Abstract
A meta-analysis was conducted to assess alterations in measures of diffusion tensor imaging (DTI) in the patients of cervical spondylotic myelopathy (CSM), exploring the potential role of DTI as a diagnosis biomarker. A systematic search of all related studies written in English was conducted using PubMed, Web of Science, EMBASE, CINAHL, and Cochrane comparing CSM patients with healthy controls. Key details for each study regarding participants, imaging techniques, and results were extracted. DTI measurements, such as fractional anisotropy (FA), apparent diffusion coefficient (ADC), and mean diffusivity (MD) were pooled to calculate the effect size (ES) by fixed or random effects meta-analysis. 14 studies involving 479 CSM patients and 278 controls were identified. Meta-analysis of the most compressed levels (MCL) of CSM patients demonstrated that FA was significantly reduced (ES -1.52, 95% CI -1.87 to -1.16, P < 0.001) and ADC was significantly increased (ES 1.09, 95% CI 0.89 to 1.28, P < 0.001). In addition, a notable ES was found for lowered FA at C2-C3 for CSM vs. controls (ES -0.83, 95% CI -1.09 to -0.570, P < 0.001). Meta-regression analysis revealed that male ratio of CSM patients had a significant effect on reduction of FA at MCL (P = 0.03). The meta-analysis of DTI studies of CSM patients clearly demonstrated a significant FA reduction and ADC increase compared with healthy subjects. This result supports the use of DTI parameters in differentiating CSM patients from health subjects. Future researches are required to investigate the diagnosis performance of DTI in cervical spondylotic myelopathy.
Introduction
Cervical spondylotic myelopathy (CSM) is a common disease caused by chronic compression of the spinal cord secondary to spondylosis or disc degeneration [1]. It is the most common form of spinal cord dysfunction and the leading cause of spinal cord injury in individuals older than 55 years [2]. Its diagnosis is based primarily on clinical manifestations and imaging evidences. The compressed part of the spinal cord shows a specific high signal intensity (HSI) on T2-weighted MR image [3]. T2-weighted imaging alone, however, has low sensitivity for detecting the subtle structural damage of the cord in myelopathy, especially in patients with chronic onset of symptoms [4–6]. Therefore, it is difficult to evaluate the condition of the compressed spinal cord with such imaging modalities.
Diffusion tensor imaging (DTI) has been widely utilized to assess nerve microstructure by tracing water molecular diffusion in the brain [7–9]. The most commonly used invariants in DTI are fractional anisotropy (FA) and apparent diffusion coefficient (ADC). FA is an anisotropic parameter wherein 0 to 1, and values closer to 1 represent a more anisotropic structure. ADC represents water diffusion magnitude, with high ADC indicating high water mobility and few boundaries to water motion. Mean diffusivity (MD) is also used to represent the degree of diffusion motion of water molecules (regardless of direction) [10]. Although DTI is not in routine clinical use, it has been proven to be a non-invasive tool for detecting subtle damage to spinal cord that appears normal on conventional T2-weighted MR images [11]. Several recently published studies have showed that FA and ADC values changed significantly at compressive myelopathy levels compared with uncompressed levels or normal volunteers [11–13]. Besides, FA is believed to correlate with myelopathy severity and predict the postoperative neurologic improvement of CSM patients [14]. However, as clinical evidences accumulated, controversial results regarding to the DTI changes between CSM patients and healthy subjects were observed, and its diagnostic ability still remained elucidation. To determine whether DTI metrics were able to serve as candidate biomarkers of CSM, we conducted a systemic review and meta-analysis of current data to estimate the effective sizes of FA and ADC in CSM patients.
Materials and Methods
Data sources
DTI studies of CSM patients comparing with healthy control subjects were searched in three computerized database of Pubmed, Web of Scinece, EMBASE, CINAHL and Cochrane. The search was in accordance to the "preferred reporting items for systemic reviewers and meta-analysis" (PRISMA) statement [15]. The search terms used in the systemic screening were "cervical spondylotic myelopathy", "cervical pain", "cervical spinal cord", which were combined with the terms "diffusion tensor imaging", "fractional anisotropy" and "apparent diffusion coefficient". Articles were limited to those published up to December 2014. The reference lists of articles retrieved for inclusion in the review were hand searched to identify other relevant articles. Two reviewers performed independent screening of the titles and abstracts of the studies to identify the relevant studies.
Selection criteria
We imposed the following criteria for inclusion into the meta-analysis: (1) peer reviewed DTI studies in English on humans comparing patients with CSM and healthy controls; (2) reported sufficient DTI measures (FA, ADC or MD) of region of interest (ROI) for effect size calculation. If studies did not report sufficient data, we emailed the corresponding author to obtain further information. The study was excluded from our analysis if the author did not respond.
Data extraction
To perform the meta-analysis, a standardized mean difference (SMD) was defined as the effect size (ES) statistic, which was the difference between the mean of the experiment group and that of the control group. In the current study, the mean and standard deviation measures of the FA, ADC or MD were extracted from the most compressed level (MCL)/C2-C3 level in healthy controls and CSM patients. For the studies that selected multiple ROIs from different cervical levels, the weighted average effect size was calculated and integrated in the analysis. Meta-analysis of observational studies in epidemiology guidelines for conducting and reporting meta-analysis of observational studies were followed [16]. Apart from DTI measures, the following demographic, clinical and methodological variants were also extracted from the studies if available: the number of CSM and control participants, mean age, male ratio, clinical assessment, surgical treatment, scanner make, scanner field strength, numbers of diffusion gradient directions, voxel size, field of voxel, b factor and DTI post-processing software.
Assessment of study quality
The quality of the included studies was assessed using the Newcastle-Ottawa scale[17]. The scale consists of nine items that cover three dimensions: selection (4 points), comparability (2 points), and exposure (3 points). A high score out of a total of 9 points indicates high quality.
Statistical Method
We used STATA version 12.0 (STATA, College Station, TX, USA) meta package (version 1.86) for continuous data meta-analysis. I2-values were computed as a measure of in-between study heterogeneity. Thresholds for the interpretation of I2 were based on previous studies suggesting that 0% to 50% represented mild heterogeneity, 50% to 75% moderate heterogeneity, and 75% to 100% considerable heterogeneity [9]. We estimated the pooled SMD with a 95% confidence interval (CI) on the basis of both the fixed and random effects models. When low heterogeneity (I2<50%) was observed in the analysis, the pooled SMD was reported on the basis of the fixed effects models. Otherwise, random effects models were used. Publication bias was examined by visual inspection of funnel plot asymmetry and Egger test [18].
To investigate the potential modifiers of the DTI differences between CSM patients and healthy controls, meta-regression analyses were performed to exam the relationship between male ratio, mean age, scanner field strength and SMD for the FA and ADC values. The regression analyses were conducted using STATA software.
Results
Selected articles
The method used to search relevant studies was presented in the flow diagram of Fig. 1. The initial literature search yielded 1016 original articles. The search strategy was extremely liberal, in order to capture all possible articles for inclusion in this review. After discarding 86 duplicate studies, we screened 930 studies for eligibility. From the remaining 930 potential candidates, 894 were excluded according to selection criteria. 10 studies were excluded because they did not compare CSM patients with healthy controls. 7 studies were discarded because they did not provide sufficient data to calculate the effect size. 4 studies comparing the DTI changes in specific ROIs or tracts instead of the whole spinal cord were excluded. 1 study investigating other causes of chronic spinal cord compression was excluded. Finally, 10 studies were included in our meta-analysis, involving 479 CSM patients and 278 healthy controls [12,19–31]. Demographic details of the included studies were listed in Table 1, and relevant technical factors were presented in Table 2. Assessment of study quality was summarized in Table 3.
Fig 1. Flow of identification of relevant studies.
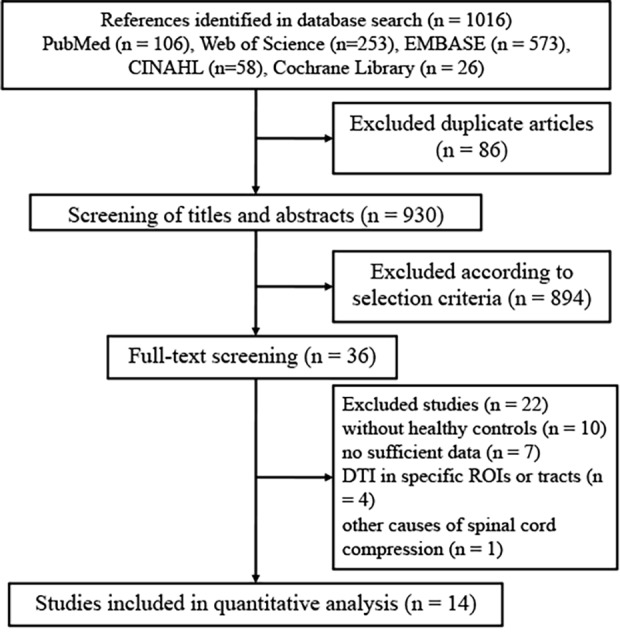
Table 1. Demographic and clinical characteristics of DTI studies of CSM in meta-analysis.
| Study | Year | Design | Level of evidence | Number (female) | Mean age, range (year) | Clinical assessment | Surgical treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| CSM | HC | CSM | HC | ||||||
| Mamata | 2005 | Case-control | 3 | 7(3) | 11(6) | NA,26–82 | 37.7,30–48 | NA | |
| Facon | 2005 | Case-control | 3 | 7(3) | 11(3) | 48.2,30–76 | 36.7,NA | NA | |
| Xiangshui | 2010 | Case-control | 3 | 84(36) | 21(9) | 45,16–63 | 43,18–60 | NA | |
| Kim | 2010 | Case-control | 3 | 8(2) | 14(NA) | 59.5,48–78 | 34,NA | NA | |
| Kang | 2011 | Case-control | 3 | 11(5) | 10(6) | 67.2, NA | 33.4, NA | NA | |
| Lee | 2011 | Case-control | 3 | 21(7) | 26(7) | 49.6,22–67 | 49.6,22–67 | mJOA | ✓ |
| Budzik | 2011 | Case-control | 3 | 20(10) | 15(7) | 57.3,34–78 | 54.8,35–73 | Self questionnaire | |
| Song | 2011 | Case-control | 3 | 53(25) | 20(6) | 56,47–71 | 55,46–67 | NA | |
| Uda | 2013 | Case-control | 3 | 26(11) | 30(15) | 59.4,41–82 | 44.2,20–72 | NA | |
| Wen | 2013 | Case-control | 3 | 7(4) | 15(NA) | 56,45–67 | 42,36–48 | mJOA | |
| Wen | 2014 | Case-control | 3 | 45(19) | 20(10) | 64,43–86 | 52,41–62 | mJOA | ✓ |
| Banaszek | 2014 | Case-control | 3 | 132(78) | 25(14) | 53.58,18–76 | 45.78,27–80 | NA | |
| Cui | 2014 | Case-control | 3 | 23(8) | 20(NA) | 59,NA | 46,NA | mJOA | |
| Rajasekaran | 2014 | Case-control | 3 | 35(32) | 40(20) | 48,NA | 38,NA | Nurick | |
CSM, cervical spondylotic myelopathy; HC, healthy control; mJOA, modified Japanese Orthopedic Association score; NA, not available
Table 2. Technical details of DTI studies on ALS in meta-analysis.
| Study | Year | DTI measures | Scanner make | Field-str. | DTI dir. | Voxel size(mm) | DTI proc. | ROI placement | FOV (mm) | b (mm2/s) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | ADC | MD | ||||||||||
| Mamata | 2005 | ✓ | ✓ | General Electric | 1.5 T | NA | NA | NA | Sagittal | 220*110 | 1000 | |
| Facon | 2005 | ✓ | ✓ | NA | 1.5 T | 6 | 1.4*1.4 | DPTools | Sagittal | 179*179 | 500 | |
| Xiangshui | 2010 | ✓ | ✓ | General Electric | 3.0 T | 15 | NA | GE Functool | Axial | 270*270 | 1000 | |
| Kim | 2010 | Siemens | 3.0 T | 12 | 1.5*1.5 | home-made software | Sagittal | 160*40 | 500 | |||
| Kang | 2011 | ✓ | ✓ | Siemens | 1.5 T | NA | NA | Syngo software | Axial | 140*140 | 1000 | |
| Lee | 2011 | ✓ | ✓ | Philips Achieva | 3.0 T | 15 | 1.95*1.95 | NA | Axial | 250*224 | 600 | |
| Budzik | 2011 | ✓ | ✓ | Philips Achieva | 1.5 T | 25 | 1.56*1.56 | NA | Sagittal | 200*200 | 900 | |
| Song | 2011 | ✓ | ✓ | Philips Gyroscan | 1.5 T | 6 | NA | NA | Axial | 230*230 | 400 | |
| Uda | 2013 | ✓ | ✓ | Philips Achieva | 3.0 T | 15 | 1.5*1.5 | NA | Axial | 240*240 | 1000 | |
| Wen | 2013 | ✓ | Philips Achieva | 3.0 T | 15 | 0.63*0.64 | Diffusion Toolkit | Axial | 80*80 | 600 | ||
| Wen | 2014 | ✓ | Philips Achieva | 3.0 T | 15 | 1.0*1.26 | DTI Studio software | Axial | 80*80 | 600 | ||
| Banaszek | 2014 | ✓ | ✓ | General Electric | 1.5 T | 15 | 1.6*1.6 | GE Functool | Axial | 160*160 | 1000 | |
| Cui | 2014 | ✓ | ✓ | Philips Achieva | 3.0 T | 15 | 0.63*0.63 | Diffusion Toolkit | Axial | 80*36 | 600 | |
| Rajasekaran | 2014 | ✓ | ✓ | Siemens | 1.5 T | 12 | 0.86*0.86 | NA | Axial | 220*220 | 500 | |
DTI, diffusion tensor imaging; FA, fractional anisotropy; ADC, apparent diffusion coefficient; MD, mean diffusivity; ROI, region of interest; T, Tesla; FOV, field of voxel; NA, not available
Table 3. Quality assessment of studies according to Newcastle-Ottawa Scale.
| Study | Year | Selection | Comparability | Exposure | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | E1 | E2 | E3 | |||
| Mamata | 2005 | * | * | * | * | * | * | * | * | * | 9 |
| Facon | 2005 | * | * | * | * | - | - | * | * | * | 7 |
| Xiangshui | 2010 | * | * | * | * | * | - | * | * | * | 8 |
| Kim | 2010 | * | * | * | * | - | - | * | * | * | 7 |
| Kang | 2011 | * | * | * | * | * | - | * | * | * | 8 |
| Lee | 2011 | * | * | * | * | * | * | * | * | * | 9 |
| Budzik | 2011 | * | * | * | * | * | * | * | * | * | 9 |
| Song | 2011 | * | * | * | * | * | * | * | * | * | 9 |
| Uda | 2013 | * | * | * | * | * | - | * | * | * | 8 |
| Wen | 2013 | * | * | * | * | * | - | * | * | * | 8 |
| Wen | 2014 | * | * | * | * | * | - | * | * | * | 8 |
| Banaszek | 2014 | * | * | * | * | * | - | * | * | * | 8 |
| Cui | 2014 | * | * | * | * | * | - | * | * | * | 8 |
| Rajasekaran | 2014 | * | * | * | * | - | - | * | * | * | 7 |
S1: Selection1-is the case definition adequate; S2: Selection2-representativeness of the cases; S3: Selection3-selection of controls; S4: Selection4-definition of controls. C1: Comparability1-comparability of controls for most important factor; C2: Comparability2-comparability of controls for other factors. E1: Exposure1-ascertainment of exposure; E2: Exposure2-same method of ascertainment for cases and controls; E3: Exposure3-non-response rate.
Meta-analysis of CSM induced DTI changes at MCL
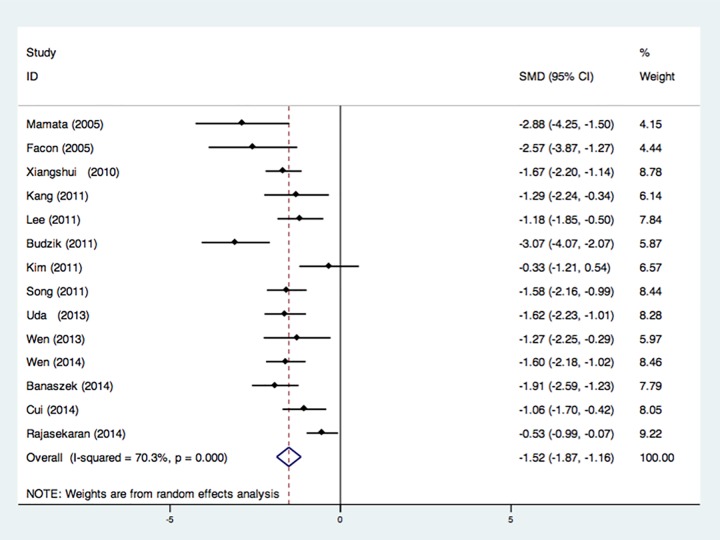
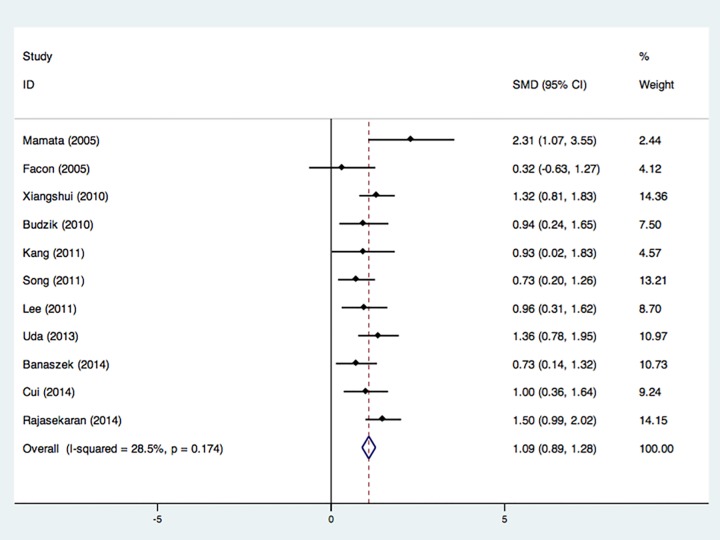
14 studies that recruited 380 CSM patients and 266 controls were integrated in the meta-analytical differences in FA value at MCL, and showed a significant FA decrease in CSM patients with an estimated weighted pooled disease effect size of -1.52 (P < 0.001) (Fig. 2) [12,19–31]. However, review of the heterogeneity measures revealed a moderate level of in-between study variation (I2 = 70.3%). 11 studies with 320 CSM patients and 215 health subjects demonstrated a significant ADC (MD) increase at the most compressed level of CSM patients (ES = 1.09, P < 0.001) with acceptable heterogeneity (I2 = 28.5%) (Fig. 3) [12,19,20,24–31].
Fig 2. Forest plot of Standardized mean differences (SMD) for FA at most compressed level between CSM patients and healthy controls.
FA was significantly reduced in CSM patients. CI indicates confidence interval.
Fig 3. Forest plot of Standardized mean differences (SMD) for ADC at most compressed level between CSM patients and healthy controls.
ADC was significantly increased in CSM patients. CI indicates confidence interval.
Meta-analysis of CSM induced DTI changes at C2-C3 level
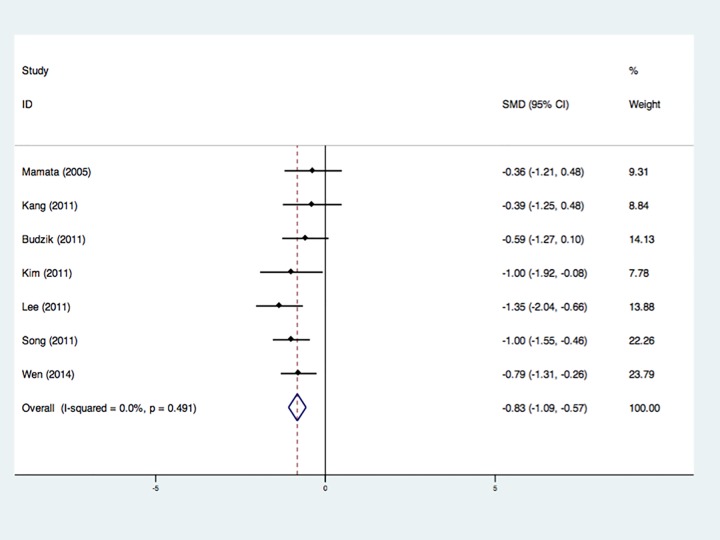
7 studies involving 165 CSM patients and 112 healthy subjects were integrated using a random effect model, and revealed a decrease of FA values at C2-C3 level of CSM patients without heterogeneity (Fig. 4) [12,19,21,23,24,26,28]. 4 datasets including 77 CSM patients and 76 controls revealed no significant ADC (MD) changes at C2-C3 level between CSM patients and controls [12,24–26].
Fig 4. Forest plot of Standardized mean differences (SMD) for FA at C2-C3 level between CSM patients and healthy controls.
FA was significantly reduced in CSM patients. CI indicates confidence interval.
Publication bias
For the FA reduction and ADC increase at MCL of CSM patients, the Egger test showed no evidence of publication bias (FA, P = 0.077; ADC, P = 0.944). For the decreased FA at C2-C3 level of CSM patients, the Egger test also showed no publication bias (P = 0.469).
Meta-regression
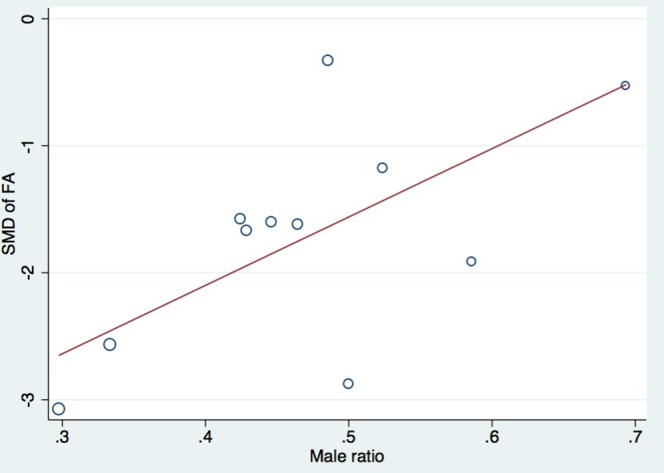
To investigate the possible modifiers of FA reduction and ADC increase in CSM patients, we conducted a meta-regression analysis with 3 modifiers: male ratio, mean age of the participants and magnetic field strength. Meta-regression analysis revealed no significant effect of mean age (P = 0.28) and magnetic field strengths (P = 0.47) on reduction of FA or increase of ADC in CSM patients. However, male ratio of CSM patients had a significant effect on reduction of FA at MCL (P = 0.03) (Fig. 5). Additionally, after reviewing all the included studies, we found a variation of ROI placement between studies. When DTI parameters were measured, the ROI were usually set to encompass the whole spinal cord on the axial plane, while four studies obtain DT images from sagittal section [12,20,23,24]. Moreover, the strongest variation of effect size in the meta-analysis was found in these studies with sagittal ROI placement. After excluding these four studies, the initial high heterogeneity was reduced in the analysis of FA values at MCL.
Fig 5. The relationship between effect size for FA and male ratio of CSM patients.
Discussion
To the best of our knowledge, this is the first meta-analysis of DTI studies between CSM patients and healthy subjects. The present meta-analysis mainly showed that CSM patients had significant FA reduction and ADC increase at both the most compressed part of cervical spinal cord and C2-C3 level. In addition, meta-regression analysis was conducted to exam the potential effects of male ratio, mean age of participants, and MRI parameters on these DTI changes. Male ratio of the CSM patients and ROI placement appeared to be potential modifiers of DTI changes in CSM.
The significantly decreased FA and increased ADC demonstrated by our meta-analysis suggest the potential diagnostic ability of DTI in CSM patients. Our findings have confirmed the assumption that previous studies were unable to demonstrate significant differences because of the small number of participants. Various microstructural conditions of the compressed cervical cord, including gliosis, microcystic degeneration, demyelination and extracellular edema, may lead to increased water mobility (ADC) and decreased anisotropy (FA) [32,33]. However, previous studies suggested that the early phases of spinal cord compression was characteristic of a focal decrease in ADC and an increase in FA [20,34]. Cheung et al. [35] conducted DTI analysis on CSM rat models and revealed the characteristic increase in ADC and decrease in FA as late as 9 months after the start of compression. In the present study, hardly could we find any correlation between DTI difference and disease duration because seldom studies provided duration of CSM patients.
In this study, a significant decrease in FA was observed not only in the part of most compressed level, but also at sites distant from MCL, like C2-C3 level. This finding reflects the fact that CSM-associated demyelination and axonal damage afflicted both the myelopathic lesion and the distal sites in the chronic course of the disease [36,37]. Thus, the diffusion indexes from the whole cervical spinal cord could be selected to comprehensively reflect overall damage in CSM patients.
Compared with conventional MRI, DTI indexes prove to be more sensitive in the detection of CSM patients, especially early myelopathy. HSI on T2-weighted images is often used to diagnose CSM, but this finding is not observed in every patient with clinical signs of myelopathy, and it sensitivity is reported to be low (between 15% and 65%) [32,38–40]. Additionally, T2 HSI is generally observed only in later stages of the disease [11]. Recent studies conducted DTI analysis on CSM patients with neurological signs but without T2 HSI, and revealed significant reduced FA and increased ADC at the stenotic levels.[41–43] Demir et al. [11] suggested that ADC value had nearly a 80% sensitivity and 53% specificity for detecting myelopathy in patients with spinal cord compression. Uda et al. [25] employed receiver-operator characteristic (ROC) analysis and suggested mean ADC as the best predictor of meyelopahty. Consistent to previous studies, our meta-analysis showed significantly increased ADC and decreased FA at both MCL and normal levels of CSM patients.
In addition to identifying abnormalities before the development of T2 HSI, quantitative analysis of DTI metrics also makes it possible to evaluate severity of myelopathy and predict the outcome of surgical treatments. Previous attempts have been made to investigate the relationship between DTI indexes and various clinical assessments in patients with CSM [14,21,25–27]. Budzik et al. [24] reported a positive correlation with FA and a self-administered questionnaire. Data from Jones et al. [44] demonstrated a strong correlation between FA and specific clinical assessments, including modified Japanese Orthopedic Association (mJOA) and Nurick scores. Recently, they also reported that severely affected CSM patients with higher FA at the compressed level tended to achieve better functional recovery after decompression surgery when compared with subjects with lower FA, indicating FA as a potential biomarker of better postoperative outcome [14]. Similarly, in the study of Wen et al. [21], FA was significantly correlated with mJOA score and enabled prediction of good surgical outcomes by Logistic regression (P = 0.030), while the presence of HSI on T2-weighted images did not (P = 0.176).
There are a number of clinical and methodological confounds that may affect DTI quantitative values. Mamata et al. [12] reported that 46% of the patients of CSM showed no elevation in ADC and no decrease in FA of the spinal cord at the narrow spinal canal level. Meanwhile, they found an increased ADC and a decreased FA with age in the spinal cord. Besides, MRI sequences, magnetic field strengths and ROI placement may also cause differences in the final DTI indexes [27]. DTI of the spinal cord can be performed in any MRI scanner that has a minimum 1.5 T magnet and at least six gradient directions [45]. The more gradient directions, the better the differentiation of nerve fibers. A 3.0 T scanner makes it possible to provide higher quality images because the signal-to-noise ratio correlates with field strength. In addition, movement artifacts are also minimized with a 3.0 T scanner because of the shortened scanning time. Hori et al. [42] investigated the line scan DTI findings in CSM patients using a 0.2-T MRI scanner, and also revealed abnormalities undetectable on T2-weighted images. In the present study, meta-analysis revealed no significant effects of these MRI parameters on the changes of DTI values in CSM patients, while strong effect size variations were found in studies that place ROIs on the sagittal plane.
In addition to improving the diagnosis of CSM and predicting surgical outcome, DTI technology can also be utilized in the controversial management of CSM patients. One possible role for this imaging evaluation would be to assess changes or progression as part of sequential follow-up of patients where the signs and conventional imaging findings are not sufficient to warrant follow-up. It has been well demonstrated that some mild CSM patients can be successfully treated by non-operative treatments [46]. DTI could possibly serve as a non-invasive tool to monitor asymptomatic or mildly affected CSM patients, which does not currently exist.
There are some methodology and data limitations to be considered for this meta-analysis. Firstly, the number of studies and the size of studies were modest, limiting the generalizability of the results. Although meta regression showed that the modest heterogeneity might result from male ratio of the participants, we failed to explore more potential modifiers of DTI changes because of the limited information from the pooled studies. In addition, the stage of CSM disease, either early or advanced, may influence the DTI findings. In the present study, diagnostic criteria of CSM patients we used were clinical manifestations and imaging evidences, which represented a relatively later stage of the disease. An early-stage DTI meta study could be interesting because it would provide stronger evidence that DTI has a better diagnostic ability of early CSM patients without abnormality on conventional MRI.
Conclusions
In conclusion, our findings from this meta-analysis of DTI studies of CSM patients clearly demonstrated a significant FA reduction and ADC increase compared with healthy subjects. This results support the use of DTI parameters in differentiating CSM patients from health subjects. Future researches are required to investigate the diagnosis performance of DTI in cervical spondylotic myelopathy.
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Crandall PH, Batzdorf U (1966) Cervical Spondylotic Myelopathy*. Journal of neurosurgery 25: 57–66. [DOI] [PubMed] [Google Scholar]
- 2. Montgomery DM, Brower RS (1992) Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am 23: 487–493. [PubMed] [Google Scholar]
- 3. Vedantam A, Jonathan A, Rajshekhar V (2011) Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy: Clinical article. Journal of Neurosurgery: Spine 15: 660–666. 10.3171/2011.8.SPINE11452 [DOI] [PubMed] [Google Scholar]
- 4.Hori M, Tsutsumi S, Yasumoto Y, Ito M, Suzuki M, et al. (2014) Cervical spondylosis: Evaluation of microstructural changes in spinal cord white matter and gray matter by diffusional kurtosis imaging. Magn Reson Imaging. [DOI] [PubMed]
- 5. Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, et al. (2000) Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: does it predict the outcome of conservative treatment? Spine 25: 677–682. [DOI] [PubMed] [Google Scholar]
- 6. Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, et al. (1991) Increased MR signal intensity due to cervical myelopathy: analysis of 29 surgical cases. Journal of neurosurgery 74: 887–892. [DOI] [PubMed] [Google Scholar]
- 7. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, et al. (2001) Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging 13: 534–546. [DOI] [PubMed] [Google Scholar]
- 8. Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H (2012) Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry 83: 870–876. 10.1007/s00415-014-7599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, et al. (2014) Diffusion tensor imaging of the spinal cord: Insights from animal and human studies. Neurosurgery 74: 1–8. 10.1227/NEU.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demir A, Ries M, Moonen CT, Vital JM, Dehais J, et al. (2003) Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 229: 37–43. [DOI] [PubMed] [Google Scholar]
- 12. Mamata H, Jolesz FA, Maier SE (2005) Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. Journal of Magnetic Resonance Imaging 22: 38–43. [DOI] [PubMed] [Google Scholar]
- 13. Maier SE, Mamata H (2005) Diffusion tensor imaging of the spinal cord. Annals of the New York Academy of Sciences 1064: 50–60. [DOI] [PubMed] [Google Scholar]
- 14. Jones JGA, Cen SY, Lebel RM, Hsieh PC, Law M (2013) Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. American Journal of Neuroradiology 34: 471–478. 10.3174/ajnr.A3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine 151: W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA SB, O’Connell D, Peterson J, Welch V, et al. (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 18. Hayashino Y, Noguchi Y, Fukui T (2005) Systematic evaluation and comparison of statistical tests for publication bias. Journal of epidemiology/Japan Epidemiological Association 15: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song T, Chen WJ, Yang B, Zhao HP, Huang JW, et al. (2011) Diffusion tensor imaging in the cervical spinal cord. European Spine Journal 20: 422–428. 10.1007/s00586-010-1587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Facon D, Ozanne A, Fillard P, Lepeintre F, Tournoux-Facon C, et al. (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. American journal of neuroradiology 26: 1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 21. Wen CY, Cui JL, Liu HS, Mak KC, Cheung WY, et al. (2014) Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy. Radiology 270: 197–204. 10.1148/radiol.13121885 [DOI] [PubMed] [Google Scholar]
- 22. Wen CY, Cui JL, Lee MP, Mak KC, Luk KD, et al. (2013) Quantitative analysis of fiber tractography in cervical spondylotic myelopathy. Spine J 13: 697–705. 10.1016/j.spinee.2013.02.061 [DOI] [PubMed] [Google Scholar]
- 23. Kim TH, Zollinger L, Shi XF, Kim SE, Rose J, et al. (2010) Quantification of diffusivities of the human cervical spinal cord using a 2D single-shot interleaved multisection inner volume diffusion-weighted echo-planar imaging technique. AJNR Am J Neuroradiol 31: 682–687. 10.3174/ajnr.A1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, et al. (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21: 426–433. 10.1007/s00330-010-1927-z [DOI] [PubMed] [Google Scholar]
- 25. Uda T, Takami T, Tsuyuguchi N, Sakamoto S, Yamagata T, et al. (2013) Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine (Phila Pa 1976) 38: 407–414. [DOI] [PubMed] [Google Scholar]
- 26. Lee JW, Kim JH, Park JB, Park KW, Yeom JS, et al. (2011) Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol 40: 1543–1551. 10.1007/s00256-011-1161-z [DOI] [PubMed] [Google Scholar]
- 27. Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, et al. (2010) 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol 65: 465–473. 10.1016/j.crad.2010.01.019 [DOI] [PubMed] [Google Scholar]
- 28.M kang EA, R Elliott, S Kalhorn, P Cooper (2010) Diffusion Tensor Imaging of the Spondylotic Cervical Spinal Cord: A Preliminary Study of Quantifiable Markers in the Evaluation for Surgical Decompression. The Internet Journal of Head and Neck Surgery 5.
- 29.Cui JL, Li X, Chan TY, Mak KC, Luk KD, et al. (2014) Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. [DOI] [PubMed]
- 30. Rajasekaran S, Yerramshetty JS, Chittode VS, Kanna RM, Balamurali G, et al. (2014) The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine (Phila Pa 1976) 39: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 31. Banaszek A, Bladowska J, Szewczyk P, Podgorski P, Sasiadek M (2014) Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J 23: 1523–1530. 10.1007/s00586-014-3323-x [DOI] [PubMed] [Google Scholar]
- 32. Baron EM, Young WF (2007) Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 60: S1–35. [DOI] [PubMed] [Google Scholar]
- 33. Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. The Spine Journal 6: S190–S197. [DOI] [PubMed] [Google Scholar]
- 34. Ford JC, Hackney DB, Lavi E, Phillips M, Patel U (1998) Dependence of apparent diffusion coefficients on axonal spacing, membrane permeability, and diffusion time in spinal cord white matter. J Magn Reson Imaging 8: 775–782. [DOI] [PubMed] [Google Scholar]
- 35. Cheung MM, Li D, Hui ES, Fan S, Ding AY, et al. In vivo diffusion tensor imaging of chronic spinal cord compression in rat model; 2009. IEEE; pp. 2715–2718. 10.1109/IEMBS.2009.5333389 [DOI] [PubMed] [Google Scholar]
- 36. Urakawa T, Matsuzawa H, Suzuki Y, Endo N, Kwee IL, et al. (2011) Analysis of ascending spinal tract degeneration in cervical spondylotic myelopathy using 3D anisotropy contrast single-shot echo planar imaging on a 3.0-T system. J Neurosurg Spine 15: 648–653. 10.3171/2011.7.SPINE10843 [DOI] [PubMed] [Google Scholar]
- 37. Fehlings MG, Arvin B (2009) Surgical management of cervical degenerative disease: The evidence related to indications, impact, and outcome. Journal of Neurosurgery: Spine 11: 97–100. 10.3171/2009.5.SPINE09210 [DOI] [PubMed] [Google Scholar]
- 38. Aota Y, Niwa T, Uesugi M, Yamashita T, Inoue T, et al. (2008) The correlation of diffusion-weighted magnetic resonance imaging in cervical compression myelopathy with neurologic and radiologic severity. Spine (Phila Pa 1976) 33: 814–820. 10.1097/BRS.0b013e318169505e [DOI] [PubMed] [Google Scholar]
- 39. Fernández de Rota JJ, Meschian S, Fernández de Rota A, Urbano V, Baron M (2007) Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. Journal of Neurosurgery: Spine 6: 17–22. [DOI] [PubMed] [Google Scholar]
- 40. Chen C-J, Lyu R-K, Lee S-T, Wong Y-C, Wang L-J (2001) Intramedullary High Signal Intensity on T2-Weighted MR Images in Cervical Spondylotic Myelopathy: Prediction of Prognosis with Type of Intensity 1. Radiology 221: 789–794. [DOI] [PubMed] [Google Scholar]
- 41. Hori M, Fukunaga I, Masutani Y, Nakanishi A, Shimoji K, et al. (2012) New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur Radiol 22: 1797–1802. 10.1007/s00330-012-2410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hori M, Okubo T, Aoki S, Kumagai H, Araki T (2006) Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging 23: 183–188. [DOI] [PubMed] [Google Scholar]
- 43. Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, et al. (2011) The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology 53: 609–616. 10.1007/s00234-011-0844-4 [DOI] [PubMed] [Google Scholar]
- 44. Jones J, Lerner A, Kim PE, Law M, Hsieh PC (2011) Diffusion tensor imaging in the assessment of ossification of the posterior longitudinal ligament: a report on preliminary results in 3 cases and review of the literature. Neurosurg Focus 30: E14 10.3171/2011.3.FOCUS1138 [DOI] [PubMed] [Google Scholar]
- 45. Rajasekaran S, Kanna RM, Shetty AP (2012) Diffusion tensor imaging of the spinal cord and its clinical applications. Journal of Bone and Joint Surgery—Series B 94 B: 1024–1031. 10.2106/JBJS.N.01130 [DOI] [PubMed] [Google Scholar]
- 46. Kadaňka Z, Bednařík J, Voháňka S, Vlach O, Stejskal L, et al. (2000) Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. European Spine Journal 9: 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.