Abstract
Purpose:
To evaluate whether prostaglandin (PG) analogue use is associated with alterations in keratocyte density and central corneal thickness (CCT) in subjects with primary open-angle glaucoma (POAG).
Materials and Methods:
Thirty-five POAG patients treated with PG analogues for >2 years and 35 control subjects without glaucoma were included in this cross-sectional study. All subjects were underwent CCT measurements using ultrasound pachymetry. Keratocyte densities of each stromal layer were determined by in vivo confocal microscopy. Student's t-test and Chi-square test were used for statistical evaluations. Correlations between keratocyte densities and CCT were analyzed using Pearson's correlation analysis.
Results:
Keratocyte densities in each stromal layer were significantly lower in glaucoma patients receiving PG analogues as compared to those of controls (P < 0.001). The mean CCT was also lower in glaucoma patients (515.2 ± 18.8 μ) than control subjects (549.6 ± 21.1 μ, P < 0.001). A positive correlation between keratocyte densities in each stromal layer and CCT was observed in POAG patients.
Conclusions:
Long-term administration of topical PG analogues may adversely influence keratocyte densities and CCT. Further prospective studies are required clarify the relationship between PG analogues and their effects on the cornea.
Keywords: In vivo confocal microscopy, keratocyte density, prostaglandin analogues
Prostaglandin (PG) analogues are efficacious hypotensive medications widely used to lower intraocular pressure (IOP) in patients with glaucoma.[1,2] It is theorized that PG analogues act primarily through activation of matrix metalloproteinases (MMPs) and reduction of different collagen types in the eye.[3,4,5] Cornea is a target tissue for topical PG analogues and may be adversely affected by chronic administration of such agents.[6,7,8,9,10] Several studies suggest that the long-term use of topical PG analogues is associated with a reduction of central corneal thickness (CCT).[6,7,11] Prior studies attributed decreased CCT to PG induced upregulation of MMPs and resultant degradation of stromal extracellular matrix. On the other hand, a recent 2-year prospective study by Bafa et al. reported increased CCT in glaucoma patients receiving bimatoprost or latanoprost therapy.[9]
In vivo confocal microscopy (IVCM) is a noninvasive imaging technique to evaluate the corneal microstructure in health and diseased states. IVCM has been utilized to evaluate conjunctival blebs following trabeculectomy as well as the corneal epithelium and subbasal nerves to assess the ocular surface toxicity profiles of topical anti-glaucoma medications in glaucoma patients.[12,13] However, corneal stromal alterations associated with topical PG drops have not been extensively studied.[8,10] Thus, the aim of this study was to evaluate whether long-term treatment with topical PG analogues had any effect on keratocyte density and CCT of patients with primary open-angle glaucoma (POAG).
Materials and Methods
Subjects
Thirty-five patients with POAG treated with PG analogues and 35 age-matched healthy control subjects were included in this cross-sectional study undertaken at a single university hospital between November 2011 and July 2012. The Tenets of the Declaration of Helsinki was followed throughout the study. Informed consent was obtained from all patients, and the study was carried out with the approval from the Institutional Review Board. Participants were divided into two groups: Group 1 was composed of patients, who had been exclusively treated with PG analogues for at least 2 years without any prior use of another class of glaucoma medication; group 2 was composed of healthy age-matched controls without any systemic or ocular disease. All patients were underwent a complete ophthalmic examination including slit-lamp examination, IOP determination and fundus examination. POAG was defined as open-angle detected by gonioscopy, an IOP >21 mmHg with Goldmann applanation tonometer and characteristic glaucomatous optic nerve head changes (rim thinning, excavation, and/or retinal nerve fiber layer defects) and/or visual field defects were detected on ≥2 consecutive tests. Subjects who used any IOP-lowering drugs except PG analogues, those with any corneal pathology on slit-lamp examination, those with a history of ocular surgery or ocular trauma, those who were using contact lenses were excluded from the study. Control subjects were recruited during routine screening visits and had no history of ocular disease and pathologic ocular findings apart from cataract, normal appearing optic discs, normal visual fields and IOP measurements below 21 mmHg.
In vivo confocal microscopy of cornea
In vivo confocal microscopy of the cornea was performed using Confoscan 3.0 (Vigonza, Italy) attached to an immersion lens (Achroplan 40x/0.75 W, Zeiss, Oberkochen, Germany). The immersion lens had a working distance of 1.98 mm, a numerical aperture of 0.75, and the front surface area of 16.61 mm2. Data were collected for both eyes, and one eye for each patient was chosen at random. Before examination, each cornea was anesthetized with propacaine hydrochloride (Alcaine 0.5%, Alcon Laboratories Inc., Belgium). After application of Viscotears (Carbomer 0.2%, Novartis, Basel, Switzerland) as a coupling agent to the applanating lens, the full thickness of the central cornea was scanned, by the same operator. For each subject, four to six complete full-layer corneal scans were obtained. The images represented an area of 450 μm × 340 μm, had a lateral resolution of 1 μm, and a depth resolution of 10 μm. The mean magnification obtained was 500x on a 15 inch display (1024 × 768 pixels).
Image analysis
The best-focused two images for each stromal layer were selected for analysis, and the mean value was calculated. Keratocyte density was determined using a manual method of counting images of cell nuclei in a predefined area of confocal images, using the software provided with Confoscan 3.0. The size of the box kept constant for all study subjects. Cells that overlapped the boundary box were counted at only the superior and the left half of the box. Keratocytes densities were measured at the anterior (0–100 μm posterior to the basal epithelium), middle (half the distance between the basal epithelium and the endothelium), and posterior (0–100 μm anterior to the endothelium) stromal layers. The observer was masked to the clinical characteristics of patients and the randomization status.
Central corneal thickness
Central corneal thickness was measured in all subjects with an ultrasonic pachymeter (NIDEK Echoscan US-4000). To avoid the effects of the diurnal variation, all measurements were carried out at the same time of day (between 9:00 am and 12:00 am). In line with previously published methodology,[6,7,9] CCT measurements were taken using ultrasound pachymetry by a single observer experienced in this field. For each patient, after instillation of a topical anesthetic (Alcaine 0.5%, Alcon Laboratories Inc., Belgium), 5 readings were taken and averaged.
Statistical analysis
The data was evaluated in collaboration with the Department of Biostatistics. Data analysis was performed by SPSS 15.0 (Statistical Package for Social Sciences, SPSS Inc. IBM, Armonk, NY, USA) software package. The data were statistically analyzed using Student's t-test and Chi-square test. Correlations between keratocytes density and CCT were analyzed using Pearson's correlation coefficients and a P = 0.05 was accepted as statistically significant.
Results
Thirty-five patients with POAG (22 female, 13 male) and 35 control subjects (14 female, 21 male) were enrolled in this study. The mean duration of the PG analogue treatment was 6.3 ± 3.4 years (range = 2–12 years) in the glaucoma group. The mean age of patients with glaucoma and control subjects were 65.2 ± 7.1 years (range = 50–77 years) and 64.4 ± 4.6 years (range = 59–82 years), respectively. There were no significant differences across the two groups with respect to age (P = 0.596) and gender (P = 0.94).
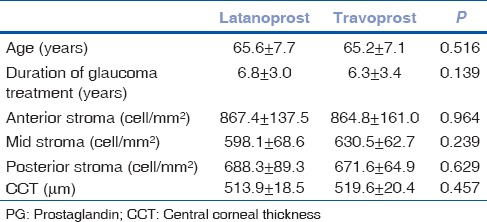
The mean keratocyte densities in three stromal layers and CCT values were significantly lower in patients with PG analogue therapy compared with control subjects [Table 1, Figs. 1 and 2]. Twenty-six patients were being treated with latanoprost and 9 with travoprost eye drops. The mean age of patients as well as the duration of topical anti-glaucoma therapy, were similar between groups [Table 2]. There were no significant differences observed in keratocyte densities and the CCT measurements between who were on latanoprost versus travoprost medication [Table 2]. A weak positive correlation between the keratocyte densities in each stromal layers and CCT was observed in patients with PG analogue therapy (anterior stroma, ρ = 0.412, P < 0.001; midstroma, ρ = 0.572, P < 0.001; posterior stroma, ρ = 0.547, P < 0.001). A negative but statistically insignificant correlation was found between the anterior stromal (ρ = −0.014, P = 0.934), mid-stromal (ρ = −0.319, P = 0.062) and posterior stromal (ρ = −0.232, P = 0.179) keratocyte densities and duration of the PG analogue treatment.
Table 1.
Comparison of the keratocyte densities and central corneal thickness measurements between glaucoma patients on PG analogue therapy and control subjects
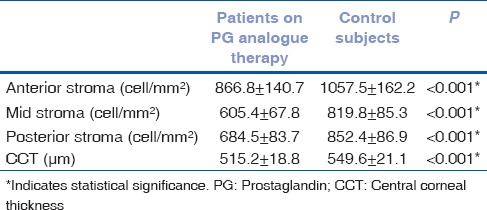
Figure 1.
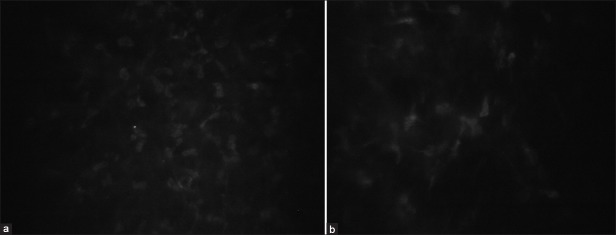
In vivo confocal microscopic images of anterior stromal keratocytes in a control (a) subject and patient treated with prostaglandin analogue (b)
Figure 2.
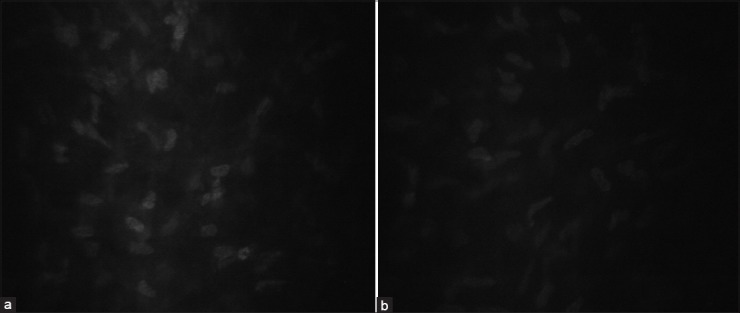
Mid-stromal section from a healthy subject (a) and a patient on prostaglandin analogue treatment (b)
Table 2.
Comparison of the keratocyte densities and central corneal thickness measurements between glaucoma patients on latanoprost versus travoprost therapy
Discussion
In this study, keratocyte densities and CCT were found to be lower in POAG patients on PG analogue therapy for at least 2 years compared with normal subjects. No differences in keratocyte densities were detected in patients receiving latanoprost versus travoprost. Overall, our results suggest a possible deleterious effect of topical PG analogues on keratocytes in the corneas of POAG patients. Our study is noteworthy in that the study subjects had only used PG analogues for the entire duration of their treatment.
There is limited data on the effects of PG analogues on the corneal microstructure. In a scanning laser confocal microscopic study by Bergonzi et al., PG analogue therapy was found to be associated with increased keratocyte densities in all corneal layers of patients with POAG independent of duration of PG analogue treatment.[8] They hypothesized decreased extracellular matrix synthesis secondary to MMP activation with resultant increased keratocyte density within the same region as an explanation for their findings though CCT measurements were not made and this hypothesis was not tested in the study.[8] In another study by Baratz et al., PG analogues, β-adrenergic blockers, and carbonic anhydrase inhibitors had no discernible effect on either keratocyte or endothelial densities of ocular hypertensive patients treated for a period of 6 years.[10] Of note, the study by Baratz et al. did not have a separate study group consisting of glaucoma subjects.[10]
In prior reports, PG analogues have been also found to be associated with reduction in tear break-up time and basal tear secretion, increased impression cytology grade of conjunctiva and altered meibomian gland function such as increased meibum score and meiboscore and tear film instability.[14,15,16] However, it is yet to be determined whether decreased tear break up time and tear secretion can lead to reduced keratocyte counts in glaucoma patients.
The relationship between prolonged treatment with PG analogues and its impact on CCT is still unclear. The Ocular Hypertension Treatment Study (OHTS) group reported that the rate of CCT reduction in ocular hypertensive patients treated with topical PG analogues over 3.8 years was more than those who were on beta-blockers.[11] OHTS group suggested that the use of topical PG analogues may be associated with a slightly higher rate of thinning in CCT. Viestenz et al., performed a nonrandomized controlled cross-sectional study of CCT among patients using topical PG analogues and found significantly thinner CCT among patients using PG analogue than among controls (539 μm vs. 539 μm).[17] In a prospective study by Sen et al., a significant reduction in CCT at 6, 12, and 24 months was observed compared to baseline levels in both POAG and patients with pseudoexfoliation glaucoma treated with PG analogues.[7] In another prospective study by Harasymowycz et al., the effect of topical travoprost on CCT was evaluated, and they found a mean decrease in CCT of 6.9 μm after 6 weeks of treatment.[18] This amount of decline was found to be similar to the 6th month decline with bimatoprost and 12th month decline with latanoprost in the study by Sen et al.[7] Zhong et al. reported a significant reduction in CCT during a mean treatment interval of 17.9 ± 15.7 months after initial diagnosis of patients with POAG and normal tension glaucoma who were treated with topical PG analogues.[6] In an experimental animal model, Park, et al. showed corneal thinning with decreased collagen type I expression in the stroma by immunohistochemical staining in rabbits treated with PG analogues.[19] In the same study, the topical use of PG analogues also resulted in increased MMP to TIMP ratio, which may be triggered by inflammatory cytokines such as interleukin-1 (IL-1) and IL-6.[19]
In an experimental study by Wu et al., they showed that the latanoprost induced morphological and biomechanical changes in cultured corneal stromal cells and the extracellular matrix, such as modifying type I collagen distribution.[20] However, Liu et al. found that latanoprost had no effect on collagen degradation by corneal fibroblasts.[21] In a prospective study by Honda et al., an increase in MMP-1 and MMP-9 and a decrease in tissue inhibitors of metalloproteinases -1 in tears collected from glaucoma patients who were on PG analogues was detected.[20] It has been shown that PG analogues increase the release of MMPs from tenon fibroblasts and ciliary smooth muscle cells.[23,24] Overall, these observations suggest that PG analogues may promote collagen degradation in the stroma.
In contrast to the findings of previous studies, a prospective study on medically treated chronic open-angle glaucoma patients reported an increase in CCT in patients who received bimatoprost or latanoprost, but no changes in travoprost group during a follow-up period of 2 years.[9] The authors of that study were not able to associate their observations with any causal factor.[9]
Although our data suggests reduction of CCT and keratocyte density with PG analogue therapy, we cannot make a distinction whether the observed changes are directly due to PG analogues or the preservative in the medication (benzalkonium chloride [BAK]). Although BAK exposure has been associated with ocular surface disease,[25] corneal epithelial loss[26] and trabecular meshwork damage,[27] to the best of our knowledge there is no data linking BAK to keratocyte loss. However, in a study by Martone et al., higher number of activated stromal keratocytes was observed in the corneas of glaucoma patients who were treated with preservative containing anti-glaucoma drops as compared to those patients on nonpreservative medication, suggesting a possible role of preservatives in stromal changes.[28]
One limitation to this study is the lack of baseline CCT measurements and keratocyte densities prior to initiation of treatment. CCT measurements and IVCM assessments were performed at least 2 years after the initiation of treatment with PG analogues. Thus, our data, as well as those obtained in other similar previous studies[8,17] cannot be used to make assumptions on time dependent changes related to PG use in corneas of glaucoma subjects. We also did not have any data on PG drop use in nonglaucomatous (i.e. ocular hypertensive) subjects. Glaucoma may be associated with corneal changes independent of topical medication use.[29] Ideally, it would have been best to include a nontreated POAG group for comparison; only then we would be able to attribute changes in keratocyte densities to the treatment. However, withholding treatment from glaucoma patients for any period is not possible on ethical grounds. Since any class of topical anti-glaucoma medication as well as drop preservatives could adversely affect keratocyte densities, inclusion of a POAG group receiving another class of medication would not have made any significant contribution to the interpretation of the keratocyte density reductions. Thus, prospective cohort studies with long follow-up intervals are required for to make causal inferences and to obtain longitudinal CCT and keratocyte data from a well-studied cohort of individuals with who were on PG analogue treatment.
In conclusion, the findings of this study suggest that the long-term treatment with PG analogues may be associated with reduced keratocyte densities and corneal thickness. Prospective studies looking into time-dependent alterations of keratocyte densities associated with PG analogue use in both glaucomatous and ocular hypertensive subjects is needed to clarify the exact relationship between topical PG analogue use and its effects on corneal stroma.
Acknowledgments
The authors thank Jale Karakaya from the Department of Biostatistics for her assistance in the statistical analysis of the data.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Alm A, Schoenfelder J, McDermott J. A 5-year, multicenter, open-label, safety study of adjunctive latanoprost therapy for glaucoma. Arch Ophthalmol. 2004;122:957–65. doi: 10.1001/archopht.122.7.957. [DOI] [PubMed] [Google Scholar]
- 2.Parrish RK, Palmberg P, Sheu WP XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(Suppl 1):S53–64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 4.Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999;117:794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- 5.Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000;11:112–5. doi: 10.1097/00055735-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y, Shen X, Yu J, Tan H, Cheng Y. The comparison of the effects of latanoprost, travoprost, and bimatoprost on central corneal thickness. Cornea. 2011;30:861–4. doi: 10.1097/ICO.0b013e3182000c27. [DOI] [PubMed] [Google Scholar]
- 7.Sen E, Nalcacioglu P, Yazici A, Aksakal FN, Altinok A, Tuna T, et al. Comparison of the effects of latanoprost and bimatoprost on central corneal thickness. J Glaucoma. 2008;17:398–402. doi: 10.1097/IJG.0b013e31815d784c. [DOI] [PubMed] [Google Scholar]
- 8.Bergonzi C, Giani A, Blini M, Marchi S, Luccarelli S, Staurenghi G. Evaluation of prostaglandin analogue effects on corneal keratocyte density using scanning laser confocal microscopy. J Glaucoma. 2010;19:617–21. doi: 10.1097/IJG.0b013e3181ca7c7a. [DOI] [PubMed] [Google Scholar]
- 9.Bafa M, Georgopoulos G, Mihas C, Stavrakas P, Papaconstantinou D, Vergados I. The effect of prostaglandin analogues on central corneal thickness of patients with chronic open-angle glaucoma: A 2-year study on 129 eyes. Acta Ophthalmol. 2011;89:448–51. doi: 10.1111/j.1755-3768.2009.01731.x. [DOI] [PubMed] [Google Scholar]
- 10.Baratz KH, Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25:1046–52. doi: 10.1097/01.ico.0000230499.07273.c5. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JD, Gordon MO, Beiser JA, Lin SC, Alexander MY, Kass MA, et al. Changes in central corneal thickness over time: The Ocular Hypertension Treatment Study. Ophthalmology. 2008;115:1550–6.e1. doi: 10.1016/j.ophtha.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Morita K, Gao Y, Saito Y, Higashide T, Kobayashi A, Ohkubo S, et al. In vivo confocal microscopy and ultrasound biomicroscopy study of filtering blebs after trabeculectomy: Limbus-based versus fornix-based conjunctival flaps. J Glaucoma. 2012;21:383–91. doi: 10.1097/IJG.0b013e3182120a08. [DOI] [PubMed] [Google Scholar]
- 13.Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 14.Terai N, Müller-Holz M, Spoerl E, Pillunat LE. Short-term effect of topical antiglaucoma medication on tear-film stability, tear secretion, and corneal sensitivity in healthy subjects. Clin Ophthalmol. 2011;5:517–25. doi: 10.2147/OPTH.S18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alagöz G, Bayer A, Boran C, Serin D, Kükner A, Elçioglu M. Comparison of ocular surface side effects of topical travoprost and bimatoprost. Ophthalmologica. 2008;222:161–7. doi: 10.1159/000126078. [DOI] [PubMed] [Google Scholar]
- 16.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Tomidokoro A, et al. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012;31:1229–34. doi: 10.1097/ICO.0b013e31823f8e7d. [DOI] [PubMed] [Google Scholar]
- 17.Viestenz A, Martus P, Schlötzer-Schrehardt U, Langenbucher A, Mardin CY. Impact of prostaglandin-F (2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness – A cross-sectional study on 403 eyes. Klin Monbl Augenheilkd. 2004;221:753–6. doi: 10.1055/s-2004-81361. [DOI] [PubMed] [Google Scholar]
- 18.Harasymowycz PJ, Papamatheakis DG, Ennis M, Brady M, Gordon KD Travoprost Central Corneal Thickness Study Group. Relationship between travoprost and central corneal thickness in ocular hypertension and open-angle glaucoma. Cornea. 2007;26:34–41. doi: 10.1097/ICO.0b013e31802e3ce4. [DOI] [PubMed] [Google Scholar]
- 19.Lopilly Park HY, Kim JH, Lee KM, Park CK. Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp Eye Res. 2012;94:13–21. doi: 10.1016/j.exer.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Wu KY, Wang HZ, Hong SJ. Effect of latanoprost on cultured porcine corneal stromal cells. Curr Eye Res. 2005;30:871–9. doi: 10.1080/02713680591006237. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Yanai R, Lu Y, Hirano S, Sagara T, Nishida T. Effects of antiglaucoma drugs on collagen gel contraction mediated by human corneal fibroblasts. J Glaucoma. 2006;15:255–9. doi: 10.1097/01.ijg.0000212210.33265.24. [DOI] [PubMed] [Google Scholar]
- 22.Honda N, Miyai T, Nejima R, Miyata K, Mimura T, Usui T, et al. Effect of latanoprost on the expression of matrix metalloproteinases and tissue inhibitor of metalloproteinase 1 on the ocular surface. Arch Ophthalmol. 2010;128:466–71. doi: 10.1001/archophthalmol.2010.40. [DOI] [PubMed] [Google Scholar]
- 23.Mietz H, Esser JM, Welsandt G, Kociok N, Hueber A, Joussen A, et al. Latanoprost stimulates secretion of matrix metalloproteinases in tenon fibroblasts both in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:5182–8. doi: 10.1167/iovs.02-0462. [DOI] [PubMed] [Google Scholar]
- 24.Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997;38:2772–80. [PubMed] [Google Scholar]
- 25.Nuzzi R, Finazzo C, Cerruti A. Adverse effects of topical antiglaucomatous medications on the conjunctiva and the lachrymal (Brit. Engl) response. Int Ophthalmol. 1998;22:31–5. doi: 10.1023/a:1006051725115. [DOI] [PubMed] [Google Scholar]
- 26.Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 27.Hamard P, Blondin C, Debbasch C, Warnet JM, Baudouin C, Brignole F. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241:1037–43. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 28.Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–735.e1. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120:2241–8. doi: 10.1016/j.ophtha.2013.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


