Abstract
The transcription factor Tbet is critical for the differentiation of Th1 CD4 T cells and is associated with the induction of multiple autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE). Herein, we demonstrate that Tbet suppresses IL-17A and Th17 differentiation both in vitro and in vivo in a cell-intrinsic manner, and that in fact, Tbet is not necessary for EAE induction. Moreover, we find that IFNγ inhibits the production of IL-17A and IL-17F in a STAT1-dependent, Tbet-independent manner. These findings illustrate multiple mechanisms utilized by developing Th1 cells to silence the Th17 program.
Keywords: CD4 T cell, Tbet, IFNγ, IL-17A, Experimental autoimmune encephalomyelitis
1. INTRODUCTION
Experimental autoimmune encephalomyelitis (EAE) is a mouse model for multiple sclerosis (MS), with similar pathology including elevated levels of the cytokines IFNγ and IL-17A in the CNS (Fletcher et al., 2010, Goverman, 2009). CD4 T cells play an essential role during EAE in which MOG35–55 peptide elicits robust CD4 T cell responses; in addition, myelin specific CD4 T cells are able to drive disease in naïve mice (Bettelli et al., 2004). Both Th1 and Th17 subsets are pathogenic populations during EAE; however, mice deficient in the signature cytokines IFNγ, IL-17A, or IL-17F are not protected from disease (Bettelli et al., 2004, Ferber et al., 1996, Gonzalez-Garcia et al., 2009, Hofstetter et al., 2005, Hu et al., 2010, Komiyama et al., 2006). Hence, the regulation and mechanism of how effector CD4 T cell subsets drive EAE remain ill-defined.
Tbet, abbreviated from T-box expressed in T cells, is expressed by a number of hematopoietic cells and is essential to initiate the gene expression program during Th1 differentiation (Lazarevic and Glimcher, 2011, Szabo et al., 2002). In addition, CD4 T cells deficient in Tbet tend to develop into either Th2 or Th17 populations depending on the stimulation conditions, indicating the suppressive property of Tbet on other lineages (Finotto et al., 2002, Lazarevic et al., 2011). During Th1 differentiation, Tbet has been reported to be phosphorylated by IL-2-inducible T-cell kinase that enables Tbet to interact with GATA-3 and inhibit Th2 differentiation (Miller et al., 2004). Likewise, Tbet is capable of suppressing Th17 development by hijacking Runx1 which promotes expression of the Th17 master transcription factor, RORγt (Lazarevic et al., 2011). Thus, Tbet functions at multiple levels to cement the Th1 differentiation program and extinguish alternative effector cell fates.
CD4 T cells are critical for the development of multiple autoimmune disorders and expression of Tbet has also been shown to be mandatory for the pathogenesis associated with certain autoimmune diseases (Alzabin and Williams, 2011, Goverman, 2009, Haskins and Cooke, 2011, Zenewicz et al., 2009). Paradoxically, while it is reported that Tbet is necessary for EAE induction (Bettelli et al., 2004), it has also been demonstrated that this molecule can suppress Th17 differentiation (Lazarevic et al., 2011), a key cell population linked with the development of EAE. It remains unclear how Tbet drives CNS inflammation and if expression of this molecule is required within the Th17 population. Interestingly, a fraction of the effector CD4 T cells isolated from the inflamed CNS during EAE are capable of producing both IFNγ and IL-17A, and these cells also express Tbet (Yeh et al., 2011). It is thought that this population is a consequence of Th17 plasticity, and, in recent years, a number of publications have correlated the presence of this cell population with the severity of disease (Ghoreschi et al., 2010, Hirota et al., 2011).
The importance of Tbet within CD4 T cells during EAE is still not understood. In this study, we interrogated the function of this transcription factor in CD4 T cells and show that it was not required for the migration of the cells to the inflamed CNS, and contrary to published reports, we did not observe a requirement for Tbet in the development of EAE. In addition, we demonstrate that Tbet actively suppressed not only IL-17A production, but also differentiation of Th17 cells by limiting expression of the master transcription factor RORγt. It is known that IFNγ can suppress Th17 differentiation and here we reveal that it signals through STAT1 to mediate this effect. Interestingly, IFNγ inhibition of RORγt requires the expression of Tbet, however IFNγ can still repress IL-17A and IL-17F in a Tbet-independent but STAT1-dependent manner. In all, our findings highlight the complex interplay between the Th1 and Th17 cell lineages and shed new information regarding the non-essential role for Tbet during EAE.
2. MATERIALS AND METHODS
2.1. Mice
The following mice were purchased from The Jackson Laboratory, Taconic Farms Inc., or were bred at the University of Alabama at Birmingham (UAB): C57BL/6 (WT), B6.129S6-Tbx21tm1Glm/J (Tbet−/−), B6.129P2-Stat1tm1 (STAT1−/−), B6.SJL-Ptprca Pep3b/BoyJ (CD45.1), B6.129S7-Rag1tm1Mom/J, and B6.129S1-Il12btm1Jm/J. All animals were bred and maintained under specific pathogen free conditions according to Institutional Animal Care and Use Committee regulations.
2.2. Generation of mixed bone marrow chimeras
Bone marrow chimeric mice were generated as previously described (Yi et al., 2009). Bone marrow was prepared from tibias and femurs of CD45.1 WT, CD45.2 WT, and CD45.2 Tbet−/− mice and injected into irradiated B6.129S7-Rag1tm1Mom/J mice. Mice were immunized for EAE after 8–10 weeks following reconstitution.
2.3. EAE induction and scoring
Experimental mice were immunized for EAE by the standard protocol described previously (Yeh et al., 2011). Disease was monitored daily by the following criteria: 0, no disease; 1, paralyzed tail; 2.0, hind limb paresis; 2.5 one hind limb paralyzed; 3.0, both hind limbs paralyzed; 4.0, forelimbs paralyzed; and 5, moribund.
2.4. Cell isolation, activation, and staining
Leukocytes were isolated from the brain (BR), spinal cord (SC), spleen (SPL), and inguinal lymph nodes (LN) and stimulated as described previously (Yeh et al., 2011).
Surface and intracellular staining was performed according to the manufacturer’s instructions (eBioscience/Life Technologies). For surface staining, cells were stained with anti-CD4 PerCP-Cy5.5, anti-CD45.1 FITC, anti-CD45.2 PE mAb. A viability dye (Life Technologies) was applied to exclude dead cells. After permeabilization, cells were stained for intracellular molecules using anti-IFNγ eFluor 450, anti-IL17A PE, and anti-Tbet eFluor 660 mAb. Samples were acquired by using an LSRII flow cytometer (BD Biosciences) followed by data analysis using Flow Jo (Tree Star).
2.5. Naïve CD4 T cell preparation and polarization
Naïve CD4+CD25−CD45RBhi T cells were sorted from WT, Tbet−/−, and STAT1−/− mice using a FACSAria or FACSAria II cell sorter (BD Biosciences). Sorted CD4 T cells were stimulated with irradiated WT feeders and 2.5 µg/ml anti-CD3 (145-11), 5 ng/ml rhTGF-β1, 20 ng/ml rmIL-6, 1 ng/ml rmIL-23 (Biolegend), 10 µg/ml anti-IL-4 (11B11) with or without 10 µg/ml anti-IFNγ (XMG1.2) and the indicated amounts of rmIFNγ (Biolegend) for 5 days.
2.6. RNA purification, cDNA synthesis, and real-time PCR
Live CD4 T cells were collected following centrifugation over a Histopaque 1083 gradient. RNA collection, cDNA synthesis, and real-time PCR analysis were performed as described previously (17).
2.7. Statistics
Statistical significance was analyzed by unpaired Student t test using Prism software (GraphPad). Statistical significance is denoted as * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
3. RESULTS
3.1. CD4 T cell entry into the CNS during EAE is independent of Tbet
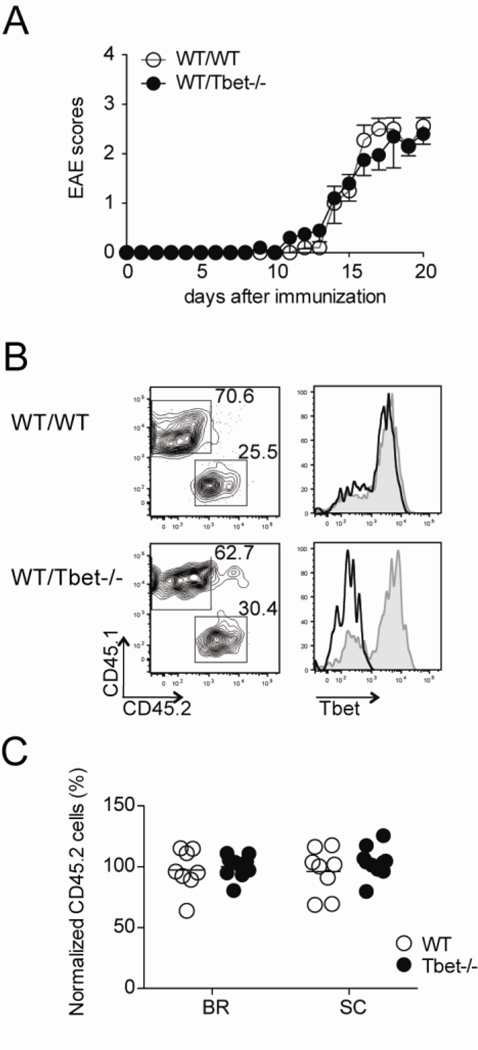
The transcription factor Tbet is termed the master regulator of Th1 differentiation, as this molecule is necessary for expression of the cardinal Th1 cytokine IFNγ, as well as other Th1-associated genes (Afkarian et al., 2002, Lord et al., 2005, Shinohara et al., 2005, Szabo et al., 2002). Interestingly, it is reported that Tbet is required for the development of EAE whereas IFNγ is not (Ferber et al., 1996), suggesting that Tbet regulates induction of CNS inflammation via a different mechanism. To interrogate the essential role of Tbet in CD4 T cells during EAE, we induced disease in mixed bone marrow chimeric mice. Irradiated mice were reconstituted with equal numbers of CD45.1 wild-type (WT) and either CD45.2 Tbet-deficient (Tbet−/−; experimental) or CD45.2 WT (control) bone marrow. No differences were observed in the disease incidence, onset, or severity between the two cohorts of mice (Fig. 1A). Hence, providing us with an experimental system to investigate the CD4 T cell intrinsic requirement for Tbet during EAE.
Figure 1. Tbet is not necessary for migration of CD4 T cells to the CNS during EAE.
Mixed bone marrow chimeric mice reconstituted with either WT CD45.1 and WT CD45.2 (WT/WT) or WT CD45.1 and Tbet−/− CD45.2 (WT/Tbet−/−) were immunized for EAE. (A) Disease severity in WT/WT (control) and WT/Tbet−/− (experimental) groups was monitored daily. Data represent two independent experiments with five mice in each group (mean ± SEM). (B) Lymphocytes were isolated from brain, spinal cord, and spleen 20 days after EAE induction. The cells were stained for CD4, CD45.1, CD45.2, and Tbet ex vivo. (left) Representative CD45.1 and CD45.2 staining of spinal cord-infiltrating CD4 T cells. (right) The expression of Tbet by infiltrating CD4 T cell populations is shown by histogram (CD45.1+ cells, gray line; CD45.2+ cells, black line). (C) The migratory capacity of CD4 T cells into CNS was determined by dividing the percent of CD45.2+ CD4 T cells in the brain (BR) and spinal cord (SC) by the percent of CD45.2+ CD4 T cells in the spleen, each dot represents an individual mouse. Collective data with two experiments are shown (n = 8–9).
We first examined the impact of Tbet deficiency on migration of CD4 T cells to the inflamed CNS. Leukocytes were isolated from both the brain and spinal cords of mice at the peak of disease severity, and the percentage of CD45.2+ CD4 T cells were compared between the experimental and control groups. The proportion of CD45.2+ CD4 T cells was similar between both groups in the brain and spinal cords, and intracellular staining verified the genetic deletion of Tbet (Fig. 1B). Moreover, when the percentages were normalized to the frequencies of CD45.2+ CD4 T cells in the spleens, the Tbet−/− CD4 T cells reached close to 100% migratory efficiency (Fig. 1C), suggesting that Tbet is not required for migration of CD4 T cells to the inflamed CNS.
3.2. Inhibition of IL-17A production by CD4 T cell intrinsic expression of Tbet
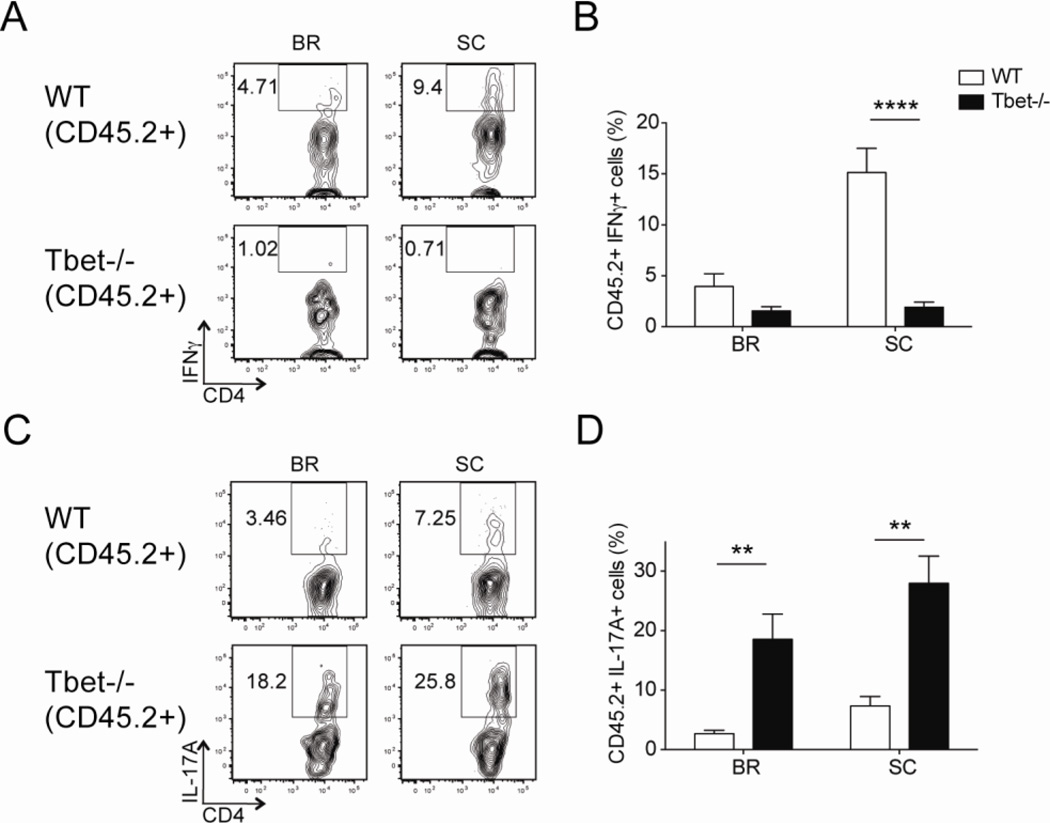
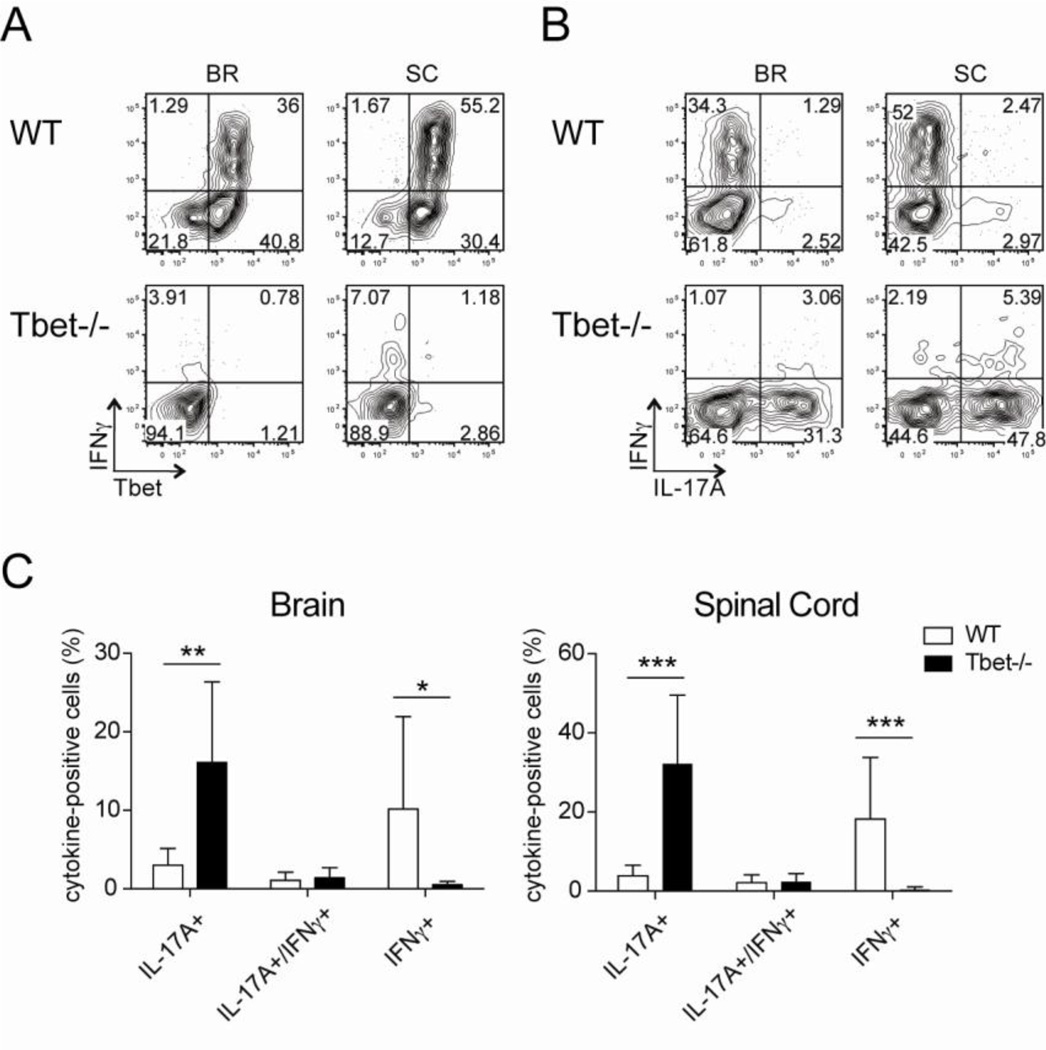
Having noted that the recruitment of CD4 T cells to the CNS was Tbet-independent, we investigated the other aspects of Tbet deficiency on CD4 T cell effector function in these mixed bone marrow chimeric mice. After a brief stimulation with either MOG35–55 peptide or PMA and ionomycin (P/I), we observed limited IFNγ production in Tbet−/− CD4 T cells (Fig. 2A–B and Fig. S1), which is consistent with previous publications (3). We did note that Tbet deficiency was associated with a marked increase in the frequency of IL-17A+ CD4 T cells, indicating Tbet suppresses IL-17A production in a cell-intrinsic manner (Fig. 2C–D and Fig. S1). Similar results were observed when we analyzed the effector CD4 T cells from intact WT and Tbet−/− immunized for EAE; WT CD4 T cells predominately produced IFNγ and some IL-17A, while the Tbet−/− CD4 T cells did not make IFNγ and produced higher levels of IL-17A (Fig. 3).
Figure 2. CD4 T cell intrinsic Tbet expression limits expansion of IL-17A-producing cells during EAE.
WT/WT and WT/Tbet−/− mixed bone marrow chimeric mice were immunized for EAE and analyzed on day 20. Single-cell suspensions from brain (BR) and spinal cord (SC) were restimulated with MOG35–55 peptide and evaluated for cytokine production by CD45.2+ CD4 T cells. (A, C) Representative flow cytometry plots gated on CD45.2+ CD4 T cells show (A) IFNγ and (C) IL-17A staining. (B, D) Cumulative frequencies of (B) IFNγ+ and (D) IL-17A+ CD45.2+ CD4 T cells in the brains and spinal cords of mixed bone marrow chimeric mice. Collective data with two experiments are shown (n = 8–9).
Figure 3. Elevated IL-17A production by CD4 T cells from intact Tbet−/− mice during EAE.
Intact WT and Tbet−/− mice were immunized for EAE and at the peak of disease CD4 T cells were isolated from the CNS and analyzed for cytokine production after a brief in vitro MOG35–55 peptide restimulation. (A–B) Representative plots gated on CD4 T cells from brain (BR) and spinal cord (SC) stained for Tbet and IFNγ IFNγ and IL-17A. (C) Cumulative data indicating the percent of CD4 T cells that were IL-17A+, IFNγ/IL-17A+, or IFNγ+. Data are representative of two experiments with nine to twelve mice total.
3.3. Tbet is not required for the induction of EAE
Our data from the mixed bone marrow chimeric mice led us to postulate if WT CD4 T cells provide a permissive environment for Tbet−/− CD4 T cells to enter the inflamed CNS. Therefore we immunized intact WT and Tbet−/− mice for EAE. Unexpectedly, we discovered that Tbet−/− mice were able to develop EAE, with the same kinetics and severity as WT mice (Table 1). Importantly, IL-12p40-deficient (IL-12p40−/−) mice remained resistant to EAE development using an identical immunization protocol, confirming that not all strains become susceptible to disease using this procedure and in our animal colony (Table 1).
Table 1.
Susceptibility of intact WT, Tbet−/−, and IL-12p40−/− mice following EAE immunization.
| Incidence | Day on onset |
Maximum score |
||
|---|---|---|---|---|
| LH colony | WT (n=19) | 100% | 12.6 ± 3.85 | 2.68 ± 0.54 |
| Tbet−/− (n=21) | 100% | 13.9 ± 3.25 | 2.65 ± 1.07 | |
| Taconic | WT (n=5) | 80% | 11 ± 1.15 | 1.3 ± 0.76 |
| Tbet−/− (n=5) | 100% | 12.2 ± 0.45 | 2.15 ± 0.55 | |
| Jackson | WT (n=5) | 100% | 13.4 ± 2.61 | 2.35 ± 0.38 |
| Tbet−/− (n=5) | 80% | 12.5 ± 1 | 2.95 ± 1.66 | |
| IL-12p40−/− (n=5) | 0% | n.a. | 0.00 | |
n.a. = not applicable
Commensal microbiota have been shown to have an impact on immune regulation (Honda and Littman, 2012) and segmented filamentous bacteria (SFB) have been reported to induce potent intestinal Th17 responses and restore susceptibility to multiple autoimmune disorders in germ-free mice (Ivanov et al., 2009, Lee et al., 2011, Stepankova et al., 2007). To investigate if the presence of SFB altered the susceptibility of the Tbet−/− mice to EAE, we immunized both WT and Tbet−/− mice obtained from The Jackson Laboratory and Taconic Farms, Inc., which have low and high levels of SFB respectively (Ivanov et al., 2009). The level of SFB was confirmed in fecal pellets at the termination of the experiments (Supplemental Fig. 2). Again, as detailed in Table 1, we noted no differences in the disease incidence, onset, and severity between the WT and Tbet−/− mice, regardless of the vendor and SFB status. Collectively, Tbet is not required for CD4 T cell pathogenecity during EAE, and the levels of SFB do not alter the susceptibility of mice (WT and Tbet-deficient mice) to EAE.
3.4. IFNγ suppresses RORγt, but not IL-17A, in a Tbet-dependent manner
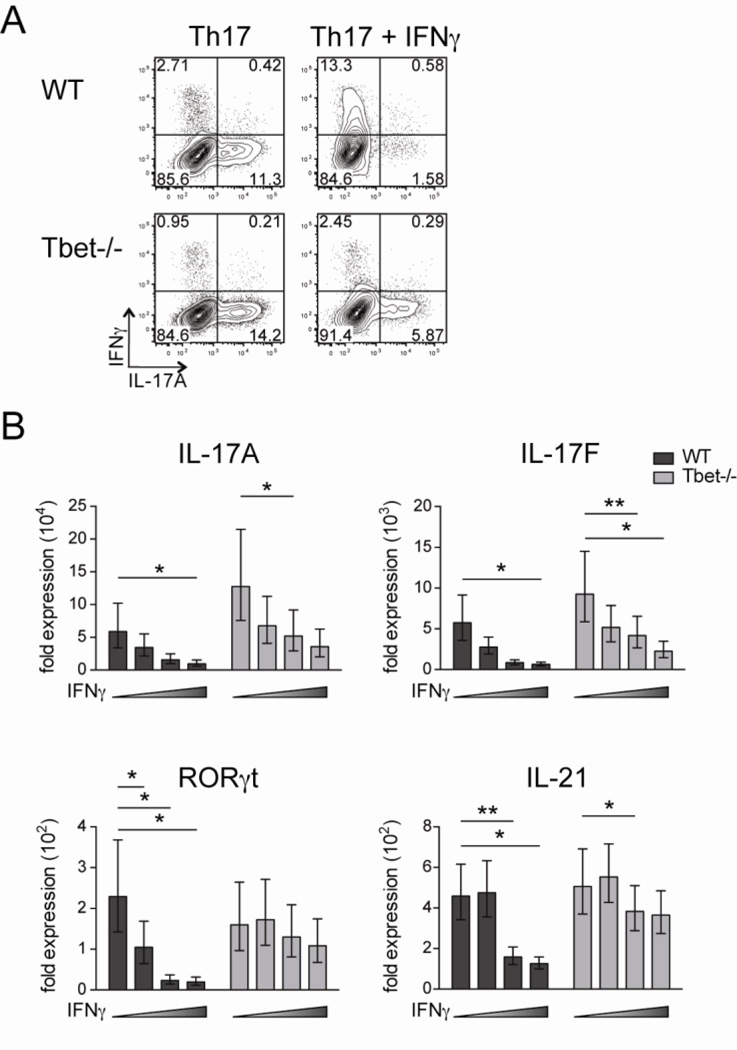
Our data indicates that Tbet suppresses IL-17A in a cell-intrinsic manner. In addition, we have previously published that mice deficient of IFNγ signaling (both IFNγ and IFNγR deficient mice) showed elevated IL-17A production in CD4 T cells during EAE (17), indicating IFNγ suppresses IL-17A. Notably, we observed that the majority of the IL-17A-producing CD4 T cells in IFNγR-deficient mice were Tbet-positive. This result raises the question as to whether IFNγ acts through Tbet for repression of IL-17A. Therefore, we assessed the mechanism by examining Tbet−/− CD4 T cells cultured under Th17 conditions with or without IFNγ and analyzing IL-17A production. We detected a reduction of IL-17A expression in Tbet−/− CD4 T cells when exogenous IFNγ was added; indicating IFNγ inhibits IL-17A in a Tbet-independent manner (Fig. 4A, B). Moreover, administration of exogenous IFNγ to WT CD4 T cells activated under Th17 conditions resulted in marked termination IL-17A production and a shift to IFNγ production (Fig. 4A, top right panel). This cumulative suppression of IL-17A by IFNγ and Tbet suggests that these two factors inhibit IL-17A production via two independent mechanisms.
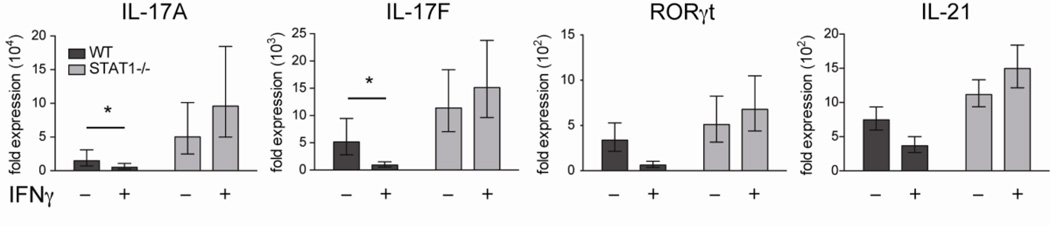
Figure 4. IFNγ suppression of IL-17A/F production independent of Tbet expression.
Naïve CD4 T cells were isolated from WT and Tbet−/− mice, stimulated under Th17 conditions in the presence of irradiated feeder cells for 5 days, and then restimulated with PMA and ionomycin prior to analysis. The cultures contained varying concentrations of IFNγ: 1) 10µg/ml anti-IFNγ mAb, 2) no anti-IFNγ mAb, 3) 100U/ml IFNγ, or 4) 1000U/ml IFNγ. (A) Representative IL-17A and IFNγ staining in CD4 T cells cultured under conditions 1 and 4. Plots are gated on live CD4 T cells. (B) Real-time PCR was performed on live CD4 T cells. Individual gene expression was normalized to β2-microglobulin and shown by the fold expression compared to naive CD4 T cells. Data are combined with three independent experiments and student t test was performed on the value of ΔΔCt.
To determine if the suppression of IL-17A by IFNγ is restricted only to this cytokine or functions universally during Th17 differentiation, we employed real-time PCR to examine the expression of other Th17-associated genes in CD4 T cells activated under Th17 conditions in the presence of varying levels of IFNγ. We found that IFNγ inhibits multiple Th17-related molecules in WT CD4 T cells including IL-17A, IL-17F, RORγt, and IL-21 in a dosage-dependent manner, indicating IFNγ has a global suppressive effect on Th17 differentiation (Fig. 4B). Next, to ascertain if IFNγ operates via the induction of Tbet to repress Th17 development, we assessed the expression of these Th17-associated genes in Tbet−/− CD4 T cells cultured under the same conditions. We observed significant inhibition of both IL-17A and IL-17F in Tbet−/− CD4 T cells by IFNγ stimulation (Fig. 4B). However, the impact of IFNγ on RORγt and IL-21 was dependent on Tbet expression, as a minimal decrease in the levels of these genes was detected when IFNγ was added to Tbet−/− CD4 T cells cultured under Th17 polarizing conditions (Fig. 4B). Taken together, we find IFNγ induces Tbet upregulation to suppress RORγt during Th17 differentiation, but that IFNγ can still repress both IL-17A and IL-17F when Tbet is absent.
3.5. IFNγ signals through STAT1 to inhibit Th17 differentiation and function
Next, we wanted to identify the mechanism by which IFNγ suppresses IL-17A and IL-17F. Both IL-27 and IFNβ have been shown to inhibit Th17 differentiation through the activation of STAT1 in several disease models (Diveu et al., 2009, Guo et al., 2008, Stumhofer et al., 2006). Since IFNγ can phosphorylate STAT1 during Th1 differentiation (Girdlestone and Wing, 1996), we sought to examine if IFNγ acts through STAT1 to suppress these Th17-associated cytokines. To this end, we polarized naïve WT and STAT1−/− CD4 T cells under Th17 conditions with or without exogenous IFNγ and determined the expression of Th17-associated genes. As noted earlier, IFNγ suppressed Th17 differentiation in WT CD4 T cells, yet IFNγ lost its ability to inhibit the expression of Th17-associated genes in STAT1−/− CD4 T cells (Fig. 5). This finding indicates that IFNγ signaling through STAT1 not only leads to the induction of Tbet (Afkarian et al., 2002) which can suppress RORγt, but also facilitates the phosphorylation of STAT1 which can inhibit production IL-17A and IL-17F independently of Tbet expression.
Figure 5. IFNγ signals through STAT1 to inhibit Th17 differentiation.
Naïve CD4 T cells were FACS sorted from WT and STAT1−/− mice and polarized under Th17 conditions with irradiated feeder cells for 5 days in the presence of either the anti-IFNγ mAb or 1000U/ml IFNγ. The expression of Th17-associated genes was analyzed by real-time PCR. Data is cumulative of 3 independent experiments.
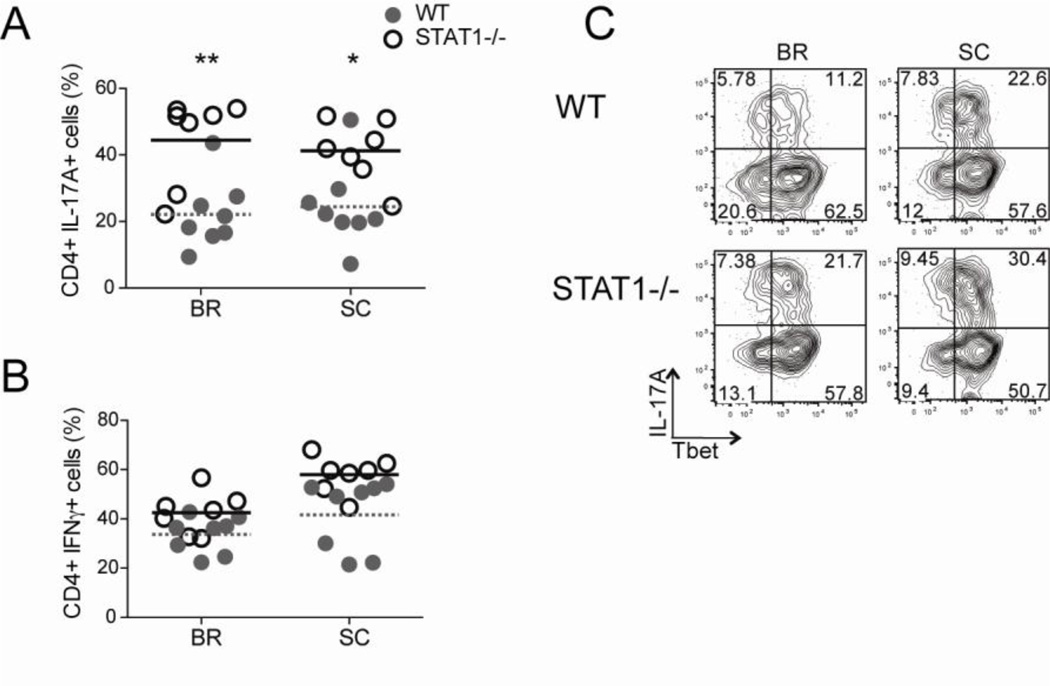
To test if this mechanism functions in vivo, we induced EAE in WT and STAT1−/− mice. At the peak of disease, CD4 T cells isolated from the CNS were evaluated for their production of IL-17A following restimulation. We found that mice deficient in STAT1 exhibited elevated frequencies of IL-17A-producing CD4 T cells compared to their WT counterparts (Fig. 6A). It was possible that this increased percentage of IL-17A+ CD4 T cells in STAT1−/− mice was the result of lower levels of IFNγ. Therefore we analyzed IFNγ production by the CNS-infiltrating CD4 T cells in the WT and STAT1−/− mice and did not detect any differences in the proportion of cells making this cytokine (Fig. 6B). Importantly, the increase in IL-17A is not due to a reduction in the amount of Tbet, as the majority of the IL-17A-producing STAT1−/− CD4 T cells co-expressed Tbet (Fig 6C), and we have previously published that high frequencies of Tbet-expressing CD4 T cells are still present in STAT1−/− mice during EAE (Yeh et al., 2011). Together, these data indicate that IFNγ signaling via STAT1 represses IL-17A in vivo and this pathway is independent of Tbet.
Figure 6. IFNγ suppresses IL-17A production in a STAT1-dependent, Tbet-independent manner during EAE.
WT and STAT1−/− mice were immunized for EAE and CNS-infiltrating CD4 T cells were analyzed at the peak of disease for cytokine production after a brief PMA and ionomycin stimulation. The frequencies of (A) IL-17A+ and (B) IFNγ+ CD4 T cells are shown. (C) Representative plots show Tbet and IL-17A staining of gated CD4 T cells recovered from the CNS. Combined data from two separate experiments are shown (n= 9–11 in each group).
4. DISCUSSION
During effector CD4 T cell development, lineage-specific transcription factors not only drive downstream gene expression, but also suppress molecules of the other lineages to guarantee full differentiation. In this study, we show that IFNγ, the principle cytokine produced by Th1 effector CD4 T cells, utilizes more than one pathway to suppress IL-17A production and Th17 development during EAE. We establish that Tbet deficiency in CD4 T cells results in elevated IL-17A in both intact Tbet−/− mice and in mixed bone marrow chimeras, demonstrating Tbet inhibits IL-17A production in a cell intrinsic manner. Moreover, Tbet deficiency in polarized Th17 cells causes augmented expression of Th17-associated genes, demonstrating Tbet abrogates Th17 differentiation universally. This result is consistent to a report by Lazarevic et al., which demonstrated Tbet interacts with Runx1 to abrogate Th17 differentiation (Lazarevic et al., 2011). Conversely, the induction of arthritis in mice which overexpress Tbet specifically in CD4 T cells showed reduced RORγt expression and amelioration of disease (Kondo et al., 2012). In addition to Tbet, our findings illustrate that IFNγ, through a STAT1-dependent and Tbet-independent mechanism, mediated suppression of the Th17-associated cytokines IL-17A and IL-17F, both in vivo and in vitro. This is in agreement with a previous report showing that IFNγ downregulated Th17-related genes in Tbet deficient, but not STAT1 deficient Th17-polarized CD4 T cells (Villarino et al., 2010).
The requirement of Tbet in CD4 T cells during autoimmunity was originally demonstrated by studying intact Tbet−/− mice in the context of EAE, type 1 diabetes, and arthritis (Bettelli et al., 2004, Esensten et al., 2009, Wang et al., 2006). The most striking and perplexing finding in this study is the susceptibility of intact Tbet−/− mice to EAE immunization. However, multiple reports have revisited the requirement of Tbet for the induction of EAE. Duhen et al. induced EAE in mice with selective deletion of Tbet in T cells and showed a delayed yet susceptible phenotype (Duhen et al., 2013). In addition, Tbet−/− MOG-specific TCR transgenic CD4 T cells cultured under alternative Th17 polarizing conditions (IL-6 and TGF-β3) elicited disease upon adoptive transfer (Lee et al., 2012), indicating Tbet expression in CD4 T cells is not necessary for EAE induction. Moreover, two recent publications demonstrated that intact Tbet−/− mice do develop EAE (Grifka-Walk et al., 2013, O'Connor et al., 2013). These results are consistent with our data regarding the dispensable role of Tbet during EAE. Importantly, we did find that this disease was still dependent on IL-23 because anti-IL-12/23p40 blockade abrogated the development of EAE in Tbet−/− mice (W. Yeh and L.E. Harrington, unpublished data).
The role of the commensal microbiota has been shown to influence adaptive immunity (Honda and Littman, 2012), and is one possible explanation for the differences observed in the susceptibility of mice to EAE. We demonstrated that the presence of SFB was not associated with the susceptibility of Tbet−/− mice to EAE, yet it is possible that differences in the microbiota could impact the development of autoimmunity. Nevertheless, Tbet−/− mice treated with both vancomycin and ampicillin to clear gram-positive bacteria remained susceptible to EAE immunization (W. Yeh and L.E. Harrington, unpublished data). Still, this does not rule out the microbiota influencing the susceptibility of Tbet−/− mice to EAE, as the impact of gram-negative bacteria to EAE induction is not yet known. Moreover, it is possible that Tbet deficiency alters the composition of the microbiota (Garrett et al., 2007), which in turn confers susceptibility and/or resistance to EAE. In all, while we are unable at this time to discern why our data contradicts previously published reports, it is clear that Tbet is not necessary for the induction of EAE.
Overall, in this report, we demonstrate that Tbet and STAT1 function independently to inhibit Th17 differentiation and function in vitro and in vivo. Expression of Tbet within CD4 T cells impeded the development of Th17 cells during EAE. Moreover, IFNγ signaling through STAT1 suppressed IL-17A production during EAE in a Tbet-independent mechanism. Taken together, these data imply that the Th1 lineage-specific molecules function in a multifaceted manner to both promote Th1 differentiation as well as inhibit the development to other effector CD4 T cell lineages.
Supplementary Material
HIGHLIGHTS.
Tbet expression is not necessary for entry of CD4 T cells into the inflamed CNS.
CD4 T cell production of IL-17A is inhibited by Tbet in a cell-intrinsic manner.
Tbet expression is not critical for the induction of EAE.
IFNγ suppression of IL-17A is STAT1 dependent but Tbet independent.
However, IFNγ repression of RORγt expression is both STAT1 and Tbet dependent.
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health Grants R01 DK084082 (to L.E.H.) and the National Multiple Sclerosis Society Award CA-1059-A-13 and the UAB Collaborative MS Research Center (W.Y.). We wish to thank the other members of the Harrington laboratory, as well as the Zajac laboratory, for helpful discussions and critical reading of this manuscript. We also wish to thank Dr. Chander Raman for providing the B6.STAT1−/− mice to us, Dr. Xiangqin Cui for assistance with statistics of real-time PCR data, Katie Alexander and the Elson laboratory for assistance with SFB PCR, and Enid Keyser of the UAB Comprehensive Arthritis, Musculoskeletal, and Autoimmunity Center Cytometry Facility and Marion Spell of the UAB Center for AIDS Research Flow Cytometry Core for cell sorting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Alzabin S, Williams RO. Effector T cells in rheumatoid arthritis: lessons from animal models. FEBS Lett. 2011;585:3649–3659. doi: 10.1016/j.febslet.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C, Mcgeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, Mcclanahan TK, De Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J Immunol. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, Mcgeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O'shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdlestone J, Wing M. Autocrine activation by interferon-gamma of STAT factors following T cell activation. Eur J Immunol. 1996;26:704–709. doi: 10.1002/eji.1830260329. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia I, Zhao Y, Ju S, Gu Q, Liu L, Kolls JK, Lu B. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifka-Walk HM, Lalor SJ, Segal BM. Highly polarized Th17 cells induce EAE via a T-bet independent mechanism. Eur J Immunol. 2013 doi: 10.1002/eji.201343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011;23:739–745. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4307–4316. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- Ivanov Ii, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Iizuka M, Wakamatsu E, Yao Z, Tahara M, Tsuboi H, Sugihara M, Hayashi T, Yoh K, Takahashi S, Matsumoto I, Sumida T. Overexpression of T-bet gene regulates murine autoimmune arthritis. Arthritis Rheum. 2012;64:162–172. doi: 10.1002/art.33335. [DOI] [PubMed] [Google Scholar]
- Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- O'connor RA, Cambrook H, Huettner K, Anderton SM. T-bet is essential for Th1-mediated, but not Th17-mediated, CNS autoimmune disease. Eur J Immunol. 2013 doi: 10.1002/eji.201343689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, Uhlig H, Read S, Rehakova Z, Benada O, Heczko P, Strus M, Bland P, Tlaskalova-Hogenova H. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–1211. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Gallo E, Abbas AK. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J Immunol. 2010;185:6461–6471. doi: 10.4049/jimmunol.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fathman JW, Lugo-Villarino G, Scimone L, Von Andrian U, Dorfman DM, Glimcher LH. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J Clin Invest. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WI, Mcwilliams IL, Harrington LE. Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signaling during experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4998–5006. doi: 10.4049/jimmunol.1100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.