Abstract
Sarcoidosis is a multisystem chronic inflammatory condition of unknown etiology that has the potential to involve every tissue in the body. Sarcoidosis in the gastrointestinal system, and particularly the colon, is very rare. Here, we report the case of a 57-year-old man with no previous diagnosis of sarcoidosis who presented with new onset of abdominal pain and constipation. A colonoscopy revealed that the abdominal pain was caused by an obstructing lesion in the cecum-ascending colon and lacked a clear histologic diagnosis. Radiologic investigation revealed concentric wall thickening of the cecum-ascending colon with multiple satellite lymphadenopathies, highly suggestive of a malignancy. The patient underwent a laparotomy and a right hemicolectomy was performed. A diagnosis of colonic sarcoidosis was made after the resected specimen was examined. Additionally, a chest computed tomography scan revealed lung involvement with atypical radiologic features in the absence of respiratory symptoms. Only histologic examination of the surgical specimen can yield a diagnosis of gastrointestinal sarcoidosis due to the non-specificity of endoscopic and radiologic findings.
Keywords: Colon, Sarcoidosis, Hemicolectomy, Systemic disease, Noncaseating granuloma
Core tip: Gastrointestinal tract involvement in systemic sarcoidosis is rare. This case report of a patient with gastrointestinal sarcoidosis is clinically relevant because the colonic location highlights an unusual cause of abdominal pain. This study provides an opportunity to clarify diagnostic criteria and therapeutic management for such a rare condition.
INTRODUCTION
Sarcoidosis is a multisystem chronic inflammatory condition of unknown etiology. The characteristic histologic lesions are noncaseating granulomas that contain multinucleated giant cells in the absence of other autoimmune diseases, infectious disease or foreign agents[1]. Sarcoidosis is more common in young and middle-aged patients and is more prevalent in females[2]. It most commonly affects African-Americans, Swedes and Danes[2]. Sarcoidosis has a prevalence range of 1-40 cases per 100000 people in the general population. In the United States, the age-adjusted annual incidence of sarcoidosis among blacks is more than triple that for Caucasians (35.5 vs 10.9 per 100000 inhabitants)[3].
Sarcoidosis is an immune-mediated multisystem disease; it is hypothesized it affects genetically susceptible hosts after interaction with a single or multiple factors. Different potential antigenic agents for sarcoidosis have been proposed, such as infectious pathogens, environmental and occupational exposures, without any definitive conclusion.
The clinical signs and symptoms for sarcoidosis are not specific and include respiratory symptoms (cough, dyspnea, bronchial hyper-reactivity), fatigue, night sweats, weight loss, and, less commonly, fever. However, approximately 50% of the sarcoidosis cases are asymptomatic and may be detected by chance during chest radiography[4,5]. Pulmonary involvement is the most common feature, with bilateral hilar adenopathy being the primary radiologic finding after a chest X-ray. Moreover, sarcoidosis in the lungs accounts for a majority of the morbidity and mortality associated with this condition. Multiple small nodules distributed along the lymphatic vessels that run within the interstitial tissues of bronchovascular bundles and the subpleural and perilobular spaces, which are potentially associated with ground-glass opacities or air-space consolidation, are also typically observed[6]. In 50% of symptomatic sarcoidosis patients, extrathoracic involvement can be an initial manifestation. Although skin and ocular lesions are very common, any organ or gland can be involved, including the liver, spleen, lymph nodes, parotid glands, central nervous system, genitourinary system, muscles and bones. Gastrointestinal involvement is quite rare, however, with a prevalence of less than 1% and may present along with systemic disease or as an isolated finding[1,7]. Although sarcoidosis has been observed in every part of the gastrointestinal tract, it most commonly affects the stomach[8]. The colon is involved less frequently[1,9,10] and sarcoidosis in the colon is difficult to diagnose preoperatively due to nonspecific symptoms and/or endoscopic findings.
Here, we report a case of a 57-year-old man in apparent good health who presented with newly onset abdominal pain and constipation due to an obstructing lesion in the cecum-ascending colon as a result of sarcoidosis. The patient underwent a right hemicolectomy and a formal diagnosis was made upon histologic examination of the surgical specimen. Simultaneously, in the absence of respiratory symptoms, lung involvement with atypical radiologic features was discovered by chance during a chest computed tomography (CT) scan. Sarcoidosis was later confirmed after histologic examination of pulmonary tissue obtained via CT-guided biopsy.
CASE REPORT
A 57-year-old white Italian male patient presented at our hospital with abdominal pain and symptoms related to colonic obstruction. The patient was healthy until three to four weeks prior to presentation, when new-onset constipation developed and he began passing pencil-like stools. His primary complaints were bloating, anorexia and a slight weight loss over the previous two months.
He had no recent travel history or family members with autoimmune disease; his sister had pulmonary tuberculosis at a young age.
A physical examination of the patient revealed mild abdominal distention and tenderness of the right lower abdominal quadrant; no peritoneal signs were found. Routine hematology and biochemistry analyses revealed a white cell count of 8.6 × 103/mL with a low lymphocyte count (16.9% and 1.4 × 103/mL). Alpha-amylase levels were elevated (114 mg/dL), whereas liver tests and serum electrolytes were normal. Iron serum levels were 42 μg/dL, with normal values of ferritin (188 ng/mL) and hemoglobin (14.5 g/dL). Serum electrophoresis revealed an albumin level of 54.4%, with a peak of alpha-1 globulin (6.2%). Carcinoembryonic antigen levels were slightly elevated (3.6 ng/mL).
A colonoscopy revealed a stenotic obstructive lesion at the cecum-ascending colon transition and we were unable to advance the colonoscope beyond this area. Biopsy specimens obtained from this site showed no evidence of a neoplastic lesion, but rather an acute inflammatory infiltration of the submucosa. An abdominal CT (Figure 1) revealed marked symmetric concentric wall thickening in the cecum-ascending colon, which notably reduced the enteral lumen and was associated with a heterogeneous hyperdensity of perivisceral fat tissue. Enlargement of the appendix characterized by a thickened wall with marked enhancement was also found. We observed multiple satellite lymphadenopathies along the ileocolic vessels, with the largest measuring approximately 1.6 cm.
Figure 1.
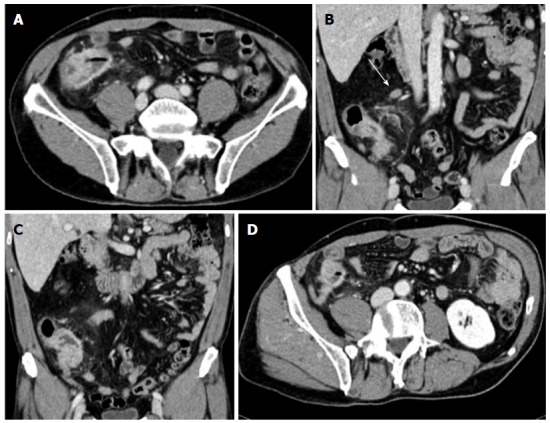
Abdominal computed tomography performed after colonoscopy. A: Transverse image of the cecum-ascending colon; B and C: Coronal images showing symmetric concentric wall thickening of the cecum-ascending colon mimicking a tumor lesion; heterogeneous hyperdensity of perivisceral fat tissue is also seen. Satellite lymphadenopathy (arrow) is observed along the ileocolic vessels; D: Enlarged appendix with a thickened wall and marked contrast enhancement.
A chest CT (Figure 2) simultaneously performed to assess patient whole-body staging revealed asymmetric discrete airspace consolidation with air bronchograms in the right upper lobe. Numerous micronodular opacities were also found distributed in the peribronchovascular interstitium in both upper pulmonary lobes. Pathologic hilar or mediastinal adenopathies were not observed, and 1.2 cm was the maximum diameter in the right hilum. A radiologist interpreted these findings as a manifestation of right-sided pneumonia associated with bilateral small airway inflammation in relation to a mild cough and slight fever in the patient the previous week. The patient was administered four weeks of oral antibiotic therapy with quinolones.
Figure 2.
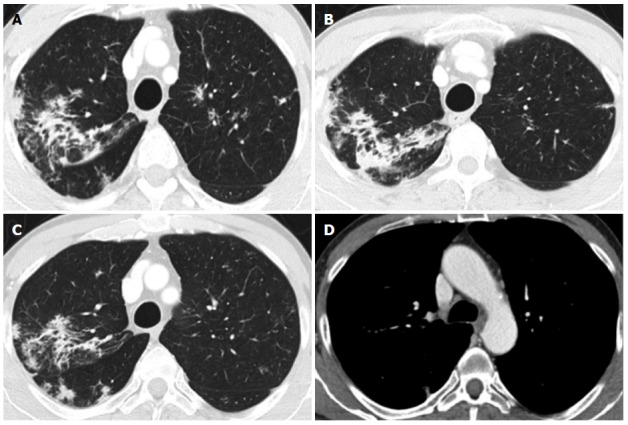
Chest computed tomography performed after colonoscopy for whole-body staging. A and B: Asymmetric discrete airspace consolidation with air bronchograms in the right upper lobe; C: Numerous micronodular opacities are present in both upper pulmonary lobes (also seen in panel A) with distribution in the peribronchovascular interstitium. These findings showed no significant change after four weeks of antibiotic therapy; D: No pathologic hilar or mediastinal lymph node enlargement was observed.
Using the findings from the endoscopy and observations via radiology, a malignant colonic lesion was suspected, despite the unclear histology results. The patient underwent an exploratory laparotomy that revealed a 4 cm stenotic lesion in the cecum-ascending colon, with numerous peritoneal micronodules and adjacent lymphadenopathies (Figure 3). The appendix was enlarged with a maximum diameter of 4 cm. A carcinologic right hemicolectomy was performed.
Figure 3.
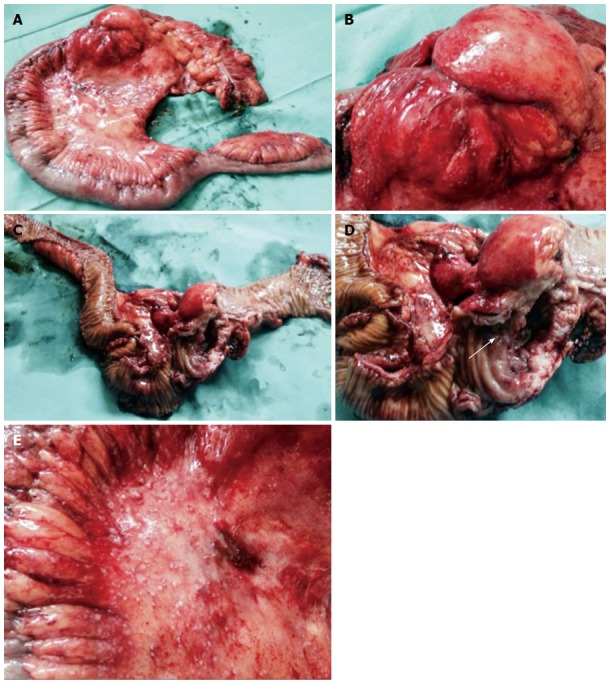
Resected specimen after laparotomy. A: Right hemicolectomy was performed; B-D: A voluminous stenotic ulcerated lesion of the colonic wall in proximity to the ileocecal valve (arrow in D) was observed; E: Numerous peritoneal micronodules near the colonic lesion are shown.
Examination of the resected specimen revealed a stenotic ulcerated lesion in the colonic wall in proximity to the ileocecal valve and microscopic examination showed multiple noncaseous granulomas composed of a central core of epithelioid cells and multinucleated giant cells surrounded by a lymphocyte cuff (Figure 4). These features were also observed in the locoregional lymph nodes and peritoneal micronodules (Figure 5). The appendix was also pathologically involved. Ziehl-Neelsen staining did not reveal acid-fast bacilli and no other organisms or foreign bodies were identified. In addition, polymerase chain reaction analysis did not reveal Mycobcaterium tuberculosis or atypical mycobacterial DNA.
Figure 4.
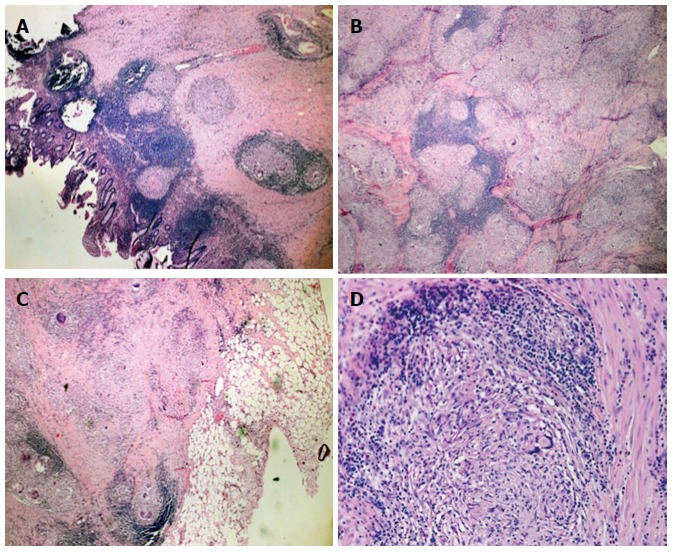
Histologic examination of the intraoperative specimen. Hematoxylin and eosin staining showed A: Noncaseating epithelioid granulomas in the colonic wall (magnification × 5); B: Confluent granulomata in the colonic wall (magnification × 10); C: Perivisceral involvement (magnification × 5); D: Microscopic aspects of the sarcoidotic granulomas (magnification × 20).
Figure 5.
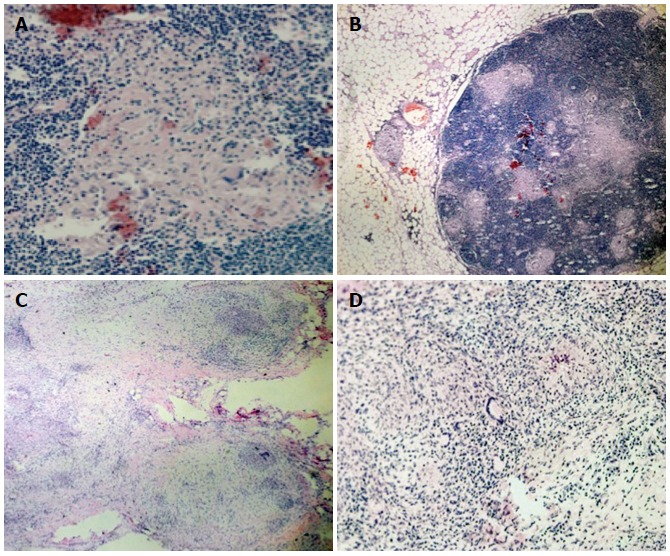
Extracolonic sarcoidosis involvement. Hematoxylin and eosin staining revealed: Sarcoidotic involvement of locoregional lymphadenopathy (A, B) (A: × 10; B: magnification × 40); Sarcoidotic granulomas in the parietal peritoneum adjacent to the colonic lesion (C, D) (C: magnification × 2; D: magnification × 20).
Following the surgery, the patient responded favorably and was discharged six days later in good condition. A chest CT performed four weeks after the antibiotic therapy revealed no change in the bilateral pulmonary manifestations indicating that the inflammatory condition was not responsive to the treatment. A CT-guided biopsy of the lung was later performed on the right upper lobe parenchymal consolidations and a final histologic diagnosis of pulmonary sarcoidosis was made.
DISCUSSION
Sarcoidosis is a multisystem disorder of unknown etiology that has the potential to affect almost every tissue in the body. Gastrointestinal involvement, however, is quite rare and accounts for less than 1% of cases. Sarcoidosis of the colon is even more rare, usually asymptomatic[11,12] and may mimic many other diseases, including colonic granulomatous disorders, such as tuberculosis, syphilis, fungal infection, inflammatory bowel disease, and malignancies.
A systematic review of the literature revealed that only a few cases for this location have been reported. Beniwal and colleagues performed a literature review and found ten cases of colonic sarcoidosis from 1966 to 2003[7]. The sigmoid colon was most frequently affected.
When tubular organs are affected in sarcoidosis, it is usually associated with concomitant pulmonary disease. Hilzenrat et al[13] published a case report of a nonoperative diagnosis and management of colonic obstruction secondary to systemic sarcoidosis. In this case, the obstructive symptoms were resolved with a moderate dose of corticosteroid therapy, thus eliminating the need for urgent surgical resection of the colonic lesion. It is important to note that pulmonary sarcoidosis had been diagnosed in this case six years earlier and endoscopic mucosal biopsies on the colonic-obstructing lesion resulted in a final diagnosis of sarcoidosis.
Nchimi et al[14] reported a case of 7-year-old boy admitted for moderate weight loss and intermittent diarrhea associated with cough and exertional dyspnea. In this case, symmetric wall thickening of the terminal ileum and cecum observed on an abdominal CT were correctly interpreted as a sarcoidotic gastrointestinal location due to an association with typical features of pulmonary sarcoidosis observed by chest radiography (bilateral and diffuse parenchymal air-space and interstitial opacities with bilateral hilar node enlargement). Moreover, gallium 67 scintigraphy revealed marked bilateral and symmetric uptake in the lungs and the parotid and lacrimal gland, definitively confirming a diagnosis of sarcoidosis.
To the best of our knowledge, this is the first case in which the sarcoidosis started with symptomatic gastrointestinal involvement that was associated with atypical pulmonary features revealed by chest CT, in the absence of clear and definite respiratory symptoms. Using these findings alone, it would have been very difficult to relate the patient’s abdominal manifestations to a systemic disease. The clinical presentation of gastrointestinal sarcoidosis is not specific, and when present, the symptoms generally resemble those of an obstructive colonic disease.
The case reported by Nchimi et al[14] showed thickening of the terminal ileum wall, which caused intermittent diarrhea. Aaronson et al[15] discussed a case of sigmoid colonic sarcoidosis presenting with constipation and hematochezia. Hilzenrat et al[13] reported a case with stricture and obstruction of the colon, resulting in abdominal pain, distention, vomiting, constipation and weight loss. Similar to these findings, our patient was symptomatic with colonic obstruction-related symptoms, such as constipation and abdominal pain with mild tenderness of the right lower abdominal quadrant. While reviewing the literature, we found only one case of rectal sarcoidosis with secondary paralytic ileus that resembled adult-onset Hirschsprung disease[16]. In this particular case, a barium enema and rectal manometry showed rectal dilatation with weak or nearly absent colonic contraction. Neither a gastrografin small bowel study nor a colonoscopy identified an organic obstruction causing a mechanical ileus.
Imaging and endoscopic findings are not diagnostically specific for sarcoidosis and may overlap with other diseases, such as chronic inflammatory bowel disease, tuberculosis, lymphoma and carcinoma. Endoscopic appearances are extremely varied and include aphthous erosions or ulcers, friable mucosa or small punctate bleeding sites mimicking colitis, plaque-like lesions, focal nodularity or segmental narrowing[17].
Veitch et al[18] reported a case with asymptomatic colonic sarcoidosis presenting as colonic polyposis where the polyps were adenomatous in appearance. Ushiki et al[9] presented another case of colonic sarcoidosis with multiple elevated lesions mimicking submucosal tumors in the colon; these lesions were sessile, soft, had a smooth surface and a diameter of 2-5 mm. Sarcoidosis lesions may also form irregular mass lesions mimicking a carcinoma[19]. In the case presented in this study, the stenotic lesion was highly suggestive of malignancy.
Histologic examination is necessary to establish or exclude diagnosis of colonic involvement. In biopsy specimens, the presence of noncaseating granulomas is the pathologic hallmark of this disease[5,7,8]. It is necessary to exclude other granulomatous disorders such as tuberculosis, fungal infections, inflammatory bowel disease, malignancy and delayed-type hypersensitivity to foreign antigens. Intestinal sarcoidosis is easy to distinguish from Crohn’s disease and Wegener granulomatosis.
It’s noteworthy that, in contrast with our case, most of authors[9,13,16,20,21] obtained definitive diagnosis of sarcoidosis on colon biopsy. Only Daldoul et al[17] revealed histology failure on endoscopic biopsy specimen of a stenotic lesion in the ascending colon; in this case the patient underwent a laparotomy and a right hemicolectomy was made.
Besides, we have verified that, when clinical presentation in association with biopsy results are suggestive of sarcoidosis, patients are started on corticosteroid therapy, with significant improvement in their symptoms[13,16,21]. Hilzenrat et al[13] showed a complete regression of two obstructive colonic lesions after steroid treatment, as seen on barium enema and colonscopy, where no granulomas were found on mucosal biopsy specimens after therapy.
From our literature review, we have confirmed that there are very few radiologic descriptions of colonic sarcoidosis. CT may show segmental or symmetric wall thickening with preserved wall stratification[14]. In our case, CT showed symmetric thickening of the colonic wall, highly suggestive of a tumor; on the other hand, pathologic enlargement of the appendix observed in the present case is rather uncommon in malignancies.
If a pseudotumor is suspected, however, and the biopsy specimens fail to demonstrate a definitive diagnosis of colonic sarcoidosis, surgical treatment with conventional surgical oncologic principles must be proposed[22]. In the other cases, systemic sarcoidosis is usually readily responsive to corticosteroid therapy and clinical resolution may be reached without surgery.
The colon is an unusual site for gastrointestinal sarcoidosis and its symptomatic involvement may represent the onset of this systemic disease. A preoperative diagnosis of sarcoidosis is difficult to make due to the overlap of imaging and radiologic findings with that of malignancies. In patients with a previous diagnosis of sarcoidosis, however, it should be suspected. In all cases, a histologic examination that reveals noncaseating granulomas allows for a definitive diagnosis. If the histology is unclear, patients will need to undergo surgery for a diagnosis.
COMMENTS
Case characteristics
A 57-year-old man in apparent good health with abdominal pain and constipation.
Clinical diagnosis
New-onset constipation with mild abdominal distention and tenderness of the right lower abdominal quadrant; subtle weight loss.
Differential diagnosis
Malignant tumor of the gastrointestinal tract.
Laboratory analysis
White blood cell count, 8.6 × 103/mL with 1.4 × 103/mL lymphocytes; hemoglobin, 14.5 g/dL; iron serum, 42 μg/dL with normal values for ferritin (188 ng/mL); carcinoembryonic antigen, 3.6 ng/mL; albumin, 54.4% with a peak of alpha-1 globulin (6.2%); alpha-amylase, 114 mg/dL; liver tests and serum electrolytes were normal.
Imaging diagnosis
An abdominal computed tomography revealed symmetric concentric wall thickening associated with heterogeneous hyperdensity of perivisceral fat tissue and satellite adenopathy at the cecum-ascending colon transition, causing a notable reduction in the enteral lumen; enlargement of the appendix was also found.
Pathological diagnosis
A colonoscopy revealed a stenotic obstructive lesion at the cecum-ascending colon transition; a biopsy showed no evidence of a neoplastic lesion, but rather an acute inflammatory infiltration of the submucosa.
Treatment
The patient underwent a right hemicolectomy.
Related reports
Very few cases of colonic sarcoidosis have been reported in the literature and most of them have been found in patients with a previous diagnosis of this systemic disease.
Term explanation
Sarcoidosis is a multisystem disorder of unknown etiology that has the potential to affect almost every tissue in the body. Polymerase chain reaction for DNA specific to Mycobacterium tuberculosis and atypical mycobacteria can be used to rule out these bacteria as the cause of the disease.
Experiences and lessons
This case report presents an unusual onset of systemic sarcoidosis with colonic involvement causing gastrointestinal obstruction-related symptoms.
Peer-review
The authors present the case of a 57-year-old man who presented with an obstructing colonic mass. Colonoscopic biopsies were non-diagnostic and he underwent hemicolectomy and was found to have colonic sarcoidosis. This is an unusual presentation of a highly condition, and a worthwhile case report.
Footnotes
Ethics approval: Si comunica che in data 29/07/2014 il Comitato Eticl dell’Azienda Sanitaria Regionale del Molisc ha csaminato cd approvato I’articolo scientifico dal titolo “Unusual onset of colonic sarcoidosis a case report” redatto dai Dottori Paola Erra, Sonia Crusco, Loredana Nugnes, Anna Maria Pollio, Gianni Di Pilla, Giuseppe Biondi, Giovanni Vigliardi Dirigenti Medici in servizio presso ASREM Ospedale “F. Veneziale” di Isernia.
Informed consent: Il sottoscritto Barile Costantino, nato a Lamusei (OG), Italia, il 22.03.1955 concede il consenso al trattamento dei propri dati personali e all’utilizzo delle immagini relative alla propria patologia rilevata presso l’Ospedale “F.Veneziale” di Isernia (IS), Italia.
Conflict-of-interest: There are no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 6, 2014
First decision: October 29, 2014
Article in press: December 16, 2014
P- Reviewer: Cologne KG, Freedberg DE, Kvasnovsky CL S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.MacArthur KL, Forouhar F, Wu GY. Intra-abdominal complications of sarcoidosis. J Formos Med Assoc. 2010;109:484–492. doi: 10.1016/S0929-6646(10)60082-4. [DOI] [PubMed] [Google Scholar]
- 2.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 3.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123:840–845. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JP, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997;18:755–785. doi: 10.1016/s0272-5231(05)70417-2. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 6.Kitaichi M. Pathology of pulmonary sarcoidosis. Clin Dermatol. 1986;4:108–115. doi: 10.1016/0738-081x(86)90039-8. [DOI] [PubMed] [Google Scholar]
- 7.Beniwal RS, Cummings OW, Cho WK. Symptomatic gastrointestinal sarcoidosis: case report and review of the literature. Dig Dis Sci. 2003;48:174–178. doi: 10.1023/a:1021711204498. [DOI] [PubMed] [Google Scholar]
- 8.Farman J, Ramirez G, Rybak B, Lebwohl O, Semrad C, Rotterdam H. Gastric sarcoidosis. Abdom Imaging. 1997;22:248–252. doi: 10.1007/s002619900182. [DOI] [PubMed] [Google Scholar]
- 9.Ushiki A, Koizumi T, Kubo K, Suzawa K, Arakura N, Suzawa H. Colonic sarcoidosis presenting multiple submucosal tumor-like lesions. Intern Med. 2009;48:1813–1816. doi: 10.2169/internalmedicine.48.2427. [DOI] [PubMed] [Google Scholar]
- 10.Vahid B, Spodik M, Braun KN, Ghazi LJ, Esmaili A. Sarcoidosis of gastrointestinal tract: a rare disease. Dig Dis Sci. 2007;52:3316–3320. doi: 10.1007/s10620-006-9448-y. [DOI] [PubMed] [Google Scholar]
- 11.Stampfl DA, Grimm IS, Barbot DJ, Rosato FE, Gordon SJ. Sarcoidosis causing duodenal obstruction. Case report and review of gastrointestinal manifestations. Dig Dis Sci. 1990;35:526–532. doi: 10.1007/BF01536930. [DOI] [PubMed] [Google Scholar]
- 12.Ryan J, Sleisenger M. Effects of systemic and extraintestinal disease on the gut. In: Sleisenger M, Fordtran J, editors. Gastrointestinal disease. 5th ed. Philadelphia: Saunders; 1993. pp. 223–224. [Google Scholar]
- 13.Hilzenrat N, Spanier A, Lamoureux E, Bloom C, Sherker A. Colonic obstruction secondary to sarcoidosis: nonsurgical diagnosis and management. Gastroenterology. 1995;108:1556–1559. doi: 10.1016/0016-5085(95)90706-8. [DOI] [PubMed] [Google Scholar]
- 14.Nchimi A, Francotte N, Rausin L, Khamis J. Case 61: ileocecal sarcoidosis. Radiology. 2003;228:452–455. doi: 10.1148/radiol.2282011952. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson HG, Meir JH, Ulin AW. A case of sarcoidosis of the colon. J Albert Einstein Med Cent (Phila) 1957;6:14–16. [PubMed] [Google Scholar]
- 16.Shimoyama Y, Kusano M, Uchiyama Y, Mori M. Education and Imaging. Gastrointestinal: rectal sarcoidosis due to paralytic ileus resembling adult-onset Hirschsprung disease. J Gastroenterol Hepatol. 2010;25:1464. doi: 10.1111/j.1440-1746.2010.06423.x. [DOI] [PubMed] [Google Scholar]
- 17.Daldoul S, Triki W, El Jeri K, Zaouche A. Unusual presentation of a colonic sarcoidosis. Case Rep Med. 2012;2012:169760. doi: 10.1155/2012/169760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veitch AM, Badger I. Sarcoidosis presenting as colonic polyposis: report of a case. Dis Colon Rectum. 2004;47:937–939. doi: 10.1007/s10350-004-0520-4. [DOI] [PubMed] [Google Scholar]
- 19.Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol. 2004;182:15–28. doi: 10.2214/ajr.182.1.1820015. [DOI] [PubMed] [Google Scholar]
- 20.Bat T, Morgan CM, Marx R, Bailey RS. Colon sarcoidosis presenting with abdominal pain. Endoscopy. 2014;46 Suppl 1 UCTN:E121. doi: 10.1055/s-0034-1364890. [DOI] [PubMed] [Google Scholar]
- 21.Esmadi M, Ahmad DS, Odum B, Diaz-Arias A, Hammad H. Sarcoidosis: an extremely rare cause of granulomatous enterocolitis. J Gastrointestin Liver Dis. 2012;21:423–425. [PubMed] [Google Scholar]
- 22.Maàmouri N, Guellouz S, Ben Hariz F, Ketari S, Belkahla N, Ouerghi H, Chelly-Enneifer I, Chouaib S, Moncef Zitouna M, Ben Mami N. [Gastrointestinal sarcoidosis] Rev Med Interne. 2010;31:262–267. doi: 10.1016/j.revmed.2009.12.003. [DOI] [PubMed] [Google Scholar]


