Abstract
Background. In Mexico and other developing countries, few reports of the survival of children with acute leukaemia exist. Objective. We aimed at comparing the disease-free survival of children with acute myeloid leukaemia who, in addition to being treated with the Latin American protocol of chemotherapy and an autologous transplant, either underwent early intensified chemotherapy or did not undergo such treatment. Procedure. This was a cohort study with a historical control group, forty patients, less than 16 years old. Group A (20 patients), diagnosed in the period 2005–2007, was treated with the Latin American protocol of chemotherapy with an autologous transplant plus early intensified chemotherapy: high doses of cytarabine and mitoxantrone. Group B (20 patients), diagnosed in the period 1999–2004, was treated as Group A, but without the early intensified chemotherapy. Results. Relapse-free survival for Group A was 90% whereas that for Group B it was 60% (P = 0.041). Overall survival for Group A (18, 90%) was higher than that for Group B (60%). Complete remission continued for two years of follow-up. Conclusions. Relapse-free survival for paediatric patients treated with the Latin American protocol of chemotherapy with an autologous transplant plus early intensified chemotherapy was higher than that for those who did not receive early intensified chemotherapy.
1. Background
The importance of acute myeloid leukaemia (AML) is evident because, although comprising only 15%–20% of childhood leukaemias, the mortality rates of AML account for up to 30% of leukaemia-related deaths [1–3]. In the past three decades, the prognosis of paediatric AML has improved significantly because of progress in the treatment of this disease, principally through the introduction of regimens of multidrug use and of intensification therapy and through improvement in postremission therapy and in support care. Despite these improvements, the accumulated risk of relapse is still approximately 30%–40% [4].
When administered alone, chemotherapy in paediatric AML has improved the probability of event-free survival (EFS); however, even in the best series published in the last decade, EFS reached only 31%–54% with the probability of overall survival (OS) between 36% and 66% [5–17]. When chemotherapy is combined with autologous haematopoietic transplantation, similar results are obtained; however, the importance of this combinatory treatment lies in its demonstrated ability to lower the risk of relapse [18–20]. Although allogeneic haematopoietic transplantation offers a higher probability of cure (up to 67%), its use in primary remission in groups with good risk is not recommended, because of its morbidity-mortality and because its use has not shown better results than chemotherapy alone [8, 12, 16, 19, 21, 22]. When the patients are stratified by independent prognostic indicators, groups with chromosomal aberrations indicative of good risk, such as t(8:21), inv (16), and t(15:17), can reach an OS of 91%, 92%, and 87%, respectively [23, 24].
Although the results with autologous transplants are similar to those with chemotherapy alone and their use is controversial, some groups that have obtained results either superior to those of chemotherapy alone or similar to those of allogeneic transplant retain their interest in the use of autologous transplants [25–30], because not only is the need for an HLA-identical donor obviated, but also the potentially fatal graft versus host disease is avoided.
However, there are disadvantages, such as a lack of effect of the graft against the leukaemia and the risk of reinfusion of leukaemia cells, bringing with them a greater risk of relapse. To reduce the latter possibility, various techniques of purge ex vivo or purge in vivo have been developed [31–34]. With the purge in vivo, together with the harvest of peripheral blood stem cells (PBSC), the risk of harvesting neoplastic cells is reduced; as a consequence, the risk of relapse from the disease is also reduced [34, 35]. When a patient previously received various cycles of intensification chemotherapy, even better results have been obtained [25, 27, 36, 37].
In the present study, the patients with AML, starting in 1999, received chemotherapy (called the Latin American protocol) that was based on the protocol of the German group (AML-Berlin-Frankfurt-Münster (BFM) 87 [38]) plus autologous transplantation in first remission, resulting in a five-year survival rate of 60%; starting in 2005, patients received, in addition to this standard treatment, a cycle of early intensification (EI) chemotherapy with high doses of cytarabine (Ara-C) and mitoxantrone (HAM) (AML-BFM 93) [39]. The objective of the present study was to compare the disease-free survival of children with AML who, in addition to being treated with the Latin American protocol of chemotherapy and an autologous transplant, either underwent EI chemotherapy or did not undergo such treatment.
2. Methods
2.1. Study Design
Cohort study for assessing survival: the Hematological Service of the General Hospital of the National Medical Center “La Raza” in Mexico City is the facility that treats the greatest number of patients with leukaemia in the whole Mexico. This project was approved by the Ethics and Research Committee of the General Hospital of the National Medical Center “La Raza,” Mexico City (number R2008-3502-63). Written consent from the parents of the patients was obtained when the children were first admitted to the hospital.
2.2. Patient Groups
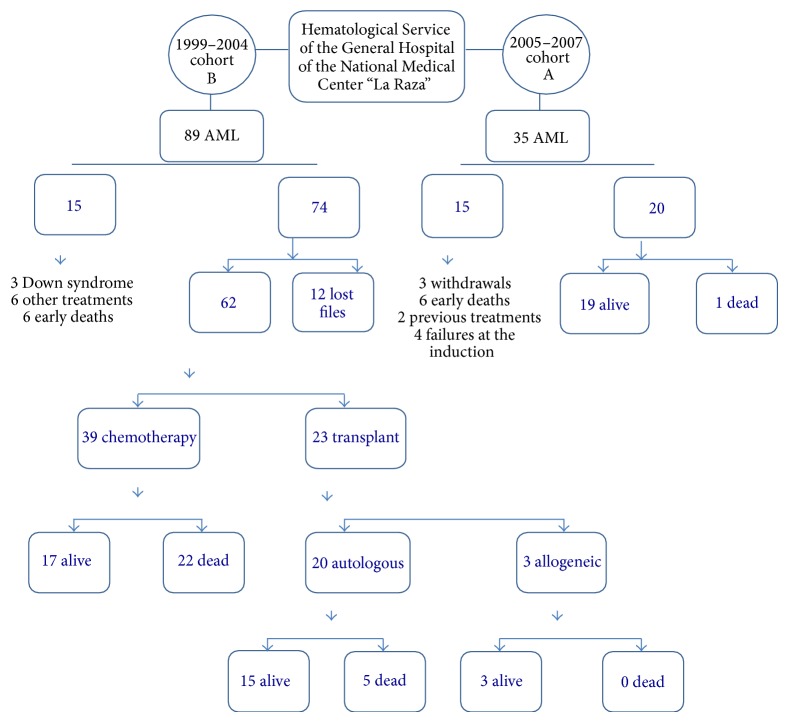
All patients were less than 16-year-old. Group A (n = 20) comprised those patients who had been diagnosed with AML for the first time from January 2005 to December 2007. Group B (n = 20) consisted of those patients diagnosed with AML for the first time from January 1999 to December 2004. The composition of the cohorts is described in Figure 1. The patients were included when they the complete remission in all subtypes since M0 to M7 was reached and only to M3 when the patients had the second remission reached or when the PLM/RARα was negative.
Figure 1.
Patient selection. Group A (20 patients), diagnosed in the period 2005–2007, was treated with the Latin American protocol of chemotherapy with an autologous transplant plus early intensified chemotherapy: high doses of cytarabine and mitoxantrone (HAM). Group B (20 patients), diagnosed in the period 1999–2004, was treated as Group A but without the early intensified chemotherapy. AML: acute myeloid leukaemia.
The French-American-British (FAB) classification was used for the initial diagnosis and the microscopy determinations were corroborated by at least three haematologist observers; the subtypes, M0 and M7, were confirmed by immunologic methods; bone marrow aspirates taken on Day 15 were examined by two or more haematologists.
2.3. Risk Classification
The classification of risk was conducted according to the criteria of group AML-BFM 93 [40]. Patients at low risk were those with morphological subtypes M1, M2 (with Auer bodies) or t(8:21), as well as M4 with eosinophilia and 16 inversion in the karyotype, and if they had <5% blast cells in the bone marrow on Day 15 of induction of remission (IR). Patients with high risk were those, that morphologically were clasified as M1, M2, or M4, additionally in patients M4 and without characteristic mentioned above. With either >5% blast cells in the bone marrow on Day 15 of IR; with monosomy 7, 5 in the karyotype; or with complex karyotypes.
2.4. Response Criteria
The criteria used were those of the International Working Group for the Diagnosis and Treatment of AML [41]. Complete haematological remission: no clinical evidence of leukaemia; bone marrow with restoration of normal haematopoiesis, with <5% blast cells; and, in the peripheral blood, ≥1 500 neutrophils/μL and ≥100 000 platelets/μL without transfusion. Early death: if death occurred when treatment was not initiated, or if death occurred less than seven days after having finished the first cycle of IR. Death during treatment: death during treatment with hypoplastic bone marrow. Therapeutic failure: persistence of leukaemia after having received two cycles of chemotherapy for IR. Relapse-free survival (DFS): the time that elapsed from complete remission until the haematological, genetic, or molecular detection of relapse of the disease. Relapse: after remission was declared, reemergence of the disease as indicated by blast cells in the peripheral blood, ≥5% of blast cells in the bone marrow, or extramedullary infiltration. Overall survival (OS): the time elapsed from the date of diagnosis until the most recent follow-up. Toxicity of the chemotherapy was evaluated in accordance with the criteria of the US National Cancer Institute (NCI) [42].
2.5. Treatment
The schematic of chemotherapy (Supplemental Figure I, see Supplementary Material available online at http://dx.doi.org/10.1155/2015/940278) which for Group B was based on the Latin American protocol (AML-BFM 87) [38] and which for Group A was based on AML-BFM 93 [39], with the following modifications: 6-thioguanine was not used in the consolidation, because this drug is not available in Mexico; prophylactic radiation of the central nervous system (CNS) was not administered, because it was not considered necessary as, historically, the number of relapses in the Hematological Service is very low for the type of patients treated. Patients in both groups underwent an autologous transplant in first complete remission (CR1). The toxicity related to this regimen was investigated by using the NCI criteria [42].
2.6. Statistical Analysis
For the quantitative variables without normal distribution, a Mann-Whitney U test was used for comparison of two independent groups; for qualitative variables, χ 2 or Fisher's exact test was used. P < 0.05 was considered statistically significant. The Kaplan-Meier method was used to construct survival plots; a log-rank test was used for comparison between groups.
We analysed the following potential variables that influence relapse: age, sex, leukocyte count at diagnosis, response on Day 15 of the IR, time from diagnosis to complete remission (CR), low or high risk, the cycles of chemotherapy required to achieve remission, time of remission to transplant, quantity of MNC × 108/kg, and quantity of CD34+ × 106/kg. For these variables, the crude relative risk (CRR), the CRR by strata, and the CRR, adjusted by Mantel-Haenszel statistics, with confidence intervals of 95%, were calculated in two-by-two tables. The statistical package SPSS version 21 was used.
3. Results
3.1. General Characteristics of the Patients
In this study, no significant differences were found between the groups (20 patients per group) for any of the general or clinical characteristics analysed (Table 1). For the two groups, the median age was nine years; the distribution by sex was similar; the median leukocyte count at diagnosis was 28 250/μL for Group A and 26 900/μL for Group B (P = 0.698). In both groups, the most common morphological subtypes were M4 and M5, which together comprised 55% of the cases in Group A and 60% in Group B. Of the 40 patients, 35% had a normal karyotype; five had a low-risk karyotype (t(8:21)), and three had a high-risk karyotype. According to the risk classification of the BFM group, 34 patients had high-risk parameters.
Table 1.
General and clinical characteristics of the paediatric patients with acute myeloid leukaemia.
| Characteristic | Patients | P | |
|---|---|---|---|
| Group A with EIa | Group B without EI | ||
| (n = 20) | (n = 20) | ||
| Age (years) | 0.718 | ||
| Median (min–max) | 9 (2–15) | 9.5 (2–15) | |
| Sex | 0.525 | ||
| Male | 11 (55%) | 11 (55%) | |
| Female | 9 (45%) | 9 (45%) | |
| Leukocyte count (103/μL) | 0.698 | ||
| Median | 28 250 | 26 900 | |
| Minimum–maximum | 9 700–125 000 | 5 800–154 700 | |
| Morphological subtype (FABb) | 0.941 | ||
| M0, M1, M2 | 6 (30%) | 4 (20%) | |
| M3 | 1 (5%) | 1 (5%) | |
| M4, M5 | 11 (55%) | 12 (60%) | |
| M6 | 1 (5%) | 1 (5%) | |
| M7 | 1 (5%) | 2 (10%) | |
| Karyotype | 0.900 | ||
| Normal | 8 (40%) | 6 (30%) | |
| t(8:21)aml/atg8 | 2 (10%) | 3 (15%) | |
| t(15:4)q+ | 0 (0%) | 1 (5%) | |
| t(9:22)abr/bcl | 1 (5%) | 1 (5%) | |
| t(9:11)p22,q23 | 1 (5%) | 1 (5%) | |
| No data | 8 (40%) | 8 (40%) | |
| Risk (by karyotype) | 0.870 | ||
| Normal | 4 (17%) | 3 (13%) | |
| Intermediate | 8 (33%) | 6 (25%) | |
| High | 2 (8%) | 1 (4%) | |
| Risk (BFMc) | 0.669 | ||
| Low | 3 (15%) | 3 (15%) | |
| High | 17 (85%) | 17 (85%) | |
aEI: early intensification.
bFAB: French-American-British classification.
cBFM: Berlin-Frankfurt-Münster study [39].
3.2. Primary Results: Response to Treatment
3.2.1. Relapse-Free Survival after an Autologous Transplant
Relapse-free survival (Figure 2) was 90% for Group A and 60% for Group B (P = 0.041); these patients were in complete continuous remission (CCR), with a median follow-up time of 405 days for Group A and 527 days for Group B. Of the 10 patients who suffered a relapse (Table 2), the relapse occurred in the bone marrow of eight, in the CNS of one, and in both sites in another; none of these 10 attained a second remission and all died from leukaemic activity.
Figure 2.
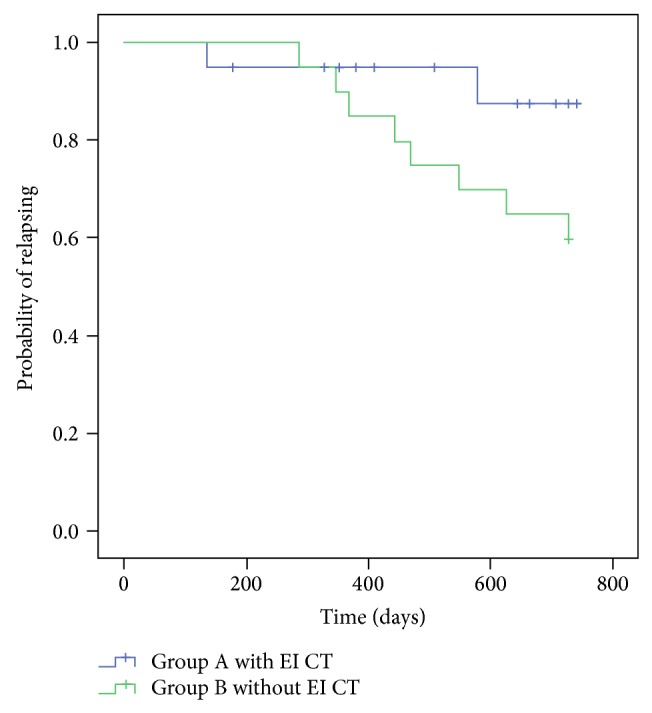
Relapse-free survival after autologous transplant for patients treated with early intensification chemotherapy or not. EI: early intensification; CT: chemotherapy. P = 0.09.
Table 2.
Outcomes for paediatric patients with acute myeloid leukaemia, treated with early intensification chemotherapy or not.
| Parameter | Patients | P | |
|---|---|---|---|
| Group A with EIa | Group B without EI | ||
| (n = 20) | (n = 20) | ||
| CCRb | 18 (90%) | 12 (60%) | 0.028 |
| Relapse | |||
| Pretransplant | 1 (5%) | 0 (0%) | |
| Posttransplant | 1 (5%) | 8 (40%) | 0.031 |
| Site of relapse | |||
| BMc | 2 (10%) | 6 (30%) | 0.030 |
| CNSd | 0 (0%) | 1 (5%) | |
| BM + CNS | 0 (0%) | 1 (5%) | |
| Current state | |||
| Alive with CCR | 18 (90%) | 12 (60%) | 0.028 |
| Dead | 2 (10%) | 8 (40%) | 0.031 |
| Cause of death | |||
| Leukaemic activity | 2 (100%) | 8 (100%) | 0.031 |
| Related to APBTe | 0 (0%) | 0 (0%) | |
aEI: early intensification.
bCCR: continued complete remission.
cBM: bone marrow.
dCNS: central nervous system.
eAPBT: autologous peripheral blood transplant.
3.2.2. Overall Survival
The overall survival (OS) (Figure 3) was 18 (90%) for Group A with a median duration of follow-up of 610 days (minimum, 226; maximum, 730). For Group B, the OS was 12 (60%), with a median follow-up of 675 days (minimum, 376; maximum, 730) (P = NS).
Figure 3.
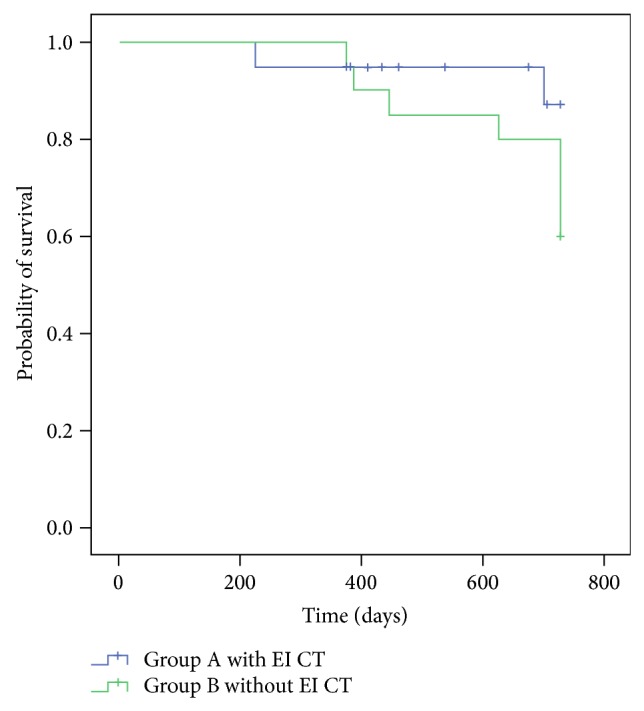
Overall survival at two years of follow-up for patients treated with early intensification chemotherapy or not. EI: early intensification; CT: chemotherapy. P = 0.13.
3.2.3. Variables Associated with Relapse
Analysis of the variables that could influence relapse showed that none of these variables affected the risk, whether calculated as CRR or as that adjusted by Mantel-Haenszel statistics (Table 3).
Table 3.
Parameters analyzed for relapse adjusted by different prognostic factors.
| Variable | Patients | ||||||
|---|---|---|---|---|---|---|---|
| Group A with EIa (n = 20) | Group B without EI (n = 20) | Crude relative risk | |||||
| No relapse | Relapse | No relapse | Relapse | ||||
| n (%) | n (%) | n (%) | n (%) | CRR × Sb (CI 95%) | RRAMHc (CI 95%) | ||
| Age (years) | |||||||
| <10 | 10 (90.9) | 1 (9.1) | 7 (58.3) | 5 (41.7) | 0.22 (0.03–1.59) | ||
| >10 | 8 (88.9) | 1 (11.1) | 5 (62.5) | 3 (37.5) | 0.30 (0.04–2.31) | 0.25 | (0.06–1.11) |
| Sex | |||||||
| Female | 8 (88.9) | 1 (11.1) | 9 (100) | 0 (0.0) | 2.00 (0.21–18.98) | ||
| Male | 10 (90.9) | 1 (9.1) | 3 (27.3) | 8 (72.7) | 0.22 (0.06–0.84) | 0.40 | (0.14–1.11) |
| Leukocytes | |||||||
| <50 000/μL | 15 (100) | 0 (0.0) | 10 (62.5) | 6 (37.5) | 0.15 (0.20–1.10) | ||
| >50 000/μL | 3 (60.0) | 2 (40.0) | 2 (50.0) | 2 (50.0) | 1.14 (0.40–3.18) | 0.47 | (0.18–1.18) |
| BMd Day 15 | |||||||
| <5% blasts | 11 (91.7) | 1 (8.3) | 10 (71.4) | 4 (28.6) | 0.29 (0.37–2.26) | ||
| >5% blasts | 7 (87.5) | 1 (12.5) | 2 (33.3) | 4 (66.7) | 0.18 (0.02–1.27) | 0.23 | (0.05–0.94) |
| Risk | |||||||
| Low | 3 (100) | 0 (0.0) | 3 (100) | 0 (0.0) | 1.00 (0.08–11.9) | ||
| High | 15 (88.2) | 2 (11.8) | 9 (52.9) | 8 (47.1) | 0.33 (0.10–1.04) | 0.40 | (0.14–1.10) |
| Time of CRe | |||||||
| <8 weeks | 14 (93.3) | 1 (6.7) | 9 (64.3) | 5 (35.7) | 0.18 (0.02–1.40) | ||
| >8 weeks | 4 (80.0) | 1 (20.0) | 3 (50.0) | 3 (50.0) | 0.40 (0.59–2.74) | 0.26 | (0.06–1.04) |
| CTf for CR | |||||||
| One cycle | 11 (91.7) | 1 (8.3) | 10 (66.7) | 5 (33.3) | 0.25 (0.03–1.86) | ||
| Two cycles | 7 (87.5) | 1 (12.5) | 2 (40.0) | 3 (60.0) | 0.20 (0.02–1.49) | 0.23 | (0.05–0.95) |
| CR upon APBTg,h | |||||||
| >10 months | 9 (90.0) | 1 (10.0) | 4 (57.1) | 3 (42.9) | 0.37 (0.87–1.61) | ||
| <10 months | 9 (100) | 0 (0.0) | 8 (61.5) | 5 (38.5) | 0.15 (0.02–1.14) | 0.26 | (0.82–0.83) |
| MNCh,i (108/kg) | |||||||
| <5 | 14 (93.3) | 1 (6.7) | 8 (66.7) | 4 (33.3) | 0.32 (0.07–1.44) | ||
| >5 | 4 (100) | 0 (0.0) | 4 (50.0) | 4 (50.0) | 0.33 (0.10–1.06) | 0.33 | (0.10–1.06) |
| CD34+ cellsh (106/kg) | |||||||
| >3 | 3 (100) | 0 (0.0) | 3 (60.0) | 2 (40.0) | 0.46 (0.06–3.23) | ||
| <3 | 15 (93.8) | 1 (6.3) | 9 (60.0) | 6 (40.0) | 0.26 (0.06–1.12) | 0.32 | (0.10–1.00) |
aEI: early intensification.
bCRR × S: crude relative risk by strata.
cCR (MH): relative risk, adjusted (Mantel-Haenszel).
dBM: bone marrow.
eCR: complete remission.
fCT: chemotherapy.
gAPBT: autologous peripheral blood transplant.
hOnly 19 patients in Group A received transplant (see text for details).
iMNC: mononuclear cell.
3.3. Secondary Results: Toxicity of EI Chemotherapy in Group A
No patient died from treatment toxicity; nor were any excluded from the study for this reason. All 20 (100%) displayed fever and neutropenia and required empirical treatment with wide-spectrum antibiotics; 12 of these patients were given amphotericin B for having persistent fever for more than five days, despite the use of the antibiotics. Two other patients suffered pneumonia; two had neutropenic enterocolitis that responded to antibiotics; and only one developed systematic candidiasis, which was resolved with amphotericin B and that did not influence the transplant. All patients presented haemorrhage of the skin and mucosa; seven had blood in the digestive tract (haematemesis or bleeding from the rectum) that was resolved with the transfusion of platelets.
3.4. Harvest of Haematopoietic Stem Cells
The characteristics of the harvested of autologous hematopoietic stem cells from pediatric patients are shown in Supplemental Table I. Neither the MNC nor the CD34+ cell count differed between groups (P = 0.70). There were no differences between groups for any of the other parameters studied.
3.5. Transplant of Haematopoietic Stem Cells
Of the 20 patients in Group A, who were programmed for an autologous transplant, a patient with AML-M2 (karyotype t(9;11)p22;q23) did not have the procedure because of a bone marrow relapse; the patient died as a result of infection and leukaemic activity eight months after diagnosis. It is for this reason that only 19 patients in Group A received transplants. (No patient died from the toxicity of the EI. One other patient died from infection at another stage of treatment because of leukaemic activity.) For these 19 patients, the median time from complete remission to transplant was 9.9 months (minimum, 6.1; maximum, 12.8), whereas for Group B (n = 20), the median was 8.6 months (minimum, 5.6; maximum 15.7) (P = 0.235); thus, EI chemotherapy did not cause a major delay in transplantation.
All these patients received an adequate transplant; the median number of days needed to reach a polymorphonuclear cell count ≥0.5 × 109/L was 18 days (minimum, 12; maximum, 35) for Group A and 18 days (minimum, 14; maximum, 38) for Group B (P = 0.749); to reach a platelet cell count ≥50 × 109/L, without transfusion, the median number of days needed was 23 (minimum, 18; maximum, 54). The patients received G-CSF after the infusion of the harvested cells; on Day +18, five patients from Group A and four from Group B did not have a neutrophil count ≥0.5 × 109/L.
3.6. Complications and Mortality Related to the Transplant
Of the 39 patients who received transplants, 12 (31%) did not present any complications; 12 (31%) developed a fever that lasted more than seven days and with neither a localized site of infection nor an isolated microorganism, thus warranting the use of wide-spectrum antibiotics (ceftazidime/amikacin or vancomycin); and eight (21%) required amphotericin B. Mortality as a result of transplantation was 0%.
4. Discussion
The study AML-BFM 93 [39] found that the combination of idarubicin in the induction and the intensification therapy with HAM reduces the risk of relapse in patients at high risk. The other factor that probably was potentiated by the EI was the prolonged average time (270 days) from remission to transplant, as was demonstrated by Locatelli et al. [43]. In that multivariate analysis, it was shown that, of the children who had transplants at ≥170 days after the first CR, 60% ± 3% had a leukaemia-free survival during five years of follow-up. This beneficial effect may be the result of two principal factors: (1) additional useful courses of consolidation to reduce the tumour burden and (2) the low risk of mortality related to the transplants in these patients, which is because of the sufficient time for organic recovery before the transplantation.
Other studies have demonstrated the effectiveness of HAM in treating AML, both in children and in adults. The GALGB study was the first to show the effect of cytarabine at a standard dose (100 mg/m2), intermediate dose (400 mg/m2), or high dose (3 g/m2) as treatment postremission [44]. Thereafter, Arlin et al. [45] reported a higher average of CR after only one course of induction with Ara-C and mitoxantrone (3 × 12 mg/m2), when these drugs were administered after a standard regimen of daunorubicin (3 × 45 mg/m2) in adult patients with newly diagnosed AML. Büchner et al. [46] demonstrated that HAM as a course for second induction in adults benefits patients at high risk; here, we had similar results for high-risk paediatric patients. In the AML-BFM 93 study, Creutzig et al. [39] evaluated HAM as a first or second cycle of postinduction treatment, with the intention of improving the results in children with AML of high risk. They found no statistically significant difference between the results obtained with the early or late administration of HAM; nevertheless, upon comparison with results of the historic group (AML-BFM 87 [38]), the differences were greater in OS and RFS of up to five years (60% ± 3%, 51% ± 2%, and 62% ± 3%, resp.). Similar values were found in the present study.
In the present study, no patient died or was excluded because of toxicity of the treatment; nor was there a significant delay in conducting the autologous transplantation. These results differ from those of AML-BFM 93 [39]. Nonetheless, the incidence of deaths related to the therapy was similar to that in AML-BFM 87 [38]. Therefore, because of the efficacy of HAM and its tolerable average toxicity in children at high risk, in the study AML-BFM 98 [47], HAM was introduced as a second course of therapy for all paediatric patients with AML, including patients with standard risk, with the objective of improving the average survival.
An important finding emerged from the present study, namely, the positive role of EI therapy, followed by a course of consolidation and two courses of late intensification before the harvesting of PBSC and autologous transplant, resulting in a significantly lower estimated probability of relapse (10%) compared with that of patients in the control group that received PBSCT without EI (40%; P = 0.04) (relative risk = 0.25, 95% CI 0.06–1.03).
Intensive chemotherapy coupled with an autologous transplant in primary remission constitutes a good option for treatment. However, the results in the literature vary and are difficult to compare because of the heterogeneity of the studies. They differ in design, number of patients, age of patients, intensity of the courses of chemotherapy before of the transplant, accumulated doses of the most important drugs (anthracyclines, cytarabine, and etoposide), prophylaxis of the CNS, difference in the stratification of risk, the time at which the transplant was performed, the conditioning regimens, the origin of the stem cells, and the use (or not) of a purge [25–37]. In the present study, with the use of EI chemotherapy, a cycle of consolidation, and two late intensifications before the harvest and autologous transplant, the RFS at two years was 90% compared with 60% for the control group that had not received such treatment (P = 0.03; Table 2). Previously, the efficacy of EI as a purge in vivo in children and adults who were newly diagnosed with AML and who received PBSCT was evaluated [36]. They reported that, for the patients who received EI before the harvesting and the transplant compared with patients who did not, the DFS rates were 68.8% ± 10.27% versus 35.5% ± 12.6%, respectively (P = 0.04). They concluded that, with the use of EI and PBSCT, the risk of relapse was reduced significantly. However, the majority of research groups do not recommend an autologous transplant as postremission therapy in paediatric patients with AML in CR1, because they have not found any benefit compared with that of chemotherapy alone [18, 39, 47].
With the best support care, the mortality related to transplant toxicity has been reduced to <5%; in the present study, it was 0%, equal to that reported in the literature [25, 26, 29]. With the use of nonpurged and noncryopreserved PBSC in this study, the toxicity and the cost were reduced without interfering with the time of implant, as has been reported by Ruiz-Argüelles et al. [48] and Gómez-Almaguer [49]. Allogeneic transplant continues to be recommended for those paediatric patients with AML at high risk in CR1, who can count on having an HLA-identical family member, because the survival is 61% at five years of follow-up [50].
The following limitations apply to this study. Because the zero point for the cohort was defined when CR was achieved, it may be that those patients who were not included for reasons such as early death, failure of the IR, and nonacceptance of treatment were probably those at higher risk of relapse. Although the two groups had similar general and clinical characteristics, the comparison with the historic group may have been biased because of improvements over time, in support care, and with greater experience in management. In Group A, the follow-up time was short: not all the patients completed the minimum time established for follow-up (two years), which is the time of greatest risk of relapse; after then the probability of cure increases up to 80%. This was observed in the historic group of this study and was recently reported by Majhail et al. [20]. Finally, it is important to emphasize that there are various groups (e.g., in Argentina (GATLA), Chile (PINDA), and Mexico [17]) that do not include EI (HAM) in their chemotherapy plan. From the results of the present work, we recommend the use of EI (HAM) in chemotherapy.
Supplementary Material
In the figure I, the schematic of chemotherapy is shown. That for Group B was based on the Latin American protocol (AML-BFM 87) [38] and that for Group A on AML-BFM 93. The treatment schedule was described with detail and the way as autologous transplant was done. In the table I the harvest of autologous haematopoietic stem cells is mentioned.
Acknowledgments
This study was partially funded by the CONACYT (SALUD-2010-1-141026) and by Mexican Institute of Social Security, Grants FIS/IMSS/PROT/PRIO/11/017 and FIS/IMSS/PROT/G12/1134 (all to Juan Manuel Mejía-Aranguré). Funding to cover the costs of the translation and publishing was provided by the División de Desarrollo de la Investigación del IMSS.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Bacher U., Schnittger S., Haferlach T. Molecular genetics in acute myeloid leukemia. Current Opinion in Oncology. 2010;22(6):646–655. doi: 10.1097/CCO.0b013e32833ed806. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S., Neglia J. P. Epidemiology of childhood acute myelogenous leukemia. Journal of Pediatric Hematology/Oncology. 1995;17(2):94–100. doi: 10.1097/00043426-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U., Zimmermann M., Reinhardt D., Dworzak M., Stary J., Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. Journal of Clinical Oncology. 2004;22(21):4384–4393. doi: 10.1200/jco.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 4.Kaspers G. J. L., Creutzig U. Pediatric acute myeloid leukemia: international progress and future directions. Leukemia. 2005;19(12):2025–2029. doi: 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 5.Armendariz H., Fernandez Barbieri M. A., Freigeiro D., Lastiri F., Felice M. S., Dibar E. Treatment strategy and long-term results in pediatric patients treated in two consecutive AML-GATLA trials. Leukemia. 2005;19(12):2139–2142. doi: 10.1038/sj.leu.2403854. [DOI] [PubMed] [Google Scholar]
- 6.Creutzig U., Zimmermann M., Ritter J., et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19(12):2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 7.Dluzniewska A., Balwierz W., Armata J., et al. Twenty years of Polish experience with three consecutive protocols for treatment of childhood acute myelogenous leukemia. Leukemia. 2005;19(12):2117–2124. doi: 10.1038/sj.leu.2403892. [DOI] [PubMed] [Google Scholar]
- 8.Entz-Werle N., Suciu S., van der Werff ten Bosch J., et al. Results of 58872 and 58921 trials in acute myeloblastic leukemia and relative value of chemotherapy vs allogeneic bone marrow transplantation in first complete remission: the EORTC Children Leukemia Group report. Leukemia. 2005;19(12):2072–2081. doi: 10.1038/sj.leu.2403932. [DOI] [PubMed] [Google Scholar]
- 9.Kardos G., Zwaan C. M., Kaspers G. J. L., et al. Treatment strategy and results in children treated on three Dutch Childhood Oncology Group acute myeloid leukemia trials. Leukemia. 2005;19(12):2063–2071. doi: 10.1038/sj.leu.2403873. [DOI] [PubMed] [Google Scholar]
- 10.Lie S. O., Abrahamsson J., Clausen N., et al. Long-term results in children with AML: NOPHO-AML Study Group—report of three consecutive trials. Leukemia. 2005;19(12):2090–2100. doi: 10.1038/sj.leu.2403962. [DOI] [PubMed] [Google Scholar]
- 11.Perel Y., Auvrignon A., Leblane T., et al. Treatment of childhood acute myeloblastic leukemia: dose intensification improves outcome and maintenance therapy is of no benefit—multicenter studies of the French LAME (Leucémie Aiguë Myéloblastique Enfant) Cooperative Group. Leukemia. 2005;19:2082–2089. doi: 10.1038/sj.leu.2403867. [DOI] [PubMed] [Google Scholar]
- 12.Pession A., Rondelli R., Basso G., et al. Treatment and long-term results in children with acute myeloid leukaemia treated according to the AIEOP AML protocols. Leukemia. 2005;19(12):2043–2053. doi: 10.1038/sj.leu.2403869. [DOI] [PubMed] [Google Scholar]
- 13.Quintana J., Advis P., Becker A., et al. Acute myelogenous leukemia in Chile PINDA protocols 87 and 92 results. Leukemia. 2005;19(12):2143–2146. doi: 10.1038/sj.leu.2403959. [DOI] [PubMed] [Google Scholar]
- 14.Ravindranath Y., Chang M., Steuber C. P., et al. Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): a review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia. 2005;19(12):2101–2116. doi: 10.1038/sj.leu.2403927. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro R. C., Razzouk B. I., Pounds S., Hijiya N., Pui C.-H., Rubnitz J. E. Successive clinical trials for childhood acute myeloid leukemia at St. Jude Children's Research Hospital, from 1980 to 2000. Leukemia. 2005;19(12):2125–2129. doi: 10.1038/sj.leu.2403872. [DOI] [PubMed] [Google Scholar]
- 16.Smith F. O., Alonzo T. A., Gerbing R. B., Woods W. G., Arceci R. J. Long-term results of children with acute myeloid leukemia: a report of three consecutive phase III trials by the Children's Cancer Group: CCG 251, CCG 213 and CCG 2891. Leukemia. 2005;19(12):2054–2062. doi: 10.1038/sj.leu.2403925. [DOI] [PubMed] [Google Scholar]
- 17.Gallegos-Castorena S., Medina-Sanson A., Gonzalez-Ramella O., Sánchez-Zubieta F., Martínez-Avalos A. Improved treatment results in Mexican children with acute myeloid leukemia using a medical research council (MRC)-acute myeloid leukemia 10 modified protocol. Leukemia and Lymphoma. 2009;50(7):1132–1137. doi: 10.1080/10428190902964768. [DOI] [PubMed] [Google Scholar]
- 18.Woods W. G., Neudorf S., Gold S., et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission: a report from the Children's Cancer Group. Blood. 2001;97(1):56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Gibson B. E. S., Wheatley K., Hann I. M., et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19(12):2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 20.Majhail N. S., Bajorunaite R., Lazarus H. M., et al. High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplantation. 2011;46(3):385–392. doi: 10.1038/bmt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horan J. T., Alonzo T. A., Lyman G. H., et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children's Oncology Group. Journal of Clinical Oncology. 2008;26(35):5797–5801. doi: 10.1200/jco.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niewerth D., Creutzig U., Bierings M. B., Kaspers G. J. L. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 23.Harrison C. J., Hills R. K., Moorman A. V., et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment Trials AML 10 and 12. Journal of Clinical Oncology. 2010;28(16):2674–2681. doi: 10.1200/jco.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 24.Von Neuhoff C., Reinhardt D., Sander A., et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. Journal of Clinical Oncology. 2010;28(16):2682–2689. doi: 10.1200/JCO.2009.25.6321. [DOI] [PubMed] [Google Scholar]
- 25.Ortega J. J., Díaz de Heredia C., Olivé T., et al. Allogeneic and autologous bone marrow transplantation after consolidation therapy in high-risk acute myeloid leukemia in children. Towards a risk-oriented therapy. Haematologica. 2003;88(3):290–299. [PubMed] [Google Scholar]
- 26.Berger M., Ferrero I., Vassallo E., et al. Stem cell transplantation as consolidation therapy for children in first-remission AML: a single-center report. Pediatric Hematology-Oncology. 2005;22(7):597–608. doi: 10.1080/08880010500198871. [DOI] [PubMed] [Google Scholar]
- 27.Jourdan E., Rigal-Huguet F., Marit G., et al. One versus two high-dose cytarabine-based consolidation before autologous stem cell transplantation for young acute myeloblastic leukaemia patients in first complete remission. British Journal of Haematology. 2005;129(3):403–410. doi: 10.1111/j.1365-2141.2005.05470.x. [DOI] [PubMed] [Google Scholar]
- 28.Anak S., Saribeyoglu E. T., Bilgen H., et al. Allogeneic versus autologous versus peripheral stem cell transplantation in CR1 pediatric AML patients: a single center experience. Pediatric Blood and Cancer. 2005;44(7):654–659. doi: 10.1002/pbc.20256. [DOI] [PubMed] [Google Scholar]
- 29.Martins C., Lacerda J. F., Lourenço F., Carmo J. A., Lacerda J. M. F. Autologous stem cell transplantation in acute myeloid leukemia: factors influencing outcome. A 13 year single institution experience. Acta Medica Portuguesa. 2005;18(5):329–338. [PubMed] [Google Scholar]
- 30.Neudorf S., Sanders J., Kobrinsky N., et al. Autologous bone marrow transplantation for children with AML in first remission. Bone Marrow Transplantation. 2007;40(4):313–318. doi: 10.1038/sj.bmt.1705680. [DOI] [PubMed] [Google Scholar]
- 31.Yeager A. M., Kaizer H., Santos G. W., et al. Autologous bone marrow transplantation in patients with acute nonlymphocytic leukemia, using ex vivo marrow treatment with 4-hydroperoxycyclophosphamide. The New England Journal of Medicine. 1986;315(3):141–147. doi: 10.1056/nejm198607173150301. [DOI] [PubMed] [Google Scholar]
- 32.Selvaggi K. J., Wilson J. W., Mills L. E., et al. Improved outcome for high-risk acute myeloid leukemia patients using autologous bone marrow transplantation and monoclonal antibody-purged bone marrow. Blood. 1994;83(6):1698–1705. [PubMed] [Google Scholar]
- 33.Smith B. D., Jones R. J., Lee S. M., et al. Autologous bone marrow transplantation with 4-hydroperoxycyclophosphamide purging for acute myeloid leukaemia beyond first remission: a 10-year experience. British Journal of Haematology. 2002;117(4):907–913. doi: 10.1046/j.1365-2141.2002.03530.x. [DOI] [PubMed] [Google Scholar]
- 34.Stein A. S., O'Donnell M. R., Chai A., et al. In vivo purging with high-dose cytarabine followed by high-dose chemotherapy and reinfusion of unpurged bone marrow for adult acute myelogenous leukemia in first complete remission. Journal of Clinical Oncology. 1996;14(8):2206–2216. doi: 10.1200/JCO.1996.14.8.2206. [DOI] [PubMed] [Google Scholar]
- 35.To L. B., Juttner C. A. Peripheral blood stem cell autografting: a new therapeutic option for AML? British Journal of Haematology. 1987;66(3):285–288. doi: 10.1111/j.1365-2141.1987.tb06911.x. [DOI] [PubMed] [Google Scholar]
- 36.Martín C., Torres A., León A., et al. Autologous peripheral blood stem cell transplantation (PBSCT) mobilized with G-CSF in AML in first complete remission. Role of intensification therapy in outcome. Bone Marrow Transplantation. 1998;21(4):375–382. doi: 10.1038/sj.bmt.1701102. [DOI] [PubMed] [Google Scholar]
- 37.Tallman M. S., Pérez W. S., Lazarus H. M., et al. Pretransplantation consolidation chemotherapy decreases leukemia relapse after autologous blood and bone marrow transplants for acute myelogenous leukemia in first remission. Biology of Blood and Marrow Transplantation. 2006;12(2):204–216. doi: 10.1016/j.bbmt.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Ritter J., Creutzig U., Schellong G. Treatment results of three consecutive German Childhood AML trials: BFM-78, -83, and -87. AML-BFM-Group. Leukemia. 1992;6:59–62. [PubMed] [Google Scholar]
- 39.Creutzig U., Ritter J., Zimmermann M., et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of study acute myeloid Leukemia-Berlin-Frankfurt-Münster 93. Journal of Clinical Oncology. 2001;19(10):2705–2713. doi: 10.1200/JCO.2001.19.10.2705. [DOI] [PubMed] [Google Scholar]
- 40.Creutzig U., Zimmerman M., Ritter J., et al. Definiont of estándar risk grop in children with AML. British Journal of Haematology. 1999;104:630–609. doi: 10.1046/j.1365-2141.1999.01304.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheson B. D., Bennett J. M., Kopecky K. J., et al. Revised Recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Journal of Clinical Oncology. 2003;21(24):4642–4649. doi: 10.1200/jco.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 42. NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE), August 2006.
- 43.Locatelli F., Labopin M., Ortega J., et al. Factors influencing outcome and incidence of long-term complications in children who underwent autologous stem cell transplantation for acute myeloid leukemia in first complete remission. Blood. 2003;101(4):1611–1619. doi: 10.1182/blood-2002-03-0764. [DOI] [PubMed] [Google Scholar]
- 44.Meyer R. J., Davis R. B., Schiffer C. H. A., et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. The New England Journal of Medicine. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 45.Arlin Z., Case D. C., Jr., Moore J., et al. Randomized multicenter trial of cytosine arabinoside with mitoxantrone or daunorubicin in previously untreated adult patients with acute nonlymphocytic leukemia (ANLL) Leukemia. 1990;4(3):177–183. [PubMed] [Google Scholar]
- 46.Büchner T., Hiddemann W., Wörmann B., et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93(12):4116–4124. [PubMed] [Google Scholar]
- 47.Ravindranath Y., Yeager A. M., Chang M. N., et al. Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. The New England Journal of Medicine. 1996;334(22):1428–1434. doi: 10.1056/nejm199605303342203. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Argüelles G. J., Ruiz-Argüelles A., Pérez-Romano B., et al. Non-cryopreserved peripheral blood stem cells autotransplants for hematological malignancies can be performed entirely on an outpatients basis. The American Journal of Hematology. 1998;58:161–164. doi: 10.1002/(sici)1096-8652(199807)58:3<161::aid-ajh1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Almaguer D. The simplification of the SCT procedures in developing countries has resulted in cost-lowering and availability to more patients. International Journal of Hematology. 2002;76(1):380–382. doi: 10.1007/bf03165288. [DOI] [PubMed] [Google Scholar]
- 50.Lange B. J., Smith F. O., Feusner J., et al. Outcomes in CCG-2961, a Children's Oncology Group Phase 3 Trial for untreated pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2008;111(3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the figure I, the schematic of chemotherapy is shown. That for Group B was based on the Latin American protocol (AML-BFM 87) [38] and that for Group A on AML-BFM 93. The treatment schedule was described with detail and the way as autologous transplant was done. In the table I the harvest of autologous haematopoietic stem cells is mentioned.