Abstract
This unit focuses on the murine model of cutaneous leishmaniasis and models of visceral leishmaniasis in mice and hamsters. Each basic protocol describes the methods used to inoculate parasites and to evaluate infections with regard to lesion progression and visceralization, and quantification of parasite load.
Introduction
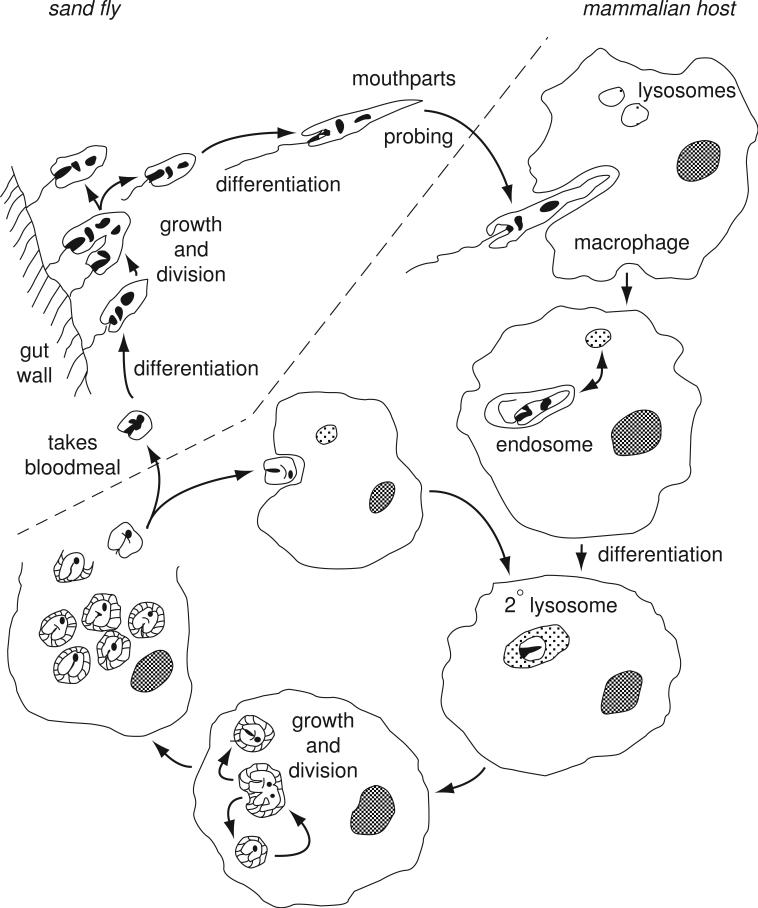
Although experimental studies on leishmaniasis have been carried out in many species (including guinea pigs, rabbits, dogs, and monkeys; (Hommel et al, 1995), this unit focuses on the frequently employed model of cutaneous leishmaniasis in mice (see Basic Protocol 1) and models of visceral leishmaniasis in mice and hamsters (see Basic Protocol 2). Each Basic Protocol describes the methods used to inoculate parasites and to evaluate infections with regard to lesion progression and visceralization, and quantification of parasite load. In order to achieve reproducible infections in these animal models, the most critical parameter to be considered is the manner in which the parasite inoculum is standardized with regard to developmental stage and dose. The life cycle of the parasite (summarized in Fig. 19.2.1) includes two stages that are well adapted to survival in the mammalian host, and each can be used to initiate infection. (1) Metacyclic promastigotes (Fig. 19.2.2A) are extracellular flagellated, highly motile promastigotes that initiate infection in nature. They develop within the sand fly midgut and initiate infection after being deposited in the skin by the bite of an infected fly. (2) Amastigotes (Fig. 19.2.2b) are non-motile intracellular forms that replicate and maintain infection in the vertebrate host.
Figure 19.2.1.
Life-cycle of Leishmania, showing the growth and development of promastigotes in the sand fly midgut, the inoculation of metacyclic promastigotes into the skin of the mammalian host, uptake by macrophages, transformation to amastigotes within phagolysosomes, intracellular replication as amastigotes, escape and reinvasion of macrophages, or transformation back to promastigotes within a sand fly infective bloodmeal.
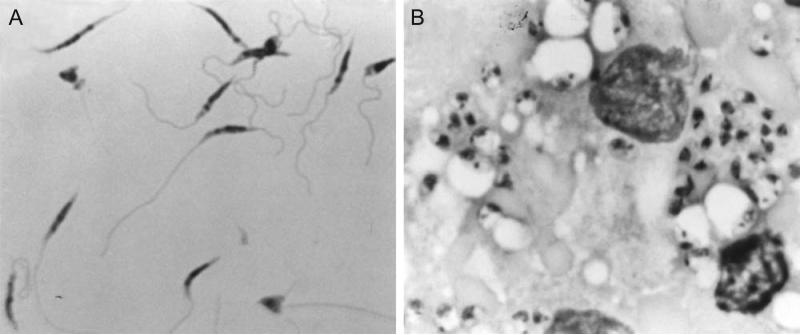
Figure 19.2.2.
(A) Light micrograph (900×) of L. major metacyclic promastigotes purified from stationary culture by negative selection using peanut agglutinin (PNA), stained with Diff-Quik solution. The purified metacyclics display a homogeneous morphology: narrow cell body with tapered anterior end, and long flagellum approximately twice the length of the cell body. (B) Light micrograph (900×) of Diff-Quik-stained impression smear of a spleen from a hamster, 8 weeks after intracardial inoculation with L. donovani amastigotes. Two host cell nuclei and many intracellular amastigotes are shown.
Five support protocols are provided in this unit, two for the growth and isolation of metacyclic promastigotes from in vitro culture (see Support Protocols 1 and 2), one for isolation of tissue amastigotes from infected animals (see Support Protocol 3), one for cryopreservation of parasites (see Support Protocol 4), and one for the preparation of blood agar plates for quantitation of parasite numbers in infected tissue (see Support Protocol 5). For discussions of the use of promastigotes versus amastigotes, fresh versus frozen stabilates of parasites, and the appropriate dose of parasites to initiate infection, see Critical Parameters and Troubleshooting.
All of the World Health Organization (WHO) reference strains of Leishmania, which include at least one of each species commonly recognized as distinct members of the genus, can be purchased as promastigotes from ATCC. Leishmania are considered to have moderate potential hazard to personnel, and Biosafety Level 2 practices and facilities should be used when working with the parasites. Laboratory-acquired infections have been reported to occur; the greatest risk is encountered when inoculating experimental animals. Laboratory personnel should have specific training related to the handling of pathogenic agents. Routine laboratory practices should include (1) wearing laboratory coats and gloves when working with the parasite, (2) using only mechanical devices for pipetting, (3) decontaminating work surfaces regularly and after spills, (4) decontaminating waste before disposal, and (5) using a biological safety cabinet (Class I or II). Care should be taken to ensure the immediate and proper disposal of needles used for experimental inoculations.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
BASIC PROTOCOL 1
MOUSE MODELS OF CUTANEOUS LEISHMANIASIS
Experimental models of cutaneous leishmaniasis in genetically susceptible and resistant inbred mouse strains (Table 19.2.1) have been used by many research groups for the dissection of the immune mechanisms implicated in resistance (protection) and susceptibility to infection. The most frequently used site for initiation of cutaneous lesions in the mouse is the left or right hind footpad. Inoculation into the ear dermis has gained increasing popularity since it is thought to better reflect the conditions of natural sand fly transmitted infection in the skin (see commentary). The advantages of these sites are that lesion development can be evaluated by simple measurement of footpad swelling or ear lesion diameter, and that sequential measurements can be made on the same lesion, because they can be obtained without having to sacrifice the animal. However, because a number of reports suggest that this measure is not necessarily an accurate reflection of parasite numbers (see Commentary), and because parasite dissemination to and growth within visceral sites might also be an important outcome to evaluate, methods are also provided for the direct quantitation of parasite numbers in infected tissue (e.g., ear, footpad, spleen, or lymph node).
Table 19.2.1.
Stage-specific lectins and antibodies for purification of metacyclic promastigotes
| Species/strain | Lectin | mAb | Reference |
|---|---|---|---|
| L. major | PNA, RCA | (Sacks et al., 1985) | |
| L. major (West Africa) | ConA | (Joshi, et al., 1998) | |
| L. donovani (Sudan) | PNA, ConA | (Sacks et al., 1995) | |
| L. donovani (India) | MnG | (Mahoney et al., 1999) | |
| L. tropica | 1H2 | (Lira et al., 1998) | |
| L. infantum/chagasi | PNA | MnG | (Soares et al., 2002) |
| L. amazonensis | 3A1 | (Courret et al., 1999) | |
| L. braziliensis | B. purpurea | (Pinto-da-Silva et al., 2002) | |
Materials
Parasite inoculum, either amastigotes or metacyclic promastigotes (see Support Protocols 1 to 4)
Dulbecco's minimal essential medium (DMEM) or HBSS without CaCl2 or MgCl2 (APPENDIX 2)
Mice (Table 19.2.1)
Complete medium 199 (C-M199; see recipe)
Caliper (Vernier, dial, or digital)
1.5-ml polypropylene microcentrifuge tubes (autoclaved, preweighed)
Pellet pestle for 1.5-ml microcentrifuge tubes (polypropylene, disposable, sterile)
Scotch double sided sticky tape
Liberase TL purified enzyme blend (Roche Diagnostic Corp.)
Ketamine
Qiagen DNeasy Blood & Tissue kit
Blood agar plates (see Support Protocol 5)
26°C incubator without CO2
Additional reagents and equipment for mouse anesthesia (UNIT 1.4) or restraint (UNIT 1.3), footpad injection (UNIT 1.6), footpad disinfection and dissection (see Support Protocol 3), and preparation of spleen or lymph node suspensions (UNIT 3.1)
Parasite inoculation in the hind footpad or ear
-
1Adjust the concentration of a parasite inoculum in DMEM or HBSS so that the desired dose is delivered in a 40- to 50-μl volume for footpad inoculation or 10-μl for ear inoculation.The typical dose range for the manifestation of the established murine susceptibility and resistance phenotypes following subcutaneous infection with L. major is 105 to 107 parasites and 103 to 105 parasites intradermal ear inoculation. For further details on dose, see Critical Parameters and Troubleshooting.
-
2a
Lightly anesthetize (UNIT 1.4) or restrain a mouse in a holding apparatus (UNIT 1.3) to stabilize the footpad. Inject the parasite suspension (UNIT 1.6).
-
2bAnesthetize a mouse with 100ul ip of Ketamine (20mg/ml). Tape the “index” finger with Scotch double-sided tape. Flatten the outside face of the ear around the index finger (see Fig. 19.2.3). Normally, for a right handed individual, the index finger of the left hand will be used, injecting with the right hand, and holding the mouse with the left hand to infect the right ear. To inject in the left ear, rest the mouse on the bench. Using a magnifying lamp, inject 10ul of the parasite suspension using a 3/10cc tuberculin syringe with a 29 1/2 gauge needle in the middle of the ear.An alternative site for the initiation of cutaneous lesions is the base of the tail. This site may need to be used if footpad or ear pathology is not permitted by animal use committees. The disadvantage with this site is the difficulty in obtaining lesional tissue for quantitation of parasite load.
Figure 19.2.3.
Needle injection of Leishmania into the ear dermis.
Assess lesion development
-
3Monitor lesion development weekly by restraining (not anesthetizing) the animal and measuring footpad width and thickness at the base of the fifth toe using a caliper. Also monitor the uninoculated contralateral footpad as a control. Express result as a single value (or score) by multiplying footpad width by thickness, both in millimeters. The size of the ear lesion is monitored by measuring the induration in two diameters with a caliper.The measurement of lesion development in the shaved rump at the base of the tail (induration in two diameters) is relatively straightforward.
Quantitate parasite load
By limiting dilution analysis
-
4aLymph node or spleen: Make a single cell suspension of draining popliteal lymph node or spleen (UNIT 3.1) and resuspend in up to 10 ml C-M199.The limiting dilution can be initiated with 1% or less of the single cell suspension, leaving ample cells for immunological assays.
Footpad: Surface disinfect the infected footpad and dissect as described (see Support Protocol 3). Remove 1 to 10 mg tissue and place in an autoclaved, preweighed 1.5-ml polypropylene microcentrifuge tube containing 0.3 ml C-M199. Close cap and invert or tap tube so that the tissue settles to the bottom. Do not allow the tissue to dry out during this processing. Weigh tube, then disrupt tissue by manual or motor-driven grinding using a pellet pestle.
Avoid placing the scalpel or scissors containing the tissue directly into the medium, as a small volume of medium may be removed, resulting in an underestimate of tissue weight.
Ear: Cut off ears, place in 70% alcohol in 15 ml tubes. Leave for 10-20 min. Leave to dry under the hood before opening dermal sheets. Place 0.5 ml of 160μg/ml of Liberase TL purified enzyme blend (Roche Diagnostic Corp.) into each well of a 24-well plate. Open ears and overturn dermal side down onto enzyme for 1 hour at 37°C. Cut all ears with scissors and homogenize using a teflon-coated microtissue grinder in a microfuge tube containing 100 μl of DMEM or RPMI. The tissue homogenates are filtered using a 50 μm-pore-size cell strainer (Falcon Products Inc, St. Louis, MO).
Alternatively, the Medimachine system (BD Biosciences), which is an automated system for mechanical disaggregation of solid tissue, can be used to process the footpad or ear. The infected footpad is surface disinfected by placing in 70% ethanol for 2-5 min, the toes and bones removed, and the remaining tissue incubated in Liberase TL purified enzyme blend as described for the ears. Place the enzymatically digested footpad or ear in a sterile Medicon (50 μm, 340592) + 1 ml DMEM + 0.05 % DNAse, and allow the machine to run for 4 minutes. The tissue homogenate is then flushed from the medicon with 10 ml RPMI media containing 0.05% DNAse and filtered using a 50 um-pore-size cell strainer, spun-down for 10 mins at 1500 rpm, and re-suspended in the appropriate media.
-
5aPerform duplicate two-fold serial dilutions of tissue parasites to extinction in blood agar plates by mixing 100 μl tissue homogenate (or spleen or lymph node suspension) and an equal volume of C-M199.The serial dilutions must be carried out to extinction. The number of wells required to achieve an end-point dilution will vary extensively depending on the tissue being examined, the parasite strain, the mouse strain, and the time elapsed since initiation of infection (see Anticipated Results).
-
6aSeal the plates and incubate 7 to 10 days at 26°C without CO2.By this time, high-density growth should have been achieved in even the last positive well, so that scoring is very easy.
-
7aScore the presence of promastigotes on an inverted microscope.If the plates containing the blood agar have been prepared so that each well contains a window in the blood agar (see Support Protocol 5), they can be examined directly on an inverted microscope. If the blood agar obscures direct microscopic examination, a small volume of liquid overlay can be transferred to a microscope slide or a 96-well plate for scoring.
-
8a
Calculate parasite load per total ear, lymph node or spleen, or per mg footpad tissue, from the highest dilution at which promastigotes can be grown out, divided by the tissue weight.
By real-time PCR
-
4b
Harvest the tissue of interest (skin, lymph node, or spleen), weigh the tissue (about 25 mg is ideal), and immediately homogenize or freeze at -80°C until use. Care should be taken to use new or decomtaminated instruments when harvesting tissue to avoid carryover of parasite DNA to other samples. Cut the tissue in small pieces with a disposable blade. Transfer the pieces to a microcentrifuge tube and add 180 μl Buffer ATL from the Qiagen DNeasy Blood & Tissue kit and grind the tissue manually with a disposable pestle. Complete homogenization of the tissue is necessary to recover a good yield of DNA.
-
5b
Add 20 ul of proteinase K from the kit, vortex and incubate the tissue at 56°C (2h- overnight) to complete the tissue homogenization. Skin samples are best incubated overnight.
-
6b
Follow the instructions of the Qiagen kit for the DNA purification. Care must be taken to when using the reagents to avoid cross-contamination. Quantify purified DNA using a NanoDrop Spectrophotometer (Thermo Scientific) and store at -80°C until assayed. Adjust DNA to 10 ng/μL (this may need to be varied depending on the source of tissue and expected parasite load).
-
7b
Perform real-time hot-start PCR using 2 μL of the DNA with 1 μl of a 100 μM of stock primers that target the conserved region of the kinetoplast DNA (forward, 5-CCTATTTTACACCAACCCCCAGT-3 [JW11]; reverse, 5-GGGTAGGGGCGTTC TGCGAAA-3 [JW12]) and SYBR green master mix in a total reaction volume of 10 μL (FastStart DNA Master SYBR Green I Kit (Roche Diagnostics) The PCR samples are initially incubated for 10 minutes at 95°C and then 40 cycles at 95°C for 15 sec and 60°C for 1 minute in 7900 HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, California).
-
8b
To quantify the number of parasites, a standard curve is generated. L. donovani amastigotes are purified from tissue and manually counted. A known quantity of parasites is then spiked into an uninfected tissue homogenate (same tissue in which the parasite load is to be determined) and 10-fold dilutions of the parasite-spiked tissue are made. The DNA is isolated from the parasite-spiked tissue dilutions and quantified by real-time PCR as described above. A standard curve is constructed by linear regression using log parasite number and CT value, and the number of parasites in the experimental samples determined by interpolation from the standard curve.
BASIC PROTOCOL 2
MOUSE AND HAMSTER MODELS OF VISCERAL LEISHMANIASIS
Visceral leishmaniasis can be studied in either mouse or hamster models; each has advantages and disadvantages. Both animals must be infected by systemic inoculation (intravenous in the case of the mouse and intracardiac in the case of the hamster). Demonstration of a consistent model of visceral disease following cutaneous inoculation has not been accomplished. Susceptible mouse strains (Table 19.2.1) develop acute infection with L. donovani but progressive clinical disease does not ensue. The outbred Syrian golden hamster provides a model of progressive visceral disease that closely mimics active human visceral leishmaniasis (VL), but has the disadvantage of having few reagents available for immunological or molecular studies. Inbred strains of hamster have been studied and do not appear to be different from the outbred animals; however, inbred hamsters are not commercially available in the U.S. In this protocol, parasite inoculation and subsequent determination of parasite burden are presented.
The parasite burden in the liver, spleen, or bone marrow can be determined by serial dilution to extinction in blood agar plates, or by real-time PCR, as described above (see Basic Protocol 1). The parasite burden in the liver or spleen can also be determined by enumeration of parasites in stained tissue imprints (touch preparations), as described here. For a discussion of the advantages and disadvantages of stained tissue imprints, see the Critical Parameters and Troubleshooting.
Materials
Parasite inoculum, either amastigotes or metacyclic promastigotes (see Support Protocols 1 to 4)
Dulbecco's minimal essential medium (DMEM) or HBSS without CaCl2 or MgCl2 (APPENDIX 2)
Mice or hamsters (Table 19.2.1)
Isoflurane anesthetic (Abbott Laboratories)
70% ethanol
LeukoStat stain kit (Fisher) containing fixative, solution I (buffered eosin), and solution II (methylene blue and AzureA)
100% methanol
1-cc Tuberculin syringes and ½-in. (1.27-cm) 26-G needles
Additional reagents and equipment for restraint (UNIT 1.3), inhalent anesthesia (UNIT 1.4), inoculation (UNIT 1.6), and cardiac puncture (UNIT 1.7)
Inoculate animals
For mice
-
1a
Resuspend parasite inoculum in DMEM or HBSS at 5 × 107/ml. Keep parasite suspension on ice until inoculation.
-
2a
Gently warm a mouse's tail under a heat lamp or by immersion in warm water to dilate the vein prior to inoculation (UNIT 1.6).
-
3aRestrain the mouse in a holding apparatus (UNIT 1.3) and inject 200 μl inoculum (107 parasites) into the lateral tail vein using a 26-G needle and a 1-cc Tuberculin syringe (UNIT 1.6).Successful injection into the vein should result in a visible, rapid “flush” of color from the vein. Inadvertent subcutaneous injection will be met with some resistance, and infiltration will cause a visible loss of skin color at the site.
For hamsters
-
1b
Resuspend parasite inoculum in DMEM or HBSS at 5 × 107/ml. Keep parasite suspension on ice until inoculation.
-
2b
Anesthetize the hamster by inhalation of isoflurane (UNIT 1.4) and lay it on its back with limbs outstretched.
-
3bWash the skin over the chest with 70% ethanol and inoculate 200 μl (107 parasites) by intracardial injection using a 1-cc Tuberculin syringe with a 26-G needle. Insert the needle through the skin just below the xyphisternum and slowly advance it in the direction of the hamster's left ear at ~15° from horizontal, while gently applying suction on the syringe. When a good blood return is obtained, slowly inject half of the inoculum. Maintain the needle position and repeat gentle suction. If a good blood return is again observed (indicating that the needle is still properly placed), slowly inject the remaining inoculum. Withdraw the needle and return the hamster to the cage.Care should be taken not to lacerate the tissue by dragging the tip of the needle across it. If the heart is missed on the first pass, the needle should be withdrawn along the same track to the subcutaneous tissue and redirected from there. The morbidity and mortality from intracardial inoculation is quite low. Where possible, the proper intracardial injection technique should be learned from observing an experienced investigator (see UNIT 1.7 for cardiac puncture).
Assess visceral parasite burden
-
4Euthanize the mouse by cervical dislocation or the hamster by CO2 asphyxiation, or anesthetize the hamster by isoflurane inhalation. Exsanguinate the animal by severing the aorta or by cardiac puncture (UNIT 1.7).Removal of red blood cells from the organ facilitates the tedious job of counting the parasites on the stained tissue imprints.
-
5
Harvest and weigh the liver or spleen.
-
6
Sharply cut a piece of the organ with a scalpel or straight-blade scissors to give a flat cut surface.
-
7
Carefully hold the tissue with forceps and gently blot the cut surface of the tissue repeatedly on filter paper to remove excess fluid. Continue blotting until only a faint imprint of the tissue is visible on the filter paper (e.g., 10 to 20 light touches).
-
8Make a tissue imprint by lightly touching the cut surface on a clean, labeled microscope slide held at an angle such that the impression left by the tissue can be seen. Touch the tissue to the slide with a vertical (perpendicular to the slide) motion so that smearing does not occur. Make multiple imprints on the slide to ensure good imprints for enumeration of the parasites.If fluid is left on the slide with the tissue imprint, either the tissue was not blotted adequately or it was pressed too firmly on to the slide.
-
9
Air dry the slide several seconds and fix it several minutes in 100% methanol.
-
10Stain the tissue impression smear with a rapid Wright-Giemsa stain (e.g., LeukoStat) by immersing in fixative for ~5 sec, followed by five 1-sec dips in solution I (buffered eosin) and three 1-sec dips in solution II (methylene blue and AzureA). Immediately remove the excess stain by dipping the slide in a beaker of water.Overstaining the tissue imprint with solution II makes visualization and enumeration of amastigotes more difficult.
-
11Air dry the slide and examine the stained tissue imprint microscopically.Large hepatocyte and mononuclear phagocyte nuclei should be stained purple and amastigotes should be visible as pink-purple with a distinct nucleus and kinetoplast.
-
12
Count the number of host cell nuclei (up to a total of 500) and amastigotes within multiple different microscopic fields. Express the parasite burden of the organ in Leishman-Donovan units (LDU), where LDU is the number of amastigotes per host nucleus multiplied by the total organ weight (milligrams).
SUPPORT PROTOCOL 1
PREPARATION OF METACYCLIC PROMASTIGOTES USING PEANUT AGGLUTININ (PNA)
Extracellular promastigotes are clearly the most convenient life cycle stage of Leishmania parasites for inoculation because they can be abundantly and cheaply grown in liquid culture. The most important issue to bear in mind when using promastigotes to initiate infection is that they are not uniform with respect to infectivity; their growth in the sand fly midgut and in axenic culture is accompanied by their sequential development from a dividing, noninfective stage to a nondividing, infective or metacyclic stage. In vitro metacyclogenesis varies considerably between strains, and can be greatly affected by the frequency of subculture and by growth conditions even within a cloned population (see Critical Parameters and Troubleshooting for a discussion of the factors affecting the ability of promastigotes to differentiate to metacyclics during growth in vitro). Thus, the standardization of promastigote inocula for animal infections should be optimized whenever possible by purification of metacyclic-stage organisms.
Metacyclic promastigotes can be identified and purified from stationary cultures of L. major and some strains of L. donovani based on their loss of agglutination with the lectin peanut agglutinin (PNA). For other Leishmania species and strains (see Table 19.2.1), other plant lectins and/or monoclonal antibodies have been used to purify metacyclic promastigotes (see Commentary for a discussion of the molecular basis of stage-specific lectin or antibody binding). The method of negative selection described below using PNA will be applicable to the lectins or antibodies listed in Table 19.2.1.
Materials
Parasites: freshly harvested or frozen stabilates of L. major or L. donovani amastigotes (see Support Protocols 3 and 4) or early passage of promastigotes (ATCC; see Support Protocol 4)
Complete medium 199 (C-M199; see recipe)
Dulbecco's minimal essential medium (DMEM) or HBSS without CaCl2 or MgCl2 (APPENDIX 2)
5 to 10 mg/ml peanut agglutinin (PNA; Vector Labs)
25-cm2 tissue culture flask
75-cm2 or 225-cm2 tissue culture flask (optional)
26°C incubator without CO2
Additional reagents and equipment for counting cells with a hemacytometer (APPENDIX 3A)
- Inoculate a 25-cm2 flask containing 10 ml C-M199 with 0.5–1 × 107 parasites.Because some parasite strains seem to require a threshold density in order to initiate growth in liquid medium, it is generally recommended that cultures be seeded at a relatively high concentration (0.5–1 × 106/ml).
Optional: Depending on the quantity of metacyclics required, expand cultures after 1 to 2 days by inoculating 10 ml of the initial logarithmic phase culture into 50 to 100 ml of C-M199 in either a 75-cm2 or a 225-cm2 tissue culture flask.
Incubate the cultures at 26°C for up to 6 days.
Wash parasites twice in 50 ml DMEM or HBSS by centrifuging 15 min at 3000 × g, 4°C, in a 50-ml polypropylene centrifuge tube.
- Resuspend in DMEM or HBSS count, and bring to 1–2 × 108/ml.By taking advantage of their tendency to attach tightly to charged surfaces via the tips of their flagellum, live parasites can be easily counted in a hemacytometer so long as the counting diluent does not contain added serum or protein, and so long as both the plane of the grid and the coverglass are included in the counts. Alternatively, a small amount of glycerol (20% v/v) can be added to the counting medium to retard parasite motility.
Add 0.01 vol of 5 to 10 mg/ml PNA (final 50 to 100 μg/ml). Incubate at room temperature for 15 to 30 min.
Centrifuge 200 × g, 4°C, for 5 min.
Collect supernatant and wash cells twice as in step 4.
Resuspend cells in a small volume of DMEM or HBSS, count, and resuspend at the desired concentration for inoculation.
SUPPORT PROTOCOL 2
PREPARATION OF METACYCLIC PROMASTIGOTES BY FICOLL DENSITY GRADIENT CENTRIFUGATION
The infective metacyclic stage of all Leishmania species is morphologically distinct from non-infective promastigotes, with a short, narrow body, and a long flagellum more than twice the body size (Sacks & Perkins, 1984; Zakai et al, 1998)(Sacks and Perkins 1984; Zakai et al. 1998). Metacyclics therefore exhibit different sedimentation properties under appropriate conditions. Highly enriched metacylic promastigotes populations of most Leishmania species and strains can be obtained by centrifugation through a discontinuous Ficoll gradient (Spath & Beverley, 2001). The advantage of this method is that it is not Leishmania species or strain restricted and does not depend on the availability of lectin or antibody reagents.
Additional Materials (also see Support Protocol 1)
20% stock solution of Ficoll Type 400 in sterile, endotoxin free water
10% Ficoll prepared using 2X PBS
15ml polypropylene centrifuge tubes
Grow promastigotes to stationary phase (4 to 6 days), harvest, and wash as described (see Support Protocol 1, steps 1 to 4). Resuspend the washed cells in 2 ml DMEM at 1- 5 × 108/ml
In 15ml conical tube, prepare a discontinouous Ficoll gradient prepared using 2 ml 20% stock solution overlayered with 10% Ficoll solution.
Overlay the parasite suspension on the 10% Ficoll centrifuge at 1300 × g for 10 min. at rm temp. Metacyclics accumulate on top of or just penetrating into the 10% Ficoll layer. Recover the parasites from the upper interface with a sterile pipette and wash x 2 in DMEM at 3,000 X g for 15 min, resuspend in a small volume of DMEM or HBSS, count, and resuspend to desired concentration for inoculation.
SUPPORT PROTOCOL 3
PURIFICATION OF TISSUE AMASTIGOTES FROM FOOTPAD OR SPLEEN
The problems associated with promastigote heterogeneity can be avoided by using inocula comprised of amastigotes purified from infected tissue, and may be especially useful when promastigote cultures yield few metacyclics. While there are a number of reports that amastigote-like organisms can be grown axenically (Doyle et al, 1991; Eperon & McMahon-Pratt, 1989; Pan, 1984)(Pan, 1984; Eperon and McMahon-Pratt, 1989; Doyle et al., 1991), these protocols have only succeeded with certain Leishmania strains (e.g., L. mexicana, L. braziliensis, L. donovani), and even in these cases the strains can require a long period of adaption to acidic pH and elevated temperature before their transformation to amastigotes will occur. Furthermore, the infectivity of the amastigote-like forms has not been confirmed in each case. Therefore, this Support Protocol only considers the manner in which amastigotes can be obtained from infected tissue, either mouse footpad (for cutaneous strains) or hamster spleen (for visceral strains). Because visceral infections in the mouse tend to be controlled, the yield of amastigotes is poor compared to hamster. When obtaining amastigotes from mouse footpad lesions, it is recommended that the footpad surface be disinfected prior to excisement of the infected tissue. For visceral strains, the infected spleen can be processed directly so long as it has been removed aseptically. In this procedure, intracellular amastigotes are released from infected macrophages by mechanical disruption of the cells using a tissue homogenizer. Host cell debris is then removed by glass-wool filtration and low-speed centrifugation. The number of viable amastigotes is determined by vital staining.
Materials
BALB/c mouse with nonulcerative footpad lesion (see Basic Protocol 1) or moribund hamster with visceral infection (see Basic Protocol 2)
1.75% (v/v) iodine solution (Wescodyne, Amsco)
70% ethanol
PBS (APPENDIX 2) containing 2 mM EDTA and 50 mM glucose
Dulbecco's minimal essential medium (DMEM) or HBSS without CaCl2 or MgCl2 (APPENDIX 2)
5 mg/ml fluorescein diacetate (FDA; Sigma) in acetone (stable up to 6 months at 4°C)
20 μg/ml propidium iodide (PI; Sigma) in PBS (stable up to 6 months at 4°C)
Sterile glass wool
15-ml Dounce or Ten Broeck tissue homogenizer
Additional reagents and equipment for counting cells with a hemacytometer (APPENDIX 3A
Purify tissue amastigotes from infected tissue
For mouse footpad
-
1a
Euthanize a BALB/c mouse with a nonulcerative footpad lesion 4 to 6 weeks postinfection by cervical dislocation. Surface disinfect the involved footpad by immersing 10 min in 1.75% iodine solution followed by 10 min in 70% ethanol. Rinse in sterile distilled water.
-
2a
Dissect away the skin from the subcutaneous lesional tissue, and remove the main lesion using a sterile scalpel or scissors.
-
3a
Grind the infected tissue lightly in a mortar containing a small volume (3 to 5 ml) of PBS containing 2 mM EDTA and 50 mM glucose. Examine homogenate under a microscope and continue grinding until most of the host cells are disrupted.
For hamster spleen
-
1b
Euthanize a moribund hamster with a visceral infection (typically 6 to 8 weeks postinfection) by CO2 asphyxiation.
-
2b
Aseptically remove the spleen and cut into smaller pieces in a small volume of PBS containing 2 mM EDTA and 50 mM glucose.
-
3b
Grind the tissue in a mortar or place in a Dounce or Ten Broeck tissue homogenizer and homogenize with sufficient strokes to disrupt the majority of cells.
-
4
Filter the resulting suspension through a small amount of sterile glass wool that has been packed into a 5-ml syringe.
-
5
Centrifuge fitrate at 200 × g, 4°C, for 10 min to remove cellular debris.
-
6
Resuspend amastigotes in 50 ml DMEM or HBSS and wash by centrifuging at 3000 × g, 4°C, for 15 min.
-
7
Resuspend in a small volume (1 to 5 ml) of DMEM or HBSS.
Count viable cells
-
8Prepare a fresh working solution of FDA by adding 0.04 ml of 5 mg/ml FDA to 10 ml PBS. Add 0.1 ml of FDA working solution (2 μg final) and 0.03 ml of 20 μg/ml PI (0.6 μg) directly to 0.1 to 1.0 ml cells.The staining volume is not critical.
-
9
Stain cells 3 min and place in a hemacytometer. Examine with a standard fluorescence microscope with epiillumination, using a filter combination that permits both green (viable) and red (nonviable) cells to be seen simultaneously.
-
10
Adjust concentration for challenge inoculum or for cryopreservation (see Support Protocol 4).
SUPPORT PROTOCOL 4
CRYOPRESERVATION AND THAWING OF PROMASTIGOTES AND AMASTIGOTES
Laboratories working with Leishmania should be able to freeze and thaw parasite stabilates to avoid the potential loss of a strain from contamination, and to avoid the decline in virulence associated with continuous in vitro passage. Clinical isolates should be cryopreserved as soon as possible after growth of promastigotes in primary explant cultures. The freezing of amastigote stabilates has considerable practical advantages, since these will provide a reproducible source of parasites without having to sacrifice an animal and isolate tissue amastigotes prior to each experiment. The viability and virulence of freshly thawed amastigotes should be confirmed before assuming that they behave exactly as the unfrozen parental stock. This Support Protocol describes how Leishmania strains can be frozen for long-term storage and thawed for in vitro cultivation, experimentation, or animal infection. It is very similar to the protocol for cryopreservation of other eukaryotic cells (APPENDIX A.3G).
Materials
Mid-logphase cultures of Leishmania promastigotes (ATCC; see Support Protocol 1, steps 1 and 2) or freshly isolated amastigotes (see Support Protocol 3)
RPMI 1640 medium, 4°C
2× freezing medium (see recipe)
70% ethanol
Complete medium 199 (C-M199; see recipe)
Freezing container (e.g., Nalgene Mr. Frosty or microprocessor controlled), prechilled with the outer chamber filled with isoproponol
75-cm2 tissue culture flask, sterile
Freeze parasite stabilates
- Prepare freshly isolated amastigotes (5 × 107 in cold RPMI; see Support Protocol 3) or cultured promastigotes prepared from fresh amastigotes (see Support Protocol 1, steps 1 and 2). Harvest cultured promastigotes at mid-log phase (2 to 3 days) by centrifuging at 1500 × g, 4°C, for 10 min. Discard supernatant, gently break up cell pellet by flicking the tube, and add cold RPMI to achieve a concentration of 5 × 107/ml.A well-growing parasite strain will reach a concentration of 1 × 107 parasites/ml at mid-log phase, so that in most cases it is acceptable to freeze an estimated number of parasites by adding 2 ml of medium to the pellet from a 10-ml culture.
Add an equal volume of cold 2× freezing medium to the parasite suspension in a dropwise fashion while gently mixing.
Transfer 1 ml parasite/freezing medium suspension to a prelabeled cryovial (~2.5 × 107 parasites/vial) and place the cryovial on ice for ~15 min.
Transfer the cryovial to a prechilled freezing container and place overnight in a – 70°C freezer.
Transfer frozen parasites to a liquid nitrogen freezer.
Thaw parasite stabilates
-
6
When cryopreserved parasites are ready to be thawed, place 10 ml RPMI in a sterile tube and warm in a 37°C water bath.
-
7
Retrieve a cryovial containing a frozen parasite stabilate from the liquid nitrogen freezer and immediately transfer to the 37°C water bath. Thaw completely while gently swirling.
-
8
Wipe the outside of the thawed cryovial thoroughly with 70% ethanol.
-
9
Transfer the parasite suspension to a labeled, sterile 15-ml polypropylene centrifuge tube and add 10 ml warm RPMI in a dropwise fashion with gentle mixing.
-
10
Centrifuge at 3000 × g, 4°C or room temperature, for 15 min and discard the supernatant.
-
11Gently break the pellet by flicking the tube and resuspend the parasites in 10 ml CM199. Transfer to a 75-cm2 culture flask for culture.Thawed amastigotes can also be directly used for infection provided the viability of the frozen-thawed parasites is determined (see Support Protocol 3).
SUPPORT PROTOCOL 5
PREPARATION OF BLOOD AGAR PLATES
Materials
Rabbit blood (UNIT 1.7)
NNN medium (see recipe)
3- to 5-mm glass beads (Thomas)
43°C water bath
96-well flat-bottom plates, sterile with cover
- Defibrinate ~5 ml rabbit blood by swirling in a 100-ml sterile glass bottle containing ~5 ml sterile 3- to 5-mm glass beads. Store at 4°C for up to 3 or 4 weeks (the beads do not need to be removed).For simplicity, add the beads to the bottle and then sterilize by autoclaving.
Liquify 70 ml NNN medium in a microwave and cool to 43°C in a water bath.
Add 30 ml defibrinated blood prewarmed to 43°C. Mix thoroughly.
- Hold 96-well flat-bottom plates at a 70° to 80° angle and dispense 50 μl/well with the aid of a repeat pipettor.Dispensing the blood agar at a slant creates a window in each well, through which the plates can later be examined using an inverted microscope.
Cover the plates and seal with parafilm or place in humidified boxes. Store at 4°C for 6 to 8 weeks before use.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2; for suppliers, see APPENDIX 5.
Complete medium 199 (C-M199)
100 ml heat-inactivated FBS (APPENDIX 2)
5 ml 10 mM adenine (mol. wt. 171.6) in 50 mM HEPES acid
1 ml 0.25% (w/v) hemin in 50% (v/v) triethanolamine
5 ml 100× penicillin/streptomycin/
5 ML 100× L-glutamine
2 ML 0.05% biopterin in 0.01N NaOH
0.5 ML Biotin in 95% EtOH
13.2 ML 10xM199, Earle's salts (Life Technologies)
368.3 ML 1× M199 containing Hanks’ salts and 25 mM HEPES acid (Life Technologies)
Store up to 4 weeks at 4°C
Adenine and hemin stock solutions can be stored up to 6 months at 4°C.
Freezing medium, 2×
20 ml heat-inactivated FBS (APPENDIX 2)
15 ml dimethyl sulfoxide (DMSO)
65 ml RPMI 1640
Store up to 2 weeks at 4°C
NNN medium
Infuse 25.0 g Bacto Beef (Difco) and 500 ml distilled water in a 56°C water bath for 1 hr. Heat mixture in an autoclave for 5 min by running temperature of the autoclave up to the sterile range and back down immediately (this partially breaks down the Bacto Beef). Filter and then add 10.0 g neopeptone (Difco), 10.0 g Bacto Agar (Difco), and 2.5 g NaCl. Adjust to pH 7.2 to 7.4 with 3 M NaOH and autoclave 20 min. Dispense 70-ml aliquots into 100-ml sterile glass bottles. Cool and keep up to 1 year at 4°C.
COMMENTARY
Background Information
The majority of leishmanial infections are zoonotic in origin, and in many cases their natural reservoirs include a variety of desert, savannah, and forest rodents. Thus it is not surprising that laboratory rodents have been found to be permissive hosts for most species of Leishmania (Hommel et al., 1995). For Leishmania species believed to be anthroponotic in origin (e.g., L. donovani, L. tropica), or for which nonrodent species such as dogs represent their natural reservoirs (e.g., L. infantum / chagasi), laboratory rodents have still been found to be permissive hosts provided that a high dose and/or systemic route of parasite inoculation is used. The disease patterns produced in experimental animals infected with Leishmania differ widely according to both the rodent species and the Leishmania species used, ranging from complete refractivity to acute fatal susceptibility. Thus, these animals provide excellent models for the spectrum of leishmanial disease that occurs in humans, and can be used to explore the immunological parameters associated with these variable disease outcomes (e.g., cutaneous versus visceral, localized versus disseminated, healing versus nonhealing).
Table 19.2.2 summarizes the disease outcomes following infection of inbred mouse strains and golden hamsters with different strains of Leishmania. After subcutaneous inoculation of different inbred mouse strains with L. major, almost the entire spectrum of disease seen in human leishmaniasis can be observed in mice. BALB/c mice exhibit progressive, ulcerating lesions at the site of inoculation and fatal visceralization (Kellina, 1965). Other mouse strains (e.g., CBA, C3H, C57BL) are relatively resistant to infection, developing only small lesions at the site of inoculation that heal after a few weeks (Preston and Dumonde, 1976). Mice with healed primary lesions are immune to further challenge with virulent parasites, and represent the gold-standard of acquired resistance against which the efficacy of experimental vaccines can be measured. In the case of mice infected with L.mexicana and L. amazonensis, (Perez et al., 1979), BALB/c mice develop progressive lesions that tend to be less ulcerating compared with L. major, while in C57Bl/6 mice their primary lesions are controlled but are unable to heal (Alexander & Kaye, 1985), and may even develop late metastatic disease in distal sites (Barral et al., 1983). Mice are in general poor or nonpermissive hosts for species of the subgenus Viannia (L. braziliensis, L. panamensis, L. guyanensis), which are responsible for most of cutaneous and mucocutaneous disease in the Americas, although high doses of some strains can be used to produce small, persisting lesions in BALB/c mice (Castilho et al, 2010). In contrast, hamsters are highly susceptible to these species, producing ulcerating, chronic cutaneous lesions (Rey et al., 1990).
Table 19.2.2.
Expected Disease Outcomes in Selected Inbred Mouse Strains and Golden Hamsters Infected with Cutaneous or Visceral Strains of Leishmania
| Host | L. MAJORa | L. MEXICANAa | L. BRAZILIENSISa | L. DONOVANIb |
|---|---|---|---|---|
| BALB/c | Progressive | Progressive | No/small lesion | Acute susceptibility/noncure |
| DBA/1 | Chronic | ND | ND | Acute susceptibility |
| DBA/2 | Chronic | No lesion | ND | Acute resistance |
| CBA | Healing | ND | ND | Acute resistance |
| C3H | Healing | Chronic | ND | Acute resistance |
| A/J | Healing | Healing | ND | Acute resistance |
| AKR | Healing | ND | ND | Acute resistance |
| NZB | Healing | ND | ND | Acute resistance |
| C57BL | Healing | Chronic | ND | Acute susceptibility/cure |
| Hamster | Chronic | Chronic | Chronic | Progressive |
Subcutaneous or intradermal infection with 104 to 107 parasites. ND, not determined.
Intravenous infection with 106 to 107 parasites.
Experimental visceral leishmaniasis (VL) has been studied in both the mouse and hamster models. The murine model can be easily manipulated to define mechanisms of disease, but has the disadvantage of lacking many of the clinicopathological features observed in active human VL. Resistance and susceptibility in inbred mouse strains is not related to a strict TH1/TH2 dichotomy as in the murine model of cutaneous leishmaniasis caused by L. major. The mouse model of VL is probably best utilized for study of the immunopathogenesis of acute infection and the immunology of chronic subclinical infection. Acutely susceptible mice (such as BALB/c or C57BL6) develop ~100-fold increases in hepatic parasite burdens within 2 weeks of infection; resistant mice (such as DBA/2 or C3H/He) show a <10-fold increase in parasite burden at 2 weeks and subsequently self-heal (Bradley, 1977). In acutely susceptible strains, acquired resistance is associated with the development of parasite-specific cell-mediated immune responses under the control of the major histocompatibility locus (Blackwell et al., 1980). Even in susceptible strains that do not heal, however, the chronic infections are not progressive and are associated with minimal pathology. The Syrian golden hamster, introduced into the laboratory by Adler and colleagues in 1930 (Adler, 1989), closely mimics the clinicopathologic features of active human disease. The visceral parasitism is uncontrolled, and results in progressive cachexia, hepatosplenomegaly, pancytopenia, hypergammaglobulinemia, and ultimately death (Pearson et al., 1990). The model is difficult to study, however, because there are few immunological reagents available, although mRNA expression profiling of relevant immune response genes can be used (Melby et al, 1998a).
By studying disease phenotypes in genetically defined inbred, recombinant inbred, and backcross mice, it has been possible to map susceptibility genes into five different regions of the genome (Blackwell, 1996). The genes include: Scl-2, controlling a no-lesion resistance phenotype to L. mexicana; Scl-1, controlling healing versus nonhealing phenotype to L. major; the TH2 cytokine gene cluster, controlling later stages of L. major infection; the MHC complex, controlling healing of visceral infections with L. donovani and L. infantum; and SLC11A1 (formerly NRAMP1), the positionally cloned murine macrophage innate resistance gene (Vidal et al, 1993), controlling the acute resistance to L. donovani and L. infantum.
In addition to genetic background, the course and outcome of disease in these murine models can be influenced by the dose of parasites, the route and site of inoculation, and intraspecies differences in parasite virulence. For example, C57Bl/6 mice that represent the classic experimental model to study acquired resistance to L. major fail to heal some strains of L. major (Anderson et al, 2005). Even within a given strain of L. major, clonal differences in virulence have been observed (Mitchell et al, 1984). The site of parasite inoculation was found to markedly influence the course of L. major cutaneous infection in genetically resistant strains. In both SWR and CB6F1 mice, inoculation into the footpad produced healing lesions, whereas inoculation into the skin at the base of the tail produced progressive, nonhealing lesions (Nabors and Farrell, 1994). Similarly, when normally resistant C57BL/6 mice were inoculated intravenously, they developed multiple nonhealing cutaneous lesions (Scott and Farrell, 1982). Sub-cutaneous high dose inoculation in the footpad remains a favored model for infection using cutaneous strains despite the fact that natural infections are initiated by deposition by infected sand flies of low numbers of parasites in the skin. The inflammatory cells recruited to the skin injection site have recently been shown to better support the initial establishment of infection as compared to the footpad (Ribeiro-Gomes et al., 2014). In the standard VL models, visceral parasitism is produced by systemic infection (i.v. inoculation in the mouse and intracardial inoculation in the hamster), unlike natural inoculation in the skin by the infected sand fly. Dissemination of L. infantum from the inoculation site in the ear dermis to the liver and spleen was observed as early as 1 week after infection, though the dissemination required a high dose inoculum (>2 million metacyclic promastigotes; Ahmed et al, 2003) and the visceral parasite burdens established were far lower compared to IV infection. By contrast, the full course of progressive, fatal VL in hamsters can be initiated by intradermal inoculation of as few as 105 L. infantum promastigotes (Gomes et al, 2008) or by the bites of L. infantum or L. donovani infected sand flies (Aslan et al, 2013). The severity of disease in the hamster caused by L. donovani also varies from strain to strain, with the size of the inoculum, and the frequency of sub-culture (Melby et al., 1998b; Giannini, 1974). Juvenile hamsters appear to be more susceptible than adult animals (Giannini, 1974) but the reason for the age-related susceptibility is unknown.
The comparison of in vivo virulence between different strains or clones, and even between the same strain or clone used for infection in different experiments, clearly requires that identical developmental stages of the parasite be used to initiate infection. This has not always been the case, particularly with inocula comprised of promastigotes. It is by now well established that extracellular promastigotes, whether obtained from vector sand flies or from axenic culture, are not uniform with respect to their ability to initiate infection in their mammalian hosts. During growth in the sand fly vector and in axenic culture, Leishmania promastigotes undergo differentiation from a dividing noninfective stage to a resting infective or metacyclic stage that is uniquely adapted for life in vertebrates (Sacks, 1989). Metacyclic promastigotes display markedly enhanced resistance to both complement-mediated and intramacrophage killing mechanisms, which presumably accounts for the ability of a small number of parasites inoculated by the fly to survive and initiate infection within a susceptible vertebrate host. The stage-specific molecules that control metacyclic survival have been described in detail for both L. major and L. donovani (McConville et al., 1992; Sacks et al, 1995). Metacyclogenesis is accompanied by structural modifications of lipophosphoglycan (LPG), which is the major surface molecule on all species of Leishmania promastigotes studied to date. The modifications are of two sorts: (1) a two- to threefold increase in size due to an increase in the number of phosphorylated saccharide repeat units expressed, and (2) a change in the nature of the terminally exposed sugars of some of these units. The downregulation of terminally exposed galactose residues on metacyclic LPG of L. major appears to permit the selective release of infective-stage organisms from adhesion receptors on the sand fly midgut, so as to make them available for subsequent transmission by bite (Sacks & Kamhawi, 2001).
While it is true that metacyclic promastigotes are normally found in stationary phase cultures, the proportion of metacyclics generated during growth can vary tremendously, from a majority of stationary cells to zero. This variability is not understood, but seems related to culture history and growth conditions. Fast-growing strains appear to differentiate at a higher rate, but even among fast-growing strains, the longer promastigotes are maintained in culture, the less they are able to differentiate into metacyclic forms during growth. The tendency for Leishmania promastigotes to become attenuated as a consequence of prolonged serial culture is well described. Thus, differences in virulence between strains or clones might simply be an artifact of the different numbers of metacyclic promastigotes contained in the inocula used. Inocula comprised of purified metacyclic promastigotes, or else defined with regard to the proportion of metacyclics, must be used for comparison of virulence between strains and between experiments.
The structural modifications in the surface LPG make it possible for metacyclic promastigotes to be identified and purified from stationary cultures of most strains of L. major based on their loss of agglutination with the lectin peanut agglutinin (PNA). Similarly, PNA and/or Concanavalin A (ConA) have been used to purify metacyclic promastigotes from stationary cultures of some strains of L. donovani and L. infantum/chagasi. Because LPG exhibits considerable interspecies polymorphisms in the nature of the terminally exposed sugars expressed (McConville et al., 1995), neither PNA nor other lectins examined have been able to discriminate between developmental stages of other species (e.g., L. tropica., L. mexicana, L. amazonensis). For these strains, stage-specific monoclonal antibodies have been generated that can be used in an identical negative selection protocol as the lectin-mediated agglutination protocol to enrich for metacyclic stage promastigotes (see Table 19.2.1). Because metacyclic promastigotes display a distinct size and morphology that is shared by most Leishmania species and strains, they exhibit different sedimentation properties that can be used to obtain highly enriched metacyclic promastigotes populations that does not depend on the availability of specific lectins or antibodies required for the negative selection protocol.
The evaluation of disease progression is best accomplished by enumeration of viable parasites in the primary footpad lesion and/or in visceral sites (e.g., draining lymph node, spleen, liver). For cutaneous lesions, measurement of footpad swelling generally correlates well with the number of viable organisms contained in the lesion. There are, however, enough examples where this correlation does not hold up that quantitation of the parasite load is routinely warranted. Once footpad lesions begin to ulcerate, the dimensions of the infected tissue changes substantially, and may not correlate with parasite number. In addition, in mice with acquired resistance, the killing of L. major occurs much earlier than is suggested by the reduction in lesion size (Hill et al, 1983; Titus et al., 1985). In immune animals, these authors noted that footpad swelling following challenge can occur without an increase in parasite numbers. Finally, these studies also confirmed that low numbers of parasites persist in the inoculation site long after the lesion appears to completely resolve. The quantitation of parasites in visceral organs is critical, not just to evaluate the course of disease in animals infected with visceral strains, but for cutaneous strains as well, because systemic spread of the parasite has been shown to have profound effects on the immune response.
Critical Parameters and Troubleshooting
Preparation of Metacyclic Promastigotes
Promastigotes grown in liquid media are the most convenient source of parasites for inoculation. For some strains of Leishmania, they may be the only source because it may not be possible to recover sufficient numbers of parasites from infected tissue to prepare amastigote inocula. Furthermore, the preparation of amastigote inocula may not be practical when many different strains are to be compared. In such cases, promastigotes will have to be grown, harvested, and characterized with respect to the number of infectious metacyclic forms that they contain. Because metacyclogenesis can be adversely affected by the frequency of promastigote subculture, the cells used to initiate the promastigote cultures should be either frozen or freshly harvested tissue amastigotes (only low numbers of amastigotes are required to initiate a promastigote culture), or else frozen stabilates prepared from an early passage of promastigotes. It is strongly advised not to maintain promastigotes in serial culture for more than a few passages.
The choice of media for generation of metacyclic promastigotes by axenic cultivation of Leishmania remains empirical. An acidic pH of 5.5 was shown to facilitate metacyclogenesis of a number of different strains (Zakai et al., 1998). and this manipulation might be considered if certain strains yield low numbers of metacyclics. It has also been shown that the depletion of nucleosides, especially adenosine, is a nutrient stress condition that triggers stage differentiation, and addition of an adenosine-receptor antagonist increased the number of metacyclics that could be recovered during growth (Serafim et al, 2012). The medium described in this unit (C-M199) is recommended based on its ability to support the high-density growth of most Leishmania species in liquid culture. In the authors’ experience, high-density growth (e.g., 0.4–1 × 108/ml) is associated with the generation of a higher percentage of metacyclics. Other basic media that have been used for promastigote cultivation include RPMI 1640, and Schneider's and Grace's insect media. Of the additives in C-M199, only the FBS is apt to be critical. New lots of FBS should be screened for their ability to support high-density promastigote growth. While a serum-free medium may support the growth of some Leishmania strains, and may be desirable because of its low cost and reproducibility, it may not support differentiation to metacyclic promastigotes.
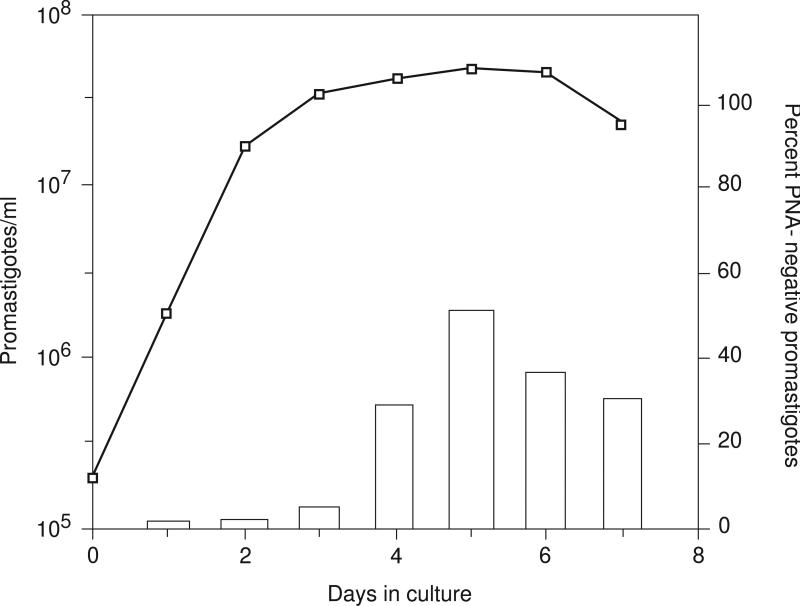
The time in culture that will optimize the purity or yield of metacyclic cells may vary according to strain and growth conditions. If the yield or purity of metacyclics is poor, the growth profiles of the strain in question may need to be determined and the selection procedure carried out on stationary populations harvested from different days in culture. A typical L. major growth curve and the percent metacyclics recovered by PNA treatment of promastigotes obtained during each day in culture is shown in Figure 19.2.4. This particular L. major strain (Friedlin/NIH), cultured from amastigotes in C-M199, yields ~30% metacyclic promastigotes by day 5. Efficient and rapid agglutination of the nonmetacyclic cells using PNA requires that they remain actively motile during the agglutination procedure; thus, if cells are harvested from late stationary cultures in which many cells have begun to die, the metacyclics may be contaminated with dead or dying cells.
Figure 19.2.4.
Growth curve of L. major promastigotes (Friedlin/NIH) in CM199, and the percent PNA-negative metacyclic promastigotes (bar graph) obtained during each day of growth.
The purity of the selected metacyclic promastigotes can be verified by staining the cells on a slide using Giemsa or LeukoStat stain and examining the cells under oil. Metacyclic promastigotes are highly motile and have a distinct and uniform morphology: slender cell body (14 to 16 μm long; 1.1 to 1.3 μm wide) with a tapered anterior end, and an elongated flagellum twice the length of cell body. Figure 19.2.2A shows a light micrograph of L. major metacyclic promastigotes purified from stationary culture using PNA.
Preparation of Tissue Amastigotes
For those Leishmania strains that differentiate poorly to metacyclic promastigotes in vitro, the problems inherent in standardizing promastigote inocula can be avoided by using amastigotes harvested from infected tissue. While this may be an unnatural way to initiate infection in the mammalian host, the advantage is that tissue amastigotes, regardless of strain, represent comparable developmental forms, and virulence comparisons between strains can therefore be legitimately made. Furthermore, inocula prepared from freshly harvested or frozen stabilates of tissue amastigotes should behave reproducibly from experiment to experiment. The procedure described for harvesting amastigotes from infected footpad or spleen yield parasites that are sufficiently clean that the small amount of contaminating host cell material should have little or no impact on the behavior of the inoculum. If cleaner material is desired (e.g., for biochemical or molecular biological studies), the amastigotes can be further purified on a discontinuous gradient of metrizamide (Saraiva et al., 1983) or more rapidly by sequential filtration through polycarbonate filters (Glaser et al., 1990).
High numbers of amastigotes (up to 108) can be harvested from a single footpad lesion of a BALB/c mouse infected with any of a number of cutaneous strains including L. major, L. mexicana, and L. amazonensis.. The footpad lesions should be harvested prior to the onset of ulceration to avoid bacterial contamination of the excised material. For typical L. major infections, ulceration can begin as early as 4 weeks, while for New World cutaneous strains, the tissue response to progressive infection tends to be much less destructive. Because growth of visceral strains (e.g., L. donovani, L. infantum/chagasi) is nonprogressive even in genetically susceptible mouse strains, the number of organisms that can be obtained from mouse spleen is limiting and may not be sufficient to yield a high-dose inoculum or to prepare frozen stabilates of amastigotes for future use. In contrast, these strains tend to produce uncontrolled visceral infections in hamsters, and 109 to 1010 splenic amastigotes can easily be recovered from a moribund animal.
Cryopreservation
The use of frozen stocks as opposed to freshly harvested amastigotes is of obvious practical advantage. In addition, there may be a rationale for minimizing the frequency of in vivo passage, insofar as this might alter the virulence phenotype of the infecting strain. The only disadvantage with the use of frozen stocks of amastigotes is that there is generally some parasite mortality associated with the freeze-thaw procedure (50% is not uncommon). This is not a serious problem so long as the viability of the cells is assessed after thawing. Since amastigotes lack motility, vital stains needs to be used. However, amastigotes stained with colored dyes such as trypan blue or eosin Y can be difficult to evaluate because of their small size. A simultaneous double-staining method using fluorescein diacetate (FDA) and propidium iodide (PI) is recommended. Viable cells stain bright green, while nonviable cells stain bright red (Jones and Senft, 1985).
Parasite Dose
The typical dose range for the manifestation of the established murine susceptibility and resistance phenotypes following subcutaneous infection with L. major is 105 to 107 parasites. There is evidence that these phenotypes will be maintained over a much broader dose range. Progressive disease has been observed in BALB/c mice with as few as 20 promastigotes (Howard et al., 1980), although in other studies using different L. major strains, 100 promastigotes failed to produce a primary lesion and the animals were resistant to reinfection with a high-dose inoculum (Bretscher et al., 1992). Low-dose infection (100 metacyclics) in resistant C57Bl/6 mice initiates sustained parasite growth in the footpad (up to 106/mg of tissue) that is associated with only minimal footpad swelling before the lesion begins to heal (Lira et al, 2000). Since infection by the sand fly vector is initiated by deposition of <10,000 metacyclic promastigotes in the skin, intradermal inoculation of 102-104 metacyclic promastigotes in the ear has become a favored infection model to try to mimic some of the key conditions of natural transmission (Belkaid et al, 2000). In the hamster, intracardial inoculation of several million parasites resulted in death in ~6 weeks, whereas inoculation of several hundred parasites was not lethal until 6 months postinfection (Stauber, 1966).
Assessment of Disease Outcome
For cutaneous infections, the measurement of footpad swelling is relatively straight forward. The most accurate measure incorporates both width and thickness. A comparison with the uninfected contralateral footpad is often included, although this comparison is not critical, especially if a baseline footpad measurement has been obtained and sequential measurements are made by the same investigator. Once a lesion begins to ulcerate it becomes more difficult to obtain a consistent measurement, and footpad dimensions no longer correlate well with numbers of viable parasites. For infections in the ear dermis, measurement of the induration in two diameters is relatively straightforward.
For visceral infections, the impression smear has been a standard method for enumerating L. donovani in infected organs. A light micrograph of a stained imprint from an infected hamster spleen is shown in Figure 19.2.2B. However, the technique cannot detect <100,000 parasites in an organ and does not directly distinguish living from dead organisms (Hill and Fahety, 1987). In addition, the impression smear cannot easily be used to enumerate parasites in footpad lesions because it is difficult to express the value in terms of a unit of organ or tissue weight. Nonetheless, the impression smear remains a reliable, straightforward assay for estimating parasite numbers in liver and spleen when the infection in these organs is well established. In other cases, more-sensitive limiting dilution assays need to be employed, which depend on the efficiency of the media to support the low-density growth of the parasite. Low-density growth has been shown to be optimal in the presence of rabbit blood agar (pating efficiencies of 61% to 81% and 58% to 149% have been described; Hill et al., 1983; Titus et al., 1985). Whereas the biphasic media has been shown to be optimal for low density parasite growth, monophasic media containing only C-M199 may produce comparable end point titrations, depending on the suitability of the growth medium, and in particular the serum component, for the strain in question. Direct comparison of the biphasic and monophasic media for end point titrations is recommended to determine if the liquid medium alone can be used.
In carrying out the limiting dilution assay for parasite quantitation, the critical choices in the method pertain to the dilution factor used and the number of serial dilutions needed to dilute the parasites to extinction. For early time points following infection in BALB/c mice with a cutaneous species, and throughoutthe course of infection in resistant strains, serial two- to threefold dilutions to 1:106 should be appropriate. For later stages of cutaneous infection in BALB/c mice, log-fold dilutions to 1:1010 may be required. For a more precise estimate of parasite numbers in infected tissue, the tissue dilutions should be plated in at least 16 replicate cultures in order to apply the Poisson probability equation, which will describe the relation between the average number of cells tested per replicate culture and the number of negatively responding cultures per group (Taswell, 1981). This method is only practical when the dilution of the tissue that will begin to yield negative cultures is known beforehand; otherwise, a high number of replicate cultures of a high number of serial dilutions is required, which may not be possible when a large series of infected tissues are being assayed.
An alternative to the relatively labor intensive limiting dilution assays is the measurement of parasite nucleic acid in the tissue. Parasite kDNA (Abassi et al., 2013; Jara et al., 2013; Nicolas et al., 2002) and RNA (Van der Meide et al., 2008; Romero et al., 2010) have been used to quantify parasite burden in blood and tissues in humans and experimental models. Quantification of parasite kDNA reflects the number of viable parasites (Prina et al., 2007), however some studies suggest that Leishmania DNA is more slowly degraded than RNA in non-viable parasites (van der Meide et al. 2008; Romero et al., 2010; van den Bogaart et al., 2014). Therefore, quantification of an RNA target may be advantageous when a short time course is being studied. For assessment of parasite load in the more chronic situation, kDNA is an ideal target because its abundance (~10,000 copies per cell) allows for detection of a low number of parasites. The parasite load is best expressed as number of parasites per tissue mass (ear, spleen or lymph node), volume (blood), or number of host cells (bone marrow or splenic aspirate).
Anticipated Results
The yield of metacyclics from cultured promastigotes is extremely species and strain dependent. For L. major strains inoculated into culture as amastigotes or early passage promastigotes, the yield of can range from 5% to 50%. The yield of metacyclics from L. donovani strains tends to be <10% and quickly diminishes with frequency of subculture.
The time course of lesion development is also strain (and dose) dependent (see Time Considerations). The parasite load in infected footpads at the time of ulceration can reach 107 to 108 amastigotes per mg of tissue, and two-fold serial dilutions of ~24 wells may be required to dilute the infected tissue to extinction. In mice and hamsters inoculated intravenously with 107 L. donovani amastigotes or metacyclic promastigotes, established infections in liver and spleen can be detected by 10 to 14 days. Fatal infections in hamsters will occur by 6 to 10 weeks.
Time Considerations
The purification of lesional amastigotes takes 2 to 3 hr; the purification of metacyclic promastigotes by PNA or density gradient centrifugation takes 1 to 2 hr. The processing of infected tissue for limiting dilution analysis requires 1 to 2 hr. The plates are read after 7 to 10 days.
The time course of lesion development is strain and dose dependent, but for typical cutaneous strains using an inoculum of 105 metacyclics, lesions will begin to appear by 2 to 3 weeks. In BALB/c mice, L. major footpad lesions will progress and begin to ulcerate by 5 to 6 weeks, while in resistant mice, the lesions will begin to resolve by 5 to 6 weeks and return to baseline by 8 to 12 weeks.
Footnotes
Contributed by David L. Sacks
National Institute of Allergy & Infectious Diseases
Bethesda, Maryland
Peter C. Melby
University of Texas Medical Branch (UTMB)
Galveston, Texas
Literature Cited
- Abbasi I, Aramin S, Hailu A, Shiferaw W, Kassahun A, Belay S, Jaffe C, Warburg A. Evaluation of PCR procedures for detecting and quantifying Leishmania donovani DNA in large numbers of dried human blood samples from a visceral leishmaniasis focus in Northern Ethiopia. BMC Infect Dis. 2013;13:153–160. doi: 10.1186/1471-2334-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler JH. The origin of the golden hamster as a laboratory animal. Isr. J. Med. Sci. 1989;25:206–209. [PubMed] [Google Scholar]
- Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infection and immunity. 2003;71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Kaye PM. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clinical and experimental immunology. 1985;61:674–682. [PMC free article] [PubMed] [Google Scholar]
- Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. Journal of immunology. 2005;174:2934–2941. doi: 10.4049/jimmunol.174.5.2934. [DOI] [PubMed] [Google Scholar]
- Aslan H, Dey R, Meneses C, Castrovinci P, Jeronimo SM, Oliva G, Fischer L, Duncan RC, Nakhasi HL, Valenzuela JG, Kamhawi S. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. The Journal of infectious diseases. 2013;207:1328–1338. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral A, Peterson E, Sacks DL, Neva FA. Late metastatic disease in the mouse. A model for mucocutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 1983;32:277–284. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- Blackwell JM. Genetic susceptibility to leishmanial infections: Studies in mice and man. Parasitology. 1996;112:S67–S74. [PubMed] [Google Scholar]
- Blackwell J, Freeman J, Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980;283:72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley DJ. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin. Exp. Immunol. 1977;30:130–140. [PMC free article] [PubMed] [Google Scholar]
- Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes susceptible mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Castilho TM, Goldsmith-Pestana K, Lozano C, Valderrama L, Saravia NG, McMahon-Pratt D. Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease. European journal of immunology. 2010;40:2816–2829. doi: 10.1002/eji.201040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret N, Prina E, Mougneau E, Saraiva EM, Sacks DL, Glaichenhaus N, Antoine JC. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 1999;29:762–773. doi: 10.1002/(SICI)1521-4141(199903)29:03<762::AID-IMMU762>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Doyle PS, Engel JC, Pimenta PF, da Silva PP, Dwyer DM. Leishmania donovani: Long-term culture of axenic amastigotes at 37 degrees C. Exp. Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- Eperon S, McMahon-Pratt D. I. Extracellular cultivation and morphological characterization of amastigote-like forms of Leishmania panamensis and L. braziliensis. J. Protozool. 1989;36:502–510. doi: 10.1111/j.1550-7408.1989.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Giannini MS. Effects of promastigote growth phase, frequency of subculture, and host age on promastigote-initiated infections with Leishmania donovani in the golden hamster. J. Protozool. 1974;21:521–527. doi: 10.1111/j.1550-7408.1974.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Glaser TA, Wells SJ, Spithill TW, Pettitt JM, Humphris DC, Mukada AJ. Leishmania major and Leishmania donovani: A method for rapid purification of amastigotes. Exp. Parasitol. 1990;71:343–345. doi: 10.1016/0014-4894(90)90039-f. [DOI] [PubMed] [Google Scholar]
- Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, de Oliveira CI, Miranda JC, Elnaiem DE, Kamhawi S, Valenzuela JG, Brodskyn CI. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Fahety JR. Leishmania spp.: Agar plating as an alternative approach to limiting dilution and impression smears for the enumeration of viable parasites in tissue. Exp. Parasitol. 1987;63:108–111. doi: 10.1016/0014-4894(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Hill JO, North RJ, Collins FM. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect. Immun. 1983;39:1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel M, Jaffe CL, Travi B, Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann. Trop. Med. Parasitol. 1995;89:55–73. doi: 10.1080/00034983.1995.11813015. [Thorough review of animal models for cutaneous and visceral leishmaniasis.] [DOI] [PubMed] [Google Scholar]
- Howard JG, Hale C, Chan-Liew WL. Immunological regulation of experimental cutaneous leishmaniasis. I. Immunological aspects of susceptibility to Leishmania tropica (major) in mice. Parasite Immunol. 1980;2:303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Jara M, Adaui V, Valencia BM, Martinez D, Alba M, Castrillon C, Cruz M, Cruz I, Van der Auwera G, et al. Real-time PCR assay for detection and quantification of Leishmania (Viannia) organisms in skin and mucosal lesions: exploratory study of parasite load and clinical parameters. J Clin Microbiol. 2013;51:1826–33. doi: 10.1128/JCM.00208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Joshi PB, Sacks DL, Modi G, McMaster WR. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63). Mol. Microbiol. 1998;27:519–530. doi: 10.1046/j.1365-2958.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- Kellina OI. A comparative study of the virulence of L. tropica major strains. Med. Parazitol. Parazit. Bolezni. 1965;34:309–316. [PubMed] [Google Scholar]
- Lira R, Mendez S, Carrera L, Jaffe C, Neva F, Sacks D. Leishmania tropica: the identification and purification of metacyclic promastigotes and use in establishing mouse and hamster models of cutaneous and visceral disease. Exp. Parasitol. 1998;89:331–342. doi: 10.1006/expr.1998.4283. [DOI] [PubMed] [Google Scholar]
- Lira R, Doherty M, Modi G, Sacks D. Evolution of lesion formation, parasitic load, immune response, and reservoir potential in C57BL/6 mice following high- and low-dose challenge with Leishmania major. Infection and immunity. 2000;68:5176–5182. doi: 10.1128/iai.68.9.5176-5182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry. 1999;38:9813–9823. doi: 10.1021/bi990741g. [DOI] [PubMed] [Google Scholar]
- McConville M, Turco S, Ferguson M, Sacks D. Developmental modification of lipophosphoglycan: during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Schnur LF, Jaffe C, Schneider P. Structure of Leishmania lipophosphoglycan Inter- and intra-specific polymorphism in Old World species. Biochem. J. 1995;310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby PC, Tryon VV, Chandrasekar B, Freeman GL. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. Infection and immunity. 1998a;66:2135–2142. doi: 10.1128/iai.66.5.2135-2142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby PC, Yang Y-Z, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect. Immun. 1998b;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Handman E, Spithill TW. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust. J. Exp. Biol. Med. Sci. 1984;62:145–153. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- Nabors GS, Farell JP. Site-specific immunity to Leishmania major in SWR mice: The site of infection influences susceptibility and the expression of the antileishmanial immune response. Infect. Immun. 1994;62:3655–3662. doi: 10.1128/iai.62.9.3655-3662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas L, Prina E, Lang T, Milon G. Real-time PCR for detection and quantitation of leishmania in mouse tissues. J Clin Microbiol. 2002;40:1666–9. doi: 10.1128/JCM.40.5.1666-1669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A. Leishmania mexicana: Serial cultivation of intracellular stages in a cell-free medium. Exp. Parasitol. 1984;58:72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- Pearson RD, Cox G, Evans T, Smith DL, Weidel D, Castracane J. Wasting and macrophage production of tumor necrosis factor/cachectin and interleukin 1 in experimental visceral leishmaniasis. Am. J. Trop. Med. Hyg. 1990;43:640–649. doi: 10.4269/ajtmh.1990.43.640. [DOI] [PubMed] [Google Scholar]
- Perez H, Labrador F, Torealba JW. Variations in the response of five strains of mice to Leishmania mexicana. Int. J. Parasitol. 1979;9:27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Pinto-da-Silva LH, Camurate M, Costa KA, Oliveira SM, da Cunha-e-Silva NL, Saraiva EM. Leishmania (Viannia) braziliensis metacyclic promastigotes purified using Bauhinia purpurea lectin are complement resistant and highly infective for macrophages in vitro and hamsters in vivo. Int. J. Parasitol. 2002;32:1371–1377. doi: 10.1016/s0020-7519(02)00137-6. [DOI] [PubMed] [Google Scholar]
- Preston PM, Dumonde DC. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infections in the mouse. Clin. Exp. Immunol. 1976;23:126–138. [PMC free article] [PubMed] [Google Scholar]
- Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes Infect. 2007;9:1307–15. doi: 10.1016/j.micinf.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Rey JA, Travi B, Valencia AZ, Saravia NG. Infectivity of the subspecies of the Leishmania braziliensis complex in vivo and in vitro. Am. J. Trop. Med. Hyg. 1990;43:89–193. doi: 10.4269/ajtmh.1990.43.623. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Roma EH, Carneiro MBH, Sacks DL, Peters NC. Site dependent recruitment of inflammatory cells determines the effective dose of Leishmania major. Infect. Immun. 2014 doi: 10.1128/IAI.01600-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero I, Tellez J, Suarez Y, Cardona M, Figueroa R, Zelazny A, Saravia N. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl Trop Dis. 2010:4–12. doi: 10.1371/journal.pntd.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- Sacks DL. Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol. 1989;69:100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Pimenta PF, McConville MJ, Schneider P, Turco SJ. Stage-specific adhesion of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. J. Exp. Med. 1995;181:685–697. doi: 10.1084/jem.181.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [Reviews promastigote developmental biology and explains the basis for metacyclic identification and purification..] [DOI] [PubMed] [Google Scholar]
- Saraiva EMB, Pimenta P, Pereira M, DeSouza W. Isolation and purification of amastigotes of Leishmania mexicana amazonensis by a gradient of metrizamide. J. Parasitol. 1983;69:627–629. [PubMed] [Google Scholar]
- Scott PA, Farrell JP. Experimental cutaneous leishmaniasis: Disseminated leishmaniasis in genetically susceptible and resistant mice. Am. J. Trop. Med. Hyg. 1982;31:230–238. doi: 10.4269/ajtmh.1982.31.230. [DOI] [PubMed] [Google Scholar]
- Serafim TD, Figueiredo AB, Costa PA, Marques-da-Silva EA, Goncalves R, de Moura SA, Gontijo NF, da Silva SM, Michalick MS, Meyer-Fernandes JR, de Carvalho RP, Uliana SR, Fietto JL, Afonso LC. Leishmania metacyclogenesis is promoted in the absence of purines. PLoS neglected tropical diseases. 2012;6:e1833. doi: 10.1371/journal.pntd.0001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares RP, Macedo ME, Ropert C, Gontijo NF, Almeida IC, Gazzinelli RT, Pimenta PF, Turco SJ. Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis. Mol. Biochem. Parasitol. 2002;121:213–224. doi: 10.1016/s0166-6851(02)00033-6. [DOI] [PubMed] [Google Scholar]
- Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- van den Bogaart E, Schoone GJ, Adams ER, Schallig HD. Duplex quantitative Reverse-Transcriptase PCR for simultaneous assessment of drug activity against Leishmania intracellular amastigotes and their host cells. Int. J. Parasitol. Drugs and Drug Resistance. 2014;4:14–9. doi: 10.1016/j.ijpddr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meide W, Guerra J, Schoone G, Farenhorst M, Coelho L, Faber W, Peekel I, Schallig H. Comparison between quantitative nucleic acid sequence-based amplification, real-time reverse transcriptase PCR, and real-time PCR for quantification of Leishmania parasites. J Clin Microbiol. 2008;46:73–8. doi: 10.1128/JCM.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Zakai HA, Chance ML, Bates PA. In vitro stimulation of metacyclogenesis in Leishmania braziliensis, L. donovani, L. major, and L. mexicana. Parasitology. 1998;116:305–309. doi: 10.1017/s0031182097002382. [DOI] [PubMed] [Google Scholar]