Abstract
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal tumours of the gastrointestinal tract, but they represent less than 3% of all gastrointestinal tract malignancies. This is a detailed case study of a 52-year-old male patient treated for very uncommon histological subtype of gastric GIST with atypical clinical presentation, asymptomatic progress and late diagnosis. The resected tumour, giant in diameters, was confirmed to represent the most rare histopathologic subtype of GISTs - sarcomatoid epithelioid GIST. We report this case and review the literature with a special focus on pathomorphological evaluation, biological aggressiveness and prognostic factors. To our knowledge this is the first report of giant GIST of very uncommon sarcomatoid epithelioid subtype. It is concluded that clinicians should pay attention to the fact that initial diagnosis may be delayed due to mildly asymptomatic and non-specific clinical presentation. Asymptomatic tumours diagnosed at a late stage, which is often the case, can be large on presentation. Prognosis for patients diagnosed with GIST depend on tumour size, mitotic rate, histopathologic subtype and tumour location. That is why early diagnosis and R0 resection, which is usually feasible and safe even in giant gastric sarcomatoid epithelioid subtype of GISTs, are the key factors for further treatment and good prognosis.
Keywords: Gastrointestinal stromal tumour, Sarcomatoid epithelioid gastrointestinal stromal tumour, Gastric gastrointestinal stromal tumour, Gastrointestinal tract tumour
Core tip: This is a detailed case study of a 52-year-old male patient treated for very uncommon histological subtype of gastric gastrointestinal stromal tumour (GIST) with atypical clinical presentation, asymptomatic progress and late diagnosis. The resected tumour, giant in diameters, was confirmed to represent the most rare histopathologic subtype of GISTs - sarcomatoid epithelioid GIST. We report this case and review the literature with a special focus on pathomorphological evaluation, biological aggressiveness and prognostic factors. To our knowledge this is the first report of giant GIST of very uncommon sarcomatoid epithelioid subtype.
INTRODUCTION
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal tumours of the gastrointestinal (GI) tract, representing only less than 3% of all primary gastrointestinal malignancies[1-3]. Interstitial cells of Cajal and stem cells of the smooth muscle tissue are known to be the precursors of GISTs[4]. Tumour size, mitotic rate, localization, tumour vascularisation and infiltration are the key prognostic factors in predicting malignancy potential. The available epidemiological studies provided diverse results, with the incidence rates of around 10-20 new cases per million population per year[2,3]. Incidence determined from data collected in 14 countries which joined the EORTC study totals 4-5 new cases per 1 million population per year[4,5]. GIST is predominantly diagnosed at an advanced stage, most patients (around 75%) are over 50 years old at diagnosis[4,5], and the peak onset is among 55-65 years old[2,4,6]. Age median for gastric GIST is 63 years, with a slight male predominance (55%)[1,7]. Stomach is the most common location for GIST, which accounts for 1%-2% of all gastric cancers[8]. Various sources report that as many as 70% of all GISTs are located in the stomach - most commonly in the gastric body (42.3%) and in the prepyloric area (28.5%)[1,2,4]. Prognosis for gastric GIST is typically better than for GISTs developed in other parts of the GI tract. Stromal tumours can be also found in the small intestine (25%-30%), in the colon and rectum (3%-10%)[2,4,5]. Less than 10% of all GISTs are estimated to be retroperitoneal GISTs or, in late-stage metastatic tumours, the location of the primary tumour can be no longer determined[5].
The molecular pathogenesis of stromal tumours involves a mutation which activates tyrosine kinase (KIT) and PDGFRA membrane receptors[1,3,4]. These receptors regulate key cell functions: proliferation, differentiation and anti-apoptotic signalling[3]. In GIST, ligand-independent activation of these receptors is observed, leading to uncontrolled cell proliferation and stimulation of downstream signalling pathways[3]. The metastatic pattern of GISTs is predominantly intra-abdominal, mainly to the liver[4,5]. Lymph nodal invasion is uncommon, therefore lymphadenectomy is not required with GIST resection[1,4].
In this paper, we study the case of a male patient treated for very uncommon histological subtype of gastric GIST with atypical clinical presentation, with a focus on pathomorphological evaluation, biological aggressiveness and prognostic factors. Authors checked the PubMed and MEDLINE databases for last ten years searching for epihelioid sarcomatoid gastric GIST and to our knowledge this is the first report of giant GIST of very uncommon sarcomatoid epithelioid subtype.
CASE REPORT
A 52 years old male patient was transferred to the general and oncological surgery department of the Medical University Teaching Hospital from a district hospital with suspicion of gastric GIST, for continuation of therapy. The patient was previously admitted for the first ever episode of fainting and loss of consciousness. A single tarry stool was reported one day before hospitalisation, and a history of bloody stools for 2 wk before presentation. During this period, the patient did not complain of nausea, vomiting or defecation disorders. Haemoglobin 8.8 g/dL, red blood cell (RBC) 2.8 million/dL, Hct 26% at admittance. No morphological disorders or sources of bleeding into the GI tract were revealed in gastroscopy and colonoscopy. Computed tomography (CT) scan showed a tumour-like mass projecting to the lumen of the stomach, adjacent to the greater curvature area, penetrating downwards and to the left (Figure 1). No interconnection between the intestinal loops and the tumour was demonstrated. Another gastroscopy was performed based on CT results, which revealed an external displacement of otherwise unchanged gastric mucosa over the area of 10 cm × 15 cm, within the angular incisure area. A biopsy sample was collected for histopathological analysis, which revealed signs of chronic inflammation, however, no GIST-type pattern was confirmed. The patient was administered 4 packed red blood cells units and 2 fresh frozen plasma (FFP) units, which improved his CBC parameters.
Figure 1.
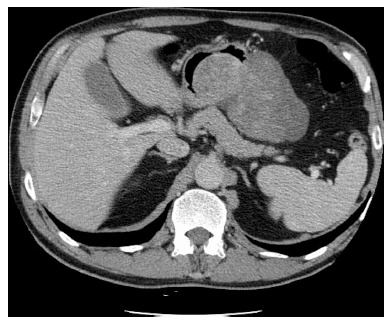
Computed tomography scan revealed a tumour-like mass projecting to the lumen of the stomach, adjacent to the greater curvature area.
On the day of admittance to the university department of surgery, the patient did not experience any pain, but reported recurring heartburn and general malaise accompanied by weight loss by around 5 kg within the preceding 2 wk. He did not complain of any other gastrointestinal discomforts. The patient had a history of appendectomy and was also treated for arterial hypertension. In a physical examination, the abdomen was soft and slightly tender in the epigastric region, where a pathological mass was detected, without any signs of peritoneal irritation. During hospitalisation patient underwent an abdominal ultrasound which revealed hypoechoic, heterogeneous tumour of 106 mm × 56 mm × 144 mm in size in the epigastric region, attached to the posterior gastric wall (Figure 2). The tumour itself demonstrated small hypoechoic and anechoic spaces of up to 21 mm. The patient was qualified for surgical treatment with suspicion of gastric GIST. A large cherry-coloured soft-structured mass of 15-18 cm diameter was revealed during surgery within the peritoneal cavity, and more specifically in lesser sac of the peritoneal cavity, attached to the posterior gastric wall of the gastric body. The patient had a wedge resection encompassing the entire tumour mass. The capsule of the tumour was intact (Figure 3). No perioperative complications were observed. The patient tolerated oral dietary intake. He was discharged home on day 7 post surgery in a good general condition. No health concerns were reported during 4 wk follow-up.
Figure 2.
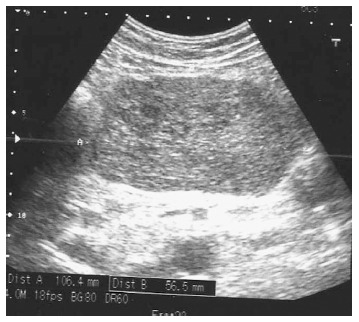
Abdominal ultrasound disclosed hypoechoic, heterogeneous mass filling the epigastrium.
Figure 3.
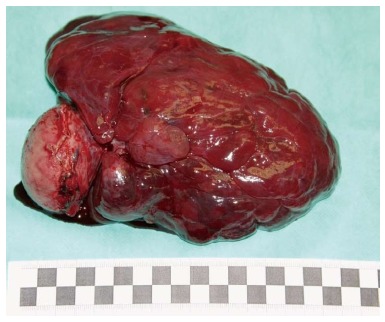
Removed gastric gastrointestinal stromal tumour. The capsule was not damaged.
In the final histopathologic analysis of a tumour sample, including a 5 cm × 4.5 cm section of gastric wall, the macroscopic appearance of the gastric mucosa was normal. In cross-section, the tumour was shown to penetrate under the gastric mucosa, without infiltrations, and projected beyond the external gastric wall, up to the size of 120 mm × 80 mm × 160 mm. The tumour was yellowish in cross-section, soft, and nested, with areas of haemorrhage. The surgical margin was preserved. Sarcomatoid epithelioid GIST was confirmed in microscopic examination. Immunohistochemical staining revealed the following: Ki67 < 5 mitoses per 50 HPFs, KIT (+/-), CKAE1 (+) AE3 (-), vimentin (+), CD34 (-), S100 (-), CD30 (-), caldesmon (-), actin (-), MCT (-), fat s(-)(Figure 4).
Figure 4.
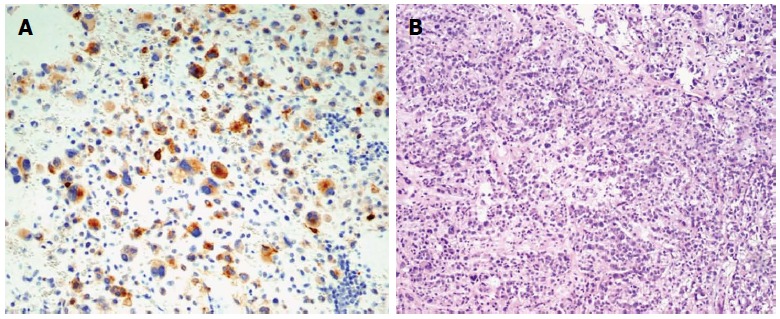
Pathology images. A: Weak immunoreactivity to KIT (CD 117; original magnification × 200); B: Gastric gastrointestinal stromal tumour (HE staining; original magnification × 100).
DISCUSSION
Gastrointestinal stromal tumours, although rare, increasingly receive the attention of clinicians. GISTs can develop along the GI tract, which may to some extent explain the broad spectrum of clinical symptoms, which include abdominal pain, gastrointestinal bleeding, anaemia, palpable abdominal mass, dyspepsia, nausea, vomiting and obstruction, constipations, diarrhoeas, swallowing difficulties, and episodes of weight loss[2,3,8]. However, some authors claim that “vague abdominal discomfort” is the most common symptom of stromal tumours, which can affect as many as 70% of all patients[2]. GIST can lead to peritonitis caused by perforation in the abdominal wall, typically accompanied by bleeding into the GI tract lumen[1-4]. Asymptomatic tumours most commonly develop in the stomach and duodenum.
GISTs, especially asymptomatic ones, pose a significant diagnostics challenge and are currently detected mainly with diagnostic imaging techniques and endoscopy. Abdominal contrast-enhanced CT or magnetic resonance (MR) are the recommended diagnostic imaging techniques in determining tumour stage and therapy planning[2,4,5]. With abdominal CT and MR, stromal tumours can be detected in 72% and 91% of patients, respectively[2]. CT images can be useful in evaluating the malignancy potential. Irregular shape, over 10 cm in size, calcification areas, cystic degeneration within the mass, and central necrosis are the main criteria for malignant behaviour[2]. However, some authors question the prognostic value of calcification and colliquative necrosis[1]. On CT images, GISTs are solid well-circumscribed tumours, and patchy enhancement by contrast medium[3,4]. Endoscopic ultrasound (EUS) is another valuable diagnostics tool in which hypoechoic lesions can be precisely marked out across individual layers of the gastrointestinal wall. EUS can be also useful in assessing the depth of invasion. The size of over 4 cm, irregular surface and heterogeneous echogenicity may point to more malignant tumours[3,4]. Endoscopy combined with biopsy and contrast-enhanced radiography is of limited diagnostic value, as it detects only around 33% of cases based on initial diagnosis[2]. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) can be considered the gold diagnostic standard (84% specificity) as it directly visualizes the tumour and provides sufficient cytological material for molecular diagnostics[3].
Gastric GIST comes in three different histological subtypes: spindle cell type (50%), epithelioid type (20%-40%) and mixed type (10%)[1,9,10]. Epithelioid GISTs are also reported to be significantly more common in the stomach[11]. There are now four different subtypes of epithelioid gastric stromal tumours distinguished: sclerosing, hypercellular, heterogeneous and sarcomatoid[1]. The last subtype is the least common subtype of epithelioid GISTs - it is present in only 2.5% of patients with gastric stromal tumours[1]. The sarcomatoid subtype can be found in both, epithelioid and spindle cell tumours[1]. The sarcomatoid pattern typically translates into statistically worse prognosis, especially in GISTs of the spindle cell type[1]. According to some sources, epithelioid stromal tumours involve larger tumour mass and shorter survival period as compared to other histological types (29.2 mo vs 96.1 mo for spindle cell tumours, and 61 mo for mixed type tumours)[12]. Pathomorphological evaluation is made up of traditional histopathology accompanied by tests for a number of tumour markers, which are of key importance in diagnosing stromal tumours. Genes responsible for increasing the density of transmembrane tyrosine kinase receptor KIT are universally overexpressed in GIST. KIT receptor can be detected by immunohistochemistry (CD117). CD117 overexpression is the most sensitive and highly specific marker of stromal tumours detected in nearly 90%-95% of cases[1,3,4,9,10]. CD117-negative gastric GISTs predominantly represent the epithelioid subtype, and only 18% of spindle cell tumours show no signs of CD117 expression[1]. Other markers such as nestin (90%) and CD34 (70%) are also representative but less specific[9]. CD34 is almost universally positive in GISTs located in oesophagus or rectum (95%-100%)[9,10]. CD34 is expressed in 80%-88%[1,10,11] of gastric GISTs, whereas α-smooth muscle actin (α-SMA) is present in around 20%-40% of patients in whom CD34 expression is absent[4,7]. The majority of CD34-negative gastric stromal tumours are conclusively identified as epithelioid-type tumours and have been shown to be more aggressive[1,11]. α-SMA (+) is present in only around 30% of patients, but is significantly more common in GISTs located in the stomach or small intestine[9]. In α-SMA-positive patients, the progression-free survival is significantly longer as compared to α-SMA-negative patients (37.7 mo vs 15.9 mo), but this correlation was demonstrated on a small population of patients and needs to be confirmed in other studies[12]. S-100 should be also mentioned in the context of pathomorphological evaluation, as it is present in only around 5% of patients and is especially common in epithelioid-type tumours, the majority of which are more malignant[1,11]. A positive relationship with the desmin antibody is rare and can be found in only 1%-3% of gastric stromal tumours, but is more common in epithelioid-type GISTs[9-11].
Biological aggressiveness of primary, resectable GISTs can be determined mainly by relying on tumour size, mitotic rate, and tumour location[4]. The National Institutes of Health (NIH) system for determining GIST malignancy potential is commonly used in daily practice and has been referred to in numerous sources. It involves two major criteria: tumour size and mitotic count[7,8]. The relationship between size and mitotic count vs malignancy potential was analysed by Miettinen et al[1]. It was based on over 1000 cases of stromal tumours. Based on this analysis, it was determined that the mortality attributed to tumours with less than 5 mitoses per 50 HPFs equals 0% for tumour with the size of less than 2 cm, 2% for tumours of 2-10 cm in size, and 11% for tumours with the size of over 10 cm. The mortality rate significantly increases in tumours showing more than 5 mitotic figures per 50 HPFs: 16% for tumours of 2-5 cm in diameter, 49% for tumours of 5-10 cm in size, and 86% for “large” tumours with the size of over 10 cm[1]. Mortality associated with primary tumour or metastasis in patients with gastric GIST > 5 cm in size and more than 5 mitotic figures per 50 HPFs amounts to 49%-86%. Epithelioid-type tumours of more than 6 cm in size and other tumour types of more than 7 cm in size can be indicative of higher malignancy and metastatic potential. More favourable prognosis for metastatic tumours can be only expected in patients with large tumours (> 10 cm) and low mitotic count (less than 5 mitotic figures/50 HPFs) - metastases are present in only 12% of cases, and the progression free survival is around 5-15 years[1]. Mean 5-year survival in patients who underwent radical surgical procedure, irrespective of the key major pathomorphological factors, is estimated at 28%-65%, or even 70%[4,7]. The largest gastric GIST reported so far was 37x24x13 cm in size and weighted 8.5 kg[13]. Size median for gastric GISTs is 6 cm. Tumours of 2-5 cm (38.2%) and 5-10 cm (29.7%) in size prevail[1,7]. Large tumours (> 10 cm) on presentation are diagnosed in only 20% of patients[1,7]. Asymptomatic disease progression is the reason of late-stage diagnosis, with metastases present in around 21% of patients on presentation[4,8]. The most malignant gastric stromal tumours are located at the fundus, entrance, and at the gastro-oesophageal junction[1]. Mortality is closely correlated with the mitotic rate and tumour size[1]. Median survival following resection is 12.4 years for all GISTs, and 14.1 years for gastric GISTs, with no signs of recurrence[1,7]. The majority of GIST recur within the first 5 years post resection[7]. Local recurrences limited to gastric walls are rare. Local recurrences result from incomplete resection of the primary tumour or new GISTs[1].
The studied case can be classified as stage II tumour according to the TNM system[14]. It is a high-risk (malignant) tumour according to the NIH system[8]. According to Miettinem, the patient should be classified to low or medium risk of death, which translates into 12%-15% risk of cancer-related death[1].
Surgery is the first choice treatment of primary gastrointestinal stromal tumours of more than 2 cm in diameter[4,6]. It is desirable to obtain 1 cm macroscopic margins of healthy tissues to secure negative microscopic margins (R0 resection) with the tumour capsule left intact[3,6]. It is imperative to avoid rupture of the tumour capsule as it can lead to tumour dissemination. Both sporadic and surgery-related ruptures are reported in only around 6% of patients[7]. According to the National Comprehensive Cancer Network guidelines, extended anatomic resection of the stomach is indicated only rarely[6]. Wedge resection and partial resection of the stomach are sufficient in 40% and 34% of cases, respectively[1]. Complete gastrectomy is necessary in only 3% of patients[1]. Infiltration into the peritoneal cavity and adjacent organs is observed in only 5.4% of patients. Laparoscopic surgery can be performed on GISTs of less than 5cm in size[6]. As compared to traditional surgery, laparoscopic approach in gastric GISTs involves reduced blood loss, fewer perioperative complications, shorter hospitalisation, and earlier resumption of oral dietary intake. However, there were no statistically significant differences between the outcomes of different surgical techniques and the number of recurrences, surgical margins, or the overall survival[15].
The key issue in effective post-surgery therapy is to inform the patient of the possibility of recurrence following many years of disease-free period. Therefore, patients should be subject to regular follow-up surveillance. Abdominopelvic contrast-enhanced CT is the mainstay for this type of follow-up. In medium and high risk patients according to NIH, follow-up surveillance should be performed at 3-4 mo intervals during the first 2 years, every 6 mo for 3 more years, and then every 12 mo (5 or more years post-resection). Patients with GISTs of very low and low aggressiveness can undergo the follow-up examinations every 12 mo.
Although surgical tumour resection is the treatment of choice in GIST, the supportive role of pharmacotherapy with tyrosine kinase inhibitors is increasingly highlighted. Adjuvant therapy remains controversial, despite the approval of imatinib in post-surgery treatment of patients with high-risk of recurrence according to NCCN-AFIP-AJCC classification. This type of treatment is most beneficial in patients classified to the highest risk of recurrence. Neoadjuvant imatinib should be also attempted in patients with borderline resectable GIST. In patients at advanced stage of GIST, inclusion criteria for imatinib are: unresectable tumour, distant metastases (liver, dissemination) and local recurrence. Second generation tyrosine kinase inhibitor - sunitinib can be also considered if there is a further disease progression. Cancer treatment is now becoming more and more personalised. However, despite the huge progress in this area, and the introduction of novel anti-cancer drugs, cancer treatment still remains a huge challenge.
COMMENTS
Case characteristics
A 52 years old male patient admitted for the first ever episode of fainting and loss of consciousness with a history of bloody stools for 2 wk before presentation.
Clinical diagnosis
In a physical examination, the abdomen was soft and slightly tender in the epigastric region, where a pathological mass was detected, without any signs of peritoneal irritation.
Differential diagnosis
Malignant tumors, benign neoplasms.
Laboratory diagnosis
Haemoglobin 8.8 g/dL, RBC 2.8 million/dL, Hct 26%, metabolic panel were within normal limits at admittance.
Imaging diagnosis
Computed tomography scan showed a tumour-like mass projecting to the lumen of the stomach, adjacent to the greater curvature area, whereas gastroscopy revealed an external displacement of otherwise unchanged gastric mucosa.
Pathological diagnosis
In the final histopathologic analysis sarcomatoid epithelioid gastrointestinal stromal tumours (GIST) was confirmed and immunohistochemical staining revealed the following: Ki67 < 5 mitoses per 50 HPFs, tyrosine kinase (KIT) (+/-), CKAE1 (+) AE3 (-), vimentin (+), CD34 (-), S100 (-), CD30 (-), caldesmon (-), actin (-), MCT (-), fats (-).
Treatment
The patient had a wedge resection encompassing the entire gastric tumour mass.
Related reports
To authors’ knowledge this is the first report of giant GIST of very uncommon sarcomatoid epithelioid subtype.
Term explanation
KIT is a receptor tyrosine kinase protein found on the surface of gastrointestinal stromal tumors cells.
Experiences and lessons
This case report not only focuses on uncommon symptoms, pathomorphological evaluation, biological aggressiveness of giant gastric sarcomatoid epithelioid GISTs, but also discuss the key factors for further treatment and good prognosis.
Peer-review
This paper includes the overviews on GISTs with clinically relevant info and concise case of uncommon sarcomatoid epithelioid subtype.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 27, 2014
First decision: September 15, 2014
Article in press: December 16, 2014
P- Reviewer: Mortensen C, Wong GLH S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 2.Scarpa M, Bertin M, Ruffolo C, Polese L, D’Amico DF, Angriman I. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J Surg Oncol. 2008;98:384–392. doi: 10.1002/jso.21120. [DOI] [PubMed] [Google Scholar]
- 3.Rammohan A, Sathyanesan J, Rajendran K, Pitchaimuthu A, Perumal SK, Srinivasan U, Ramasamy R, Palaniappan R, Govindan M. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013;5:102–112. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cichoz-Lach H, Kasztelan-Szczerbińska B, Słomka M. Gastrointestinal stromal tumors: epidemiology, clinical picture, diagnosis, prognosis and treatment. Pol Arch Med Wewn. 2008;118:216–221. [PubMed] [Google Scholar]
- 5.Rutkowski P, Wozniak A, Dębiec-Rychter M, Kąkol M, Dziewirski W, Zdzienicki M, Ptaszynski K, Jurkowska M, Limon J, Siedlecki JA. Clinical utility of the new American Joint Committee on Cancer staging system for gastrointestinal stromal tumors: current overall survival after primary tumor resection. Cancer. 2011;117:4916–4924. doi: 10.1002/cncr.26079. [DOI] [PubMed] [Google Scholar]
- 6.Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 2013;13:3–18. doi: 10.5230/jgc.2013.13.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3–24. [PubMed] [Google Scholar]
- 10.Patil DT, Rubin BP. Gastrointestinal stromal tumor: advances in diagnosis and management. Arch Pathol Lab Med. 2011;135:1298–1310. doi: 10.5858/arpa.2011-0022-RA. [DOI] [PubMed] [Google Scholar]
- 11.Bülbül Doğusoy G. Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol. 2012;23:203–211. [PubMed] [Google Scholar]
- 12.Demir L, Ekinci N, Erten C, Kucukzeybek Y, Alacacioglu A, Somali I, Can A, Dirican A, Bayoglu V, Akyol M, et al. Does immunohistochemistry provide additional prognostic data in gastrointestinal stromal tumors? Asian Pac J Cancer Prev. 2013;14:4751–4758. doi: 10.7314/apjcp.2013.14.8.4751. [DOI] [PubMed] [Google Scholar]
- 13.Cappellani A, Piccolo G, Cardì F, Cavallaro A, Lo Menzo E, Cavallaro V, Zanghì A, Di Vita M, Berretta M. Giant gastrointestinal stromal tumor (GIST) of the stomach cause of high bowel obstruction: surgical management. World J Surg Oncol. 2013;11:172. doi: 10.1186/1477-7819-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, (Eds. ). AJCC Cancer Staging Handbook. 7th ed. New York: Springer; 2010. [Google Scholar]
- 15.Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, Goh BK. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549–3560. doi: 10.1245/s10434-013-3051-1. [DOI] [PubMed] [Google Scholar]


