Abstract
The ability of antigen-specific T cells to simultaneously produce multiple cytokines is thought to correlate with the functional capacity and efficacy of T cells. These ‘polyfunctional’ T cells have been associated with control of HIV. We aimed to assess the impact of co-infection with Mycobacterium tuberculosis (MTB) on HIV-specific CD8+ and CD4+ T cell function. We assessed T cell functionality in 34 South African adults by investigating the IFN-y, IL-2, TNF-α, IL-21 and IL-17 cytokine secretion capacity, using polychromatic flow cytometry, following HIV Gag-specific stimulation of peripheral blood mononuclear cells. We show that MTB is associated with lower HIV-specific T cell function in co-infected as compared to HIV mono-infected individuals. This decline in function was greatest in co-infection with active Tuberculosis (TB) compared to co-infection with latent MTB (LTBI), suggesting that mycobacterial load may contribute to this loss of function. The described impact of MTB on HIV-specific T cell function may be a mechanism for increased HIV disease progression in co-infected subjects as functionally impaired T cells may be less able to control HIV.
Introduction
HIV and Tuberculosis (TB) are severe global dual-epidemics. Data suggest that co-infection with HIV and Mycobacterium tuberculosis (MTB) increases disease progression of both diseases[1]. For example, higher HIV viral loads are observed in MTB co-infection and increased HIV replication occurs in MTB infected macrophages [2, 3]. The high levels of inflammation and immune activation, as present in TB, may create an optimal cytokine milieu for HIV replication[4]. Whilst immunological impairment is likely to contribute to the increased morbidity and mortality associated with co-infection, the specific mechanisms remain largely unknown. Several studies have reported an impact of HIV on MTB-specific T cell immunity [5,6, 7]. For example, increased infection and lysis of MTB-specific T cells has been accredited to HIV infection [5, 6]. Day et al showed that HIV infection impairs MTB-specific responses in HIV co-infection with LTBI, demonstrating that the proportion of IL-2 secreting MTB-specific CD4+ T cells inversely correlated with HIV viral load [7].
The ability of antigen-specific T cells to simultaneously produce multiple cytokines is believed to correlate with the functional capacity and efficacy of T cells. Frequency of these ‘polyfunctional’ T cells in blood samples from infected subjects has been associated with clinical control of HIV and TB [8, 9]. For example, higher bacterial load has been shown to decrease MTB-specific T cell functionality and mono-functional T cells have been shown to dominate functionality profiles in TB as compared to LTBI [10]. Harari et al have reported that greater proportions of TNF-α single-positive CD4 T cells are present in individuals with active TB as compared with LTBI [9]. If and how MTB co-infection affects HIV-specific T cell function and polyfunctionality is unknown.
Methods
Participants and Study Samples
We enrolled 13 HIV positive individuals with active TB, 9 HIV positive individuals with latent MTB (LTBI), and 11 HIV positive individuals without evidence of LTBI or active TB (Table 1). All were chronically infected HIV positive South-African adults and were CD4 T cell count matched. Viral loads did not significantly differ between patient groups (p = 0.978). TB was identified by a positive sputum acid-fast bacillus smear or sputum culture. LTBI was defined as a positive ESAT-6/CFP-10 IFN-gamma ELISPOT, in the absence of signs and symptoms of TB [11]. Ethical approval and written informed consent from participants was obtained (University of KwaZulu-Natal Biomedical Research Ethics Committee: E028/99 and H020/06). Patients were anti-retroviral treatment naive and not receiving anti-TB treatment.
Table 1. Viral load and CD4 count information for study participants.
| HIV+ | HIV+/LTBI | HIV+/TB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID. | CD4 Count (cells/ul) | Viral Load | Sex | Patient ID. | CD4 Count (cells/ul) | Viral Load | Sex | Patient ID. | CD4+ count (cells/ul) | Viral Load | Sex |
| SK 010 B21 | 218 | 5822 | F | SK 141 B20 | 278 | 2676 | F | PID 1079 | 209 | 2900 | M |
| SK 114 B14 | 274 | 29100 | F | SK 195 B20 | 207 | 10077 | M | PID 1120 | 460 | 3700 | M |
| SK 139 B20 | 184 | 47547 | F | SK 236 B9 | 184 | 272227 | F | PID 1135 | 253 | 470000 | M |
| SK 142 B12 | 275 | 186858 | F | SK 351 B15 | 350 | 285891 | F | PID 1224 | 345 | 3850 | M |
| SK 208 B11 | 126 | 25523 | F | SK 359 B15 | 466 | 9449 | F | PID 1225 | 366 | 224137 | M |
| SK 212 B22 | 233 | 19670 | F | SK 364 B15 | 285 | 3140 | M | PID 821 | 110 | 20000 | F |
| SK 278 B17 | 443 | 26036 | F | SK 391 B13 | 247 | 1936 | F | PID 683 | 195 | 430000 | F |
| SK 324 B14 | 353 | 144252 | F | SK 397 B14 | 332 | 198266 | M | PID 863 | 204 | 49000 | M |
| SK 373 B15 | 184 | 58237 | F | SK 425 B13 | 192 | 101600 | F | PID 917 | 239 | 66000 | F |
| SK 410 B11 | 495 | 2570 | F | PID 929 | 270 | 540000 | M | ||||
| SK 444 B13 | 331 | 21447 | F | PID1024 | 239 | 88000 | M | ||||
| PID1046 | 267 | 17000 | M | ||||||||
Flow cytometry
We assessed T cell functionality using a multi-parameter flow cytometry panel: Viability marker, CD3, CD4, CD8, IFNγ, IL-2, TNF-α, IL-21 and IL-17. Intracellular cytokine staining (ICS) of peripheral blood mononuclear cells (PBMC) was performed following a 6 hour stimulation with either Staphylococcal enterotoxin B (SEB), an HIV Gag peptide pool, or an MTB-specific ESAT-6/CFP-10 peptide pool. FlowJo (version 8.3.3; Treestar) and GraphPad Prism (V.5.5) software were used to analyze the data. A positive antigen-specific response was defined as greater than or equal to 0.05% of the T cell subset analyzed, and 3 times above background.
Statistical analysis
GraphPad Prism (V.5.5) was used to perform all statistical analysis. Mann-Whitney test was used to compare continuous outcomes between two groups. For more than two groups comparison, Kruskall-Wallis test with Dunn’s post hoc analyses was used. F Fisher’s exact test was used to compare categorical outcomes (i.e., pie charts). All p values are two sided and a p-value<0.05 was considered significant.
Results
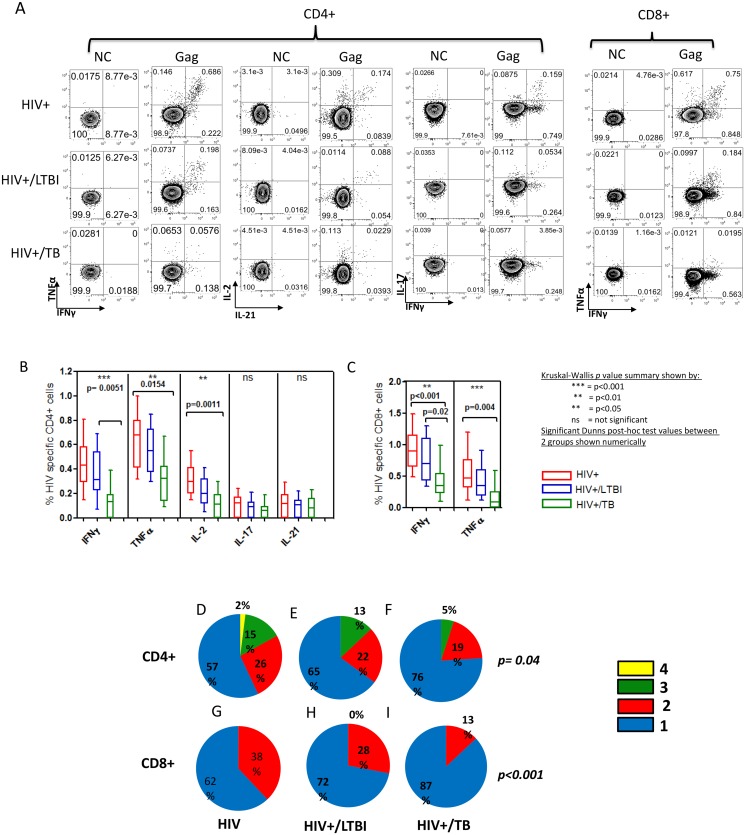
HIV-specific CD4 T cells were readily detectable in mono-infected individuals (Fig. 1A and 1B). HIV-specific CD4+ cell release of IFN-γ was significantly lower in HIV+/TB as compared to HIV+/LTBI (p = 0.005)(Fig. 1B). HIV-specific CD4+ cell release of TNF-α (p = 0.01) and IL-2 (p<0.001) were significantly lower in HIV+/TB as compared to HIV mono-infected individuals. In summary, HIV co-infection with MTB was associated with a decrease in the amount of cytokine (IFN-γ, TNF-α and IL-2) secreted for HIV-specific CD4+ T cells.
Fig 1. HIV specific CD4+ and CD8+ T cell functionality is reduced in HIV infected individuals co-infected with latent Mycobacterium tuberculosis infection (LTBI) and active Tuberculosis (TB) disease.
(A) Representative flow cytometry plots showing cytokine responses for HIV (gag), control (no antigen) for HIV mono-infected subjects, and subjects co-infected with latent MTB infection (LTBI) and active tuberculosis (TB) (B) HIV-specific CD4+ release of IFNγ (p<0.001), TNFα (p<0.01) and IL-2 (p<0.01) were significantly different between all groups (Kruskal-Wallis). (C) HIV-specific CD8+ release of IFNγ (p<0.01) and TNFα (p<0.001) was significantly lower from HIV mono-infected subjects, to those co-infected with active TB. We additionally assessed the polyfunctionality profile of HIV specific CD4+ and CD8+ T cells in all patient groups. The polyfunctionality profiles between groups differed significantly for both CD4+ (p = 0.04) and CD8+ (p<0.001).(D) HIV-specific CD4+ T cells from HIV mono-infected subjects displayed a polyfunctional CD4+ T cell profile with a maximum of four functions being present (IFN+IL-2+IL-17+TNFα (2%)).(E) HIV-specific four function CD4+ T cells were not present in subjects co-infected with LTBI (F) Further decreases in HIV-specific CD4+ T cell polyfunctionality were observed in HIV positive subjects co-infected with TB, being replaced by a largely mono-functional profile with a decreased amount of triple cytokine cells (5% as compared to 13% in HIV/LTBI and 15% in HIV mono-infection). Additionally, single positive TNF-α cells dominated the profile (48%). (G) HIV-specific CD8+ T cells in HIV mono-infected subjects displayed a polyfunctional profile with a maximum of 2 functions being present (IFN+ TNFα (2%). (H) A maximum of 2 functions (IFN+TNFα+ (28%)) were present in HIV-specific CD8+ cells from subjects co-infected with LTBI. (I) 87% mono-functional cells were present in the HIV-specific CD8+T cell profile from subjects co-infected with TB, suggesting a complete loss of polyfunctionality.
HIV-specific CD8+ release of IFN-γ (p<0.001) and TNFα (p = 0.004) were significantly lower in HIV/TB co-infected subjects as compared to HIV mono-infected subjects (Fig. 1C). Additionally, IFN-γ secretion was found to be significantly lower in the HIV+/TB group as compared to the HIV+/LTBI group (p = 0.02). No significant antigen-specific production of IL-2, IL-17 or IL-21 was observed for CD8+ T cell responses. In summary, HIV co-infection with MTB was associated with a decrease in the amount of cytokine (TNF-α and IFN-γ) secreted for HIV-specific CD8+ T cells.
Significant differences were also observed between the polyfunctionality profiles for CD4+ (p = 0.04) and CD8+ (p<0.001) T cells (Fig. 1 D-I). HIV mono-infected subjects displayed a polyfunctional HIV-specific CD4+ T cell profile with a maximum of four functions (IFN-γ+IL-2+IL-17+TNF-α+ (2% of HIV-specific CD4+ T cells)(Fig. 1d). 15% of HIV-specific CD4 T cells were able to secrete 3 cytokines simultaneously (IFN-γ+IL-2+TNF-α+), 26% secreted 2 cytokines (IFN-γ+TNF-α+(15%),IFN-γ+IL-2+(6%), IL-2+IL-21+(5%))while 57% were mono-functional (IFN-γ+(31%), TNF-α+(26%)). HIV-specific four-function CD4+ T cells were not present in those co-infected with LTBI and this was accompanied by a higher proportion of mono-functional T cells: 65%(IFN-γ+(29%),TNF-α+(36%))(Fig. 1E).Further changes in HIV-specific CD4+ T cell polyfunctionality were observed in HIV positive subjects co-infected with TB, with only 5% being able to co-secrete 3 cytokines (IFN-γ+IL-2+TNF-α+) and 76% with mono-functional secretion capacity (TNF-α only (47%), IL-2 only (2%), or IFN-γ only (26%))(Fig. 1G). In summary, HIV co-infection with MTB was associated with a decrease in the number of cytokines secreted from HIV-specific CD4+ T cells.
In HIV mono-infected individuals the HIV-specific CD8+ T cell profile consisted of dual cytokine secreting cells IFN-γ+TNF-α+ (38% of total HIV-specific CD8+ T cells) and mono-functional cells (62% of total HIV-specific CD8+ T cells)(Fig. 1G).Bi-functional T cells were found to be present at progressively lower frequencies from HIV mono-infection, to co-infection with LTBI, to co-infection with TB (Fig. 1 G-1). 87% mono-functional HIV-specific CD8+ T cells were present in subjects co-infected with TB (Fig. 1I). In summary, HIV co-infection with MTB was associated with a decrease in the number of cytokines secreted from HIV-specific CD8+ T cells.
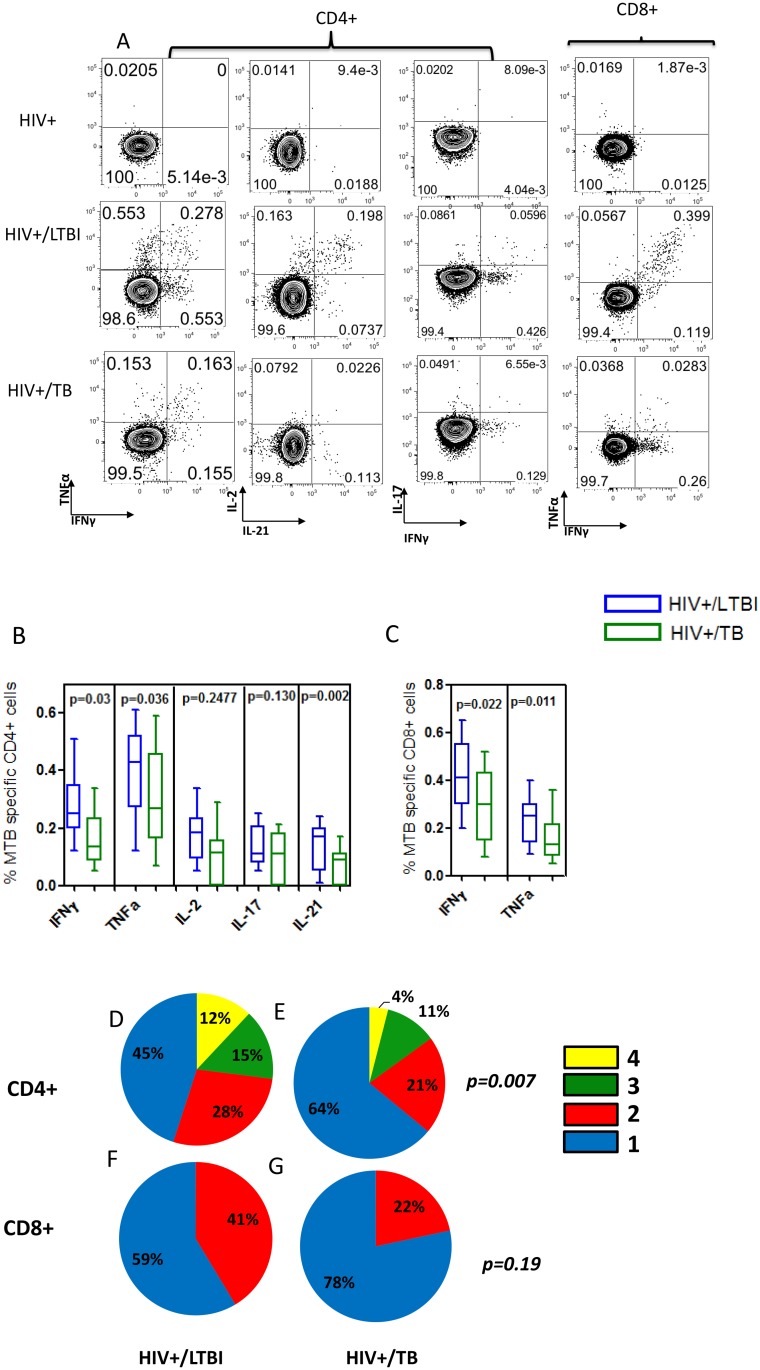
Interestingly, when we assessed MTB-specific T cells functionality, we observed a decline in functionality (both in amount and number of cytokines secreted) in HIV-TB co-infected individuals compared to those with HIV-LTBI (Fig. 2). Mono-functional IFN-γ and TNF-α producing cells dominated the profile of MTB-specific CD8+ cells in HIV infected subjects co-infected with TB (at 78%) (Fig. 2I).
Fig 2. MTB-specific CD4+ and CD8+ T cell functionality is reduced in HIV infected individuals co-infected with latent Mycobacterium tuberculosis infection (LTBI) and active tuberculosis (TB) disease.
(A) Representative flow cytometry plots showing cytokine IFN-gamma and TNF-α response for Mycobacterium tuberculosis (MTB) (CFP-10 and ESAT-6). Corresponding negative controls (no antigen) are the same as shown in Fig. 1B. (B) MTB-specific CD4+ release of IFNγ (p = 0.03), TNFα (p<0.03) and IL-21(p<0.002) were observed to be significantly lower from subjects co-infected with LTBI to those co-infected with TB. (C) MTB-specific CD8+ release of IFNγ (p = 0.022) and TNFα (p<0.011), were lower subjects co-infected with TB as compared co-infected with LTBI.
We next assessed the polyfunctionality profile of Mycobacterium tuberculosis (MTB)—specific CD4+ and CD8+ T cells in HIV mono-infection and co-infection with LTBI or TB. (D). We observed significant difference between the polyfunctionality profiles of HIV+/LTBI and HIV+/TB groups for both CD4+ (p = 0.007) and CD8+ (p = 0.19). A highly polyfunctional MTB-specific CD4+ T cell cytokine profile was observed in HIV positive subjects co-infected with LTBI, which including the capacity to secrete four cytokines by 12% of MTB-specific T cells (IFN+IL-2+IL-17+TNFα (9%), or IFN+IL-21+IL-17+TNFα (3%)). (E) A decrease in the polyfunctional profile of MTB-specific CD4+ T cells was observed in HIV co-infection with TB, with an increased dominance in mono-functional TNFα producing cells (from 12% to 31%). (F) MTB-specific CD8+ T cells from HIV infected subjects co-infected with LTBI displayed a profile with a maximum of 2 functions being present (IFN+TNFα (41%)). (G) Mono-functional IFNγ and TNFα producing cells dominated the profile of MTB-specific CD8+ cells in HIV infected subjects co-infected with TB (at 78%). There was total loss of IL-2 function but IFNγ+TNF+ double positive cells were present (IFN+TNFα+ (22%)). An increased dominance in mono-functional TNFα producing cells was also observed (47%).
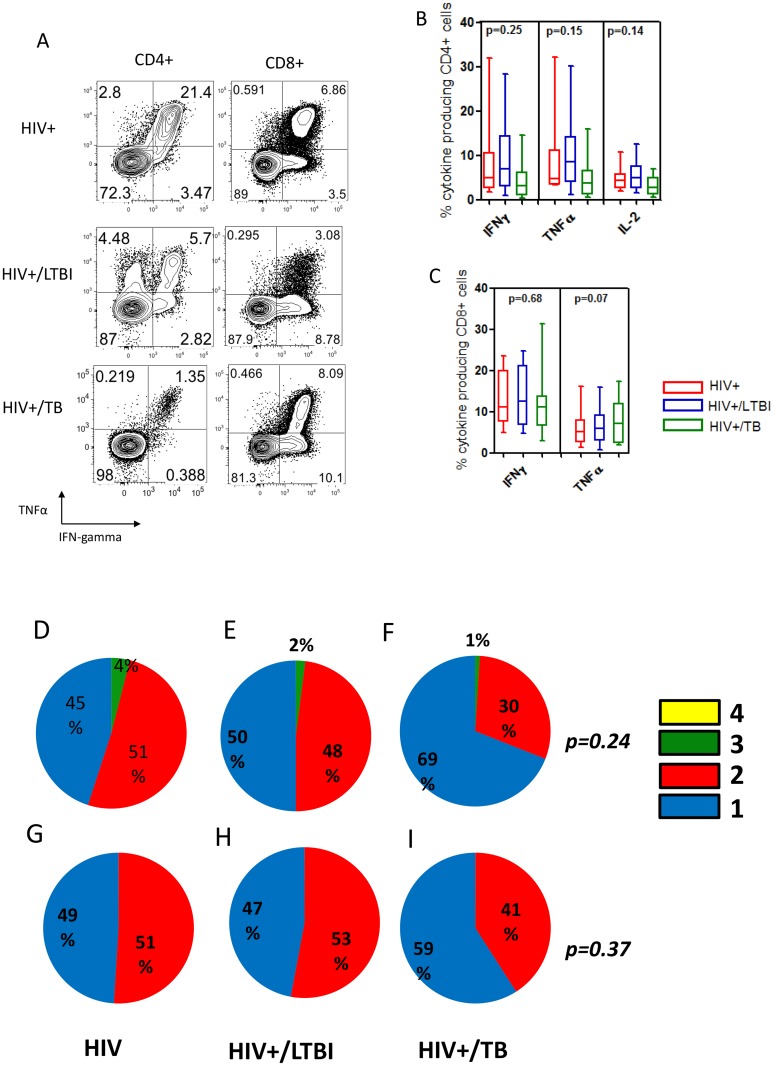
We additionally assessed non-specific cytokine production following SEB stimulation (Fig. 3A-I). Whilst no significant quantitative differences were observed, HIV/TB co-infected subjects appeared to produce less cytokine in response to SEB as compared to HIV/LTBI and HIV mono-infected subjects. Analysis of non-specific CD4+ T cell polyfunctionality revealed a higher quantity in mono-functional cells from HIV mono-infection (Fig. 3D) to HIV/LTBI (Fig. 3E) to HIV+/TB (Fig. 3F). Analysis of non-specific CD8+ T cell polyfunctionality indicated no significant changes between the groups (Fig. 3G-I).
Fig 3. Non-specific CD4+ and CD8+ T cell functionality is reduced in HIV infected individuals co-infected with latent Mycobacterium tuberculosis infection (LTBI) and active tuberculosis (TB) disease.
We assessed non-specific cytokine production following 6 hour SEB stimulation. (A) Representative flow cytometry plots showing cytokine responses for SEB stimulated CD4 and CD8 T cells from all patient categories. (B, C) No quantitative differences were observed when non-specific cytokine production was compared amongst patient groups. Whilst no significant differences were observed, HIV/TB co-infected subjects appeared to produce less cytokine in response to SEB. (D, E, F) Analysis of non-specific CD4 polyfunctionality revealed an increase (p = 0.007) in mono-functional cells from HIV+ mono-infection to HIV/LTBI to HIV/TB. Analysis of non-specific CD8+ T cell polyfunctionality showed no significant difference between the groups (p = 0.19) (G, H, I).
Discussion
HIV-specific CD4+ and CD8+ T cell functionality was found to be lower in co-infection with LTBI, and to a greater extent TB, as compared to HIV mono-infection. The observed changes in HIV-specific T cell single cytokine release may not necessarily be a loss in functional capacity but rather a loss of HIV-specific T cells. However, the polyfunctionality data are rather striking, showing that MTB co-infection resulted in a reduced ability of HIV-specific T cells to co-secrete multiple cytokines, suggesting that MTB infection augments HIV- specific T cell dysregulation.
In HIV mono-infection, several studies have highlighted the relationship between high-levels of T cell polyfunctionality and the control of HIV disease progression, with increased viral load being linked to a decrease in HIV-specific T cell function [8],[12]. HIV-specific CD4+ T cells in our HIV mono-infected subjects secreted IFN-y, IL-2, TNF-α, IL-21 and IL-17. The loss of each of these functions individually could have a significant impact on HIV control. For example, IL-21 has been strongly associated with mechanisms of viral control in elite controllers [13]. HIV-specific CD8+ T cells in our HIV mono-infected subjects were shown to secrete predominantly TNF-α and IFN-γ withextremely low amounts of IL-2[8]. A loss in the ability of CD8+ T cells to produce IL-2 has been shown to be associated with T cell exhaustion [8].
For both HIV-specific CD4+ and CD8+ T cells we observed a decline in polyfunctional capacity in MTB co-infection suggesting that mycobacterial load may a role.This is supported by lower MTB-specific T cell functionality in TB co-infection with HIV compared to LTBI. Day et al, (2011) have previously demonstrated that the functional capacity (specifically IL-2, TNF-α and IFNγ) of MTB-specific CD4+ T cells is decreased in TB compared to LTBI mono-infection. Our data extend these findings by additionally assessing IL-21 and IL-17 secretion capacity in HIV and MTB co-infection. Our data suggest that the loss of T cell function associated with TB may extend beyond HIV and MTB-specific responses, as our preliminary data reveals impairment in SEB-specific responses in this co-infection state. The high levels of inflammation and immune activation present in TB may enhance general immune exhaustion and T cell anergy [14].
Importantly, cytokine T cell profiles may be able to act as biomarkers of specific disease states. Mono-functional TNF-α producing cells dominated the MTB-specific T cell polyfunctionality profiles in those co-infected with TB as compared to those co-infected with LTBI. This confirms and extends the predictive model by Harari et al which showed that TNF-α single positive CD4+ T cells can differentiate between LTBI and TB in MTB mono-infected individuals [9]. More importantly, we show that this may hold true in HIV co-infection where diagnosing TB is problematic using currently available assays. Mono-functional TNF-α secreting T cells may therefore be an effective diagnostic biomarker for active TB in HIV positive populations. Interestingly, TNF-α generated in response to MTB infection has been shown to increase HIV viral replication suggesting that the cytokine profiles and dominance of TNF-α single producing T cells in TB, may contribute to increased HIV replication and disease progression[15]. In addition, our data suggests that the loss of T cell function associated with TB may extend beyond HIV and MTB-specific responses, as our preliminary data revealed impairment in SEB-specific responses in this co-infection state. The high levels of inflammation and immune activation present in TB may enhance general immune exhaustion and T cell anergy [14].
In conclusion, our results indicate that MTB infection is linked to lower HIV-specific CD8+ and CD4+ T cell polyfunctionality. As HIV-specific T cells are most defective in HIV co-infection with TB, as compared to LTBI, mycobacterial load may contribute to the loss of T cell function. Decreased T cell function may be a contributing factor to increased HIV disease progression in co-infection as functionally defective HIV-specific T cells may be less able to control HIV.
Acknowledgments
We would like to thank Sr Kesia Ngwenya and the staff and participants of the Sinikithimba Cohort at McCord Hospital as well as Dr Faieza Sahid and the String Study clinical team at King Edward Hospital. We also thank Daniel Kaufmann and Bruce Walker for helpful discussions. We would like to thank the following funders: the Howard Hughes Medical Institute (support for T.N., V.O.K),the Harvard University CFAR grant (P30 AI060354), the South African Medical Research Council (MRC) and The CU-SA Fogarty AITRP programme (S.C), the National Heart Lung and Blood Institute of the National Institutes of Health (RO1 HL-092565 and R37 AI067073 and P01 AI-080192). F.P. is supported by fellowship grants of the Massachusetts General Hospital Executive Committee on Research and the Harvard Global Health Institute (HGHI). T.N. is also supported by the South African Research Chairs Initiative and the Victor Daitz Foundation. Open access publication of this article has been made possible through support from the Victor Daitz Information Gateway, an initiative of the Victor Daitz Foundation and the University of KwaZulu-Natal. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors would like to thank the following funders: the Howard Hughes Medical Institute (support for T. N., V. O. K.),the Harvard University CFAR grant (P30 AI060354), the South African Medical Research Council (MRC) and The CU-SA Fogarty AITRP programme (S. C.), the National Heart Lung and Blood Institute of the National Institutes of Health (RO1 HL-092565 and R37 AI067073 and P01 AI-080192). F. P. is supported by fellowship grants of the Massachusetts General Hospital Executive Committee on Research and the Harvard Global Health Institute (HGHI). T. N. is also supported by the South African Research Chairs Initiative and the Victor Daitz Foundation. Open access publication of this article has been made possible through support from the Victor Daitz Information Gateway, an initiative of the Victor Daitz Foundation and the University of KwaZulu-Natal. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Karim SSA, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. The Lancet 2009; 374:921–933 10.1016/S0140-6736(09)60916-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goletti D, Weissman D, Jackson RW, Collins F, Kinter A, Fauci AS. The in vitro induction of human immunodeficiency virus (HIV) replication in purified protein derivative-positive HIV-infected persons by recall antigen response to Mycobacterium tuberculosis is the result of a balance of the effects of endogenous interleukin-2 and proinflammatory and antiinflammatory cytokines. J Infect Dis 1998;177:1332–8 [DOI] [PubMed] [Google Scholar]
- 3. Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse D, Shioda T, et al. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 2002;195:495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stacey AR, Norris PJ, Qin L, Haygreen E, Taylor E, Heitman J, et al. Induction of a Striking Systemic Cytokine Cascade prior to Peak Viremia in Acute Human Immunodeficiency Virus Type 1 Infection, in Contrast to More Modest and Delayed Responses in Acute Hepatitis B and C Virus Infections. J Virol 2009;83:3719–3733 10.1128/JVI.01844-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geldmacher C, Schuetz A, Ngwenyama N, Casazza J, Sanga E, Saathoff E, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis 2008;198:1590–8 10.1086/593017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave E, et al. Preferential infection and depletion of Mycobacterium tuberculosis—specific CD4 T cells after HIV-1 infection. J Exp Med 2010;207:2869–2881 10.1084/jem.20100090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis 2008;197:990–9 10.1086/529048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 2011;17:372–6 10.1038/nm.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'rie T, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 2011;187:2222–32 10.4049/jimmunol.1101122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell JE, Chetty S, Govender P, Pillay M, Jaggernath M, Kasmar A, et al. Prospective Monitoring Reveals Dynamic Levels of T Cell Immunity to Mycobacterium Tuberculosis in HIV Infected Individuals. PLoS ONE;7:e37920 10.1371/journal.pone.0037920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007;81:8468–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, et al. HIV-1-Specific Interleukin-21+ CD4+ T Cell Responses Contribute to Durable Viral Control through the Modulation of HIV-Specific CD8+ T Cell Function. Journal of Virology;85:733–741 10.1128/JVI.02030-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. Journal of immunology (Baltimore, Md: 1950) 2009;182:5891–7 10.4049/jimmunol.0803771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrait V, Cadranel J, Esvant H, Herry H, Morinet P, Mayaud C, et al. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. Journal of immunology 1997;159:2824–30 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.