Abstract
AIM: To evaluate the incidence and risk factors of Korean tuberculosis (TB) infection in patients with inflammatory bowel disease (IBD) undergoing anti-TNF treatment.
METHODS: The data of IBD patients treated with anti-TNFs in 13 tertiary referral hospitals located in the southeastern region of Korea were collected retrospectively. They failed to show response or were intolerant to conventional treatments, including steroids or immunomodulators. Screening measures for latent TB infection (LTBI) and the incidence and risk factors of active TB infection after treatment with anti-TNFs were identified.
RESULTS: Overall, 376 IBD patients treated with anti-TNF agents were recruited (male 255, mean age of anti-TNF therapy 32.5 ± 13.0 years); 277 had Crohn’s disease, 99 had ulcerative colitis, 294 used infliximab, and 82 used adalimumab. Before anti-TNF treatment, screening tests for LTBI including an interferon gamma release assay or a tuberculin skin test were performed in 82.2% of patients. Thirty patients (8%) had LTBI. Sixteen cases of active TB infection including one TB-related mortality occurred during 801 person-years (PY) follow-up (1997.4 cases per 100000 PY) after anti-TNF treatment. LTBI (OR = 5.76, 95%CI: 1.57-21.20, P = 0.008) and WBC count < 5000 mm3 (OR = 4.5, 95%CI: 1.51-13.44, P = 0.007) during follow-up were identified as independently associated risk factors.
CONCLUSION: Anti-TNFs significantly increase the risk of TB infection in Korean patients with IBD. The considerable burden of TB and marked immunosuppression might be attributed to this risk.
Keywords: Tuberculosis, Anti-TNF, Korea, Inflammatory bowel disease, Latent tuberculosis infection, Risk factor
Core tip: Anti-TNF antagonist therapy implies significant tuberculosis (TB) risk in inflammatory bowel disease (IBD) patients residing in areas with an intermediate burden of TB infection. The risk of susceptibility to TB under anti-TNF treatment is associated with latent TB infection and considerable immunosuppression. Rigorous and sustained assessment of TB infection should be implemented in Korean IBD patients undergoing anti-TNF therapy.
INTRODUCTION
Anti-TNF agents are able to favorably change the natural course of inflammatory bowel disease (IBD), and these drugs are currently the most effective treatment to achieve sustained clinical remission and mucosal healing[1]. Korea and other East Asian countries, once considered being a region in which IBD was extremely rare, have had a rapidly increasing number of patients with IBD. The population-based Korean data showed that the mean annual incidence rates of Crohn’s disease (CD) and ulcerative colitis (UC) increased from 0.05 and 0.34 per 100000 persons, respectively, in 1986-1990 to 1.34 and 3.08 per 100000, respectively, in 2001-2005[2]. Anti-TNF agents such as infliximab and adalimumab have been used with gradually increasing frequency since they were approved for IBD treatment in the mid 2000s in Korea[3-5].
One of the primary concerns regarding anti-TNF agents is an increased risk of tuberculosis (TB) infection. Despite a wide variation in TB rates among different countries, it has been reported that there was an approximately four-fold increased risk of TB in patients with rheumatoid arthritis (RA) treated with anti-TNF agents compared with those not treated with anti-TNF agents[6,7]. For IBD patients, the current incidence of active TB after treatment with an anti-TNF inhibitor is approximately 1%-2%[8,9]. The majority of TB cases occurred within 3-4 mo after anti-TNF therapy, suggesting that TB develops as a result of reactivation of latent disease rather than as a new infection[9,10]. Furthermore, the clinical characteristics of TB infection in anti-TNF treated patients are markedly atypical, presenting a greater chance of disseminated and extra-pulmonary diseases[11,12].
Although the incidence rate of TB infection in South Korea has been declining over recent decades, it remains one of the most common infectious diseases in the country. According to the World Health Organization, the incidence rate of TB in South Korea was 108 per 100000 inhabitants in 2012[13]. Whereas the risk of TB in Korean patients with RA treated with infliximab has been reported[14], there have been no reports on the incidence of TB infection in Korean IBD patients using anti-TNFs. Given that TB risks vary in different countries and with different diseases[15], it would be noteworthy to identify the risk of TB infection due to anti-TNF therapy in Korean patients with IBD. The aim of this study was to evaluate the incidence of active TB infection and associated risk factors in Korean IBD patients treated with anti-TNF agents. Additionally, the clinical characteristics of TB infection in these subjects were estimated.
MATERIALS AND METHODS
This study was conducted in 13 referral hospitals in Gyeongsang province in the southeastern region of Korea. Two anti-TNF agents, infliximab and adalimumab, are currently approved to treat IBD in South Korea. Candidates approved for the use of anti-TNFs by the National Health Insurance Service were patients with a moderate to severe stage of IBD who failed to show response or were intolerant to conventional treatments, including steroids or immunomodulators[4,5]. IBD patients treated with either of these TNF antagonists from June 2003 to January 2014 were included in this study. Information regarding clinical and demographic characteristics such as sex, age of IBD diagnosis, disease duration, anti-TNF drug exposure period, location and behavior of CD, and extent of UC were obtained from medical records. For the risk factors for TB, diabetes mellitus (DM), previous TB infection, latent TB infection (LTBI), concomitant immunosuppressant at the start of anti-TNF therapy, and WBC count measured around the last follow-up day were recorded. When active TB infection developed after anti-TNF therapy, WBC counts at the time of TB diagnosis were counted. However, history regarding contact with active TB patients was not obtained. The study was approved by the ethics review committee of the Institutional Review Board of all of the hospitals participating in the study.
Definition of TB infection and screening modalities
According to the Korean Guidelines for Tuberculosis published in 2011, chest radiography, a tuberculin skin test (TST), and an interferon gamma release assay (IGRA) should be performed for LTBI screening before the initiation of anti-TNF therapy[16]. Abnormal findings on chest radiography included apical densities, pleural scarring, and calcified granulomas. TST was performed according to the Mendel-Mantoux method using purified protein derivative (PPD). Skin induration with a diameter ≥ 10 mm at 48-72 h after the PPD inoculation on the forearm was considered positive[17]. Two methods for IGRA are available in Korea: QuantiFERON®-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, VIC, Australia) and T-SPOT®.TB (T-SPOT; Oxford Immunotec, Abingdon, UK). LTBI was defined as (1) cases of an abnormal chest X-ray without previous complete TB treatment or (2) positive results with TST or IGRA[18]. The criteria for active TB infection were as follows: (1) typical symptoms with isolation of Mycobacterium tuberculosis from a clinical specimen or (2) typical symptoms with radiological or histological findings of TB without culture or when a culture sample could not be obtained[18]. Although there was no bacterial confirmation, these cases were regarded as active TB when the clinical symptoms and the radiological or histological findings improved with anti-TB therapy[18]. The patients diagnosed with active TB before the initiation of anti-TNF therapy were not counted as the TB cases in the study.
Statistical analysis
The incidence rate of active TB was calculated using person-years (PY) and was expressed as new cases per 100000 PY. Differences in the categorical variables between the groups were assessed with the χ2 test or Fisher’s exact test. For comparisons of continuous variables, the Mann-Whitney test was used. To investigate the independent risk factors associated with active TB infection during anti-TNF therapy, a logistic regression analysis was performed using variables with statistically significant associations identified in a univariate analysis. Age and sex were also included as variables for a multivariate analysis because these are considered important risk factors of TB infection[19,20]. A two-tailed P value < 0.05 was considered significant. The statistical analysis was performed with SPSS version 14.0 (SPSS, Chicago, IL, United States).
RESULTS
In total, 376 IBD patients using anti-TNF agents were included in the study (255 males, mean age at the start of anti-TNF therapy of 32.5 ± 13.0 years, with 277 patients with CD and 99 patients with UC). The ileocolon (157, 56.7%) and non-stricturing non-penetrating type disease (102, 36.8%) were the most common location and behavior of CD, respectively. The majority of the UC patients had extensive disease (46, 46.5%). Eight patients (2.1%) had a previous TB infection history with successful anti-TB treatment. Infliximab and adalimumab were used in 294 (78.2%) and 82 (21.8%) patients, respectively. The baseline characteristics of the patients are described in Table 1.
Table 1.
Baseline characteristics of patients treated with anti-TNF agent
| n = 376 | |
| Sex (male:female) | 255:121 |
| Age of diagnosis (yr) | 27.9 ± 12.6 |
| Age at the start of anti-TNFs (yr) | 32.5 ± 13.0 |
| Follow-up (mo) | 81.6 ± 58.9 |
| Diseases | |
| Crohn’s disease | 277 (73.7) |
| L1/L2/L3/L1+L4/L3+L4 | 48/60/157/4/8 |
| B1/B2/B3 | 102/87/88 |
| Perianal disease | 160 (42.5) |
| Ulcerative colitis | 99 (26.3) |
| E1/E2/E3 | 8/45/46 |
| Previous anti-TB treatment | 8 (2.1) |
| Diabetes Mellitus | 12 (3.2) |
| Anti-TNF agents | |
| Infliximab | 294 (78.2) |
| Adalimumab | 82 (21.8) |
Data are espressed as n (%) or mean ± SD. TB: Tuberculosis; UC: Ulcerative colitis.
Screening for latent TB infection before anti-TNF therapy
The screening outcomes prior to anti-TNF therapy are summarized in Table 2. A chest X-ray was taken before anti-TNF therapy in the majority of patients (356, 94.7%); 8 (2.2%) of the chest x-rays showed abnormal appearances, suggesting old pulmonary TB. Among these patients, 4 had a history of a complete course of anti-TB treatment for pulmonary TB infection. IGRA was performed in 276 (73.4%) patients, and the positivity rate was 5.8% (16/276). One hundred and thirty-one patients (34.8%) underwent TST before anti-TNF therapy, and the positivity rate was 9.2% (12/131). Both IGRA and TST were performed in 98 patients (26.7%). The use of IGRA increased considerably from 34% in 2009 to 90.2% in 2013, whereas there was no significant change in the use of TST during the same period, with TST being performed in 30% and 39.8% of patients in 2009 and 2013, respectively. Using chest X-ray, IGRA and TST as screening measures, LTBI was confirmed in 30 patients (8.0%). Of these LTBI cases, 16 patients received prophylactic anti-TB medications. The patient flow diagram is shown in Figure 1. Immunosuppressants, such as steroids or thiopurine, were being administered at the time of IGRA and TST screening in 77.5% and 79.4% of patients, respectively.
Table 2.
Screening outcomes for latent tuberculosis infection before anti-TNF agent
| n = 376, n (%) | |
| Chest X-ray | |
| Done | 356 (94.7) |
| Old tuberculosis | 8/356 (2.2) |
| Negative | 348/356 (97.8) |
| IGRA | |
| Unknown | 9 (2.4) |
| Done | 276 (73.4) |
| Positive | 16/276 (5.8) |
| Negative | 241/276 (87.3) |
| Indeterminate | 19/276 (6.9) |
| Steroid or thiopurine at IGRA | 214/276 (77.5) |
| QuantiFERON | 247 (62.9) |
| Positive | 14/247 (5.7) |
| Negative | 218/247 (88.3) |
| Indeterminate | 15/247 (6) |
| T-SPOT | 29 (12.6) |
| Positive | 2/29 (6.9) |
| Negative | 23/29 (79.3) |
| Indeterminate | 4/29 (13.8) |
| TST | |
| Unknown | 10 (2.7) |
| Done | 131 (34.8) |
| Positive | 12/131 (9.2) |
| Negative | 117/131 (89.3) |
| Steroid or thiopurine at TST | 104/131 (79.4) |
| Screening tests for LTBI | |
| 1 test (IGRA or TST) | 211 (56.1) |
| 2 tests (IGRA and TST) | 98 (26.1) |
| Neither IGRA or TST | 59 (15.7) |
| Latent tuberculosis infection | 30 (8.0) |
IGRA: Interferon gamma release assay; TST: Tuberculin skin test; LTBI: Latent tuberculosis infection.
Figure 1.
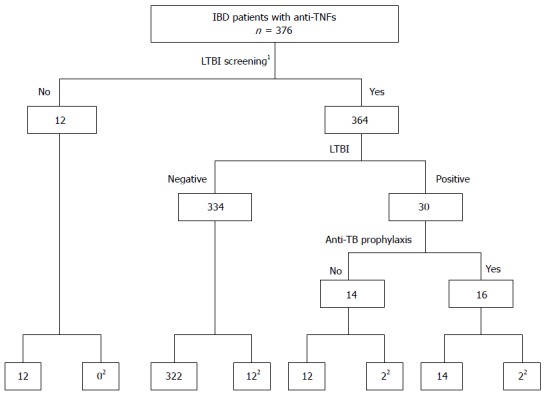
Flow diagram of patients, illustrating screening outcomes. 1Chest X-ray, tuberculin skin test, or interferon gamma release assay; 2Development of active tuberculosis infection after anti-TNF therapy. LTBI: Latent tuberculosis infection.
Incidence and risk factors of active TB infection after anti-TNF agents
Sixteen cases of active TB infection occurred during the 801 PY follow-up period after anti-TNF exposure (1997.4 per 100000 PY). The median time from anti-TNF initiation to active TB infection was 28.7 wk (range, 8-142). The clinical characteristics of these patients are summarized in Table 3. Infliximab was used in 15 patients, whereas adalimumab was used in 1 patient. All of the patients except one (who had a chest X-ray only) underwent LTBI screening tests of IGRA (75%, 12/16) or TST (31.2%, 5/16) before initiating anti-TNF treatment. Fifteen patients (93.8%) were taking immunosuppressants, including steroids or azathioprine, within 1 wk of the screening tests, and 4 patients had LTBI. Among the 4 LTBI cases, 2 patients received prophylactic treatment of either isoniazid for 9 mo or isoniazid plus rifampicin for 3 mo, and one patient had a previous complete anti-TB medication history for active pulmonary TB. The majority of the patients (15/16, 93.8%) were taking steroids or azathioprine when the active TB diagnosis was made. We conducted a univariate analysis to identify the risk factors of TB infection during anti-TNF treatment in IBD patients (Table 4). The positive rate of screening for LTBI in the TB infection group was higher than in the non-TB infection group (25% vs 7.2%, P = 0.031). A WBC count < 5000 mm3 was more often observed in the TB infection group than in the non-TB infection group (62.5% vs 31.9%, P = 0.015). There was no significant difference between the groups regarding disease type, DM, anti-TNF agents, and immunomodulator use at the start of anti-TNF treatment. A multivariate analysis using logistic regression after adjustment for age and sex demonstrated that LTBI (OR = 5.76, 95%CI: 1.57-21.20, P = 0.008) and white blood cell (WBC) count < 5000 mm3 during follow-up (OR = 4.5, 95%CI: 1.51-13.44, P = 0.007) were significant independent risk factors for active TB infection during anti-TNF agent therapy (Table 5).
Table 3.
Clinical characteristics of patients developing active tuberculosis infection after anti-TNF agent
| No. | IBD | Sex | Age of anti-TNFs (yr) | Anti-TNFs | Previous TB treatment | Screening IGRA | Screening TST | IS at screening | Prophylaxis | Interval to TB infection (wk) | IS at TB diagnosis | Extra-pulmonary TB | No. of involvement organ |
| 1 | CD | F | 21 | IFX | No | ND | ND | None | ND | 83 | CS + AZA | No | 1 |
| 2 | CD | M | 24 | ADA | No | + | ND | CS | INH | 22 | None | Yes | 1 |
| 3 | CD | M | 24 | IFX | No | - | ND | CS +AZA | ND | 35 | AZA | Yes | 3 |
| 4 | CD | M | 42 | IFX | Yes | - | ND | CS | ND | 46 | CS + AZA | Yes | 6 |
| 5 | CD | F | 34 | IFX | Yes | - | ND | AZA | ND | 23 | AZA | Yes | 1 |
| 6 | CD | M | 29 | IFX | No | - | ND | AZA | ND | 10 | AZA | Yes | 5 |
| 7 | CD | M | 41 | IFX | No | ND | - | AZA | ND | 12 | AZA | Yes | 1 |
| 81 | CD | M | 24 | IFX | No | ND | - | CS | ND | 8 | CS | Yes | 4 |
| 9 | CD | M | 27 | IFX | No | ND | - | AZA | ND | 22 | AZA | No | 1 |
| 10 | CD | M | 23 | IFX | Yes | + | - | AZA | ND | 8 | CS + AZA | Yes | 1 |
| 11 | CD | M | 21 | IFX | No | - | Unknown | CS +AZA | ND | 129 | CS + AZA | Yes | 3 |
| 12 | UC | M | 32 | IFX | No | + | ND | AZA | INH + RFP | 40 | AZA | Yes | 4 |
| 13 | UC | M | 70 | IFX | No | - | ND | AZA | ND | 142 | AZA | No | 1 |
| 14 | UC | M | 21 | IFX | No | - | ND | CS +AZA | ND | 52 | AZA | Yes | 3 |
| 15 | UC | M | 56 | IFX | No | - | ND | AZA | ND | 47 | AZA | Yes | 2 |
| 16 | UC | F | 25 | IFX | No | + | - | AZA | ND | 12 | AZA | Yes | 2 |
This patient died 55 d after miliary tuberculosis diagnosis due to devastating acute renal failure. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IFX: Infliximab; ADA: Adalimumab; ND: Not done; TB: Tuberculosis; IGRA: Interferon gamma release assay; TST: Tuberculin skin test; IS: Immunosuppressant; CS: Corticosteroid; AZA: Azathioprine; INH: Isoniazid; RFP: Rifampin.
Table 4.
Univariate analysis of risk factors for active tuberculosis infection after anti-TNF therapy n (%)
| TB infection + (n = 16) | TB infection - (n = 360) | P value | |
| Age at anti-TNF (yr) | 26 (21-70) | 30 (11-76) | 0.808 |
| Female | 3 (18.8) | 118 (32.8) | 0.287 |
| Diseases | 0.772 | ||
| Crohn’s disease | 11 (68.8) | 266 (73.9) | |
| Ulcerative colitis | 5 (31.3) | 94 (26.1) | |
| Diabetes mellitus | 1 (6.3) | 11 (3.1) | 0.411 |
| Anti-TNF agent | 0.212 | ||
| Infliximab | 15 (93.8) | 279 (77.5) | |
| Adalimumab | 1 (6.3) | 81 (22.5) | |
| LTBI | 4 (25) | 26 (7.2) | 0.031 |
| IM at anti-TNF agent | 13 (81.3) | 228 (63.3) | 0.187 |
| WBC count < 5000 mm3 | 10 (62.5) | 115 (31.9) | 0.015 |
LTBI: Latent tuberculosis infection; IM: Immunomodulator.
Table 5.
Multivariate analysis of risk factors for active tuberculosis infection after anti-TNF therapy
| OR | 95%CI | P value | |
| Age at anti-TNF | 0.98 | 0.94-1.02 | 0.346 |
| Female | 1.95 | 0.53-7.18 | 0.328 |
| LTBI | 5.76 | 1.57-21.20 | 0.008 |
| WBC count < 5000 mm3 | 4.50 | 1.51-13.44 | 0.007 |
LTBI: Latent tuberculosis infection; WBC: White blood cell.
One 24-year-old male patient with CD undergoing infliximab treatment died 55 d after miliary TB diagnosis because of devastating acute renal failure. His chest X-ray had been normal, and TST had shown a negative result. He was taking steroids at the time of the TST.
Clinical features of active tuberculosis infection during treatment with anti-TNF agents
Fever (43.8%) was the most common clinical manifestation, followed by cough, dyspnea, abdominal distension, fatigue and chest pain. The most frequent sites of infection were the lung, lymph node and pleura, in that order. Miliary TB was observed in 6 (37.5%) patients. The majority of the patients (81.3%) showed extra-pulmonary TB, indicating an atypical presentation of TB infection. The clinical characteristics of active TB infections are shown in Figures 2A and 2B.
Figure 2.
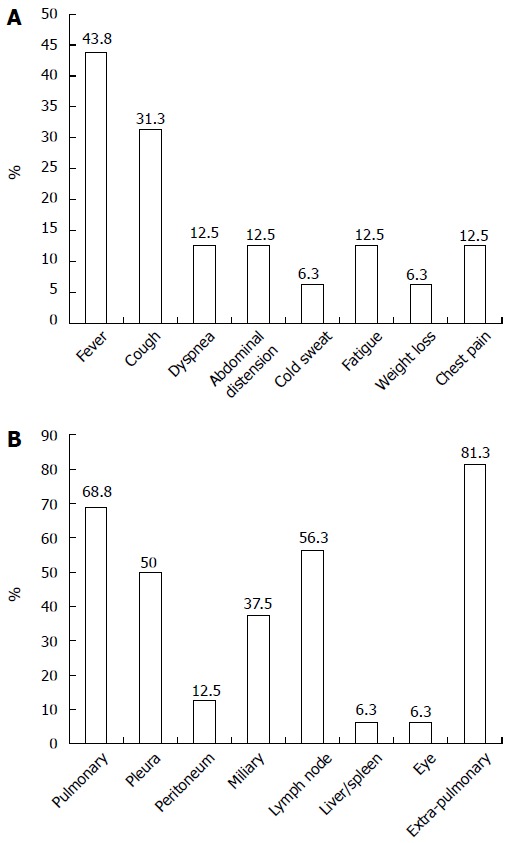
Clinical symptoms (A) and involvement locations (B) of active tuberculosis infection after anti-TNF therapy in patients with inflammatory bowel disease.
DISCUSSION
In this study, the incidence rate of TB infection was 1997.4 per 100000 PY in IBD patients exposed to anti-TNF inhibitors, and this risk was associated with LTBI confirmed by the screening tests and with leukopenia (WBC < 5000 mm3) during the follow-up period. These rates are much higher than the rates found in Western studies[7,9,11]. The remarkably high incidence of TB in this study might be related to the large burden of TB in the general population. South Korea has been reported to have an intermediate burden of TB with an incidence, mortality and prevalence of 108, 5.4 and 146 per 100000 persons, respectively, in 2012[13]. For reference, the incidence, mortality, and prevalence of TB in the United States were 3.6, 0.14, and 4.7 per 100000 persons, respectively, in the same year[13]. The incidence rates were also considerably high in Korean ankylosing spondylitis (AS) or RA patients using anti-TNF agents, with the rates ranging from 540 to 2558 per 100000 PY, which is similar to the observations in our study[14,18]. To the best of our knowledge, this is the first study to evaluate the risk of TB infection in a large number of Korean IBD patients undergoing anti-TNF therapy.
Although the Korean Guidelines for TB in 2011 recommend TST or IGRA as screening tests before initiating anti-TNF agents, TST and IGRA were used in only 34.8% and 73.4% of patients, respectively, in the present study. This low compliance of screening tests might be partly attributed to the patients who were taking anti-TNF agents in the early period when LTBI screening before the use of TNF blockers was not strictly performed in Korea. A study from the USA also showed a low rate (65%) of LTBI screening prior to initiating anti-TNF therapy in IBD patients, and the authors reported that the initiation of treatment prior to 2006 was a risk factor for screening failure[21]. In our study, the use of IGRA rose significantly over time from 34% in 2009 to 90.2% in 2013. However, the rate of TST remained low throughout the study period, reflecting the low preference for TST due to insufficient accuracy of TST for LTBI screening, particularly in Korea, where Bacille Calmette-Guerin (BCG) vaccination is mandatory, which might influence TST results. A lack of specificity for pathogenic Mycobacterium tuberculosis is a limitation of TST, and this might be due to cross-reactivity with the BCG vaccination and environmental mycobacteria[22,23]. Therefore, IGRA might be more appropriate as a LTBI screening test in countries using routine BCG vaccination, such as Korea[24].
One important finding of the present study was the low positivity rates of each screening test (5.8% for IGRA and 9.2% for TST). There appear to be different positivity rates of LTBI screening tests between patients with different diseases. For example, the TST and IGRA positivity rates of RA patients were relatively high, up to 23% and 31.6%, respectively[25], whereas the rates of IBD patients were 12.5%-16% and 7.2%-9%, respectively[26,27], which are similar to our results. Although the cause of the difference between the studies is unclear, the different patient age ranges and the varying use of concomitant immunosuppressants during screening might be plausible explanations. IBD patients are typically younger than RA patients, and age is strongly associated with the positivity of TST as a result of longer exposure to Mycobacterium tuberculosis[26]. Additionally, approximately 80% of patients in the present study were taking steroids or thiopurine during the screening tests, which could lead to low positive results for the tests. Immunosuppression has been known to negatively affect the outcomes of TST and IGRA, resulting in low sensitivity of these screening tests[26]. Therefore, the ideal time for LTBI screening would be prior to the initiation of immunosuppressant therapy.
There has been no study on the risk factors for the development of TB infection during anti-TNF therapy because the number of TB cases is too small for a precise assessment. We identified positive LTBI and a WBC count < 5000 mm3 as independent risk factors for active TB infection during anti-TNF therapy. Patients with LTBI were more likely to have active TB infection than patients without LTBI (OR = 5.76, 95%CI: 1.57-21.20, P = 0.008) (Table 5). This result is not surprising because LTBI is likely to progress to active TB in immunocompromised patients, such as those taking anti-TNF inhibitors[17]. There is clear evidence suggesting that chemoprophylaxis with screening for LTBI considerably reduces the TB reactivation rate[28,29]. However, we should consider that chemoprophylaxis for suspected LTBI prior to anti-TNF therapy does not entirely avoid the development of active disease[30]. It has been reported that chemoprophylaxis is only moderately effective[28,30]. In the present study, we could not find a prophylactic effect of anti-TB medications; 12.5% (2/16) of patients with LTBI who took prophylactic anti-TB medication still developed active TB, whereas 14.3% (2/14) of patients with LTBI who did not take prophylaxis had active TB during anti-TNF therapy (Figure 1). Although the exact reason for the lack of efficacy of prophylaxis in this study is unclear, the emergence of drug-resistant TB in Korea might be a possible explanation[31,32].
Guidelines recommend delaying to begin anti-TNF for at least 3 wk when LTBI is confirmed[16,33]. Anti-TNF agents can be started early in some inevitable cases for disease control. Given that LTBI positivity is a significant risk factor for the development of active TB and there seems to be the lack of efficacy of chemoprophylaxis for that, we should consider seriously undertaking the balance between the risk and the benefit before initiating anti-TNF agents in IBD patients with positive result for LTBI. If anti-TNF is used in these patients, more strict and complete anti-TB prophylaxis measures should be followed by rigorous monitoring for the development of active TB.
Our result showing leukopenia as an independent factor for active TB infection might indicate the synergistic risk of the substantial level of immunosuppression in anti-TNF users. Anti-TNF blocker plus azathioprine combination therapy has been shown to be the most effective treatment for IBD[34,35]. A significant proportion (87.5%, 14/16) of patients with active TB infection had concomitant azathioprine treatment at the time of TB diagnosis (Table 3). However, we are not certain that the relative leukopenia at the time of TB diagnosis is entirely a result of immunomodulator therapy alone because leukopenia can be observed in some patients with disseminated TB infection, such as miliary TB[36]. Mert et al[36] observed leukopenia in 26% of miliary TB patients, and this was considered to be a poor prognostic sign, although the exact cause was not clear[37]. Therefore, the results of our study should be interpreted cautiously.
In contrast to TB in immunocompetent individuals, where pulmonary infection is the main manifestation, patients who received anti-TNF therapy showed a significantly high percentage of extrapulmonary disease, at 57%-75%, and 25% had disseminated disease[7,38]. Similarly, we determined that the rates of extrapulmonary manifestations and miliary TB were considerably high at 81.3% and 37.5%, respectively (Figure 2B). Furthermore, these patients presented a variety of non-specific symptoms, including fever, fatigue, abdominal distension, dyspnea, and chest pain (Figure 2A). These results suggest that the potential diagnosis of active TB infection should be vigilantly evaluated when any patient taking an anti-TNF inhibitor has these constitutional symptoms aside from coughing because the TB manifestations under anti-TNF therapy are remarkably atypical.
Most cases of TB related to anti-TNFs are considered as reactivations of LTBI because it has been reported that TB develops early after the initiation of TNF blockade, usually within 3-4 mo[9,10]. In the present study, however, the median time from the first anti-TNF dose to TB diagnosis was 28.7 wk, which was longer than previous studies, and a quarter of TB cases occurred 1 year after the initiation of anti-TNF agents. We presumed that some TB cases in our study might represent de novo infection from exposure to other TB-infected persons during the course of anti-TNF treatment instead of representing reactivation of LTBI. This finding is in accordance with another Korean study evaluating the TB incidence in AS patients taking anti-TNFs with a long median time of 21.5 mo[18]. These results highlight the recommendation that information regarding close contact with TB-infected individuals should be rigorously and continuously assessed in patients receiving anti-TNF therapy, particularly in countries with a significant TB burden such as South Korea. Further prospective studies are needed to clarify whether periodically repeated screening tests for LTBI are effective in this population.
This study has several limitations. The retrospective design is the major limitation. Important information regarding contact with active TB patients could not be obtained. The WBC count was not systematically collected for the analysis of risk factors. The WBC counts of non-TB patients were obtained at the last follow-up visit, whereas the WBC counts of the TB patients were obtained at TB diagnosis.
In conclusion, anti-TNF inhibitors imply significant TB risk in IBD patients residing in areas with an intermediate burden of TB infection. The risk of susceptibility to TB under anti-TNF therapy is significantly associated with LTBI and considerable immunosuppression. Rigorous and sustained assessment of TB infection should be performed in IBD patients undergoing anti-TNF therapy. Further studies to establish the strategy of effective TB monitoring in this population are required.
COMMENTS
Background
Anti-TNF agents are able to favorably change the natural course of inflammatory bowel disease (IBD), and these drugs are currently the most effective treatment to achieve sustained clinical remission and mucosal healing. However, Anti-TNF agents considerably increase the risk of tuberculosis (TB) infection.
Research frontiers
Although the use of anti-TNF agents in Korean patients with IBD has recently increased, there have been no reports on the risk of active TB infection in this population.
Innovations and breakthroughs
In this study, the incidence rate of TB infection was 1997.4 per 100000 person-years in IBD patients exposed to anti-TNF inhibitors and these rates are much higher than the rates found in Western studies. The risk was associated with LTBI confirmed by the screening tests and with leukopenia (WBC < 5000 mm3) during the follow-up period.
Applications
Rigorous and sustained assessment of TB infection should be performed in IBD patients undergoing anti-TNF therapy. Further studies to establish the strategy of effective TB monitoring in patients residing in areas with an intermediate burden of TB infection are required.
Terminology
Latent TB infection was defined as (1) cases of an abnormal chest X-ray without previous complete TB treatment or (2) positive results with tuberculin skin test or interferon gamma release assay.
Peer-review
This article presents important data concerning the safety of anti-TNF therapy in IBD patients who live in countries with a significant burden of TB infection.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 11, 2014
First decision: October 14, 2014
Article in press: December 1, 2014
P- Reviewer: Fujimori S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.van Assche G, Vermeire S, Rutgeerts P. Mucosal healing and anti TNFs in IBD. Curr Drug Targets. 2010;11:227–233. doi: 10.2174/138945010790309902. [DOI] [PubMed] [Google Scholar]
- 2.Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 3.Lee KM, Jeen YT, Cho JY, Lee CK, Koo JS, Park DI, Im JP, Park SJ, Kim YS, Kim TO, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013;28:1829–1833. doi: 10.1111/jgh.12324. [DOI] [PubMed] [Google Scholar]
- 4.Ye BD, Yang SK, Shin SJ, Lee KM, Jang BI, Cheon JH, Choi CH, Kim YH, Lee H. [Guidelines for the management of Crohn’s disease] Korean J Gastroenterol. 2012;59:141–179. doi: 10.4166/kjg.2012.59.2.141. [DOI] [PubMed] [Google Scholar]
- 5.Choi CH, Kim YH, Kim YS, Ye BD, Lee KM, Lee BI, Jung SA, Kim WH, Lee H. [Guidelines for the management of ulcerative colitis] Korean J Gastroenterol. 2012;59:118–140. doi: 10.4166/kjg.2012.59.2.118. [DOI] [PubMed] [Google Scholar]
- 6.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52:1986–1992. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- 7.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 8.Mañosa M, Domènech E, Cabré E. Current incidence of active tuberculosis in IBD patients treated with anti-TNF agents: still room for improvement. J Crohns Colitis. 2013;7:e499–e500. doi: 10.1016/j.crohns.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Jauregui-Amezaga A, Turon F, Ordás I, Gallego M, Feu F, Ricart E, Panés J. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 12.Desai SB, Furst DE. Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol. 2006;20:757–790. doi: 10.1016/j.berh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Global Tuberculosis Report 2013. [Accessed on Sep 2, 2014] Available from: http://www.who.int/tb/publications/global_report/ed/
- 14.Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, Kim TH, Jun JB, Yoo DH, Lee JT, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007;34:706–711. [PubMed] [Google Scholar]
- 15.Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:19–30. doi: 10.1111/j.1365-2036.2007.03553.x. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Practice Guidelines for Tuberculosis, Seoul, Korea: Korea Centers for Disease Control and Prevention, 2011. Accessed July 16, 2014. Available from: http://www.lungkorea.org/image/mail/file 11017.pdf/
- 17.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 18.Kim EM, Uhm WS, Bae SC, Yoo DH, Kim TH. Incidence of tuberculosis among korean patients with ankylosing spondylitis who are taking tumor necrosis factor blockers. J Rheumatol. 2011;38:2218–2223. doi: 10.3899/jrheum.110373. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Li T, Tan S. Males, ages ≥ 45 years, businessperson, floating population, and rural residents may be considered high-risk groups for tuberculosis infection in Guangzhou, China: a review of 136,394 tb confirmed cases. Rev Inst Med Trop Sao Paulo. 2013;55:366–368. doi: 10.1590/S0036-46652013000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memish ZA, Bamgboye EA, Abuljadayel N, Smadi H, Abouzeid MS, Al Hakeem RF. Incidence of and risk factors associated with pulmonary and extra-pulmonary tuberculosis in Saudi Arabia (2010-2011) PLoS One. 2014;9:e95654. doi: 10.1371/journal.pone.0095654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012;18:1057–1063. doi: 10.1002/ibd.21824. [DOI] [PubMed] [Google Scholar]
- 22.Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004;2:309–313. doi: 10.1016/s1542-3565(04)00060-6. [DOI] [PubMed] [Google Scholar]
- 23.Ponce de León D, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, Perich R, Pastor C, Harrison J, Sánchez-Schwartz C. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005;64:1360–1361. doi: 10.1136/ard.2004.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 25.Song GG, Bae SC, Lee YH. Interferon-gamma release assays versus tuberculin skin testing in patients with rheumatoid arthritis. Int J Rheum Dis. 2013;16:279–283. doi: 10.1111/1756-185X.12098. [DOI] [PubMed] [Google Scholar]
- 26.Papay P, Eser A, Winkler S, Frantal S, Primas C, Miehsler W, Novacek G, Vogelsang H, Dejaco C, Reinisch W. Factors impacting the results of interferon-γ release assay and tuberculin skin test in routine screening for latent tuberculosis in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:84–90. doi: 10.1002/ibd.21427. [DOI] [PubMed] [Google Scholar]
- 27.Kim BJ, Choi YS, Jang BI, Park YS, Kim WH, Kim YS, Jung SA, Han DS, Kim JS, Choi JH, et al. Prospective evaluation of the clinical utility of interferon-γ assay in the differential diagnosis of intestinal tuberculosis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:1308–1313. doi: 10.1002/ibd.21490. [DOI] [PubMed] [Google Scholar]
- 28.Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, Montero D, Pascual-Gómez E, Mola EM, Carreño L, Figueroa M. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–1772. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 29.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006;2:602–610. doi: 10.1038/ncprheum0336. [DOI] [PubMed] [Google Scholar]
- 30.Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127–1132. [PubMed] [Google Scholar]
- 31.Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Lee NY, Park YK, Bai GH, Koh WJ. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007;22:677–681. doi: 10.3346/jkms.2007.22.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009;24:592–595. doi: 10.3346/jkms.2009.24.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D’Haens G, Domènech E, Eliakim R, Eser A, Frater J, et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 36.Mert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, Aki H, Seyhan N, Karayel T, Aktuglu Y. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001;6:217–224. doi: 10.1046/j.1440-1843.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 37.Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;89:291–296. doi: 10.1016/0002-9343(90)90340-j. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]


