Abstract
Atlanto-occipital dislocation (AOD) is being increasingly recognized as a potentially survivable injury as a result of improved prehospital management of polytrauma patients and increased awareness of this entity, leading to earlier diagnosis and more aggressive treatment. However, despite overall improved outcomes, AOD is still associated with significant morbidity and mortality. The purpose of this paper is to review the biomechanical aspects, clinical features, radiologic criteria, and treatment strategies of AOD. Given that the diagnosis of AOD can be very challenging, a high degree of clinical suspicion is essential to ensure timely recognition and treatment, thus preventing neurological decline or death.
Keywords: Atlanto-occipital dislocation, Cervical spine, Craniocervical junction, Occipitocervical fusion, Trauma
Core tip: Atlanto-occipital dislocation (AOD) is being increasingly recognized as a potentially survivable injury as a result of improved prehospital management, increased awareness, and more aggressive management. However, despite overall improved outcomes, AOD is still associated with significant morbidity and mortality. Given that the diagnosis can be very challenging, a high degree of clinical suspicion is essential to ensure timely recognition and treatment, thus preventing neurological decline or death.
INTRODUCTION
In a recent study of 300 patients with cervical spine trauma, 30% of injuries were located between the occiput and C2. Among these, acute spondylolysis of C2 (hangman’s fracture), C1 ring fractures, odontoid fractures, and atlanto-occipital dislocation (AOD) were the most common[1].
AOD is a highly unstable craniocervical injury, resulting from damage to ligaments and/or bony structures connecting the skull to the cervical spine. It is historically associated with significant neurological morbidity and mortality secondary to brainstem and upper cervical spinal cord injury. Although AOD represents roughly only 1% of all cervical spine injuries in the acute care setting, it has been reported to be the most common cervical spine injury in motor vehicle accident (MVA) fatalities. Modern case reports, however, have documented improved neurological outcomes, likely as a result of earlier diagnosis and surgical stabilization[2,3].
EPIDEMIOLOGY
AOD was first described by Blackwood[4] in 1908 and was long held to be a rare entity in comparison with other cervical spine injuries. Although rarely encountered and treated by spine surgeons, the incidence of AOD becomes much higher than historically assumed when non survivors of trauma are taken into account. In fact, AOD has been identified in 6%-10% of fatal cervical spine injuries from any mechanism[5,6]. When MVAs specifically are considered, the incidence of AOD among fatal cervical spine injuries may be as high as 35%[7,8].
AOD is more common among children and young adults. In fact, the injury is 3 times more common in children than in adults. This is thought to be secondary to a more horizontal plane of the articular surfaces and a relative laxity of the ligamentous structures, combined with the presence of a relatively large head and a higher effective fulcrum in the cervical spine[9].
AOD is generally associated with high-energy trauma, including high-speed MVAs or falls from heights, and thus should be considered as a possibility in any trauma involving large acceleration and deceleration forces[10]. As a result, AOD is frequently associated with severe traumatic brain injury, which can complicate initial identification of the injury as well as rehabilitation efforts after stabilization[10,11].
The advent of specialized emergency response systems and the evolution of Emergency Medicine as a specialty have served to alter the epidemiology of AOD over the last 3 decades. Improvements in field resuscitation, cervical immobilization, rapid transport, and increased recognition have resulted in more survivors of AOD, which is now being seen more often by clinicians in the acute care setting[3,8].
ANATOMY OF THE CRANIOCERVICAL JUNCTION
AOD is primarily an injury of the ligaments between the occiput and upper cervical spine, often without accompanying bony fractures. Thus, it can be missed more easily than traumatic fractures of the cervical spine. Proper identification and treatment of this injury requires a good understanding of the anatomy of the craniocervical junction (CCJ).
The junction between the skull and the cervical spine is stabilized by ligaments joining the axis and atlas to the clivus, occipital bone, and occipital condyle. The craniocervical junction must accommodate a wide variety of motions, which require many ligaments for stabilization (Figure 1).
Figure 1.
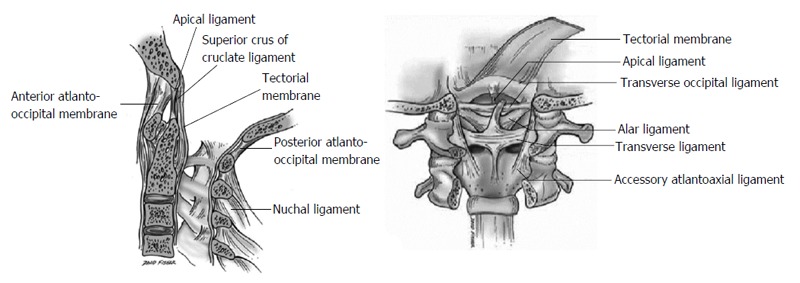
Anatomy of the craniocervical junction (reproduced with permission from Ref. [13]).
The atlanto-occipital joint is formed by the superior articular facet of the atlas and the occipital condyle, which are stabilized by an articular capsule. This joint allows for 25 degrees of flexion and extension and 5 degrees of axial rotation[12,13].
The atlantoaxial segment consists of 3 joints, which together allow for 15 degrees of flexion and extension and 30 degrees of axial rotation. These include 2 lateral mass articulations and an atlantodental joint. The latter resists excessive extension, allowing only 10 degrees of extension in the average person[12,13].
The anterior atlanto-occipital membrane attaches from the anterior arch of the atlas to the anterior aspect of the clivus. It is a continuation of the anterior longitudinal ligament and serves to prevent excessive neck extension[12,13].
The alar ligaments attach from the lateral aspect of the odontoid process to the medial occipital condyle on each side. These ligaments limit contralateral flexion and axial rotation at the atlanto-occipital joint[12-14].
The apical ligament attaches from the tip of the odontoid process to the basion, lying posterior to the alar ligaments and anterior to the superior band of the cruciate ligament. This ligament may be absent in 20% of cases and is often a rudimentary structure, with limited contribution to mechanical stability of the CCJ[12-14].
The Barkow ligament connects the tip of the dens to the occipital condyle, lying anterior and parallel to the alar ligaments. This ligament may assist in preventing excessive neck extension[12,13].
The transverse occipital ligament spans the foramen magnum, attaching to the medial aspect of the occipital condyles. This ligament sometimes joins the alar ligaments and may help prevent excessive lateral bending, flexion, and axial rotation[12,13].
The cruciform or cruciate ligament consists of a superior, transverse, and inferior bands centered just posterior to the odontoid. The superior band stabilizes the odontoid to the basion. The transverse band is the strongest portion of the cruciform ligament and stabilizes the odontoid to the lateral masses of the atlas. It limits lateral motion of C1 relative to the dens and prevents posterior displacement of the dens, thus limiting anterior C1-2 subluxation to 3-5 mm. The inferior band is a continuation of the superior band, which further strengthens the connection between the body of C2 and basion[12,13].
The tectorial membrane lies immediately posterior to the cruciate ligament. It attaches to the clivus lateral to the hypoglossal canals and continues through the spinal canal as the posterior longitudinal ligament. This ligament limits both excessive flexion and extension[12,13].
The accessory atlantoaxial ligament attaches from the posterior aspect of the body of C2 to the lateral masses of C1, lying anterior to the tectorial membrane. The role of this ligament is unclear[12,13].
The posterior atlanto-occipital membrane attaches from the occipital bone to the posterior arch of the atlas. It is a continuation of the ligamentum flavum[12,13].
The ligamentum nuchae is a continuation of the supraspinous ligament and spans from the external occipital protuberance to the spinous process of C7. This ligament serves to limit excessive neck flexion[12,13].
BIOMECHANICAL CONSIDERATIONS
AOD may be caused by different traumatic mechanisms, all having in common the transmission of excessive force to the CCJ, leading to widespread ligamentous disruption. Such mechanisms include hyperextension, hyperflexion, lateral flexion, or a combination of these[15-17].
Several predisposing conditions, including inflammatory, neoplastic, and congenital disorders, may increase the risk of AOD in the face of relatively minor trauma. Rheumatoid arthritis may involve the spine, particularly the CCJ, and cause weakening of the transverse ligament, thus increasing the risk of C1 subluxation. Down syndrome is associated with laxity of craniocervical ligaments in up to 30% of cases. Congenital cervical vertebral fusion syndromes may also predispose to AOD by creating a fulcrum-like effect[13].
CLINICAL FEATURES
Because of the relatively wide cross-sectional area of the spinal canal at the CCJ, spinal cord injury is less common than expected. However, when present, neurological injury from AOD can be devastating, often leading to sudden death secondary to brainstem injury. Neural injury may be direct, as a result of traction or compression mechanisms, or indirect, secondary to cerebrovascular injury leading to ischemia.
Survivors of AOD often have neurological impairment, including lower cranial nerve deficits, unilateral or bilateral weakness, or even quadriplegia. However, there is a wide range of presentations, with some patients being completely asymptomatic and others being dependent on advanced life support measures. Concomitant traumatic injuries to the brain, chest, abdomen, and extremities can further blur the clinical picture, masking weakness, apnea, or neurogenic shock.
Up to 20% of patients with AOD may have normal neurological examination at presentation. Severe neck pain may be the only symptom in such patients[18]. The lack of localizing neurological findings can delay the diagnosis of AOD. However, the majority of patients present with unconsciousness and respiratory arrest. Lower cranial nerves, such as abducens, vagus and hypoglossal, may also be involved in AOD.
More severe cases of AOD can present with spinal cord injury, including sensory and motor deficits, hyperreflexia with clonus, positive Babinski sign, and abnormal sphincter tone. Neurological deficits may be unilateral or bilateral, and typically include the entirety of the affected side from shoulder to foot. Reflex examination should be interpreted cautiously, given the possibility of spinal shock.
Autonomic dysregulation, including neurogenic shock, may also be a presenting symptom. The ensuing hemodynamic instability may cause trauma teams to undertake negative exploratory laparotomies, which may increase the risk of neurological deterioration during transfers and may delay the diagnosis of AOD.
Finally, symptoms of AOD may be caused by cerebrovascular injury. It is fairly common for vertebral dissections to occur with AOD, as well as carotid dissections. These injuries can lead to ischemic strokes, further clouding the clinical presentation[11].
Thus, until radiologic evaluation with computed tomography (CT) or magnetic resonance imaging (MRI) can be performed, any patient involved in high-energy trauma should be suspected of having AOD, irrespective of clinical findings, and appropriate precautionary measures should be taken.
RADIOLOGIC CRITERIA
Given the complex anatomical and biomechanical factors involved in AOD, a single measurement or abnormality on imaging studies cannot universally define AOD. Over the years, many different and complementary methods have been developed to help diagnose this often overlooked entity, each having its own strengths and weaknesses. All methods seek to assess for damage to the structures stabilizing the occipital-atlanto-axial unit. These include the Traynelis classification[19], Powers’ ratio[20], X-line method[21], basion-dens interval (BDI) and basion-axis interval (BAI) (i.e., Harris lines)[22,23], and occipital condyle-C1 interval (CCI)[24,25]. Care should be taken when applying these various techniques to adult vs pediatric patients, as there are significant anatomic and biomechanical differences between these 2 populations.
The Traynelis classification[8,19] (Figure 2) divides AOD into 3 groups: (1) Type I is an anterior displacement of the occiput relative to the atlas; (2) Type II is a distraction of the occiput from the atlas; and (3) Type III is a posterior displacement of the occiput relative to the atlas.
Figure 2.
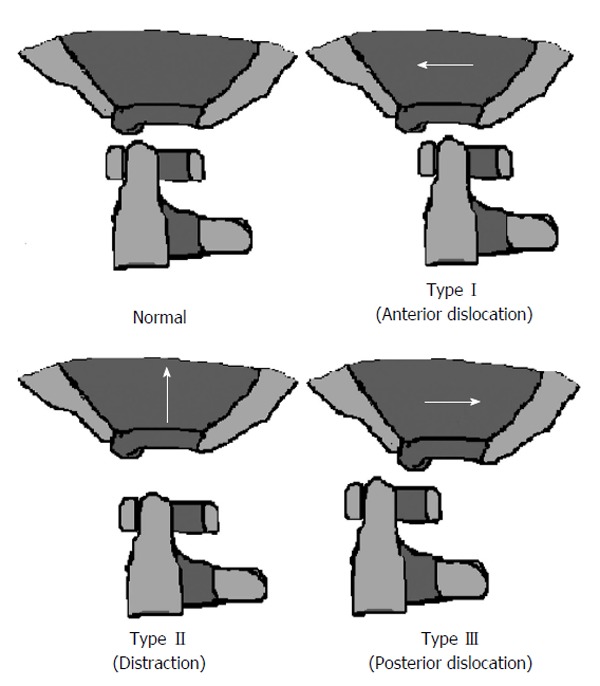
The Traynelis classification.
Traction is sometimes used to realign types I and III, but remains controversial. Unfortunately, this classification scheme does not take into consideration the presence of coronal misalignment. Thus, a clinician depending solely on this method may miss an AOD with pure coronal distraction. Nevertheless, this system still provides a useful framework when assessing for AOD and may help guide management.
Powers’ ratio[8,20] compares measurements relating the skull base to C1 (Figure 3A). The distance from the basion (B) to the midpoint of the anterior cortex of the posterior arch of C1 (C) is measured. The distance from the opisthion (O) to the midpoint of the posterior cortex of the anterior arch of C1 (A) is measured. If BxC/OxA exceeds 1, then AOD should be suspected. Normal values are typically < 0.9. Powers’ ratio was originally described to detect anterior dislocation injuries and, as such, is less sensitive to distraction or posterior dislocation injuries, i.e., Traynelis types II and III. Nevertheless, it remains one of the earliest reliable and reproducible published methods and often stands as the benchmark to which other methods are compared.
Figure 3.
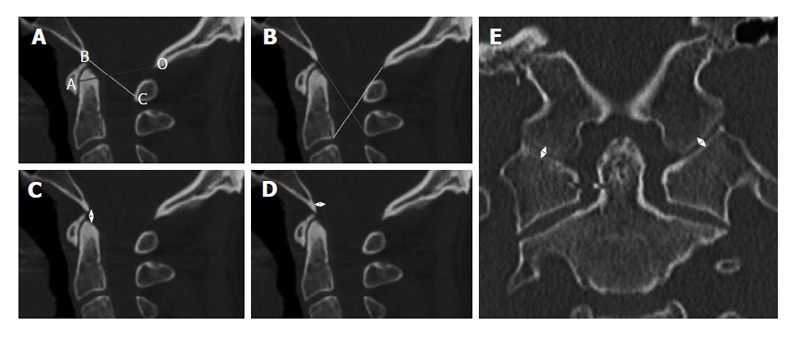
Diagnostic methods for atlanto-occipital dislocation (see text for details). A: Powers’ ratio; B: X-line method; C: Basion-dens interval; D: Basion-axis interval; E: Occipital condyle-C1 interval.
The X-line method[8,21] involves drawing a line from the basion to the spinolaminar junction of C2 and a line from the opisthion to the posteroinferior corner of the body of C2 (Figure 3B). The result is considered abnormal if both the first line does not intersect C2 and the second line does not intersect C1. Because of its more anatomic definition of normality, the X-line method is more sensitive than Powers’ ratio in detecting Traynelis types II and III injuries.
The Harris method[8,22,23] combines 2 previously developed measures: the BDI and the BAI (Figure 3C and D). BDI measures the distance between the basion and the tip of the dens. Values above 10 mm in adults and 12 mm in children are considered abnormal. BDI is particularly sensitive to Traynelis type II injuries. BAI measures the distance between a line drawn tangentially to the posterior cortical surface of C2, i.e., the posterior axial line, and a second parallel line drawn through the basion. Normal values range from 12 mm (basion anterior to dens) to -4 mm (basion posterior to dens) in adults and from 12 mm to 0 mm in children. BAI is most sensitive to Traynelis type I and III injuries. Using BDI and BAI in combination, Harris et al[23] demonstrated increased diagnostic accuracy compared with Powers’ ratio.
The CCI or condylar gap method is a measurement used and validated in the pediatric population[8,24,25]. It is the only method that directly assesses structural elements of the atlanto-occipital joint. Specifically, the distance between the occipital condyle and its articulating surface on C1 is measured (Figure 3E), making this technique highly sensitive for Traynelis type II injuries. The measurement is made on coronal CT images. A distance of more than 2 mm in adults or more than 5 mm in children, or gross asymmetry between the 2 joints is highly sensitive and specific for AOD, with good interrater reliability[24,25]. While this technique was initially validated in the pediatric population, it is rapidly becoming the gold standard for diagnosing AOD in adults as well, as recent studies suggest similar accuracy in that population[26].
Based on level III evidence, the “Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries”[27] recommend applying the BDI-BAI (Harris lines) method on a plain lateral cervical X-ray in adults. If this is nondiagnostic and there is high clinical suspicion or significant prevertebral soft tissue swelling, CT and/or MRI are recommended. In children, the CCI determined on CT has the highest diagnostic sensitivity and specificity for AOD[27]. Signs that should raise concern for AOD include: enlargement of the predental space, high cervical spinal cord deficits, respiratory dysfunction or apnea, subarachnoid hemorrhage at the CCJ, cranial nerve deficits, and signal abnormalities affecting the tectorial membrane, alar and transverse ligaments, or occipitoatlantal joint capsule on MRI[25].
Ultimately, none of these diagnostic methods is perfect and each has limitations. For instance, none maximally tests for all 3 Traynelis types and few assess for coronal plane displacement. Also, the diversity of these methods and measurement techniques may cause confusion among clinicians. There is still no gold standard technique to diagnose AOD and the large number of available methods reinforces the notion that this diagnosis can be easily missed. We recommend utilizing at least 2 complementary methods to help compensate for the shortcomings of any single method. Also, clinicians should take into account the patient’s clinical presentation and suspected mechanism of injury when assessing for AOD, since no radiographic measures can completely rule out the diagnosis.
TREATMENT
Treatment of AOD begins in the field. Hemodynamic and respiratory instability should be immediately dealt with at the scene and given utmost priority. Inline stabilization of the neck and cervical spine injury precautions, including the proper application of a rigid cervical collar at the trauma scene, are also critical to prevent this potentially recoverable injury from becoming a lethal one. In the emergency department, careful documentation of the patient’s neurological exam may help raise clinical suspicion for AOD. A rapid, yet thorough assessment, followed by appropriate radiologic imaging, is essential to ensure timely diagnosis and treatment of this injury. Once the diagnosis of AOD is confirmed, halo immobilization should be performed, followed by internal occipitocervical fixation and fusion. Cervical traction should be avoided, since it is associated with a 10% risk of neurological deterioration[27].
Anterior approaches to the CCJ have been well described and are typically used for pathology anterior to the spinal cord. Such approaches are more suited for decompression rather than stabilization and are often technically challenging. Because of this, they tend to be less useful for AOD, where stabilization is the major goal of surgery and where patients are often critically ill and unable to tolerate long and morbid procedures. Conversely, the posterior approach can be used to achieve both decompression and stabilization of the CCJ. While the approach itself has not changed over time, fusion technology has evolved from cable wiring to laminar clamps to screw fixation, resulting in improved stabilization results[28-31].
Posterior fixation was historically accomplished with sublaminar wiring. C1-2 sublaminar wiring and facet fusion was described as early as 1939[28]. In the 1980s, techniques combining C1-2 cable wiring with occipital bone wiring through burr holes were developed[29]. Cable-wired metal rods, such as modified Steinmann pins, were introduced as a way to maximize stabilization[30]. These craniocervical stabilization techniques were often used in adjunction with spinal traction and halo immobilization throughout the 1980s[29]. As experience and technology progressed, wiring techniques were largely abandoned in favor of screw fixation techniques, which provide better biomechanical stability[31].
In the modern era, patients with strictly occipitoatlantal joint instability and no other associated cervical injuries may be treated with an O-C1 or O-C2 screw fixation. The former has the benefit of sparing the atlantoaxial motion segment. Occipitocervical fusion can be performed using either transarticular or lateral mass screws, although C2 pedicle or laminar screws have also been successfully used[8,11,29]. At the cranial level, instrumentation options include bicortical occipital screws and occipital condyle screws. Transarticular screws have excellent purchase when placed bicortically, leading to high biomechanical stability, and can be placed even when posterior elements are absent or incompetent. However, transarticular techniques place the vertebral artery at risk for injury. Thus, the patient’s individual anatomy must be taken into account[8,29]. In our institution, C1-2 transarticular screws have been largely abandoned in favor of C1 lateral mass and C2 pars screws, given the lower risk of vascular injury with this technique (Figure 4).
Figure 4.
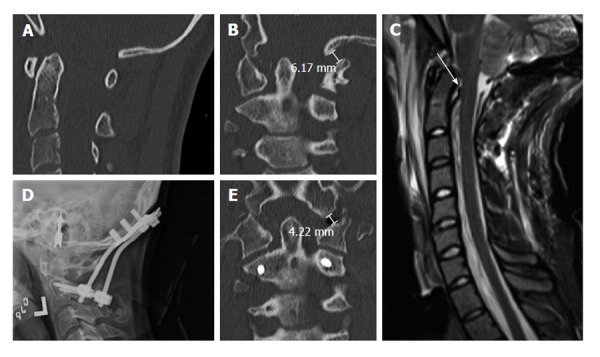
Nineteen years old woman with traumatic atlanto-occipital dislocation following high-speed motor vehicle accident. A and B: CT of the cervical spine demonstrates no significant abnormalities in the midsagittal plane (A), but clear asymmetry of the occipito-atlantal joints in the coronal plane (B). The left occipital condyle-C1 interval is increased, measuring 6 mm; C: MRI of the cervical spine (T2WI) reveals abnormal signal suggesting disruption of the cruciate ligament; D: Post-operative cervical radiographs show O-C2 fusion using bicortical occipital screws and C2 pedicle screws; E: Post-operative CT of the cervical spine demonstrates reduction of the left occipital condyle-C1 interval to 4 mm. CT: Computed tomography; MRI: Magnetic resonance imaging.
AOD is often associated with concomitant cervical injuries below C1, necessitating the extension of fusion to the lowest disrupted level[32]. When such an extension is needed, lateral mass or pedicle screws can be placed at the remaining levels[8]. An important factor in the viability of these constructs is ensuring a strong fusion. Involved facet joints should be abraded and exposed bony surfaces should be decorticated in preparation for autograft application. Appropriate options for autograft include: iliac crest, locally harvested bone (lamina, spinous process), and rib graft. In addition, calvarial bone may be used in children[8].
Even in this modern era, halo fixation may still play a role in the treatment of AOD. In fact, some authors have proposed halo immobilization as a treatment option for patients with normal CT findings, i.e., no bony distraction, but equivocal changes on MRI, including mild signal changes at the occipitoatlantal joint[11]. However, halo devices are not frequently used for a number of reasons. First, the halo device is often ineffective in adequately stabilizing the CCJ. Second, halo immobilization is a cumbersome and potentially morbid procedure. It prevents early patient mobilization and limits daily activities, and has been associated with increased mortality rates in the elderly. Finally, AOD is an essentially ligamentous injury and, as such, is unlikely to spontaneously heal well over time, even after prolonged external immobilization[11,33]. In our institution, the use of halo immobilization for AOD is essentially limited to temporary preoperative stabilization pending definitive occipitocervical fusion. In such cases, care is always taken to avoid significant cephalad traction during halo application, which could easily exacerbate the injury and lead to neurological decline.
Special considerations apply when treating AOD in children, including smaller dimensions, syndromic anatomic variations, and the risk of limiting normal bony development and growth[8,34]. In a review of over 750 CCJ fusions in children, Ahmed et al[34] found lower morbidity rates with rib grafts compared with iliac crest grafts. The authors recommended using rib grafts alone in children 6 years of age or less, contoured rod-wire constructs in children between 7 and 10 years of age, and rigid instrumentation in children over the age of 10 years. No cervical spine growth abnormalities were observed in patients fused before the age of 5 years[34]. However, alternative fixation methods at C1 and C2, such as transarticular screws, lateral mass screws, and translaminar screws, have been successfully used in pediatric patients with atlantoaxial and occipitocervical instability, ranging in age from 1 to 17 years[35]. Moreover, in a recent pediatric study (age range 1-19 years), O-C2 fusion without C1 instrumentation had similar fusion rates compared with constructs incorporating C1[36]. Therefore, it seems that, irrespective of age, a successful fusion in children with upper cervical instability can be obtained using a variety of different instrumentation methods.
OUTCOME
Previous autopsy reports have documented AOD as cause of death in 6%-8% of traffic fatalities[8]. However, not every case of AOD will result in fatality or severe disability. Unfortunately, outcome analysis is biased by the high rate of misdiagnosis, particularly among asymptomatic patients. Outcome analysis is also complicated by the heterogeneity of treatment groups in the published literature, including variable mechanisms of injury, concurrent injuries, and comorbidities.
The natural history of asymptomatic AOD is unknown, since current standards of care mandate immediate stabilization. Earlier studies had documented a 54% rate of neurological worsening and 15% mortality among untreated AOD patients[27]. Some information is also available on initially asymptomatic patients that exhibit subsequent neurological worsening. A literature review in 2002 identified 9 such patients with an initially missed diagnosis of AOD. Half of these never returned to their baseline neurological condition, even after surgical stabilization[33].
Early aggressive surgical stabilization is associated with improved outcomes after AOD[33]. A study of 40 patients treated conservatively with external immobilization alone demonstrated a 30% rate of continued craniocervical instability and neurological worsening on follow-up[33]. Following early occipitocervical fusion in 19 patients, neurological improvement was seen in 15 patients, clinical stability in 3, and a new cranial nerve palsy in only 1[33]. Likewise, no neurological worsening was observed in a group of 8 patients who underwent delayed occipitocervical fusion after temporary external immobilization without traction[33].
CONCLUSION
AOD is an uncommon, yet increasingly recognized traumatic injury, which can be difficult to diagnose and may be easily overlooked on routine cervical spine radiographs. Despite advances in pre-hospital and hospital care and improved overall outcomes, AOD remains a potentially lethal and disabling injury. A high index of suspicion is critical for early diagnosis and to avoid neurological deterioration secondary to delay in care. AOD may initially be temporarily treated with external halo immobilization, but early surgical stabilization is required to confer long-term craniocervical stability and facilitate neurological recovery.
Footnotes
P- Reviewer: Anderson RCE, Singh H S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 14, 2014
First decision: March 12, 2014
Article in press: October 27, 2014
References
- 1.Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119–1142. [PubMed] [Google Scholar]
- 2.Payer M, Sottas CC. Traumatic atlanto-occipital dislocation: presentation of a new posterior occipitoatlantoaxial fixation technique in an adult survivor: technical case report. Neurosurgery. 2005;56:E203; discussion E203. doi: 10.1227/01.neu.0000144171.37158.f0. [DOI] [PubMed] [Google Scholar]
- 3.Jeszenszky D, Fekete TF, Lattig F, Bognár L. Intraarticular atlantooccipital fusion for the treatment of traumatic occipitocervical dislocation in a child: a new technique for selective stabilization with nine years follow-up. Spine (Phila Pa 1976) 2010;35:E421–E426. doi: 10.1097/BRS.0b013e3181c91fa1. [DOI] [PubMed] [Google Scholar]
- 4.Blackwood NJ. III. Atlo-Occipital Dislocation: A Case of Fracture of the Atlas and Axis, and Forward Dislocation of the Occiput on the Spinal Column, Life being Maintained for Thirty-four Hours and Forty Minutes by Artificial Respiration, during which a Laminectomy was Performed upon the Third Cervical Vertebra. Ann Surg. 1908;47:654–658. doi: 10.1097/00000658-190805000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alker GJ, Oh YS, Leslie EV, Lehotay J, Panaro VA, Eschner EG. Postmortem radiology of head neck injuries in fatal traffic accidents. Radiology. 1975;114:611–617. doi: 10.1148/114.3.611. [DOI] [PubMed] [Google Scholar]
- 6.Bucholz RW, Burkhead WZ, Graham W, Petty C. Occult cervical spine injuries in fatal traffic accidents. J Trauma. 1979;19:768–771. doi: 10.1097/00005373-197910000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Fisher CG, Sun JC, Dvorak M. Recognition and management of atlanto-occipital dislocation: improving survival from an often fatal condition. Can J Surg. 2001;44:412–420. [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett M, Consiglieri G, Kakarla UK, Chang SW, Dickman CA. Occipitoatlantal dislocation. Neurosurgery. 2010;66:48–55. doi: 10.1227/01.NEU.0000365802.02410.C5. [DOI] [PubMed] [Google Scholar]
- 9.Bucholz RW, Burkhead WZ. The pathological anatomy of fatal atlanto-occipital dislocations. J Bone Joint Surg Am. 1979;61:248–250. [PubMed] [Google Scholar]
- 10.Labler L, Eid K, Platz A, Trentz O, Kossmann T. Atlanto-occipital dislocation: four case reports of survival in adults and review of the literature. Eur Spine J. 2004;13:172–180. doi: 10.1007/s00586-003-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn EM, Feiz-Erfan I, Lekovic GP, Dickman CA, Sonntag VK, Theodore N. Survivors of occipitoatlantal dislocation injuries: imaging and clinical correlates. J Neurosurg Spine. 2007;6:113–120. doi: 10.3171/spi.2007.6.2.113. [DOI] [PubMed] [Google Scholar]
- 12.Tubbs RS, Dixon J, Loukas M, Shoja MM, Cohen-Gadol AA. Ligament of Barkow of the craniocervical junction: its anatomy and potential clinical and functional significance. J Neurosurg Spine. 2010;12:619–622. doi: 10.3171/2009.12.SPINE09671. [DOI] [PubMed] [Google Scholar]
- 13.Tubbs RS, Hallock JD, Radcliff V, Naftel RP, Mortazavi M, Shoja MM, Loukas M, Cohen-Gadol AA. Ligaments of the craniocervical junction. J Neurosurg Spine. 2011;14:697–709. doi: 10.3171/2011.1.SPINE10612. [DOI] [PubMed] [Google Scholar]
- 14.Yuksel M, Heiserman JE, Sonntag VK. Magnetic resonance imaging of the craniocervical junction at 3-T: observation of the accessory atlantoaxial ligaments. Neurosurgery. 2006;59:888–892; discussion 892-893. doi: 10.1227/01.NEU.0000232661.24547.06. [DOI] [PubMed] [Google Scholar]
- 15.Adams VI. Neck injuries: III. Ligamentous injuries of the craniocervical articulation without occipito-atlantal or atlanto-axial facet dislocation. A pathologic study of 21 traffic fatalities. J Forensic Sci. 1993;38:1097–1104. [PubMed] [Google Scholar]
- 16.Montane I, Eismont FJ, Green BA. Traumatic occipitoatlantal dislocation. Spine (Phila Pa 1976) 1991;16:112–116. [PubMed] [Google Scholar]
- 17.Yüksel KZ, Yüksel M, Gonzalez LF, Baek S, Heiserman JE, Sonntag VK, Crawford NR. Occipitocervical vertical distraction injuries: anatomical biomechanical, and 3-tesla magnetic resonance imaging investigation. Spine (Phila Pa 1976) 2008;33:2066–2073. doi: 10.1097/BRS.0b013e31817e2cfc. [DOI] [PubMed] [Google Scholar]
- 18.Harmanli O, Koyfman Y. Traumatic atlanto-occipital dislocation with survival: a case report and review of the literature. Surg Neurol. 1993;39:324–330. doi: 10.1016/0090-3019(93)90015-s. [DOI] [PubMed] [Google Scholar]
- 19.Traynelis VC, Marano GD, Dunker RO, Kaufman HH. Traumatic atlanto-occipital dislocation. Case report. J Neurosurg. 1986;65:863–870. doi: 10.3171/jns.1986.65.6.0863. [DOI] [PubMed] [Google Scholar]
- 20.Powers B, Miller MD, Kramer RS, Martinez S, Gehweiler JA. Traumatic anterior atlanto-occipital dislocation. Neurosurgery. 1979;4:12–17. doi: 10.1227/00006123-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lee C, Woodring JH, Goldstein SJ, Daniel TL, Young AB, Tibbs PA. Evaluation of traumatic atlantooccipital dislocations. AJNR Am J Neuroradiol. 1987;8:19–26. [PMC free article] [PubMed] [Google Scholar]
- 22.Harris JH, Carson GC, Wagner LK. Radiologic diagnosis of traumatic occipitovertebral dissociation: 1. Normal occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol. 1994;162:881–886. doi: 10.2214/ajr.162.4.8141012. [DOI] [PubMed] [Google Scholar]
- 23.Harris JH, Carson GC, Wagner LK, Kerr N. Radiologic diagnosis of traumatic occipitovertebral dissociation: 2. Comparison of three methods of detecting occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol. 1994;162:887–892. doi: 10.2214/ajr.162.4.8141013. [DOI] [PubMed] [Google Scholar]
- 24.Pang D, Nemzek WR, Zovickian J. Atlanto-occipital dislocation: part 1--normal occipital condyle-C1 interval in 89 children. Neurosurgery. 2007;61:514–521; discussion 521. doi: 10.1227/01.NEU.0000290897.77448.1F. [DOI] [PubMed] [Google Scholar]
- 25.Pang D, Nemzek WR, Zovickian J. Atlanto-occipital dislocation--part 2: The clinical use of (occipital) condyle-C1 interval, comparison with other diagnostic methods, and the manifestation, management, and outcome of atlanto-occipital dislocation in children. Neurosurgery. 2007;61:995–1015; discussion 1015. doi: 10.1227/01.neu.0000303196.87672.78. [DOI] [PubMed] [Google Scholar]
- 26.Gire JD, Roberto RF, Bobinski M, Klineberg EO, Durbin-Johnson B. The utility and accuracy of computed tomography in the diagnosis of occipitocervical dissociation. Spine J. 2013;13:510–519. doi: 10.1016/j.spinee.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Theodore N, Aarabi B, Dhall SS, Gelb DE, Hurlbert RJ, Rozzelle CJ, Ryken TC, Walters BC, Hadley MN. The diagnosis and management of traumatic atlanto-occipital dislocation injuries. Neurosurgery. 2013;72 Suppl 2:114–126. doi: 10.1227/NEU.0b013e31827765e0. [DOI] [PubMed] [Google Scholar]
- 28.Gallie WE. Fractures and dislocations of cervical spine. Am J Surg. 1939;46:495–499. [Google Scholar]
- 29.Vale FL, Oliver M, Cahill DW. Rigid occipitocervical fusion. J Neurosurg. 1999;91:144–150. doi: 10.3171/foc.1999.6.6.11. [DOI] [PubMed] [Google Scholar]
- 30.Apostolides PJ, Dickman CA, Golfinos JG, Papadopoulos SM, Sonntag VK. Threaded steinmann pin fusion of the craniovertebral junction. Spine (Phila Pa 1976) 1996;21:1630–1637. doi: 10.1097/00007632-199607150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hurlbert RJ, Crawford NR, Choi WG, Dickman CA. A biomechanical evaluation of occipitocervical instrumentation: screw compared with wire fixation. J Neurosurg. 1999;90:84–90. doi: 10.3171/spi.1999.90.1.0084. [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulos SM. Manual of cervical spine internal fixation. Philadelphia: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 33.Diagnosis and management of traumatic atlanto-occipital dislocation injuries. Neurosurgery. 2002;50:S105–S113. doi: 10.1097/00006123-200203001-00018. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R, Traynelis VC, Menezes AH. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209–1224. doi: 10.1007/s00381-008-0607-7. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RC, Ragel BT, Mocco J, Bohman LE, Brockmeyer DL. Selection of a rigid internal fixation construct for stabilization at the craniovertebral junction in pediatric patients. J Neurosurg. 2007;107:36–42. doi: 10.3171/PED-07/07/036. [DOI] [PubMed] [Google Scholar]
- 36.Hankinson TC, Avellino AM, Harter D, Jea A, Lew S, Pincus D, Proctor MR, Rodriguez L, Sacco D, Spinks T, et al. Equivalence of fusion rates after rigid internal fixation of the occiput to C-2 with or without C-1 instrumentation. J Neurosurg Pediatr. 2010;5:380–384. doi: 10.3171/2009.10.PEDS09296. [DOI] [PubMed] [Google Scholar]


