. doi: 10.1016/j.ijrobp.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 10.Peschaud F, Benoist S, Julié C, Beauchet A, Penna C, Rougier P, Nordlinger B. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–1073. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 11.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 12.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 13.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rullier A, Laurent C, Capdepont M, Vendrely V, Belleannée G, Bioulac-Sage P, Rullier E. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 15.Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–5440. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 16.Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426–431. doi: 10.1016/j.ijrobp.2004.06.259. [DOI] [PubMed] [Google Scholar]
- 17.de la Fuente SG, Manson RJ, Ludwig KA, Mantyh CR. Neoadjuvant chemoradiation for rectal cancer reduces lymph node harvest in proctectomy specimens. J Gastrointest Surg. 2009;13:269–274. doi: 10.1007/s11605-008-0717-2. [DOI] [PubMed] [Google Scholar]
- 18.Thorn CC, Woodcock NP, Scott N, Verbeke C, Scott SB, Ambrose NS. What factors affect lymph node yield in surgery for rectal cancer? Colorectal Dis. 2004;6:356–361. doi: 10.1111/j.1463-1318.2004.00670.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Safar B, Wexner S, Zhao R, Cruz-Correa M, Berho M. Lymph node harvest after proctectomy for invasive rectal adenocarcinoma following neoadjuvant therapy: does the same standard apply? Dis Colon Rectum. 2009;52:549–557. doi: 10.1007/DCR.0b013e31819eb872. [DOI] [PubMed] [Google Scholar]
- 20.Morcos B, Baker B, Al Masri M, Haddad H, Hashem S. Lymph node yield in rectal cancer surgery: effect of preoperative chemoradiotherapy. Eur J Surg Oncol. 2010;36:345–349. doi: 10.1016/j.ejso.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Derwinger K, Carlsson G, Gustavsson B. A study of lymph node ratio as a prognostic marker in colon cancer. Eur J Surg Oncol. 2008;34:771–775. doi: 10.1016/j.ejso.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–1608. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 23.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]


