Abstract
Background: Hyperglycemia associated with insulin resistance is common among critically ill patients. Interleukin (IL)-18 has been linked with hyperglycemia and insulin resistance in chronic disease, but the relation between IL-18 and insulin resistance during critical illness was unexplored. This study investigated whether IL-18 modulates hyperglycemia and insulin resistance during acute inflammation.
Methods: We injected lipopolysaccharide (LPS) 40 mg/kg into wild-type (WT) and IL-18 knockout (KO) mice to induce endotoxemia and examined insulin resistance and insulin-dependent signaling pathways during the acute phase.
Results: During the first hour after LPS treatment, IL-18 KO mice showed higher blood glucose and insulin and less insulin receptor substrate-1 and less phosphorylated Akt in the liver compared with WT mice. Interleukin-18 KO mice exhibited better survival after LPS treatment.
Conclusions: The findings suggest that endogenous IL-18 may attenuate hyperglycemia and modulate insulin signaling in liver. Accordingly, IL-18 may modulate glucose tolerance during acute inflammation.
Hyperglycemia during critical illness, such as multiple injuries, extensive burns, major surgical trauma, or infection, is noted as a universal finding even in patients without a history of diabetes mellitus [1–3]. Acute insulin resistance induces hyperglycemia, and, thus, this condition is referred to as critical diabetes. Van den Berghe et al. [4,5] showed that critical diabetes could be remedied in patients in intensive care units (ICU) by intensive insulin therapy with the goal of normoglycemia (80–110 mg/dL; Leuven I and Leuven II studies). In the Leuven studies, intensive insulin therapy reduced mortality and morbidity in ICU patients suggesting that hyperglycemia is a strong independent risk factor for death. However, because intensive insulin therapy carries a risk of hypoglycemia, additional studies, including the Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study were conducted [6,7]. In the NICE-SUGAR study, the patients who received intensive insulin therapy surprisingly exhibited slightly increased mortality compared with patients permitted to remain hyperglycemic until the blood glucose levels threatened kidney function (>80 mg/dL). Thus, the 2012 Surviving Sepsis Campaign guidelines state that blood glucose should be controlled to be lower than 180 mg/dL to reduce the risk of inducing hypoglycemia [8].
Pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, are elevated in acute inflammation and promote hyperglycemia by inhibiting insulin signaling pathways [9,10]. Interleukin-18 is a pro-inflammatory cytokine and is also elevated dramatically in acute inflammation [11–13]. In addition, some studies suggest that circulating IL-18 concentrations are elevated during chronic insulin resistance, such that seen as a result of obesity and diabetes [14,15]. However, an association between IL-18 and acute insulin resistance had not been reported. In this study, we used IL-18 knockout (KO) mice to investigate the role of IL-18 during acute lethal endotoxemia.
Materials and Methods
Animals
Twenty-week-old male C57BL/6J (wild-type, WT) mice (Clea Japan, Tokyo, Japan) and IL-18 KO mice (B6.129P2-IL18tm1Aki/J; The Jackson Laboratory, Bar Harbor, ME) were studied. All mice were kept at 22°C under a 12-h light/dark cycle and allowed access to a standard diet and water ad libitum. Mice were anesthetized with isoflurane and then injected intraperitoneally with lipopolysaccharide (LPS) 40 mg/kg (Escherichia coli O111: B4, Sigma-Aldrich, St Louis, MO) or phosphate buffered saline (PBS). Either 1 h or 12 h after injection, the mice were killed by cardiac puncture under general anesthesia. Samples of blood, liver, and lung were harvested immediately and homogenized in lysis buffer A (100 mM HEPES [pH 7.4], 1% Triton, 10% glycerol, 150 mM NaCl, 1 mM sodium pyrophosphate, 100 mM sodium fluoride, 2 mM ethylenediaminetetraacetic acid [EDTA], 5 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mcg/mL aprotinin, and 5 mcg/mL leupeptin) for protein isolation or TRIzol Reagent (Invitrogen, Carlsbad, CA) for total RNA isolation, and stored at −80°C until analysis. All experiments involving mice were conducted in accordance with the approval of the Animal Welfare Committee of Kobe University Graduate School of Health Sciences.
Glucose, hormones, and cytokines
Venous blood was obtained from the tail vein before LPS injection and 15, 30, and 60 min and 12 h after LPS injection. Blood glucose concentrations were measured using a portable glucose meter (Glutest Sensor; Sanwa Kagaku Kenkyusho, Nagoya, Japan). Plasma IL-18 concentrations were assessed using an enzyme-linked immunosorbent assay (ELISA) kit (MBL, Nagoya, Japan) specific for the 18 kDa bioactive form of IL-18 according to the manufacturer's instructions. Plasma glucagon (WAKO, Osaka, Japan) and insulin (Shibayagi, Gunma, Japan) concentrations were also measured using ELISA according to the manufacturer's instructions. Plasma TNF-α and IL-6 concentrations were assayed using Cytometric Bead Array (CBA) flex sets (BD Pharmingen Corp., San Diego, CA). Flow cytometric analysis was performed using a FACS Array flow cytometer (BD Immunocytometry Systems, Franklin Lakes, NJ). Data were acquired and analyzed by BD FACS Array software and FCAP Array software, version 1.0 (BD Immunocytometry Systems). The limit of detection for TNF-α was 17.1 pg/mL and the limit for detection of IL-6 was 6.5 pg/mL.
Western blotting
Anti-insulin receptor substrate (IRS)-1, anti-phosphorylated IRS-1 (Tyr895), anti-Akt and anti-phosphorylated Akt (Ser473) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-β-actin antibody was purchased from Sigma-Aldrich. Western blotting was performed as described previously (16). Membranes were incubated overnight at 4°C with primary antibodies diluted in Can Get Signal immunoreaction enhancer solution 1 (Toyobo, Osaka, Japan), then with horseradish peroxidase-conjugated anti-rabbit secondary antibodies diluted in Can Get Signal solution 2 for 1 h at room temperature. Antibody binding was detected using enhanced chemiluminescence plus reagents (GE Healthcare, Buckinghamshire, United Kingdom) and exposed on Hyperfilm (GE Healthcare, Fairfield, CT). The band intensity was quantified by Image J software version 1.47 (National Institutes of Health, Bethesda, MD).
Hematoxylin and eosin staining
To evaluate liver and lung injury 12 h after LPS or PBS injection, we performed hematoxylin and eosin (H&E) staining. The liver and lung samples were immersed in fixative solution (4% paraformaldehyde in PBS) for 24 h, dehydrated through graded alcohol dilutions, and embedded in paraffin. Tissue was sectioned and stained with H&E using standard protocols.
Statistical analysis
The data were expressed as mean±standard error (SEM). The blood glucose and plasma IL-18 concentrations were examined using two-factor analysis of variance (ANOVA). For other experiments, ANOVA with the Tukey-Kramer post-hoc test was performed. Survival was examined by Kaplan-Meier analysis and the log-rank test. A probability level of p<0.05 was considered statistically significant.
Results
Blood glucose and plasma insulin
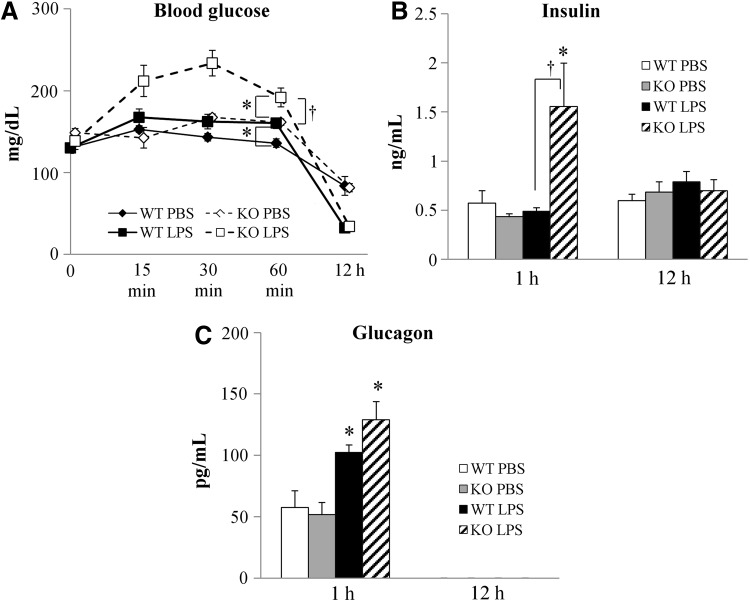
To assess the effects of IL-18 alteration on glucose metabolism, we measured blood glucose, plasma insulin, and glucagon concentrations in WT and IL-18 KO mice after LPS administration to induce endotoxemia. High-dose LPS induced transient hyperglycemia within 1 h of administration, followed by hypoglycemia 12 h after administration in both WT and IL-18 KO mice (Fig. 1A). Blood glucose concentrations in the IL-18 KO mice were significantly increased compared with WT during the first hour after LPS injection (Fig. 1A). Plasma insulin concentrations were increased in the IL-18 KO mice 1 h after LPS administration then returned to baseline concentrations after 12 h (Fig. 1B). In contrast, plasma insulin concentrations in WT were not altered by LPS injection. Plasma glucagon concentrations increased during the first hour after LPS injection in both the WT and IL-18 KO mice and were beneath the assay detection threshold in all animals 12 h after LPS administration (Fig. 1C).
FIG. 1.
Blood glucose and plasma hormones concentrations after lipopolysaccharide (LPS) injection. (A) LPS induced early hyperglycemia and late hypoglycemia in interleukin (IL)-18 knockout (KO) mice; n=8–10 per time point. (B) Plasma insulin concentrations in wild-type (WT) and IL-18 KO mice; n=5–8 per time point. (C) Plasma glucagon concentrations in WT and IL-18 KO mice; n=6 per time point. *p<0.01 vs. phosphate-buffered saline (PBS) group; †p<0.01 vs. WT+LPS group.
Cytokine levels in plasma
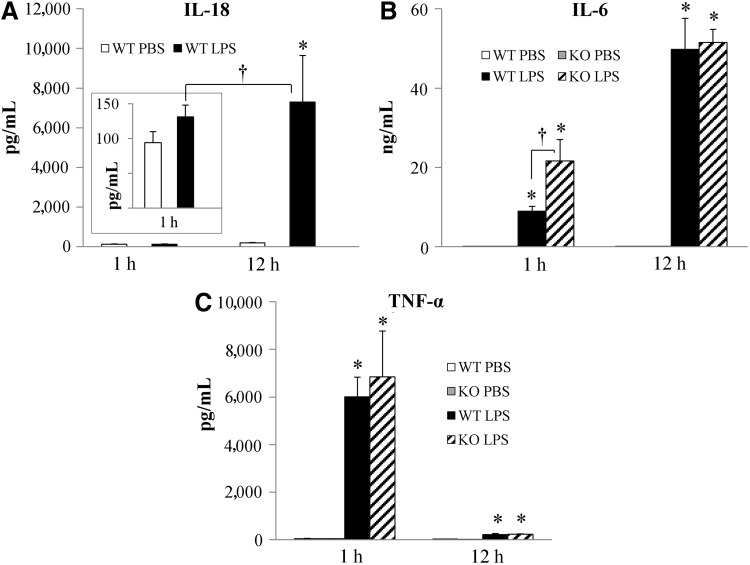
To assess cytokine expression during hyperglycemia induced by endotoxemia, we measured plasma concentrations of IL-18, IL-6, and TNF-α by ELISA or CBA. In WT mice, IL-18 concentrations were not elevated 1 h after LPS administration (100 pg/mL in both PBS- and LPS-treated mice) but increased dramatically to ∼7,000 pg/mL 12 h after LPS administration (Fig. 2A). IL-6 and TNF-α were elevated significantly after LPS administration in both WT and IL-18 KO (Fig. 2B and 2C). Concentrations of IL-6 were much higher 12 h after LPS injection than 1 h after injection, but 1 h after injection the IL-18 KO mice expressed more IL-6 than the WT mice (Fig. 2B). In contrast, TNF-α concentrations were much higher 1 h after LPS injection than 12 h after LPS injection and did not differ between WT and IL-18 KO mice (Fig. 2C).
FIG. 2.
Plasma cytokine concentrations after lipopolysaccharide (LPS) injection. (A) Plasma interleukin (IL)-18 concentrations; n=6. (B) Plasma IL-6 concentrations; n=5–8. (C) Plasma tumor necrosis factor (TNF)-α concentrations; n=5–8. *p<0.01 vs. phosphate-buffered saline (PBS) group; †p<0.01 vs. WT+LPS 1 h group.
Activation of IRS-1 and Akt in the liver
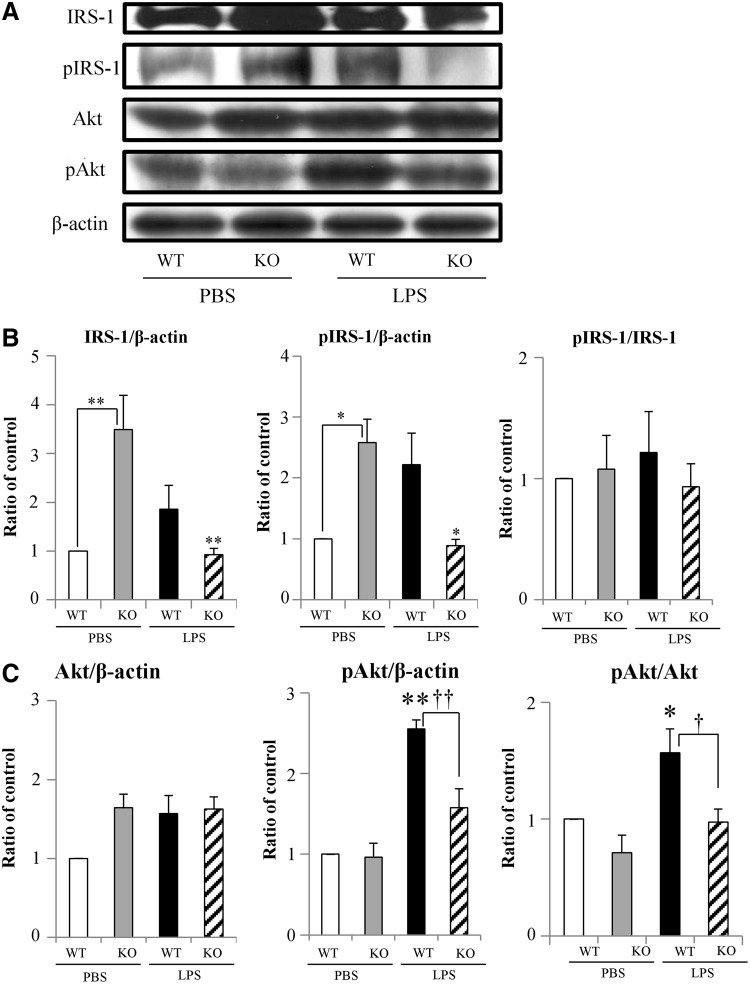
To assess the effects of IL-18 deletion on insulin signaling in response to acute endotoxemia, we analyzed the activation of IRS-1 and Akt. Because the blood glucose and plasma insulin and IL-6 concentrations differed between WT and IL-18 KO 1 h after LPS administration and did not differ 12 h after LPS injection, we chose to analyze the effect of IL-18 deletion on insulin receptor signaling only at the 1-h time point. In PBS-treated mice, total IRS-1 expression was higher in IL-18 KO mice compared with WT mice (Fig. 3A and 3B), and high concentrations of IRS-1 phosphorylation were observed, indicating higher baselines concentrations of activated IRS-1 in the absence of IL-18. After LPS treatment, total IRS-1 and pIRS-1 tended to increase in WT mice but decreased significantly in IL-18 KO mice compared with PBS-treated WT or IL-18 KO mice (Fig. 3A and 3B). The differences likely reflected changes in total IRS-1 protein concentrations, as the pIRS-1/IRS-1 ratio did not differ significantly between WT and KO or in response to LPS administration (Fig. 3A and Fig. 3B, right panel). Total akt expression was not altered significantly by either IL-18 deletion or LPS injection (Fig. 3A and Fig. 3C, left panel). Akt phosphorylation (pAkt) was increased significantly by LPS in WT mice, but did not increase significantly in the IL-18KO mice after LPS treatment. (Fig. 3A and Fig 3C, center and right panel)
FIG. 3.
Activation of the insulin signaling pathway. (A) The protein expression of insulin signaling pathway 1 h after lipopolysaccharide (LPS) injection in interleukin (IL)-18 knockout (KO) and wild-type (WT) mice. (B) The band intensity of total IRS-1 (left panel) and pIRS-1 (center panel) relative to β-actin and the pIRS-1/IRS-1 ratio (right panel); n=5. (C) The band intensity of Akt (left panel) and pAkt (center panel) relative to β-actin and the pAkt/Akt expression ratio (right panel); n=5. *p<0.05; **p<0.01 vs. PBS group; †p<0.05; ††p<0.01 vs. WT+LPS group.
Liver and lung morphology
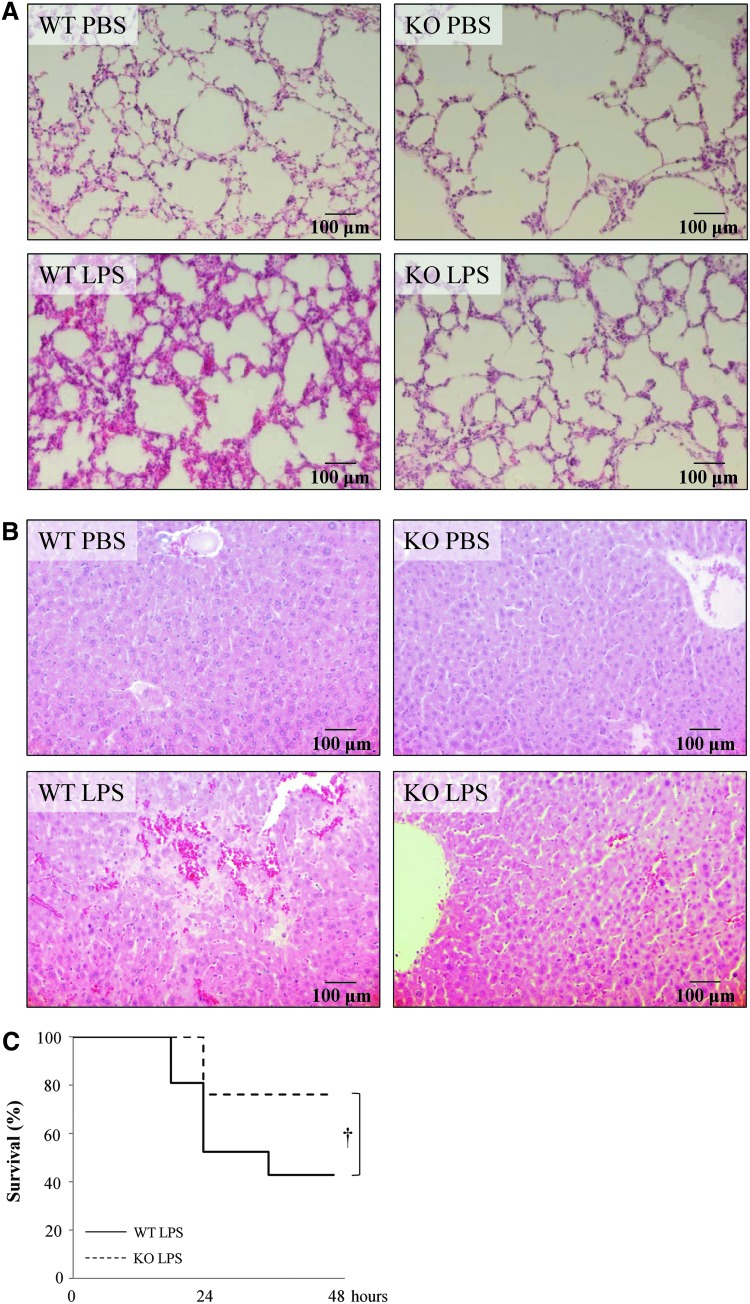
Because clinical sepsis can lead to dysfunction of multiple organs, we examined lung and liver morphology after LPS administration in presence and absence of IL-18. Lipopolysaccharide-treated IL-18 KO mice experienced less severe lung injury, such as alveolar septal thickening and neutrophil accumulation, after LPS administration than WT mice (Fig. 4A). The lungs of PBS-treated WT and IL-18 KO mice were similar morphology.
FIG. 4.
Morphologic changes in lung and liver and mouse survival after lipopolysaccharide (LPS) adminstration. (A) Hematoxylin and eosin (H&E) staining of the lungs of mice 12 h after LPS injection (×200). (B) H&E staining of the livers of mice 12 h after LPS injection (×200). (C) Survival of mice with LPS-induced lethal endotoxemia. The solid line shows the survival ratio of wild-type (WT) mice (n=21) and the dashed line shows the survival ratio of interleukin (IL)-18 KO mice (n=21). †p<0.05 vs. LPS group. Color image is available online at www.liebertpub.com/sur
In the livers of WT mice, many hemorrhagic changes and large necrosis were observed (Fig. 4B). In contrast, the livers of IL-18 KO mice had few hemorrhagic changes and small necrosis in response to LPS administration. Hemorrhagic changes and necrosis were rarely observed in the livers of WT or IL-18 mice injected with PBS only.
The mortality of lethal endotoxemia
Endotoxemia induced by LPS injection is frequently lethal. More than one-half of the WT mice (60%) died within 2 d of injection. The IL-18 KO mice exhibited better survival with 70% surviving more than 2 d (Fig. 4C).
Discussion
This study showed that a deficiency of IL-18 promoted hyperglycemia and hyperinsulinemia during the hyperacute phase (first hour) of endotoxemia. In addition, IL-18 deficiency inhibited insulin signaling pathway activation in the liver during acute endotoxemia. These data suggest that IL-18 modulates glucose tolerance during endotoxin-induced acute inflammation. Surprisingly, IL-18 deficiency limited lung and liver damage and improved survival after LPS-induced endotoxemia.
Our observations of LPS-induced hyperglycemia 1 h after LPS injection and hypoglycemia 12 h after injection in this study correlate well with previous reports [16]. During the first hour after LPS injection, plasma glucagon, IL-6, and TNF-α concentrations increased compared with mice given a sham injection of PBS only. Because glucagon is an inducer of hyperglycemia and the cytokines are upregulated in response to hyperglycemia, we believe that these compounds may contribute to the transient hyperglycemia after LPS administration.
Our results demonstrating changes in insulin signaling in the liver in response to LPS-induced acute endotoxemia also support the findings of previous studies. Acute insulin resistance with hyperglycemia and hyperinsulinemia has been observed in hepatocytes in response to trauma and hemorrhage with corresponding downregulation of the insulin signaling pathway [17,18]. Additionally, inflammatory cytokines, such as TNF-α and IL-6, are elevated during acute and chronic inflammation, can inhibit insulin signaling pathways, and are associated with insulin resistance [9,10,19]. Although we hypothesized that IL-18 deletion would decrease acute insulin resistance and hyperglycemia, in fact, blood glucose, insulin and IL-6 concentrations were significantly higher in IL-18 KO mice than in WT mice. There are a few experimental studies suggesting that IL-18 is necessary for glucose tolerance in chronic inflammation [20,21]. The present study does not contradict these reports. In our study, the insulin signaling pathway was dampened in the liver of IL-18 KO mice, but not in that of WT mice. Insulin receptor substrate-mediated insulin signaling was altered in the IL-18 KO compared with the WT because the total cellular pool of IRS-1 differed between the IL-18 KO mice and the WT mice both before and after LPS administration. These data suggest that endogenous IL-18 plays an important role in modulating IRS-1 expression. Endogenous IL-18 might suppress hyperinsulinemia by maintaining insulin signaling pathway activity during the hyperacute phase (first hour) of endotoxemia, and IL-18 deletion might accelerate the inhibition of insulin signals. High plasma IL-6 in IL-18 KO mice may have promoted hyperglycemia, because IL-6 induces gluconeogenesis and glycogenolysis in hepatocytes and inhibits the phosphorylation of IRS-1 [10,22]. Maintenance of the insulin signaling pathway by IL-18 may be through the inhibition of IL-6, however, it is unclear how IL-18 might suppress IL-6 production. Negative feedback signaling could play a role because IL-18 can activate signal transducer and activator of transcription (STAT)-3, which is downstream of IL-6 [22,23].
Some reports have shown that increased circulating IL-18 or genetic variation of IL-18 is linked with insulin resistance in patients with type 2 diabetes mellitus, obesity, or polycystic ovary syndrome [14,15,24,25]. By contrast, our study identified insulin resistance during acute endotoxemia only in the absence of IL-18. This contradiction between our results and these reports may derive from differences in IL-18 sensitivity during acute and chronic inflammation. Zilverschoon et al. [26] reported that leukocytes from patients with obesity or type 2 diabetes mellitus had low responses after stimulation with IL-18, despite having high serum IL-18 concentrations. This suggests that IL-18 sensitivity is decreased in chronic inflammation. High IL-18 concentrations in patients with chronic insulin resistance may be compensatory reaction to reverse insulin resistance. Alternatively, the role of IL-18 in insulin resistance in response to chronic or acute inflammation may be understood insufficiently. This is a complex pathway with many redundancies to ensure adequate regulation. Studies of single elements of the pathway should be interpreted conservatively, and care must be taken not to imply causality when in fact only correlations are proven.
Our previous study suggested that endogenous IL-18 promoted lung edema and contributed to poor outcomes during endotoxemia [27]. The present study showed that in the absence of IL-18 (IL-18 KO) lung and liver injury were less severe and a survival was better after LPS injection compared with WT mice. We speculate that dramatically elevated IL-18 may promote organ dysfunction and contribute to a poor prognosis. Additionally, it is possible that the high blood glucose concentrations in the IL-18 KO mice preserved intravascular volume and perfusion and led to better survival.
There were some limitations of this study. First, the LPS-induced sepsis model does not model all aspects of clinical sepsis. We used a lethal endotoxemia model, because we believe it more closely mimics clinical septic shock than non-lethal models. However, we could only reproduce hyperglycemia for a short time. Second, KO mice are known to develop compensatory mechanisms that allow proper development and organ function in the absence of the targeted gene product. We cannot exclude the possibility of compensatory reactions in this study. Thus, the current findings may not be applicable directly to clinical sepsis, and further study is warranted.
In summary, this report revealed new aspects of the relation between IL-18 and glucose tolerance during acute inflammation. In this study, we discovered that IL-18 KO mice exhibited hyperglycemia and hyperinsulinemia after LPS injection. Because overinduction of IL-18 may contribute to high mortality, the possibility that an IL-18 blocking agent could be developed to treat patients with acute inflammation is intriguing. However, this report should also raise an alarm that perfect block of IL-18 may be undesirable and may induce acute insulin resistance and hyperglycemia.
Acknowledgments
The authors thank Dr. Shannon L Wyszomierski for editing the manuscript.
This research was supported by JSPS KAKENHI Grant Number 23659852.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systemic overview. Lancet 2000;355:773–778 [DOI] [PubMed] [Google Scholar]
- 2.Ikezu T, Okamoto T, Yonezawa K, et al. Analysis of thermal injury-induced insulin resistance in rodent. Implication of postreceptor mechanisms. J Biol Chem 1997;272:25289–25295 [DOI] [PubMed] [Google Scholar]
- 3.Carter EA. Insulin resistance in burns and trauma. Nutr Rev 1998;56:S170–S176 [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe , Pieter W, Frank W, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 6.Vriesendorp TM, van Santen S, DeVries JH, et al. Predisposing factor for hypoglycemia in the intensive care unit. Crit Care Med 2006;34:96–101 [DOI] [PubMed] [Google Scholar]
- 7.NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Preraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665–668 [DOI] [PubMed] [Google Scholar]
- 10.Senn JJ, Klover PJ, Nowak IA, et al. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002;51:3391–3399 [DOI] [PubMed] [Google Scholar]
- 11.Endo S, Inada K, Yamada Y, et al. Interleukin 18 (IL-18) levels in patients with sepsis. J Med 2000;31:15–20 [PubMed] [Google Scholar]
- 12.Zaki Mel-S, Elgendy MY, El-Mashad NB, et al. IL-18 level correlates with development of sepsis in surgical patients. Immunol Invest 2007;36:403–411 [DOI] [PubMed] [Google Scholar]
- 13.Aoyama M, Kotani J, Usami M. Gender difference in granulocyte dynamics and apoptosis and the role of IL-18 during endotoxin-induced systemic inflammation. Shock 2009;32:401–409 [DOI] [PubMed] [Google Scholar]
- 14.Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: Results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 2005;54:2932–2938 [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, et al. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: Relationship to insulin resistance and to obesity. J Clin Endocrinol Metab 2004;89:806–811 [DOI] [PubMed] [Google Scholar]
- 16.Raetzsch CF, Brooks NL, Alderman JM, et al. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor kappa b pathway. Hepatology 2009;50:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, Wang P, Kuebler JF, et al. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 2003;284:G107–G115 [DOI] [PubMed] [Google Scholar]
- 18.Li L, Thompson LH, Zhao L, et al. Tissue-specific difference in the molecular mechanisms for the development of acute insulin resistance after injury. Endocrinology 2009;150:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 2004;30:748–756 [DOI] [PubMed] [Google Scholar]
- 20.Yang YS, Li XY, Hong J, et al. Interleukin-18 enhances glucose uptake in 3T3-L1 adipocytes. Endocr 2007;32:297–302 [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, Joosten LAB, Lewis E, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 2006;12:650–656 [DOI] [PubMed] [Google Scholar]
- 22.Blumberg D, Hochwald S, Brennan MF, et al. Interleukin-6 stimulates gluconeogenesis in primary cultures of rat hepatocytes. Metabolism 1995;44:145–146 [DOI] [PubMed] [Google Scholar]
- 23.Kalina U, Kauschat D, Koyama N, et al. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol 2000;165:1307–1313 [DOI] [PubMed] [Google Scholar]
- 24.Zilverschoon GRC, Tack CJ, Joosten LAB, et al. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:1407–1414 [DOI] [PubMed] [Google Scholar]
- 25.Smart MC, Dedoussis G, Yiannakouris N, et al. Genetic variation within IL18 is associated with insulin levels, insulin resistance and postprandial measures. Nutr Metab Cardiovasc Dis 2011;21:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JW, Lee MH, Park JE, et al. Association of IL-18 genotype with impaired glucose regulation in Korean women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2012;161:51–55 [DOI] [PubMed] [Google Scholar]
- 27.Takahara M, Aoyama-Ishikawa M, Shuno K, et al. The role of endogenous IL-18 in the lung during endotoxin-induced systemic inflammation. Acute Med Surg 2014;1:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]