Abstract
AIM: To investigate the relationship between the iron-metabolism-related gene expression profiles and efficacy of antiviral therapy in chronic hepatitis C patients.
METHODS: The hepatic expression profile of iron-metabolism-related genes was analyzed and its association with virological response to pegylated-interferon plus ribavirin combination therapy was evaluated. A hundred patients with chronic hepatitis C (genotype1b, n = 50; genotype 2, n = 50) were enrolled and retrospectively analyzed. Liver biopsy samples were subjected to quantitative polymerase chain reaction for iron-metabolism-related genes and protein expression (Western blotting analysis) for ferroportin. As a control, normal liver tissue was obtained from 18 living donors of liver transplantation. Serum hepcidin level was measured by sensitive liquid chromatography/electrospray ionization tandem mass spectrometry.
RESULTS: Iron overload is associated with liver damage by increasing oxidative stress and hepatitis C virus (HCV) is reported to induce iron accumulation in hepatocytes in vivo. Conversely, iron administration suppresses HCV replication in vitro. Therefore, the association between HCV infection and iron metabolism remains unclear. Compared with controls, patients had significantly higher gene expression for transferrin, iron-regulatory proteins 1 and 2, divalent metal transporter 1, and ferroportin, but similar for transferrin receptors 1 and 2, and hepcidin. When the expression profiles were compared between sustained virological response (SVR) and non-SVR patients, the former showed significantly lower transcription and protein expression of hepcidin and ferroportin. Expression of hepcidin-regulating genes, BMPR1, BMPR2, and hemojuvelin, was significantly increased, whereas BMP2 was decreased in HCV-infected liver. BMPR2 and hemojuvelin expression was significantly lower in the SVR than non-SVR group. HCV infection affects the expression of iron-metabolism-related genes, leading to iron accumulation in hepatocytes.
CONCLUSION: Decreased expression of hepcidin and ferroportin in SVR patients indicates the importance of hepatocytic iron retention for viral response during pegylated-interferon plus ribavirin treatment.
Keywords: Chronic hepatitis C, Iron-metabolism, Hepcidin, Ferroportin, Interferon
Core tip: The first showing the relationship between expression of iron-metabolism-related genes and response to pegylated-interferon (PEG-IFN) and ribavirin (RBV) therapy in patients with chronic hepatitis C. The expression of hepcidin and ferroportin in the liver before therapy was significantly lower in sustained virological response (SVR) patients than non-SVR patients. The expression of hepcidin was positively correlated with that of ferroportin. The variation in hepatic expression profile in iron-metabolism-related genes is important for the response to PEG-IFN + RBV treatment.
INTRODUCTION
Hepatitis C virus (HCV) is a major pathogen of chronic hepatitis and subsequent liver cirrhosis and hepatocellular carcinoma. Approximately 170 million people are infected with HCV worldwide and, according to natural history studies, 5%-20% of patients develop cirrhosis after about 20 years of infection[1]. Pegylated-interferon (PEG-IFN) plus ribavirin (RBV) combination therapy has developed as a basic antiviral treatment for chronic hepatitis C because it can bring patients into sustained virological response (SVR) at a high rate. Nowadays, an inhibitor of HCV NS3/4A protease, telaprevir or simeprevir, is added to PEG-IFN and RBV to achieve higher SVR rates[2,3]. However, some relevant adverse events such as severe anemia still hinder the effect of the treatment by leading to dose reduction and cessation of treatment.
Iron is an essential biometal, and mammalian cells require sufficient amounts of iron to satisfy metabolic needs and accomplish some specialized functions. In humans, the vast majority of iron (> 2 g) is distributed to hemoglobin and is involved in oxygen transport. A significant amount of iron is also present in macrophages (≤ 600 mg) and in the myoglobin of muscles (≤ 300 mg), whereas excess body iron (about 1 g) is stored in the liver. Mammals lose iron by sloughing off their mucosal and skin cells, but do not possess any regulated mechanism for iron excretion from the body. Therefore, the iron balance needs to be regulated tightly, although the amount of iron uptake from nutrition and iron excretion is relatively low (1-2 mg)[4]. Liver iron overload is a well-described but not fully understood feature of HCV infection, which can induce liver damage by increasing oxidative stress. More than 30% of patients with chronic HCV infection have shown increased serum and hepatic iron concentrations[5-9]. Elevated iron index is correlated with progression of liver disease, while iron administration in vitro suppresses HCV replication[10]. Although the mechanism of disordered iron metabolism has not been fully elucidated, the recent discovery of hepcidin, a liver-derived iron-regulatory protein (IRP), has changed the philosophy of iron metabolism[1,11,12]. Iron is absorbed by intestinal villous cells through divalent metal transporter (DMT) 1 and transported into blood through ferroportin expressed on the basal membrane of villous cells. Serum iron is bound to transferrin and imported into hepatocytes via the function of transferrin receptor (TFR) 1 and 2, and DMT1 expressed on hepatocytes. Absorbed iron is stored with ferritin and ferroportin excretes iron into blood. Hepcidin, a hepatic peptide hormone, is a primary regulator of systemic iron status by blocking iron release from villous cells into the blood through binding to and driving degradation of ferroportin[1]. Recent studies have shown that the function of hepcidin is reduced in patients with chronic hepatitis C, leading to the pathogenesis of hepatic iron overload[13,14].
In some studies, hepatic iron accumulation was associated with resistance to IFN-based antiviral therapy and iron depletion before therapy improved SVR rates in patients with chronic hepatitis C[15-24]. Conversely, another study showed that hepatic iron storage was predominant in treatment responders and useful as a predictive marker for efficacy of IFN-based therapy[25]. Some studies have shown an association between iron overload and virological response to IFN + RBV therapy[26-28], but others have shown that iron content is not correlated with response to antiviral therapy[29-36]. The relationship between iron metabolism and response to antiviral therapy is still confused. In this study, we analyzed the hepatic expression of iron-metabolism-related genes and evaluated its association with virological response to PEG-IFN+RBV therapy.
MATERIALS AND METHODS
Study population
In Kyushu Medical Center, a standard protocol in Japan [subcutaneous PEG-IFNα2a (180 μg) or PEG-IFNα2b (median dose of 1.5 μg/kg, range 1.3-1.7) weekly, along with oral RBV daily for 48 wk] was adopted for chronic hepatitis C. The dose of RBV was adjusted according to body weight: 600 mg for patients weighing < 60 kg; 800 mg for patients weighing 60-80 kg; and 800 mg for patients weighing > 80 kg. In these protocols, 48-wk and 24-wk regimens were applied to patients infected with HCV genotype 1b (HCV-1b) and those infected with HCV genotype 2 (HCV-2), respectively[37]. The study protocol was approved by the Ethics Committee of the National Hospital Organization, and written informed consent was obtained from all patients. HCV-1b patients (n = 50) and HCV-2 patients (n = 50) were enrolled and retrospectively analyzed. The background of the patients is shown in Table 1. Blood biochemistry was examined 1 or 2 d before biopsy. For real-time reverse transcription polymerase chain reaction (RT-PCR), tissue samples were obtained by liver biopsy. As a control, normal liver tissue was obtained from 18 living donors of liver transplantation whose liver function and histological findings were normal. Written informed consent was obtained from these donors for this investigation.
Table 1.
Demographic and clinical characteristics of the patients
| Number of patients | 100 |
| SVR/non-SVR | 63/37 |
| Age (yr) | 56.3 ± 7.4 |
| Male/female | 39/61 |
| Genotype (1b/2a/2b) | 50/17/33 |
| HCV RNA (log IU/mL) | 5.87 ± 0.95 |
| Aspartate aminotransferase (< 30 IU/L) | 51.1 ± 35.9 |
| Alanine aminotransferase (< 30 IU/L) | 61.8 ± 53.0 |
| γ-glutamyl transpeptidase (< 50 IU/L) | 50.7 ± 58.9 |
| Fe (male, 55-200 μg/dL; female, 45-180 μg/dL) | 130.3 ± 64.2 |
| Unsaturated iron binding capacity (105-300 μg/dL) | 215.1 ± 84.2 |
| WBC (4000-9000/μL) | 4987 ± 1303 |
| Hemoglobin (male, 13-17; female, 11-15 g/dL) | 13.6 ± 1.5 |
| Platelet (11 × 104-35 × 104/μL) | 19.6 ± 6.7 |
| Dose reduction of ribavirin (%) | 47 |
Normal range is presented in parenthesis. Measured values are shown in mean ± SD. SVR: Sustained virological response; HCV: Hepatitis C virus; WBC: White blood cell.
Laboratory data
Hematological, biochemical and virological parameters were determined by the clinical laboratory at Kyushu Medical Center. Serum HCV RNA concentrations were measured by the COBAS TaqMan PCR HCV test (Roche Diagnostics, Tokyo, Japan). SVR was defined as undetectable HCV RNA at 24 wk after therapy completion.
Real-time RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and cDNA was synthesized from 1.0 μg RNA using GeneAmp RNA PCR (Applied Biosystems, Branchburg, NJ, United States) with random hexamers. Real-time RT-PCR was performed using LightCycler-FastStart DNA Master SYBR Green 1 (Roche, Basel, Switzerland) according to the manufacturer’s instructions[38]. The reaction mixture (20 μL) contained LightCycler-FastStart DNA Master SYBR Green 1, 4 mmol/L MgCl2, 0.5 μmol/L upstream and downstream PCR primers, and 2 μL first-strand cDNA as a template. To control for reaction variations, all PCR data were normalized against the expression of retinoblastoma binding protein 6, which was also selected in previous studies[37,39]. Primer sets used for real-time PCR are shown in Table 2.
Table 2.
Primer sets used for real-time polymerase chain reaction
| Gene | Forward primer | Reverse primer |
| 5’ 3’ | 5’ 3’ | |
| Ferritin | CAGGTGCGCCAGAACTACCA | CCACATCATCGCGGTCAAAG |
| Transferrin | CGAAGACTGCATCGCCAAGA | ACACTTGCCCGCTATGTAGACAAAC |
| Hepcidin | AGCCTGACCAGTGGCTCTGT | TTCGCCTCTGGAACATGG |
| Ferroportin | AAGGGCAAGAATCCCAATTTAACTC | TGCCAGGCTGAAGGCTTACAC |
| TFR1 | GCATGTGGCATGTTCATCGTATAA | TCTCAAGACCAGGAGCTTGTCACTA |
| TFR2 | GCGACTGACACGCATGTACAAC | CCATGAAGATGTGGCGGAAC |
| DMT1 | CTTGCGAGGCAATCTCAGGA | CTGAGACAGTGAACTTTGCAACCA |
| IRP1 | GAAACAGTCCTGCTGCTCGCTAC | GAGCCATAGGAGTTGAATTCTCGTG |
| IRP2 | TTTATCTCCAGGCAGTGGGATG | CTGCGTCTGATAAGGGTGCTGTA |
| BMPR1 | TGGGAGTTGCTGCATTGCTGACC | ATGTAGCGTTTGGTGCCCACCC |
| BMPR2 | GCCACAAATGTCCTGGATGGCA | GAGGGGCGCCACCGCTTAAG |
| BMP2 | TTGCGCCAGGTCCTTTGACCAG | ACCTGGGGAAGCAGCAACGCTA |
| Hemojuvelin | TGCCAGACGGCTGTGCAAGG | CGGGCATCCTCCAGTGCTGC |
| RBBP6 | GCGACCTGCAGATCACCAA | TGCCATCGCTGGTTCAGTTC |
TFR: Transferrin receptor; DMT: Divalent metal transporter; IRP: Iron-regulatory protein; BMP: Bone morphogenetic protein; BMPR: BMP receptor; RBBP: Retinoblastoma binding protein.
Protein expression
Liver biopsy samples were lysed in phosphate-buffered saline containing 1% Triton X-100. Forty-microgram aliquots of total tissue lysate were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto Immobilon-P polyvinylidene fluoride membranes (Millipore, Billerica, MA, United States). The membranes were incubated with primary antibodies to ferroportin (ab85370: rabbit polyclonal to SLC40A1, Abcam, Tokyo, Japan) and β-actin (ab3280: mouse monoclonal to actin, Abcam), followed by incubation with peroxidase-labeled anti-rabbit and anti-mouse IgG antibodies (170-5046 and 170-5047, Bio-Rad, Tokyo, Japan), respectively. The bands were visualized by chemiluminescence using the ECL Western blotting analysis system (Amersham Biosciences, Little Chalfont, Bucks, United Kingdom).
Measurement of hepcidin
Serum hepcidin level was measured by sensitive liquid chromatography/electrospray ionization tandem mass spectrometry using an AB Sciex Triple Quad 5500 system (AB Sciex, Foster City, CA, United States) equipped with a Prominence UFLCXR system (Shimadzu Corporation, Kyoto, Japan), as reported previously[40,41]. Hepcidin exists in three isoforms, the iron bioactive 25-amino acid peptide (Hep-25) and its two amino-terminal truncated isoforms (Hep-20 and -22). In mass spectrometry-based studies, Hep-25 and Hep-20 can be measured in serum, while Hep-22 is found only in urine[42].
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC, United States). Mann-Whitney U test was used for continuous variables including the difference in gene expression. A value of P ≤ 0.05 was considered to be statistically significant.
RESULTS
Expression of iron-metabolism-related genes in hepatitis C patients
We examined the expression profile of the genes associated with iron metabolism by quantitative real-time RT-PCR to investigate disorders of iron metabolism in the liver of HCV-infected patients (HCV liver) (Figure 1). mRNA levels of transferrin, ferroportin, DMT1, IRP1 and IRP2 were significantly increased in HCV-1b or HCV-2 liver compared with normal controls. The levels of ferritin and TFR1 were significantly increased in HCV-1b liver, but not in HCV-2 liver. Expression levels of hepcidin and TFR2 in HCV-1b and HCV-2 liver were similar to the control level. The expression profile was consistent regardless of HCV genotype, except for ferritin, ferroportin and TFR1.
Figure 1.
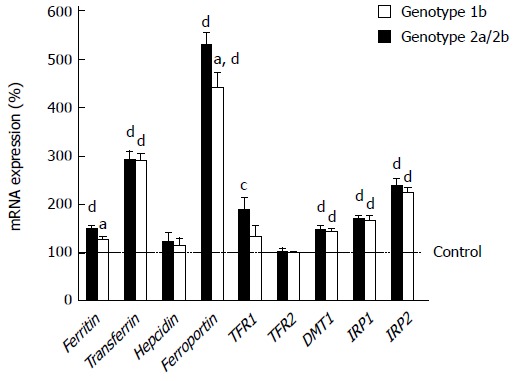
Expression levels of iron-metabolism-related genes in hepatitis C virus-infected liver in each genotype. The levels were measured by real-time reverse transcription polymerase chain reaction. aP < 0.05 vs genotype1b, bP < 0.01 vs genotype1b, cP < 0.05 vs control, dP < 0.01 vs control.
Expression of iron-metabolism-related genes and treatment outcome
We studied the involvement of iron metabolism in outcomes of PEG-IFN + RBV combination therapy. SVR patients showed significantly lower expression of hepcidin and ferroportin than non-SVR patients (Figure 2A), but no significant difference was found in other iron-metabolism-related genes (data not shown). Anemia is one of the critical adverse events during therapy and often compels a reduction in total RBV dose. It is possible that the changes in expression of hepcidin and ferroportin might affect iron metabolism and anemia. Furthermore, HCV genotype might be involved in the expression of the genes and treatment response. We compared the expression of hepcidin and ferroportin between the treatment outcomes for each HCV genotype, and between patients with and without RBV dose reduction. The expression of hepcidin and ferroportin was still lower in the SVR group both in HCV-1b and HCV-2 liver, although the difference was significant only in HCV-2 (Figure 2B). SVR patients, who did not need RBV reduction, showed significantly lower hepcidin and ferroportin expression than non-SVR patients showed, while expression did not differ significantly between SVR and non-SVR patients in the group with RBV dose reduction (Figure 2C). Serum hepcidin levels were also lower in SVR patients, although the difference was not significant (Figure 3A). Hepcidin is the protein regulating ferroportin expression through binding and degrading ferroportin, and might influence the protein level of ferroportin in hepatocytes as well as villous cells. We examined the expression of ferroportin protein in the liver. Hepatic levels of ferroportin protein, as well as RNA, were significantly lower in SVR patients than non-SVR patients (Figure 3B).
Figure 2.
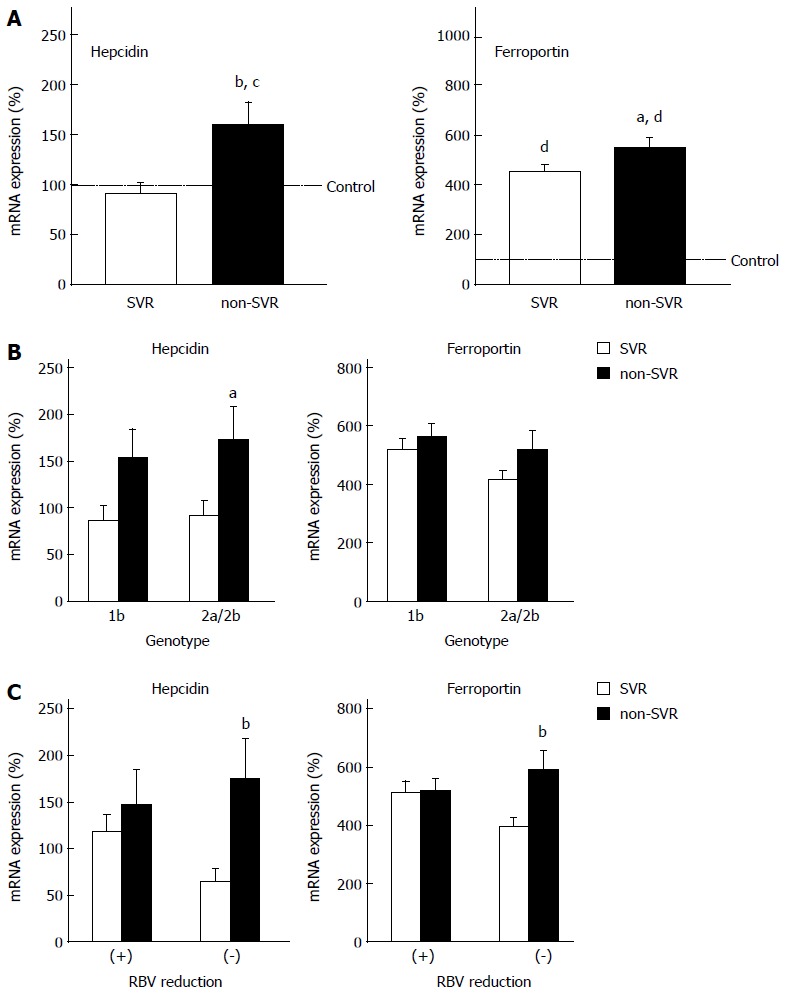
Expression levels of hepcidin and ferroportin genes. A: Sustained virological response (SVR) vs non-SVR; B: Genotype 1b vs 2a/2b; C: RBV dose reduction (+) vs (−). The levels were measured by real-time reverse transcription polymerase chain reaction. aP< 0.05 vs SVR, bP < 0.01 vs SVR, cP < 0.05 vs control, dP < 0.01 vs control.
Figure 3.
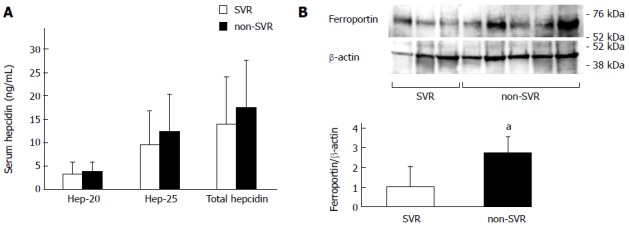
Protein expression of hepcidin and ferroportin. A: Serum hepcidin levels measured by sensitive liquid chromatography/electrospray ionization tandem mass spectrometry (total hepcidin and its two isoforms, Hep-20 and Hep 25); B: Ferroportin levels in the liver measured by Western blotting. aP < 0.05 vs sustained virological response (SVR).
Relationship between hepcidin-expression-associated gene expression and treatment outcome
We examined the gene expression of known regulators of hepcidin expression. Although the regulation of hepcidin expression is not completely clear, a few pathways are known to control hepcidin expression: (1) TFR2 for sensing serum iron and saturated transferring; (2) IL-6 receptor (IL-6R) and signal transducer and activator of transcription (STAT) pathway for reflecting infection and inflammation; and (3) bone morphogenetic protein receptor (BMPR) and hemojuvelin, which are receptor and co-receptor for BMP2 and 6, respectively[43-45]. Hepatic expression of TFR2 and IL-6R was similar in SVR and non-SVR patients, and control subjects (data not shown). The levels of BMPR2 and hemojuvelin were significantly lower in SVR liver, and the same trend was found for BMPR1 and BMP2 expression, although the difference was not significant (Figure 4).
Figure 4.
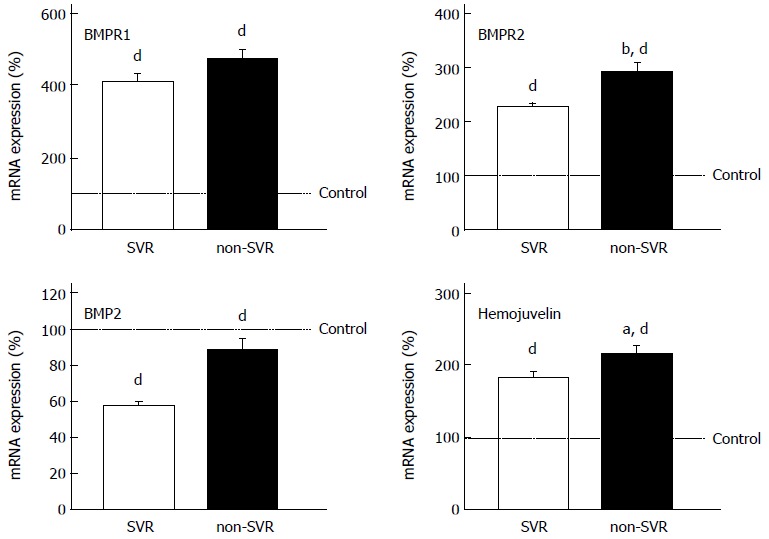
Expression levels of genes regulating hepcidin expression. The levels were measured by real-time reverse transcription polymerase chain reaction. aP < 0.05 vs sustained virological response (SVR), bP < 0.01 vs SVR, dP < 0.01 vs control.
DISCUSSION
In this study, we examined the hepatic expression of the genes involved in iron metabolism and compared the expression levels between SVR and non-SVR patients. In HCV liver, expression levels of transferrin, TFR1, DMT1, ferritin, ferroportin, IRP1 and IRP2 were upregulated, while those of hepcidin and TFR2 were similar to the control levels. Transcription of ferritin and ferroportin was higher in patients with HCV-1b than HCV-2a/2b. It is possible that the change of expression might have affected iron deposition in the liver, although the mechanism was unclear. HCV genotype 2a/2b patients are known to achieve higher viral clearance during IFN treatment than patients with genotype 1b. The difference in gene expression might be affected by innate immunity reaction or influence viral response through iron metabolism.
It is reported that iron administration in vitro suppresses HCV replication[10]. Furthermore, hepatic iron storage is reported to be predominant in treatment responders and useful as a predictive marker for efficacy of IFN-based antiviral therapy[25]. Conversely, iron overload and HFE gene mutations are associated with resistance to IFN therapy, and iron depletion before therapy is effective in patients with chronic hepatitis C[15-24]. In other studies, no association was found between iron overload and the response to IFN and RBV therapy[29-36]. Therefore, the relationship between iron metabolism and response to antiviral therapy is still unclear and controversial. The present study is believed to be the first showing the relationship between expression of iron-metabolism-related genes and response to PEG-IFN and RBV therapy. The expression of hepcidin and ferroportin in the liver before therapy was significantly lower in SVR patients than non-SVR patients regardless of HCV genotype and RBV dose reduction. Serum hepcidin and hepatic ferroportin protein were also lower in SVR patients. Jaroszewicz et al[27] have also reported that a decrease in serum prohepcidin level was associated with successful treatment using PEG-IFN and RBV. When we checked iron storage in the liver by staining biopsy tissue, we could not detect much difference between the liver of SVR and non-SVR patients (data not shown). These findings indicate the following: (1) Patients with enough capacity for accumulating iron in the liver achieve viral clearance during PEG-IFN+RBV therapy; (2) The amount of hepatic iron deposition at the beginning of treatment might not influence the therapeutic response to PEG-IFN and RBV; (3) Patients who have higher hepcidin and ferroportin expression in the liver could store more hepatic iron released from red blood cells via RBV-induced hemolysis; and (4) Oxidative stress from accumulated iron might inhibit viral replication and help completion of viral clearance.
Transcriptional regulation of hepcidin might be mediated by the BMP-BMPR pathway but not the TFR2 or IL-6 pathway. It is possible that decreased hepcidin expression in SVR patients is affected by decreased iron in serum or liver tissue. However, serum iron concentration, as well as iron deposition in the liver, was similar between SVR and non-SVR patients. In addition, we could not detect any major difference in the expression of TFR2 that could play a role in tracking iron concentration between SVR and non-SVR patients. These findings indicate that downregulation of hepcidin in the liver of SVR patients might not reflect iron insufficiency, and that patients with decreased hepatic hepcidin via expression change in the BMP-BMPR pathway have greater capacity to absorb iron into the body and liver, and higher capacity for viral clearance. Moreover, the expression of hepcidin showed parallel change with the expression of ferroportin, and we found that these expressions were positively correlated with each other. It is possible that these expressions are affected by HCV infection or iron demand via a shared mechanism.
The variation in hepatic expression profile in iron-metabolism-related genes in patients with chronic hepatitis C is important for the response to PEG-IFN + RBV treatment. As an adverse event, anemia is more serious during triple therapy with telaprevir or boceprevir in combination with PEG-IFN and RBV. Therefore, characterization of iron metabolism during triple therapy has become more important. Further studies for controlling iron balance and metabolism could not only prevent dose reduction during therapy, but also enhance the therapeutic effect.
COMMENTS
Background
Iron overload is associated with liver damage by increasing oxidative stress and hepatitis C virus (HCV) is reported to induce iron accumulation in hepatocytes in vivo. Conversely, iron administration suppresses HCV replication in vitro. Therefore, the association between HCV infection and iron metabolism remains unclear.
Research frontiers
The association between iron metabolism/accumulation in the liver and viral response to antiviral treatments has been scarcely investigated.
Innovations and breakthroughs
The authors investigated the iron-metabolism-related gene expression profiles in HCV-infected liver, and the relationship between the profiles and therapeutic efficacy of pegylated-interferon (PEG-IFN) and ribavirin (RBV) treatment.
Applications
A decrease in hepcidin and ferroportin levels was associated with successful treatment using PEG-IFN and RBV. Patients with enough capacity for accumulating iron in the liver may achieve viral clearance during PEG-IFN + RBV therapy.
Terminology
HCV infection affects the expression of iron-metabolism-related genes, leading to iron accumulation in hepatocytes. Decreased expression of hepcidin and ferroportin in SVR patients, which can be regulated via the BMP-BMPR pathway, indicates the importance of hepatocytic iron retention for viral response during PEG-IFN + RBV treatment.
Peer-review
The authors present novel data and have presented a good context for the findings. The results indicating the importance of hepatic iron retention for viral response were interesting for readers.
Footnotes
Supported by grants from Research Program of Intractable Disease provided by the Ministry of Health, Labor and Welfare of Japan, and a Grant-in-Aid for Clinical Research from the National Hospital Organization of Japan.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 4, 2014
First decision: October 14, 2014
Article in press: December 8, 2014
P- Reviewer: Georgopoulou U, Goll R, Kakizaki S S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. doi: 10.1016/j.jhep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Aghemo A, Rumi MG, Colombo M. Pegylated IFN-alpha2a and ribavirin in the treatment of hepatitis C. Expert Rev Anti Infect Ther. 2009;7:925–935. doi: 10.1586/eri.09.70. [DOI] [PubMed] [Google Scholar]
- 3.Kumada T, Toyoda H, Honda T, Kuzuya T, Katano Y, Nakano I, Goto H. Treatment of chronic hepatitis C with interferon alone or combined with ribavirin in Japan. Intervirology. 2006;49:112–118. doi: 10.1159/000087273. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108–2113. doi: 10.1016/0016-5085(92)90339-z. [DOI] [PubMed] [Google Scholar]
- 6.Bonkovsky HL, Banner BF, Rothman AL. Iron and chronic viral hepatitis. Hepatology. 1997;25:759–768. doi: 10.1002/hep.510250345. [DOI] [PubMed] [Google Scholar]
- 7.Bonkovsky HL. Iron as a comorbid factor in chronic viral hepatitis. Am J Gastroenterol. 2002;97:1–4. doi: 10.1111/j.1572-0241.2002.05390.x. [DOI] [PubMed] [Google Scholar]
- 8.Price L, Kowdley KV. The role of iron in the pathophysiology and treatment of chronic hepatitis C. Can J Gastroenterol. 2009;23:822–828. doi: 10.1155/2009/290383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hörl WH, Schmidt A. Low hepcidin triggers hepatic iron accumulation in patients with hepatitis C. Nephrol Dial Transplant. 2014;29:1141–1144. doi: 10.1093/ndt/gft467. [DOI] [PubMed] [Google Scholar]
- 10.Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, Kato N. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother. 2007;51:2016–2027. doi: 10.1128/AAC.01426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 12.Aoki CA, Rossaro L, Ramsamooj R, Brandhagen D, Burritt MF, Bowlus CL. Liver hepcidin mRNA correlates with iron stores, but not inflammation, in patients with chronic hepatitis C. J Clin Gastroenterol. 2005;39:71–74. [PubMed] [Google Scholar]
- 13.Fujita N, Sugimoto R, Urawa N, Tanaka H, Konishi M, Kobayashi Y, Iwasa M, Watanabe S, Kaito M. Influence of phlebotomy on iron-related gene expression levels in the livers of patients with chronic hepatitis C. J Gastroenterol. 2007;42:326–327. doi: 10.1007/s00535-007-2004-5. [DOI] [PubMed] [Google Scholar]
- 14.Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, et al. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fargion S, Fracanzani AL, Rossini A, Borzio M, Riggio O, Belloni G, Bissoli F, Ceriani R, Ballarè M, Massari M, et al. Iron reduction and sustained response to interferon-alpha therapy in patients with chronic hepatitis C: results of an Italian multicenter randomized study. Am J Gastroenterol. 2002;97:1204–1210. doi: 10.1111/j.1572-0241.2002.05705.x. [DOI] [PubMed] [Google Scholar]
- 16.Carlo C, Daniela P, Giancarlo C. Iron depletion and response to interferon in chronic hepatitis C. Hepatogastroenterology. 2003;50:1467–1471. [PubMed] [Google Scholar]
- 17.Van Thiel DH, Friedlander L, Molloy PJ, Kania RJ, Fagiuoli S, Wright HI, Gasbarrini A, Caraceni P. Retreatment of hepatitis C interferon non-responders with larger doses of interferon with and without phlebotomy. Hepatogastroenterology. 1996;43:1557–1561. [PubMed] [Google Scholar]
- 18.Fong TL, Han SH, Tsai NC, Morgan TR, Mizokami M, Qian D, Phan C, Goad K, Redeker AG. A pilot randomized, controlled trial of the effect of iron depletion on long-term response to alpha-interferon in patients with chronic hepatitis C. J Hepatol. 1998;28:369–374. doi: 10.1016/s0168-8278(98)80308-5. [DOI] [PubMed] [Google Scholar]
- 19.Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, Levine RA, Fiarman G, Thiim M, Tavill AS, et al. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31:730–736. doi: 10.1002/hep.510310325. [DOI] [PubMed] [Google Scholar]
- 20.Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M, Horiike S, Tanaka H, Iwasa M, Kobayashi Y, et al. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1886–1893. doi: 10.1111/j.1440-1746.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- 21.Franchini M, Targher G, Capra F, Montagnana M, Lippi G. The effect of iron depletion on chronic hepatitis C virus infection. Hepatol Int. 2008;2:335–340. doi: 10.1007/s12072-008-9076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin TJ, Liao LY, Lin CL, Chang TA, Liu SO. Hepatic iron influences responses to combination therapy with peginterferon alfa and ribavirin in chronic hepatitis C. Hepatogastroenterology. 2008;55:1412–1415. [PubMed] [Google Scholar]
- 23.Gentile I, Viola C, Paesano L, D’Onofrio M, D’Agostino E, Cerini R, Borrelli F, Piazza M, Borgia G. Iron depletion before HCV antiviral therapy: a pilot, randomized, controlled trial. J Clin Apher. 2009;24:190–196. doi: 10.1002/jca.20210. [DOI] [PubMed] [Google Scholar]
- 24.Sikorska K, Stalke P, Izycka-Swieszewska E, Romanowski T, Bielawski KP. The role of iron overload and HFE gene mutations in the era of pegylated interferon and ribavirin treatment of chronic hepatitis C. Med Sci Monit. 2010;16:CR137–CR143. [PubMed] [Google Scholar]
- 25.Akiyoshi F, Sata M, Uchimura Y, Suzuki H, Tanikawa K. Hepatic iron stainings in chronic hepatitis C patients with low HCV RNA levels: a predictive marker for IFN therapy. Am J Gastroenterol. 1997;92:1463–1466. [PubMed] [Google Scholar]
- 26.Fargion S, Fracanzani AL, Sampietro M, Molteni V, Boldorini R, Mattioli M, Cesana B, Lunghi G, Piperno A, Valsecchi C, et al. Liver iron influences the response to interferon alpha therapy in chronic hepatitis C. Eur J Gastroenterol Hepatol. 1997;9:497–503. doi: 10.1097/00042737-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Jaroszewicz J, Rogalska M, Flisiak I, Flisiak R. Successful antiviral therapy is associated with a decrease of serum prohepcidin in chronic hepatitis C. World J Gastroenterol. 2010;16:1747–1752. doi: 10.3748/wjg.v16.i14.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carneiro MV, Souza FF, Teixeira AC, Figueiredo JF, Villanova MG, Secaf M, Passos AD, Ramalho LN, Carneiro FP, Zucoloto S, et al. The H63D genetic variant of the HFE gene is independently associated with the virological response to interferon and ribavirin therapy in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2010;22:1204–1210. doi: 10.1097/MEG.0b013e32833bec1e. [DOI] [PubMed] [Google Scholar]
- 29.Herrera JL. Iron depletion is not effective in inducing a virologic response in patients with chronic hepatitis C who failed to respond to interferon therapy. Am J Gastroenterol. 1999;94:3571–3575. doi: 10.1111/j.1572-0241.1999.01648.x. [DOI] [PubMed] [Google Scholar]
- 30.Sievert W, Pianko S, Warner S, Bowden S, Simpson I, Bowden D, Locarnini S. Hepatic iron overload does not prevent a sustained virological response to interferon-alpha therapy: a long term follow-up study in hepatitis C-infected patients with beta thalassemia major. Am J Gastroenterol. 2002;97:982–987. doi: 10.1111/j.1572-0241.2002.05550.x. [DOI] [PubMed] [Google Scholar]
- 31.Pianko S, McHutchison JG, Gordon SC, Heaton S, Goodman ZD, Patel K, Cortese CM, Brunt EM, Bacon BR, Blatt LM. Hepatic iron concentration does not influence response to therapy with interferon plus ribavirin in chronic HCV infection. J Interferon Cytokine Res. 2002;22:483–489. doi: 10.1089/10799900252952271. [DOI] [PubMed] [Google Scholar]
- 32.Hofer H, Osterreicher C, Jessner W, Penz M, Steindl-Munda P, Wrba F, Ferenci P. Hepatic iron concentration does not predict response to standard and pegylated-IFN/ribavirin therapy in patients with chronic hepatitis C. J Hepatol. 2004;40:1018–1022. doi: 10.1016/j.jhep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Rulyak SJ, Eng SC, Patel K, McHutchison JG, Gordon SC, Kowdley KV. Relationships between hepatic iron content and virologic response in chronic hepatitis C patients treated with interferon and ribavirin. Am J Gastroenterol. 2005;100:332–337. doi: 10.1111/j.1572-0241.2005.41112.x. [DOI] [PubMed] [Google Scholar]
- 34.Souza RM, Freitas LA, Lyra AC, Moraes CF, Braga EL, Lyra LG. Effect of iron overload on the severity of liver histologic alterations and on the response to interferon and ribavirin therapy of patients with hepatitis C infection. Braz J Med Biol Res. 2006;39:79–83. doi: 10.1590/s0100-879x2006000100009. [DOI] [PubMed] [Google Scholar]
- 35.Jurczyk K, Karpińska E, Wawrzynowicz-Syczewska M, Morańska I, Noceń I, Chlubek D, Boroń-Kaczmarska A. State of the iron metabolism in patients with chronic hepatitis C type C does not influence antiviral treatment with interferon and ribavirin. Hepatogastroenterology. 2008;55:557–561. [PubMed] [Google Scholar]
- 36.Pereira Pda S, Silva IS, Uehara SN, Emori CT, Lanzoni VP, Silva AE, Ferraz ML. Chronic hepatitis C: hepatic iron content does not correlate with response to antiviral therapy. Rev Inst Med Trop Sao Paulo. 2009;51:331–336. doi: 10.1590/s0036-46652009000600004. [DOI] [PubMed] [Google Scholar]
- 37.Kohjima M, Enjoji M, Yoshimoto T, Yada R, Fujino T, Aoyagi Y, Fukushima N, Fukuizumi K, Harada N, Yada M, et al. Add-on therapy of pitavastatin and eicosapentaenoic acid improves outcome of peginterferon plus ribavirin treatment for chronic hepatitis C. J Med Virol. 2013;85:250–260. doi: 10.1002/jmv.23464. [DOI] [PubMed] [Google Scholar]
- 38.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–511. [PubMed] [Google Scholar]
- 39.Nakamuta M, Fujino T, Yada R, Aoyagi Y, Yasutake K, Kohjima M, Fukuizumi K, Yoshimoto T, Harada N, Yada M, et al. Expression profiles of genes associated with viral entry in HCV-infected human liver. J Med Virol. 2011;83:921–927. doi: 10.1002/jmv.22042. [DOI] [PubMed] [Google Scholar]
- 40.Murao N, Ishigai M, Yasuno H, Shimonaka Y, Aso Y. Simple and sensitive quantification of bioactive peptides in biological matrices using liquid chromatography/selected reaction monitoring mass spectrometry coupled with trichloroacetic acid clean-up. Rapid Commun Mass Spectrom. 2007;21:4033–4038. doi: 10.1002/rcm.3319. [DOI] [PubMed] [Google Scholar]
- 41.Hosoki T, Ikuta K, Shimonaka Y, Sasaki Y, Yasuno H, Sato K, Ohtake T, Sasaki K, Torimoto Y, Saito K, et al. Heterogeneous expressions of hepcidin isoforms in hepatoma-derived cells detected using simultaneous LC-MS/MS. Proteomics Clin Appl. 2009;3:1256–1264. doi: 10.1002/prca.200900112. [DOI] [PubMed] [Google Scholar]
- 42.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53:620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 43.Deicher R, Hörl WH. New insights into the regulation of iron homeostasis. Eur J Clin Invest. 2006;36:301–309. doi: 10.1111/j.1365-2362.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson GJ, Darshan D, Wilkins SJ, Frazer DM. Regulation of systemic iron homeostasis: how the body responds to changes in iron demand. Biometals. 2007;20:665–674. doi: 10.1007/s10534-006-9030-2. [DOI] [PubMed] [Google Scholar]
- 45.Core AB, Canali S, Babitt JL. Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front Pharmacol. 2014;5:104. doi: 10.3389/fphar.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]


