Abstract
Strongyloides stercoralis, a soil transmitted helminth infection, affects millions with varying prevalence worldwide. A large number of affected hosts are asymptomatic. Symptoms pertaining to pulmonary and gastrointestinal involvement may be present. Manifestations of involvement beyond lung and intestine can be seen with dissemination of infection and lethal hyperinfection. Immunosuppression secondary to use of steroids or other immunosuppressants and coexistence of human T-lymphotropic virus type-1 are the known risk factors for dissemination and hyperinfection. Diagnostic modalities comprise stool examination, serology and molecular testing. Stool tests are inexpensive but are limited by low sensitivity, whereas serologic and molecular tests are more precise but at the expense of higher cost. Treatment with Ivermectin or Albendazole as an alternative is safe and efficacious. We present a rare case of acute pancreatitis secondary to Strongyloides. High index of suspicion in patients specifically from endemic countries of origin and lack of other common etiologies of acute pancreatitis may help in early diagnosis and prompt treatment of this potentially fatal infection.
Keywords: Strongyloides, Pancreatitis, Autoinfection, Helminth, Eosinophilia
Core tip: Strongyloides affects millions of people worldwide. Large numbers of infected hosts are asymptomatic or have non-specific gastrointestinal and/or pulmonary symptoms. Infected hosts, especially in the setting of human T-lymphotropic virus type-1 infection and immunosuppressant or steroid use, may develop overwhelming infection in the form of dissemination or hyperinfection. Peripheral eosinophilia may be the only non-specific finding. Diagnostic methods range from simple stool examination to serologic tests and molecular techniques based on nucleic acid amplification. Endoscopic examination may be needed which may provide evidence of infection on pathological exam. Treatment options are both safe and efficacious with oral Ivermectin being superior to Albendazole.
INTRODUCTION
Strongyloidiasis, an infection caused by Strongyloides stercoralis, is endemic in tropical and sub-tropical regions. Poor sanitary conditions favor its propagation. Infection with Strongyloides is usually asymptomatic which leads to underestimated figures of its prevalence. The most commonly affected organs are lung and intestines with mild non-specific symptoms like abdominal pain, vomiting, diarrhea, tracheal irritation and cough. Hyperinfection or disseminated infection due to unique feature of autoinfection may occur. We present a rare case of Strongyloides associated acute pancreatitis and a review of four reported cases.
CASE REPORT
A 48-year-old man presented to Bronx Lebanon Hospital Center with abdominal pain after he had been admitted to another hospital for epigastric pain of ten days duration. He described the pain as intermittent, non-radiating and worsening with food intake. The patient denied nausea, vomiting, fever or dysphagia. An upper endoscopic exam had reportedly revealed normal esophagus, gastric erythema and normal duodenum. On the 4th day of hospitalization, the patient reported slight improvement in his symptoms and he was discharged home on omeprazole 20 mg oral daily.
Two days after discharge from the other hospital, on 16th day of illness he presented to Bronx Lebanon Hospital Center due to persistent epigastric pain.
He had hypertension, and he was taking losartan 50 mg daily. He had had surgery for kidney stones. He had no known drug or food allergies. He did not smoke, drink alcohol, or use illicit drugs. He was married and had children. He had immigrated to United States from the Dominican Republic 5 years ago, lived in Pennsylvania and worked in a New York factory that produced cleaning liquids. His brother had diabetes mellitus; his parents and children were healthy.
On examination, temperature was 97.9°F, blood pressure 134/95 mmHg, pulse 68 beats per minute and regular, weight 81.6 kg with a body-mass index of 30. The patient looked stable but was uncomfortable due to pain. The abdomen was non-distended and had normal bowel sounds on auscultation; but it was soft with moderate tenderness in the epigastrium region. The rest of the examination was normal.
The white blood cell count was 18700 per mm3 with an eosinophil percentage of 42.2 and absolute eosinophil count of 7900 per mm3. Serum amylase and lipase levels were 78 units per liter and 83 units per liter respectively. Other test results were unremarkable as shown in Table 1. Computed tomography (CT) of the abdomen and pelvis, performed after the administration of intravenous and oral contrast material was normal. An upper gastrointestinal endoscopy was performed which showed normal appearing esophagus and multiple erosions in the stomach and duodenum (Figure 1A, B). Pathological exam of random esophageal biopsy specimen revealed hyperplastic squamous mucosa with mucosal congestion. Gastric biopsy specimen showed increased eosinophils in lamina propria, intraepithelial eosinophils, and eosinophilic cryptitis; and duodenal specimen showed increased eosinophils in lamina propria (more than 20 per high power field), intraepithelial eosinophilic infiltrates, eosinophilic cryptitis and no shortening of villi or crypt distortion. A diagnosis of eosinophilic gastroenteritis was entertained. Omeprazole was administered, test for stool ova and parasite was ordered before considering steroid treatment for eosinophilic gastroenteritis while awaiting results of endoscopic biopsies. The patient was discharged home after three days of hospitalization with instructions to follow up in outpatient clinic in 1 wk.
Table 1.
Results of blood tests
| Blood tests | Labs on first admission Day 16 of illness | Labs on second admission Day 22 of illness |
| Hemoglobin (g/dL) | 16.4 | 15.9 |
| Hematocrit (%) | 48.3 | 46.0 |
| Platelet count | 237000 | 286000 |
| (/μL) | ||
| White blood cell count | 18700 | 23300 |
| (/mm3) | ||
| Differential count (%) | ||
| Neutrophils | 35.2 | 33.5 |
| Lymphocytes | 18.4 | 16.5 |
| Eosinophils | 42.2 | 46.5 |
| INR | 1.1 | 1.0 |
| Blood urea nitrogen (mg/dL) | 10 | 10 |
| Creatinine (mg/dL) | 1.0 | 0.9 |
| Albumin (g/dL) | 3.4 | 3.8 |
| Alanine aminotransferase | 26 | 65 |
| (U/L) | ||
| Aspartate aminotransferase (U/L) | 31 | 39 |
| Alkaline phosphatase | 144 | 284 |
| (U/L) | ||
| Total bilirubin (mg/dL) | 0.2 | 0.3 |
Figure 1.
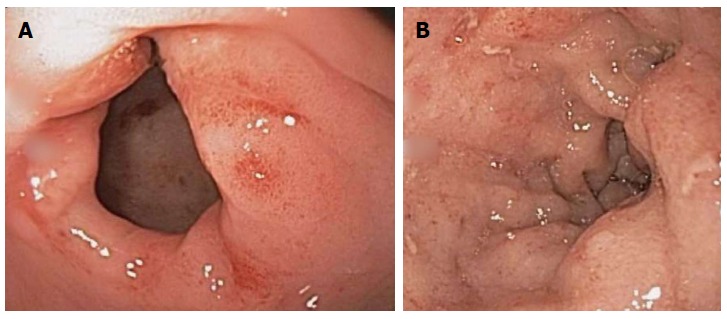
Endoscopic images of gastric antrum and duodenal bulb erosions (A and B).
Three days later, on the 22nd day of his illness, he returned to this hospital with worsening epigastric pain that he rated at 10 out of 10. It was constant, radiating to his back and associated with one episode of non-bilious vomiting. He also reported about 11 pounds of unintentional weight loss over last three weeks. On examination he was anxious and uncomfortable. His temperature was 97.0°F, blood pressure 166/107 mmHg, pulse 60 beats per minute and respiratory rate 15 per minute. His abdomen was non-distended with normal bowel sounds, it was soft but there was severe tenderness in the epigastrium region. The remainder of the examination was normal. The white blood cell count level was again found to be elevated at 23300 per mm3 with an eosinophil percentage of 46.5 and absolute eosinophil count of 10900 per mm3. Serum levels of amylase and lipase were 263 and 476 units per liter. The other blood test results are shown in Table 1. Radiography of the abdomen was normal. CT of the abdomen and pelvis following the administration of oral and intravenous contrast was normal. Ultrasonography of abdomen revealed gall bladder sludge, a normal common bile duct and no gallstone.
A diagnosis of acute pancreatitis was made. The patient was managed conservatively with aggressive intravenous fluid administration. Several possibilities were entertained in the search for an etiology. Etiologies of acute pancreatitis, like gallstones and hypertriglyceridemia, were excluded based on the normal abdominal sonogram and serum triglyceride level. Pancreatitis secondary to alcohol and drug were also excluded based on history. The differential diagnosis, especially in the presence of eosinophilia, also included aggressive systemic mastocytosis with lymphadenopathy and eosinophilia, hypereosinophilic syndrome, occult parasitic infection and hypoadrenalism. Hematology consult was obtained and bone marrow biopsy was performed.
Bone marrow biopsy revealed normocellular marrow with trilineage maturation but increase in both mature and immature eosinophils to about 30 percent of nucleated hematopoietic cells. Serum levels of aldosterone, tryptase and immunoglobulin E level were all normal. Stool for ova and parasite was reported negative on multiple occasions. Strongyloides immunoglobulin G (IgG) tested by Enzyme Linked Immunosorbent Assay (ELISA) level was reported positive. Careful review of the endoscopic biopsy specimens again revealed a Strongyloides stercoralis larva in the duodenal biopsy specimen (Figure 2A, B).
Figure 2.
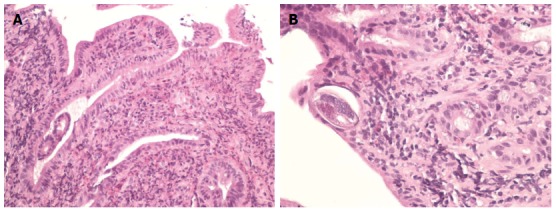
Hematoxylin and Eosin staining of the biopsy specimen. A: Hematoxylin and eosin (HE) stained section of small bowel biopsy showing prominent eosinophilia associated with Strongyloides infection; B: HE stained longitudinal cross section of a small bowel biopsy showing Strongyloides worm lying within the crypt.
A single dose of Ivermectin 200 microgram per kilogram bodyweight was administered orally and 24 h later the patient reported complete resolution of his symptoms. A repeat dose of Ivermectin 200 microgram per kilogram bodyweight was administered orally two weeks later during an outpatient follow up visit.
DISCUSSION
Background
Strongyloides are soil-transmitted helminths. They are vertebrate parasites and the genus comprises about fifty species[1]. They infect different hosts like amphibians, birds and mammals including humans. The latter can be infected by three different species of Strongyloides, namely Strongyloides stercoralis, Strongyloides fuelleborni fuelleborni and Strongyloides fuelleborni kelleyi[2]. Strongyloides stercoralis is the most prevalent species worldwide, whereas Strongyloides fuelleborni fuelleborni is prevalent in Africa and Strongyloides fuelleborni kelleyi in Papua New Guinea.
Epidemiology
Strongyloides was first discovered by Louis Normand in 1876 in the stools of French soldiers returning from Indochina[3]. The two different forms of Strongyloides larvae were initially considered as two different species and were named as Anguillula stercoralis and Anguillula intestinalis. Later they were classified under genus Strongyloides and the worm was renamed as Strongyloides stercoralis. Since its discovery more than a century ago, it has been found in all parts of world except Antarctica.
Strongyloides stercoralis is most prevalent in tropical and sub-tropical regions but also occurs in temperate regions when conditions are favorable. It is endemic in Southeast Asia, Sub Saharan Africa and Latin America. Due to the dearth of epidemiologic studies, worldwide prevalence is unknown but about 30 to 100 million people are estimated to be infected[4].
A recent literature review aimed at estimating prevalence of Strongyloides in different countries of the world exhibited the diversity in its worldwide prevalence. On the African continent, prevalence as high as 99% in Namibia, 92% in Gabon and 80% in Kenya was reported. In Latin America, prevalence was found to be 98% in the Dominican Republic and 75% in Peru. Prevalence in New Papua Guinea was reported as 99%[5].
Historically, in the United States, Strongyloides stercoralis has been considered endemic in southern states and Appalachia. With the last high quality study from the United States having been published three decades ago[6], information about most recent prevalence trends of Strongyloides stercoralis is lacking. Due to improvement in sanitary conditions and hygienic practices, these prior studies had shown marginal decline in prevalence from 3.8% to 3%[7]. In studies among veterans returning from endemic regions and immigrants, respective prevalence as high as 37%[8] and 40%[5] has been recorded. Since the initiative from the Centers for Disease Control and prevention to presumptively treat parasitic infections in immigrants prior to their arrival in the United States, there has been a significant decline in Strongyloides stercoralis prevalence among immigrants[9].
Life cycle
The life cycle for Strongyloides is unique and features like parthenogenesis (development of larvae from unfertilized eggs without sexual reproduction), release of larvae in feces and not eggs, and autoinfection make it stand out from the rest. Strongyloides life cycle comprises two forms - free-living form and parasitic form. Rhabditiform larvae upon their release in the feces of an infected host can molt to form either free living adult worms or the infective filariform larvae. The infective filariform larvae can be transmitted to another host through exposure to contaminated soil. After penetrating skin of the new host, these infective filariform larvae are carried by blood to the lungs where they break out of lung capillaries into alveoli and then ascend up the tracheobronchial tree to be swallowed eventually into the gastrointestinal tract. In the gastrointestinal tract, larvae molt into adult female worms that settle down in the lamina propria of the small intestine and release eggs without involvement of male for reproduction (parthenogenesis). Rhabditiform larvae hatch out of these eggs and are eventually passed in feces. These rhabditiform larvae, instead of being released into feces can also re-penetrate the gastrointestinal mucosa or the perianal skin (autoinfection)[10].
Immunology
Overall understanding on human immune responses to Strongyloides stercoralis is limited. The limitation is partly from the complex nature of the Strongyloides as well as inability to reproduce the pathophysiology of the Strongyloides stercoralis human infection in the currently available rat model studies. Nevertheless, these rat models have demonstrated an armamentarium of immune responses that interplay to fight against Strongyloides. Innate immune responses begin by recruitment of neutrophils and eosinophils. Neutrophils need myeloperoxidase and toll like receptors to kill the larvae. On the other hand, eosinophils mediate larval killing by supplying cytokines needed for immunoglobulin IgM production. Eosinophils, as antigen presenting cells, also play a key role in activating adaptive immune responses. Once adaptive immunity is activated, helper T cells activate B cells through interleukin-5 and initiate production of IgM and IgG. Immunoglobulin IgM and IgG not only recognize different antigens of Strongyloides stercoralis but also work through different mechanisms. IgG needs neutrophils and complement activation for larval killing (antibody dependent cellular cytotoxicity), whereas IgM action is independent of neutrophils and cytokines[11,12].
Clinical features
Clinical manifestations of Strongyloides stercoralis infection vary depending on the worm burden. Most of the immunocompetent hosts infected with Strongyloides stercoralis are asymptomatic which is one of the reasons for the underestimation of its true prevalence. After the skin penetration by infective filariform larvae, a rash may be recognized in some but not all patients. The rash, which may last for several weeks, can present in the form of multiple wheels like urticarial reaction or a serpiginous creeping rash created by movement of larva under the skin (larva currens). In patients with autoinfection, where larvae re-enter the peri-anal skin as soon as they exit from the anal canal, the rash may be seen on the buttocks and thighs.
With involvement of respiratory system, it can manifest as cough, tracheal irritation and shortness of breath. Gastrointestinal manifestations, which develop about two weeks after the entry of the larvae, include abdominal pain, nausea, vomiting and diarrhea. Fever, malaise, anorexia and weight loss can also be seen frequently[13]. Rarely, association with acute pancreatitis as in our case has been described in the medical literature. Prior to our case, there have been only four cases of acute pancreatitis related to Strongyloides reported in the English and Spanish medical literature (Table 2)[14-17]. Likely mechanism of pancreatitis is involvement of duodenal ampulla and then pancreatic duct leading to intense inflammation and edema. A rare case report of Strongyloides association with cystadenocarcinoma of pancreas and pancreatic head mass has also been described[18,19].
Table 2.
Reported cases of acute pancreatitis associated with Strongyloides stercoralis
| Case | Age (yr)/gender | Place of origin | Presentation | Eosinophil count (per mm3) | Amylase (U/L) | Lipase (U/L) | Diagnostic method | Treatment | Ref. |
| 1 | 44/F | West Indies (Caribbean) | Abdominal pain Fever Pruritus | 210 | Not provided | Not provided | Examination of biliary fluid obtained through percutaneous biliary drain | Thiabendazole | Delarocque Astagneau et al[14], 1994 |
| 2 | 45/F | Ecuador | Abdominal pain Nausea Vomiting | 900 | 259 | 1574 | Endoscopic duodenal biopsy Stool examination | Albendazole Thiabendazole Ivermectin | Núñez et al[15], 2003 |
| 3 | 81/F | Kentucky, USA | Abdominal pain Nausea Vomiting | 1254 | 2100 | >2000 | Examination of biliary fluid obtained through ERCP | Albendazole | Perez-Jorge et al[16], 2008 |
| 4 | 64/M | Puerto Rico | Abdominal pain Nausea Vomiting Dyspnea Confusion | 776 | 4367 | >396 | Clinical history and stool examination | Ivermectin | Jones et al[17], 2009 |
| 5 (our case) | 48/M | Dominican Republic | Abdominal pain | 10900 | 263 | 476 | Endoscopic duodenal biopsy and serology | Ivermectin | Makker et al, 2015 |
ERCP: Endoscopic retrograde cholangio pancreatography.
After the establishment of Strongyloides stercoralis infection, factors that lead to autoinfection are unclear. The immune system constantly attempts to contain the infection, however in the presence of specific risk factors, Strongyloides infection supersedes and severe forms of infection known as hyperinfection and disseminated infection emerge. Well-established risk factors are treatment with steroids[20] or immunosuppressants[21] and coexistence of human T-lymphotropic virus type-1 (HTLV-1)[22]. Hyperinfection is characterized by enormous burden of parasite in the lungs and gastrointestinal tract, leading to the hallmark appearance of multiple larvae in sputum and/or feces. As the parasitic burden increases with autoinfection, these larvae can go beyond the normally infected tissues - lungs and gastrointestinal tract. Dissemination to other organs can manifest as meningitis, atrial fibrillation, hemoptysis, pneumothorax, intestinal hemorrhage, intestinal obstruction, protein losing enteropathy and intestinal ulcerations with subsequent gram-negative septicemia[23].
Diagnostic methods
Diagnosis of Strongyloides stercoralis infection in the absence of a gold standard remains a challenge. Various methods as discussed below are available but none seem to be ideal. Many patients with low parasite burden and non-specific symptoms may have peripheral eosinophilia as the only finding, which in itself is a non-specific indicator. Stool examination for larvae, one of the oldest methods available, in such patients may be completely negative, especially if only one sample is tested. Testing multiple samples increases the yield of stool examination[24]. Stool examination can be done by two different methods - direct stool smear examination or stool culture. Historically, different methods have been described to increase the detection rate of stool examination. These include formalin-ethyl acetate concentration, Baermann method that relies on the ability of larvae to convert to free-living stage, and Harada-Mori filter paper method that relies on water tropism of larvae[25]. Stool culture on agar plate requires stool inoculation and incubation for at least 2-3 d, following which larvae can be seen on the agar plate with the help of a microscope. Even if larvae are not seen, specific track marks on agar plate created by larval movement can be seen and also be differentiated from hookworm larvae tracks[26,27]. Agar plate culture has a high sensitivity of 90% but is time consuming, expensive and a health hazard to laboratory workers[28].
The next available diagnostic studies are serological tests (Table 3), which depend on the detection of the larval antigen or antibody generated in response to their antigens. Indirect immunofluorescence antibody test for Strongyloides stercoralis has been shown to have a sensitivity of 97% and a specificity of 98%[29]. ELISA to detect antibody against crude Strongyloides larva antigen has also been widely used, with sensitivity and specificity of 93% and 95% respectively[30]. The methods using ELISA have been hampered by the constant need for crude antigen, which must be prepared from feces of heavily infected patients or experimental animals. Hence interest in recombinant antigens was born, leading to the emergence of recombinant antigen NIE based ELISA test[31]. More recently a newer technique called luciferase immunoprecipitation system has been utilized to detect IgG against recombinant antigen NIE with sensitivity equal to that of NIE-ELISA test and a specificity of 100%[32]. Detection of antibodies does not differentiate between past and current infection, as these antibodies may last for years after the infection has cleared. Moreover, there may be cross-reactivity between Strongyloides antibodies and other helminths.
Table 3.
Various diagnostic tests available for Strongyloides stercoralis
| Test | Sensitivity and specificity | Remarks |
| Stool smear | 30% sensitivity for single stool sample[35] | Simple test but insensitive |
| Agar plate culture | 90% sensitivity[28] | Sensitive and simple |
| But needs 2 d for results | ||
| Health hazard to lab workers | ||
| Serologic tests | ||
| IFAT | IFAT: 97% sensitivity and 98% specificity[29] | Cross reactivity except with LIPS |
| ELISA | ELISA: 93% sensitivity and 95% specificity[30] | Inability to differentiate past and current infection |
| LIPS | LIPS: 97% sensitivity and 100% specificity[32] | Expensive |
| Molecular tests | ||
| PCR | PCR: 99%-100% sensitivity and 15%-100% specificity[34] | Low specificity with low parasite burden |
| Expensive | ||
| LAMP | Not widely available | |
ELISA: Enzyme Linked Immunosorbent Assay; IFAT: Indirect immunofluorescence antibody test; LAMP: Loop-mediated isothermal amplification; LIPS: Immunoprecipitation system; PCR: Polymerase chain reaction.
Molecular tests based on nucleic acid amplification by polymerase chain reaction (PCR) have also been used. Real time PCR technique using the 18S ribosomal DNA has been shown to have a specificity of 99%-100% but varying sensitivity based on the parasitic burden. With moderate to high burden, a sensitivity of 100% was found, however with low parasite burden it drastically dropped to only 15%[33]. A newer technique of nucleic acid amplification named Loop-mediated isothermal amplification has been introduced but needs further validation[34]. Table 3 above summarizes the sensitivity and specificity of these various diagnostic tests available[28-30,32,34,35].
Invasive methods like endoscopy with duodenal aspirate or biopsy can also be used in patients with strong clinical suspicion of infection. Upper endoscopic exam may show normal looking mucosa with eosinophilic infiltration on pathological examination or it may show abnormal mucosa in the form of erosions and ulcerations with pathological examination characterized by cryptitis, crypt abscess and eosinophilic infiltration[36,37]. Similarly the colon, particularly right-sided colon, may get involved in the presence of overwhelming parasite burden. Colonoscopy may show colonic mucosa inflammation or nodular mucosa with pathological examination revealing larvae, eosinophilic infiltration or granulomas[38].
Treatment
Ideally, the improvement of sanitary conditions and provision of better hygienic conditions should be targeted to control or eliminate Strongyloides infection. However, in the real world huge economic investments especially in developing nations are needed to achieve this goal. In the absence of such perfect sanitary conditions, reliance on anti-helminthic drugs is a reasonable option. The treatment of Strongyloides has come a long way since the days when intravenous gentian violet was introduced in 1950[39]. Currently, three drugs have been approved for the treatment of Strongyloides: Albendazole, Mebendazole and Ivermectin. Albendazole and Mebendazole belong to a group of benzimidazole antihelminthic drugs, which were originally developed as plant fungicides[40]. Ivermectin, a macrocyclic lactone is derived from avermectins, which is produced by the bacterium Streptomyces avermitilis.
Albendazole acts by interfering with the microtubular system of the parasite. The usual dose of oral Albendazole is 400 mg twice a day for three to seven days[41]. In a large review of its efficacy, a cure rate of 62.2% was seen with a 400 mg daily dosing[40]. In one study its side effect profile was similar to that of a placebo[42]. It is a pregnancy class C drug but neither the reports from its inadvertent use during the first trimester nor a large randomized controlled trial have shown any congenital defects associated with its use[43,44].
Mebendazole, another member of the benzimidazole group, also acts by interfering with the microtubular system of the parasite. Since October 2011, it has not been available in the Unites States. Mebendazole was used in doses of 100 mg twice a day for five days followed by repeated doses at weeks 1, 3 and 4. Is has been reported to achieve cure rates of 87% after 15 mo of treatment completion[45].
Ivermectin, a semi synthetic derivative of macrocyclic lactone, mediates parasite paralysis and killing through glutamate activated chloride channels[46]. In a comparison of single dose Ivermectin (200 microgram per kilogram of bodyweight) orally with three-day regimen of Albendazole 400 mg orally daily, cure rates of 83% with Ivermectin were seen as compared to 45% with Albendazole[47]. Single dose of Ivermectin (200 microgram per kilogram of bodyweight) orally was also shown to have superior cure rate of 97% vs 63% with high dose Albendazole 800mg orally daily for seven days[41]. Currently, oral Ivermectin in the dose of 200 microgram per kilogram of bodyweight repeated on two consecutive days or after 2 wk is the preferred drug for treatment of Strongyloides stercoralis (Table 4)[48,49]. In patients who cannot tolerate it orally, alternate routes like subcutaneous administration of Ivermectin has been advised[50]. Ivermectin is usually well tolerated unless the patient has concomitant Loa loa infection. Loa associated encephalopathy, especially with high Loa microfilaremia, has been observed after mass treatment with Ivermectin[51]. Ivermectin is a pregnancy class C drug, but in a randomized controlled trial involving more than 800 pregnant patients in their second trimester, it did not show any significant effect on mean birth weight, pregnancy outcomes or congenital defects[44].
Table 4.
Treatment of Strongyloidiasis
| Drug | Dose | Pregnancy class |
| Ivermectin | 200 μg/kg of bodyweight orally repeated on two days consecutively or after 2 wk | C |
| (preferred drug of treatment) | ||
| Albendazole | 400 mg orally two times a day for 3-7 d | C |
| Hyperinfection syndrome and disseminated Strongyloides infection | ||
| 200 μg/kg of bodyweight orally until stool and/or sputum tests are negative for two wk (duration of auto-infective cycle) | ||
The resolution of hyperinfection syndrome and disseminated Strongyloides infection requires prolonged treatment. Since the autoinfection cycle of Strongyloides takes two weeks, it has been recommended to give daily oral Ivermectin in the doses of 200 microgram per kilogram of bodyweight until stool and/or sputum tests are negative for two weeks[21].
In conclusion, we present here an uncommon case of Strongyloides stercoralis infection in an immunocompetent adult male associated with acute pancreatitis. Infection was associated with eosinophilia and negative repeated stool examinations, but was eventually diagnosed on pathological examination of duodenal biopsies.
Helminth Strongyloides affects millions of people worldwide with endemicity in the tropical and sub-tropical regions. Life cycle comprises two different forms of larvae, and is unique with features like parthenogenesis and autoinfection. Large numbers of infected hosts are asymptomatic or have non-specific gastrointestinal and/or pulmonary symptoms. Both innate and adaptive immune mechanisms get activated with infection. Infected hosts, especially in the setting of HTLV-1 infection and immunosuppressant or steroid use, may develop overwhelming infection in the form of dissemination or hyperinfection. Peripheral eosinophilia may be the only non-specific finding. Diagnostic methods range from simple stool examination to serologic tests and molecular techniques based on nucleic acid amplification. Endoscopic examination may be needed which may provide evidence of infection on pathological exam. Treatment options are both safe and efficacious with oral Ivermectin being superior to Albendazole.
COMMENTS
Case characteristics
A 48-year-old man presented with peripheral eosinophilia and recurrent epigastric pain, aggravated by food intake, not associated with nausea, vomiting and fever.
Clinical diagnosis
On examination patient appeared uncomfortable due to abdominal pain and the abdominal examination revealed moderate epigastric tenderness.
Differential diagnosis
Differential diagnosis of epigastric pain: Acute pancreatitis, peptic ulcer disease; Differential diagnosis of acute pancreatitis: gallstone, alcohol, triglyceride, drugs, and parasitic infections; Differential diagnosis of eosinophilia with gastrointestinal symptoms: aggressive systemic mastocytosis with lymphadenopathy and eosinophilia, hypereosinophilic syndrome, occult parasitic infection and hypoadrenalism.
Laboratory diagnosis
Patient had elevated lipase level as well as peripheral eosinophilia. Stool ova and parasite testing was negative repeatedly. Strongyloides immunoglobulin G tested by Enzyme Linked Immunosorbent Assay was positive.
Imaging diagnosis
Computed tomography and ultrasound abdomen were unremarkable. Upper endoscopic exam showed multiple erosions in the stomach and duodenum.
Pathological diagnosis
Endoscopic biopsy revealed a Strongyloides stercoralis larva in the duodenum.
Treatment
Two doses of Ivermectin 200 microgram per kilogram bodyweight were administered orally at two weeks interval.
Related reports
Very few cases related to acute pancreatitis secondary to Strongyloidiasis infection have been reported in literature. All the cases were associated with peripheral eosinophilia.
Term explanation
Strongyloidiasis is a soil transmitted helminth infection caused by Strongyloides stercoralis that initiates after infective larvae penetrate the host skin.
Experiences and lessons
This case report presents the rare association of Strongyloidiasis and acute pancreatitis. In the absence of other common etiologies of acute pancreatitis, the authors recommend considering this parasitic infection as one of the etiologies, especially in patients from endemic regions and who demonstrate peripheral eosinophilia.
Peer-review
The authors have described a case of acute pancreatitis secondary to Strongyloides stercoralis infection. The article highlights the diagnostic methods and treatment options available for this parasitic infection.
Footnotes
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: Authors have no conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 14, 2014
First decision: November 14, 2014
Article in press: January 16, 2015
P- Reviewer: Luo HS, Zambudio N S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Viney ME, Lok JB. Strongyloides spp. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.141.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorris M, Viney ME, Blaxter ML. Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int J Parasitol. 2002;32:1507–1517. doi: 10.1016/s0020-7519(02)00156-x. [DOI] [PubMed] [Google Scholar]
- 3.Grove DI. Who discovered that intestinal worm infections could be diagnosed by finding eggs in the faeces? J R Soc Med. 1986;79:670–673. doi: 10.1177/014107688607901118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol. 2010;73:197–230. doi: 10.1016/S0065-308X(10)73008-6. [DOI] [PubMed] [Google Scholar]
- 5.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walzer PD, Milder JE, Banwell JG, Kilgore G, Klein M, Parker R. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am J Trop Med Hyg. 1982;31:313–319. doi: 10.4269/ajtmh.1982.31.313. [DOI] [PubMed] [Google Scholar]
- 7.Starr MC, Montgomery SP. Soil-transmitted Helminthiasis in the United States: a systematic review--1940-2010. Am J Trop Med Hyg. 2011;85:680–684. doi: 10.4269/ajtmh.2011.11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelletier LL. Chronic strongyloidiasis in World War II Far East ex-prisoners of war. Am J Trop Med Hyg. 1984;33:55–61. doi: 10.4269/ajtmh.1984.33.55. [DOI] [PubMed] [Google Scholar]
- 9.Swanson SJ, Phares CR, Mamo B, Smith KE, Cetron MS, Stauffer WM. Albendazole therapy and enteric parasites in United States-bound refugees. N Engl J Med. 2012;366:1498–1507. doi: 10.1056/NEJMoa1103360. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoud AA. Strongyloidiasis. Clin Infect Dis. 1996;23:949–952; quiz 953. doi: 10.1093/clinids/23.5.949. [DOI] [PubMed] [Google Scholar]
- 11.Bonne-Année S, Hess JA, Abraham D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res. 2011;51:205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriemenam NC, Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF. Strongyloides stercoralis and the immune response. Parasitol Int. 2010;59:9–14. doi: 10.1016/j.parint.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 14.Delarocque Astagneau E, Hadengue A, Degott C, Vilgrain V, Erlinger S, Benhamou JP. Biliary obstruction resulting from Strongyloides stercoralis infection. Report of a case. Gut. 1994;35:705–706. doi: 10.1136/gut.35.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Núñez E, Montero J, García-Picazo L, Ramón y Cajal S. [Recurrent pancreatitis after cholecystectomy] Enferm Infecc Microbiol Clin. 2003;21:461–462. doi: 10.1016/s0213-005x(03)72983-8. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Jorge EV, Burdette SD. Association between acute pancreatitis and Strongyloides stercoralis. South Med J. 2008;101:771–772. doi: 10.1097/SMJ.0b013e31817a8b24. [DOI] [PubMed] [Google Scholar]
- 17.Jones N, Cocchiarella A, Faris K, Heard SO. Pancreatitis associated with Strongyloides stercoralis infection in a patient chronically treated with corticosteroids. J Intensive Care Med. 2010;25:172–174. doi: 10.1177/0885066609359992. [DOI] [PubMed] [Google Scholar]
- 18.Setia U, Bhatia G. Pancreatic cystadenocarcinoma associated with strongyloides. Am J Med. 1984;77:173–175. doi: 10.1016/0002-9343(84)90456-x. [DOI] [PubMed] [Google Scholar]
- 19.Pijls NH, Yap SH, Rosenbusch G, Prenen H. Pancreatic mass due to Strongyloides stercoralis infection: an unusual manifestation. Pancreas. 1986;1:90–93. doi: 10.1097/00006676-198601000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Fardet L, Généreau T, Cabane J, Kettaneh A. Severe strongyloidiasis in corticosteroid-treated patients. Clin Microbiol Infect. 2006;12:945–947. doi: 10.1111/j.1469-0691.2006.01443.x. [DOI] [PubMed] [Google Scholar]
- 21.Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25:458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487–497. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 23.Keiser PB, Nutman TB. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen PB, Mojon M. Improved diagnosis of strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;263:616–618. doi: 10.1016/s0176-6724(87)80207-9. [DOI] [PubMed] [Google Scholar]
- 25.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jongwutiwes S, Charoenkorn M, Sitthichareonchai P, Akaraborvorn P, Putaporntip C. Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999;93:398–400. doi: 10.1016/s0035-9203(99)90132-3. [DOI] [PubMed] [Google Scholar]
- 27.Inês Ede J, Souza JN, Santos RC, Souza ES, Santos FL, Silva ML, Silva MP, Teixeira MC, Soares NM. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120:206–210. doi: 10.1016/j.actatropica.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Arakaki T, Iwanaga M, Kinjo F, Saito A, Asato R, Ikeshiro T. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. J Parasitol. 1990;76:425–428. [PubMed] [Google Scholar]
- 29.Boscolo M, Gobbo M, Mantovani W, Degani M, Anselmi M, Monteiro GB, Marocco S, Angheben A, Mistretta M, Santacatterina M, et al. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin Vaccine Immunol. 2007;14:129–133. doi: 10.1128/CVI.00278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, Sarfati C, Vervoort T, van Gool T. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45:438–442. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol. 2002;125:73–81. doi: 10.1016/s0166-6851(02)00214-1. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sultana Y, Jeoffreys N, Watts MR, Gilbert GL, Lee R. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. Am J Trop Med Hyg. 2013;88:1048–1051. doi: 10.4269/ajtmh.12-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, Verweij JJ, Iredell JR, Chen SC, Lee R. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am J Trop Med Hyg. 2014;90:306–311. doi: 10.4269/ajtmh.13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 36.Santos RB, Fonseca LE, Santana AT, Silva CA, Guedes JC. Clinical, endoscopic and histopathological profiles of parasitic duodenitis cases diagnosed by upper digestive endoscopy. Arq Gastroenterol. 2011;48:225–230. doi: 10.1590/s0004-28032011000400002. [DOI] [PubMed] [Google Scholar]
- 37.Kakati B, Dang S, Heif M, Caradine K, McKnight W, Aduli F. Strongyloides duodenitis: case report and review of literature. J Natl Med Assoc. 2011;103:60–63. doi: 10.1016/s0027-9684(15)30246-7. [DOI] [PubMed] [Google Scholar]
- 38.Minematsu H, Hokama A, Makishi T, Arakaki K, Kinjo F, Fujita J. Colonoscopic findings and pathologic characteristics of Strongyloides colitis: a case series. Digestion. 2011;83:210–214. doi: 10.1159/000321812. [DOI] [PubMed] [Google Scholar]
- 39.BROWNE DC, CONTACOS PG, WELCH GE, McHARDY G. Treatment of Strongyloides stercoralis infection with intravenous gentian violet. Am J Trop Med Hyg. 1957;6:1066–1067. doi: 10.4269/ajtmh.1957.6.1066. [DOI] [PubMed] [Google Scholar]
- 40.Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121 Suppl:S113–S132. doi: 10.1017/s0031182000007290. [DOI] [PubMed] [Google Scholar]
- 41.Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl Trop Dis. 2011;5:e1044. doi: 10.1371/journal.pntd.0001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pene P, Mojon M, Garin JP, Coulaud JP, Rossignol JF. Albendazole: a new broad spectrum anthelmintic. Double-blind multicenter clinical trial. Am J Trop Med Hyg. 1982;31:263–266. doi: 10.4269/ajtmh.1982.31.263. [DOI] [PubMed] [Google Scholar]
- 43.Bradley M, Horton J. Assessing the risk of benzimidazole therapy during pregnancy. Trans R Soc Trop Med Hyg. 2001;95:72–73. doi: 10.1016/s0035-9203(01)90338-4. [DOI] [PubMed] [Google Scholar]
- 44.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. Am J Trop Med Hyg. 2008;79:856–863. [PubMed] [Google Scholar]
- 45.Zaha O, Hirata T, Kinjo F, Saito A. Strongyloidiasis--progress in diagnosis and treatment. Intern Med. 2000;39:695–700. doi: 10.2169/internalmedicine.39.695. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda T. Pharmacological effects of ivermectin, an antiparasitic agent for intestinal strongyloidiasis: its mode of action and clinical efficacy. Nihon Yakurigaku Zasshi. 2003;122:527–538. doi: 10.1254/fpj.122.527. [DOI] [PubMed] [Google Scholar]
- 47.Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55:477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- 48.Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, Sánchez-Sánchez P, Matogo-Oyana J, Rodríguez-Calabuig D. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615–2619. doi: 10.1517/14656566.5.12.2615. [DOI] [PubMed] [Google Scholar]
- 49.Zaha O, Hirata T, Kinjo F, Saito A, Fukuhara H. Efficacy of ivermectin for chronic strongyloidiasis: two single doses given 2 weeks apart. J Infect Chemother. 2002;8:94–98. doi: 10.1007/s101560200013. [DOI] [PubMed] [Google Scholar]
- 50.Turner SA, Maclean JD, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am J Trop Med Hyg. 2005;73:911–914. [PubMed] [Google Scholar]
- 51.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux JP. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003;2 Suppl 1:S4. doi: 10.1186/1475-2883-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]


