Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen in both community and healthcare-related settings worldwide. Current knowledge regarding the epidemiology of S. aureus and MRSA in Gaza is based on a single community-based carriage study. Here we describe a cross-sectional analysis of 215 clinical isolates collected from Al-Shifa Hospital in Gaza during 2008 and 2012.
Methods
All isolates were characterized by spa typing, SCCmec typing, and detection of genes encoding Panton-Valentine leukocidin (PVL) and toxic shock syndrome toxin (TSST-1). Representative genotypes were also subjected to multilocus sequence typing (MLST). Antibiotic susceptibility testing was performed using VITEK2 and MicroScan.
Results
MRSA represented 56.3% of all S. aureus strains, and increased in frequency from 2008 (54.8%) to 2012 (58.4%). Aside from beta-lactams, resistance was observed to tetracycline, erythromycin, clindamycin, gentamicin, and fluoroquinolones. Molecular typing identified 35 spa types representing 17 MLST clonal complexes (CC), with spa 998 (Ridom t223, CC22) and spa 70 (Ridom t044, CC80) being the most prevalent. SCCmec types I, III, IV, V and VI were identified among MRSA isolates, while type II was not detected. PVL genes (lukF/S-PV) were detected in 40.0% of all isolates, while the TSST-1 gene (tst) was detected in 27.4% of all isolates, with surprisingly high frequency within CC22 (70.4%). Both PVL and TSST-1 genes were found in several isolates from 2012.
Conclusions
Molecular typing of clinical isolates from Gaza hospitals revealed unusually high prevalence of TSST-1 genes among CC22 MRSA, which is noteworthy given a recent community study describing widespread carriage of a CC22 MRSA clone known as the ‘Gaza strain’. While the latter did not address TSST-1, tst-positive spa 998 (Ridom t223) has been detected in several neighboring countries, and described as endemic in an Italian NICU, suggesting international spread of a ‘Middle Eastern variant’ of pandemic CC22 strain EMRSA-15.
Introduction
Staphylococcus aureus is one of the most prevalent human pathogens isolated from hospitalized patients worldwide, and its importance in community settings continues to increase. S. aureus causes a broad variety of diseases ranging from skin and soft-tissue infections to bacteremia, osteomyelitis, infective endocarditis, and necrotizing pneumonia [1–3]. In recent decades, MRSA has emerged as the most frequently identified antibiotic-resistant pathogen in many parts of the world, including North Africa and the Middle East [4]. While hospital-acquired MRSA (HA-MRSA) strains remain endemic in most of these regions, in recent years community-acquired (CA-MRSA) strains have emerged as a cause of invasive and life-threatening infections in young, healthy patients with no significant healthcare exposure [2,5–8]. Within the past few years, livestock-associated MRSA (LA-MRSA) have posed an additional threat [9–11]. Fortunately, whereas HA-MRSA isolates are generally multi-drug resistant, CA-MRSA and LA-MRSA tend to be resistant primarily to beta-lactam antibiotics and, in the case of LA-MRSA CC398, to tetracycline as well.
Molecular typing techniques are indispensable for understanding the evolution and epidemiology of S. aureus and MRSA. The most widely used techniques include staphylococcal protein A (spa) typing [12,13], staphylococcal cassette chromosome (SCC) mec typing [14], multilocus sequence typing (MLST) [15], and pulsed-field gel electrophoresis (PFGE) [16]. Other markers of interest include virulence factors such as Panton-Valentine leukocidin (PVL) and TSST-1, the principal cause of staphylococcal toxic shock syndrome [17]. Approximately 20% of S. aureus isolates possess the gene encoding TSST-1 (tst) [18], which is harbored by a family of mobile staphylococcal pathogenicity islands (SaPI) [19–21]. The distribution of TSST-1 appears limited to a handful of clonal lineages, and is most frequently associated with methicillin-susceptible S. aureus (MSSA) strains belonging to MLST clonal complex (CC) 30. More recently, a tst-positive strain of CC5 MRSA has been documented in France [22], while several reports have described tst-positive CC22 MRSA strains in Abu Dhabi [23], Jordan [24,25], Kuwait [26], Saudi Arabia [27], India [3], Italy [28,29], the United Kingdom [30], and the United States [31]. Several recent studies have also described tst-positive CC30 [25] and CC80 [24,32] MRSA in Jordan.
Molecular epidemiological data about S. aureus and MRSA in the Middle East, including the Palestinian Territories, are generally scarce and insufficient [4,33–35], with current knowledge regarding the epidemiology of S. aureus in Gaza based on a single recent community-based carriage study [36]. The Gaza Strip (geographic coordinates 31°25′ N, 34° 20′ E) is a narrow territory (41 km long and 6–12 km wide) along the eastern Mediterranean coast, with tightly-controlled borders abutting Israel and the Sinai Peninsula of Egypt. It is considered one of the most densely populated areas in the world, with a population of about 1.7 million inhabitants (37.5% of the total estimated Palestinian population), and a population density (4,073/km2) nearly ten-fold greater than that of the West Bank (433/km2) [37]. Over the last decade, the socioeconomic situation in Gaza has declined steadily, leaving nearly 80% of the population dependent on international assistance. The healthcare infrastructure within Gaza suffers accordingly from the long-term effects of war, economic isolation, and border closures, with marked scarcity of medical equipment and instrumentation. Recent events such as war, border fighting and regional political unrest have only served to exacerbate the situation [38].
Here we describe the molecular characteristics and antibiotic susceptibilities of clinical MRSA and MSSA isolates collected from the largest medical complex and primary hospital in Gaza (Al-Shifa Hospital) in 2008 and 2012. To the best of our knowledge, this is the first report describing clinical S. aureus infection within the Gaza Strip. We note the high prevalence of a tst-positive CC22 MRSA clone, which is likely related to the ‘Gaza strain’ described in the aforementioned community-based carriage study [36], as well as the ‘Middle Eastern variant’ of EMRSA-15 from a recent NICU outbreak in Sicily, Italy [28].
Methods
Bacterial isolates
For the present study, 215 unique S. aureus isolates associated with diverse clinical infections were collected from the largest public tertiary referral hospital (Al-Shifa Hospital) in Gaza City. Of the 215 unique S. aureus isolates, 126 were collected in 2008, out of a total unique 1091 bacterial isolates (March 1–July 31); while 89 were collected in 2012, out of a total unique 1121 bacterial isolates (March 1–August 11). Isolates were obtained directly from the clinical laboratory of Al-Shifa Hospital, and represent complete capture of all S. aureus isolates during the stated collection periods. Although a defined sampling strategy was not employed, the collected strains likely reflect the clinical epidemiology of S. aureus in Gaza, since Al-Shifa Hospital is the primary referral hospital for patients from all areas of the Gaza Strip.
Identification of S. aureus and MRSA
S. aureus isolates were identified phenotypically using one or more of the following methods: catalase test, tube coagulase test, Pastorex Staph Plus latex agglutination (Bio-Rad, Hercules, California), and the Staph ID 32 API system and VITEK 2 Compact Gram Positive Card (bioMérieux, Paris, France). Multiplex PCR was also used for detection of the nuc (S. aureus species-specific) and mecA (methicillin-resistance) genes [39,40]. DNA for molecular typing was isolated as described previously, with different methods used for the 2008 [18] and 2012 [41] isolates.
Antimicrobial susceptibility testing
Antibiotic susceptibility testing for the 2008 isolates was performed with the automated VITEK 2 Compact automated system, using the VITEK 2 Compact Gram Positive Card (bioMérieux, Paris, France); antimicrobials tested included amoxicillin/clavulanic acid, ampicillin, ampicillin/sulbactam, azithromycin, cefaclor, cefotaxime, ceftriaxone, cefuroxime, clarithromycin, clindamycin, erythromycin, fosfomycin, fusidic acid, gentamicin, imipenem, levofloxacin, linezolid, moxifloxacin, mupirocin, nitrofurantoin, oxacillin, penicillin, rifampin, teicoplanin, tetracycline, tigecycline, tobramycin, trimethoprim/sulfamethoxazole, and vancomycin. Susceptibility testing for the 2012 isolates was performed using the MicroScan WalkAway Plus System (Siemens Healthcare, Erlangen, Germany); antimicrobials tested included amoxicillin/clavulanic acid, ampicillin, ampicillin/sulbactam, cefazolin, cefoxitin, ciprofloxacin, clindamycin, daptomycin, erythromycin, gentamicin, levofloxacin, linezolid, moxifloxacin, oxacillin, penicillin, rifampin, synercid, tetracycline, trimethoprim/sulfamethoxazole, and vancomycin. Due to the different selection of antimicrobials in each panel, only antibiotics common to each system were compared in this report.
Molecular typing
All 215 S. aureus isolates were characterized by staphylococcal protein A (spa) typing [12]; 2008 isolates were spa typed using the Ridom StaphType software [13,42], while 2012 isolates were spa typed using eGenomics software [12,41], with Ridom assignments made using the Spa Server website (http://spa.ridom.de/). In order to avoid confusion, both designations are used throughout this manuscript, with eGenomics spa types prefaced by the term “spa”, and Ridom spa types shown in parentheses. All MRSA isolates were subjected to staphylococcal cassette chromosome (SCC)mec typing; 2012 isolates were typed using multiplex real-time PCR [43], while 2008 isolates were typed using both multiplex conventional [44] and real-time [43] PCR. Multilocus sequence typing (MLST) was performed as described previously [15] on a representative subset of 42 isolates, with clonal complexes inferred via eBURST analysis [45]; all other clonal complexes were inferred from spa typing data as described previously [41], using both the Ridom Spa Server website and our own internal eGenomics database. Clonal complex sub-groups with distinct genotypic signatures were classified as individual CCs [8], e.g. ST239 strains were classified as CC239 rather than CC8; ST34 strains were classified as CC34 rather than CC30; and ST291 strains were classified as CC291 rather than CC398.
Detection of PVL and TSST-1 genes
All isolates were tested for the genes encoding Panton-Valentine leukocidin (PVL) and toxic shock syndrome toxin (TSST-1). The 2008 isolates were initially tested for PVL (lukS-PV and lukF-PV) and TSST-1 (tst) using previously described methods [39,46], then re-tested along with the 2012 isolates as follows. The 2012 isolates were tested for PVL (lukF-PV) using a previously described real-time PCR assay [47], and tested for TSST-1 (tst) using a novel real-time PCR assay (this study). The novel assay uses the following oligonucleotide sequences: a) forward primer tst.sy-F1, CCCTTTGTTGCTTGCG; b) reverse primer tst.sy-R1 CGTTTGTAGATGCTTTTGC; and c) molecular beacon probe tst-MB, 5′-6-HEX d(cgcgtCCCCTGTTCCCTTATCATCTacgcg)-DABCYL-3′, where stem sequences are underlined, HEX is 6-hexachlorofluorescein, and DABCYL is 4-[4-(dimethylamino)phenylazo]benzoic acid. PCR cycling conditions for the novel assay were as described previously [43].
Production of TSST-1 toxin by 17 representative tst-positive strains was assayed via reversed passive latex agglutination (RPLA), using the Oxoid TST-RPLA Toxin Detection Kit from Thermo Fisher Scientific (Oxoid Ltd., United Kingdom), according to the manufacturer’s instructions.
Sequencing of TSST-1 and SaPI element
Long-range PCR assays were employed to partially amplify the SaPI region of three tst-positive isolates. Known SaPI sequences were downloaded from GenBank and used as templates for primer design. Forward primers were designed to target the SaPI integrase gene, while the reverse primer is located within tst and is universally conserved among all known SaPIs. Primer oligonucleotide sequences were as follows: a) Int-F1, GCGTGATGTTTAGCGTCTGA (targets the SaPI integrase in strains N315, Mu50, and RN3984); b) Int-F2, TTTGAAGTTTTGCCTCAGCA (targets the SaPI integrase in strains RF122 and ED133); c) Int-F3, TTCCCTACAAGAGATGCAACA (targets the integrases in SaPI68111 and SaPIj50); d) Int-F4, CCACTCATCACTTGCAGCAT) (targets the integrase sequence in Tn557); and e) reverse primer Tst-Rv, GTAAGCCCTTTGTTGCTTGC (conserved in all tst-harboring SaPI elements). Genomic bacterial DNA was isolated using a previously-described method [48], and long-range PCR was performed using the Advantage GC Genomic LA PCR Polymerase Kit (Clontech Laboratories, Inc., Mountain View, CA), according to the manufacturer’s protocol. The resulting amplicons were then sequenced by primer walking as described previously [48]. One of the three identical SaPI sequences was submitted to GenBank (NCBI) as accession number KP334121.
Statistical analysis
Comparisons of selected data from 2008 and 2012 were performed by Chi-square analysis using GraphPad Software (San Diego, California). Chi-square tests (two-tailed) with p-values ≤ 0.05 were considered statistically significant.
Ethics Statement
The study protocol and data handling were approved by the ethical committee at the laboratory medicine department of Al Azhar University-Gaza, which does not have an Institutional Review Board (IRB) at this time. The study was performed in accordance with the ethical standards established in the 1964 and 1975 Declaration of Helsinki, and the modifications thereafter.
Results
S. aureus and MRSA prevalence
A total of 215 unique S. aureus clinical isolates were collected from individual patients. Of these, 126 were collected in 2008 (out of a total 1091 bacterial isolates), while 89 were collected in 2012 (out of a total 1121 bacterial isolates). The majority of the isolates were from skin (44.2%), surgical site (30.7%), urogenital (14.9%), and bloodstream (8.4%) infections. Unfortunately, patient demographic data were very limited, and could not be addressed in this report. S. aureus represented 9.7% of all bacterial isolates (215/2212), decreasing significantly (p = 0.005) in prevalence from 11.5% in 2008 (126/1091) to 7.9% in 2012 (89/1121). MRSA (defined both phenotypically and by the presence of mecA) represented 5.5% of all bacterial isolates (121/2212) and 56.3% (121/215) of all S. aureus strains, with MRSA prevalence increasing from 54.8% in 2008 (69/126) to 58.4% in 2012 (52/89).
Antimicrobial resistance
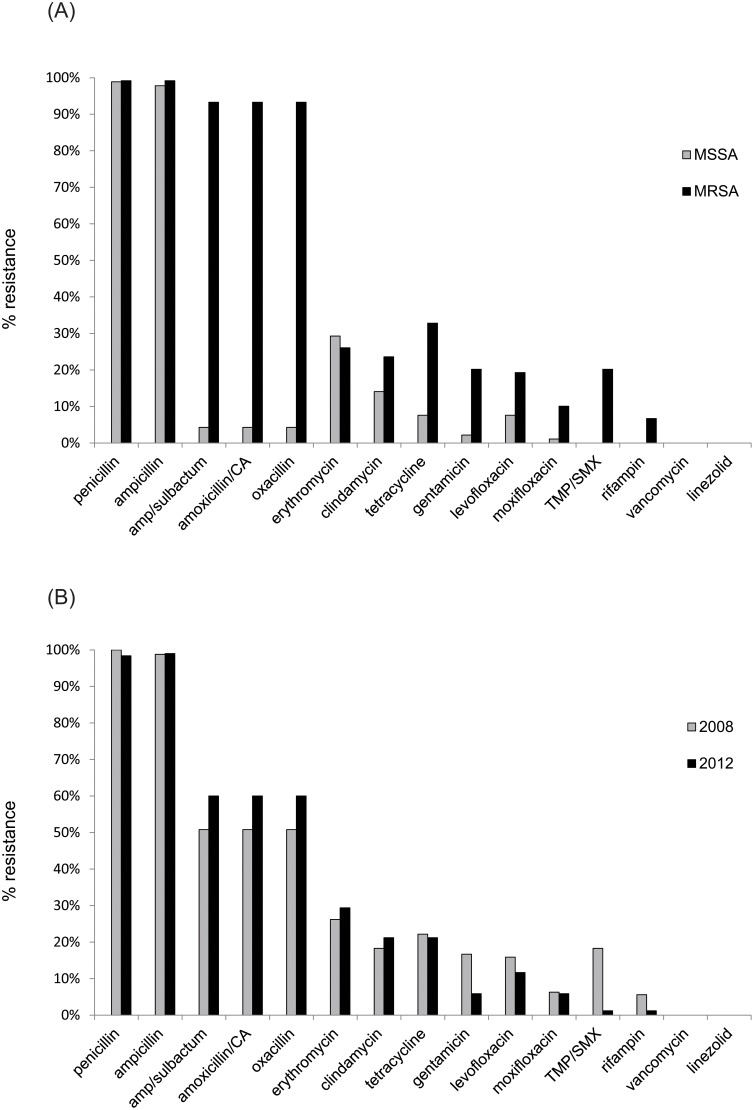
Resistance to multiple classes of antimicrobials was observed among both MRSA and MSSA, including aminoglycosides, beta-lactams, cephalosporins, fluoroquinolones, fusidic acid, macrolides, and tetracycline. Since different susceptibility testing methods were used in 2008 (VITEK 2) and 2012 (MicroScan), direct comparisons were only possible for 15 of the total 35 antimicrobials tested. Fig. 1 shows the prevalence of resistance to these 15 antimicrobials for: a) MRSA vs. MSSA, and b) 2008 vs. 2012, respectively. Whereas resistance to beta-lactams increased from 2008 to 2012, resistance to various other classes of antimicrobials decreased, associated primarily with the apparent replacement of several multi-resistant clones by less resistant ones. MSSA strains exhibited nearly complete resistance to penicillin (98.9%) and ampicillin (97.8%), but were mostly susceptible to the remaining beta-lactams. In general, susceptibility of S. aureus isolates from both 2008 and 2012 was greater than 75% to common anti-staphylococcal agents such as erythromycin, fluoroquinolones, gentamicin, and tetracycline. No resistance was observed to any of the following antibiotics: linezolid and vancomycin (both 2008 and 2012); fosfomycin, mupirocin, nitrofurantoin, teicoplanin, and tigecycline (2008 only); and daptomycin and synercid (2012 only).
Fig 1. Antimicrobial resistance frequencies among S. aureus isolates in this study.
Vertical bars denote relative resistance to selected antimicrobials described in the text. a. Black bars denote percent resistance among MRSA isolates; gray bars denote resistance among MSSA isolates. b. Gray bars denote percent resistance among S. aureus isolates from 2008; black bars denote resistance among S. aureus isolates from 2012. Only antimicrobials tested in both 2008 (VITEK 2) and 2012 (MicroScan) are depicted. Amp, ampicillin; CA, clavulanic acid; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; TMP-SMX, trimethoprim-sulfamethoxazole.
SCCmec typing
Among the MRSA isolates, SCCmec types I (2.5%), III (7.4%), IV (79.3%), V (7.4%) and VI (3.3%) were identified; SCCmec type II was not detected in this collection (Table 1). SCCmec type IV increased in prevalence from 75.4% (52/69) in 2008 to 84.6% (44/52) in 2012, with subtypes IVa and IVc accounting for 61.5% (59/96) and 36.5% (35/96) of all SCCmec IV isolates, respectively; two SCCmec IV isolates could not be sub-typed. SCCmec type V was only detected in 2008, while SCCmec types I and VI were only detected in 2012. Notably, only the SCCmec type III isolate from 2012 appeared to harbor SCCmercury (III-Hg), as indicated by the presence of ccrC [14,43].
Table 1. Molecular characteristics of S. aureus isolates described in this study.
| Clonal complex | Sequence type a | spa type (Ridom) | spa repeats (eGenomics) | spa type (eGenomics) | SCCmec type b | PVL (lukF) | TSST-1 (tst) | Year of isolation | Clinical source d | No. of isolates |
|---|---|---|---|---|---|---|---|---|---|---|
| CC 1 | ST 1278 | t3643 | ZMBME2JQQQ | 1598 | - | 2008 | P, SA, SS, SW | 5 | ||
| - | t3643 | ZMBME2JQQQ | 1598 | - | 2012 | SE | 1 | |||
| CC 5 | ST 228 | t001 | TO2MBMDMGMK | 385 | I | 2012 | SW | 2 | ||
| - | t688 | TJMBMK | 176 | - | 2008 | SU | 1 | |||
| - | t688 | TJMBMK | 176 | - | 2012 | SW | 1 | |||
| ST 5 | t688 | TJMBMK | 176 | VI | 2012 | SW | 1 | |||
| CC 6 | ST 6 | t14480 | YC2FMBQBBQBLO | 1592 | IVa | 2012 | SW | 1 | ||
| ST 6 | t304 | YC2FMBQBLO | 91 | - | 2008 | SN, U | 2 | |||
| CC 8 | ST 8 | t008 | YHGFMBQBLO | 1 | IVa | 2008 | B, P, S, SV | 6 | ||
| - | t008 | YHGFMBQBLO | 1 | - | 2012 | SE, SW, U | 3 | |||
| ST 8 | t008 | YHGFMBQBLO | 1 | VI | 2012 | SW | 1 | |||
| ST 8 | t2229 | YHGFO | 943 | VI | 2012 | SW | 2 | |||
| ST 8 | t4229 | YHGFMBH5BLO | 1222 | IVa | + | 2012 | SW | 1 | ||
| CC 15 | - | t084 | UJGBBGGJAGJ | 21 | - | 2008 | P, SW | 3 | ||
| ST 2434 | t084 | UJGBBGGJAGJ | 21 | - | 2012 | B, P, SW, U | 4 | |||
| - | t346 | UJGBGGJAGJ | 155 | - | 2012 | SW | 2 | |||
| CC 22 | ST 737 | t005 | TJEJNCMOMOKR | 113 | - | + | 2008 | SN | 1 | |
| ST 22 | t005 | TJEJNCMOMOKR | 113 | - | + | 2012 | B, SE, SW | 5 | ||
| - | t005 | TJEJNCMOMOKR | 113 | - | 2008 | B, P, S, SA, SE, SU, SV, U | 13 | |||
| ST 22 | t223 | TJEJCMOMOKR | 998 | IVa | + | + | 2012 | SW | 1 | |
| - | t223 | TJEJCMOMOKR | 998 | IVa | + | 2008 | B, P, Sp, SN, U | 13 | ||
| - | t223 | TJEJCMOMOKR | 998 | IVa | + | 2012 | B, SE, SW, U | 15 | ||
| ST 22 | t223 | TJEJCMOMOKR | 998 | IVa | 2008 | DV, P | 2 | |||
| - | t223 | TJEJCMOMOKR | 998 | IVa | 2012 | SE | 1 | |||
| ST 22 | t223 | TJEJCMOMOKR | 998 | V | + | 2008 | SU | 1 | ||
| ST 22 | t223 | TJEJCMOMOKR | 998 | - | + | 2008 | P, SS, U | 3 | ||
| ST 22 | t309 | TJCMOMOKR | 66 | IVa | + | 2012 | SE, SW | 2 | ||
| ST 22 | t541 | TMOMOKR | 846 | IVa | + | 2012 | SW | 2 | ||
| ST 22 | t5485 | TJEJCMOKKR | 1601 | IVa | + | 2008 | SW, U | 2 | ||
| ST 22 | t7063 | TJEJCLMMOKR | 1599 | IVa | + | 2008 | SW | 2 | ||
| ST 22 | t7063 | TJEJCLMMOKR | 1599 | V | + | 2008 | P, SS, SW, U | 8 | ||
| CC 30 | ST 30 | t012 | WGKAKAOMQQ | 33 | - | + | 2012 | SE | 1 | |
| - | t019 | XKAKAOMQ | 19 | - | + | 2008 | P, SW | 2 | ||
| ST 30 | t019 | XKAKAOMQ | 19 | - | + | 2012 | SW | 1 | ||
| ST 30 | t021 | WGKAKAOMQ | 43 | - | + | 2008 | P, DN, DU, SN, SS, U | 16 | ||
| - | t021 | WGKAKAOMQ | 43 | - | + | 2012 | SW | 1 | ||
| - | t021 | WGKAKAOMQ | 43 | - | 2012 | SW | 2 | |||
| - | t096 | WGKKAMQ | 1591 | - | + | 2008 | U | 1 | ||
| ST 30 | t096 | WGKKAMQ | 1591 | - | + | 2012 | SW | 1 | ||
| ST 30 | t253 | WGKAKAOMQQQQ | 204 | - | + | 2008 | B, P, U | 5 | ||
| ST 30 | t318 | WGKKAKAOMQ | 251 | IVa | + | 2008 | B, DU, P, SA | 4 | ||
| - | t318 | WGKKAKAOMQ | 251 | IVa | + | 2012 | P, SW | 6 | ||
| - | t318 | WGKKAKAOMQ | 251 | - | + | 2012 | SW | 3 | ||
| CC 34 | ST 34 | t166 | ZZ2PNGKBKGOLB | 295 | - | + | 2012 | SW | 1 | |
| CC 80 | ST 80 | t044 | UJGBBPB | 70 | IVc | + | + | 2012 | SW | 1 |
| ST 80 | t044 | UJGBBPB | 70 | IVc | 2008 | B, P, Sp, SE, SN, SS, SV, U | 23 | |||
| - | t044 | UJGBBPB | 70 | IVc | 2012 | SE, SW, U | 12 | |||
| ST 80 | t458 | T | 1269 | IV | + | 2012 | SW | 1 | ||
| CC 88 | ST 88 | t4103 | TGFMBBBBPB | 1270 | - | 2012 | SW | 3 | ||
| CC 97 | ST 97 | t2112 | TJGFMBBBBPB | 1135 | - | 2008 | DU | 1 | ||
| CC 121 | ST 121 | t314 | XMJH2M | 454 | - | + | 2008 | P, SA, Sp | 3 | |
| - | t7793 | XMJQM | 1594 | IV | + | 2012 | SW | 1 | ||
| ST 121 | t7793 | XMJQM | 1594 | - | + | 2012 | SW | 1 | ||
| CC 133 | ST 133 | t1166 | D2KFMJEMMMJQ | 1593 | - | 2012 | SW | 2 | ||
| CC 239 | ST 239 | t037 | WGKAOMQ | 3 | III | + | 2008 | P | 1 | |
| ST 239 | t037 | WGKAOMQ | 3 | III | 2008 | B, CSF, P, SA, SS, U | 7 | |||
| ST 239 | t037 | WGKAOMQ | 3 | III-Hg c | 2012 | SW | 1 | |||
| CC 291 | ST 291 | t1149 | XKBQBMM | 718 | - | 2012 | SW | 3 | ||
| - | t1614 | XKBQBBMMM | 117 | - | 2012 | SW | 1 | |||
| CC 398 | ST 580 | t6527 | UAOAOQOMO | 1600 | - | 2008 | SW | 1 | ||
| CC 1153 | ST 1153 | t903 | TLHMMDMG | 824 | I | + | 2012 | SW | 1 | |
| - | t903 | TLHMMDMG | 824 | - | + | 2012 | SW | 1 |
S. aureus isolates are classified hierarchically in the following order, from left-to-right: MLST clonal complex, MLST sequence type, spa type (both Ridom and eGenomics), SCCmec type, presence of genes encoding PVL (lukF/S-PV) and/or TSST-1 (tst), and year of isolation. Clonal complexes were inferred from spa repeat analysis and MLST typing of representative isolates of each genotypic pattern (except as noted). “No. of isolates” refers to the number of strains with the characteristics described in the preceding columns. CC, clonal complex; MLST, multilocus sequence typing; PVL, Panton-Valentine leukocidin; ST, sequence type; spa, staphylococcal protein A; SCCmec, staphylococcal cassette chromosome mec; TSST-1, toxic shock syndrome toxin.
aMultilocus sequence typing (MLST) was performed for one representative isolate from each clonal complex. For each row where a sequence type (ST) value appears, only one isolate was chosen, regardless of the number of isolates listed in the final column. A dash (-) indicates that MLST was not performed.
bA dash (-) indicates that no SCCmec targets were detected, and the strain is methicillin-susceptible (MSSA).
c“III-Hg” refers to SCCmec type III with SCCmercury (ccrC).
dClinical isolate sources are abbreviated as follows: B, blood; CSF, cerebrospinal fluid; DN, nipple discharge; DU, urethral discharge; DV, vaginal discharge; P, pus; S, semen; Sp, sputum; SA, abscess swab; SE, ear swab; SN, nasal swab; SS, skin swab; SU, umbilical swab; SV, vaginal swab; SW, wound swab.
spa typing and MLST
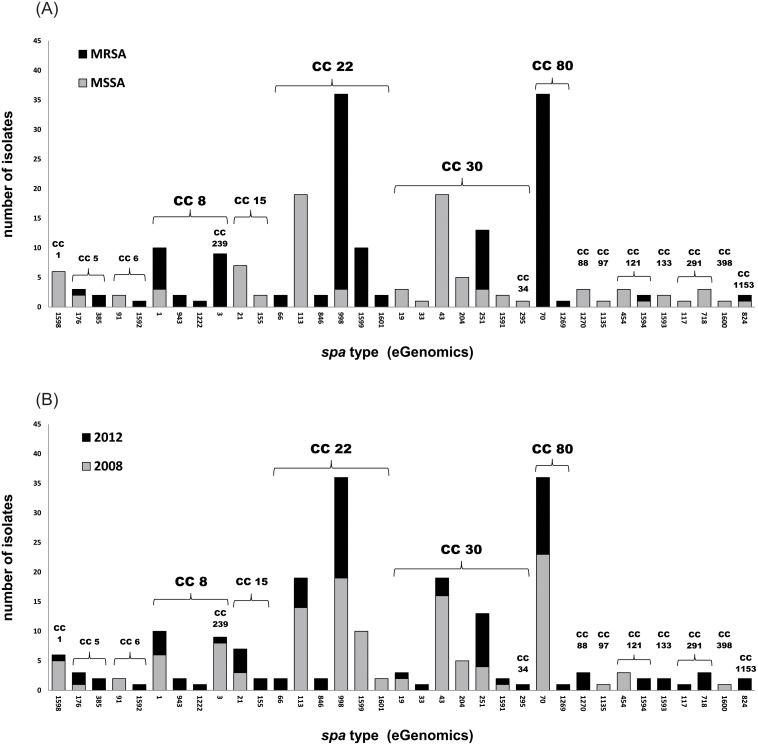
A total of 35 unique spa types were detected among the 215 isolates (Table 1). As shown in Fig. 2, 11 spa types were associated exclusively with MRSA, 18 exclusively with MSSA, and 6 with both MRSA and MSSA. The spa type diversity was greater in 2012 than 2008, with 28 total spa types observed in 2012, vs. only 19 in 2008. The most prevalent spa types overall were spa 998 (Ridom t223) and spa 70 (Ridom t044), each of which accounted for 16.7% (36/215) of the total number of isolates. The prevalence of spa 998 (t223) increased from 15.1% (19/126) in 2008 to 19.1% (17/89) in 2012, while that of spa 70 (t044) decreased from 18.3% (23/126) in 2008 to 14.6% (13/89) in 2012.
Fig 2. Relative frequencies of spa types among S. aureus isolates in this study.
Vertical bars denote the relative frequencies of 35 spa types observed among S. aureus isolates in this study. a. Black bars denote spa types observed among MRSA isolates (n = 17); gray bars denote spa types observed among MSSA isolates (n = 24). b. Gray bars denote spa types observed among isolates from 2008 (n = 19); black bars denote spa types observed among isolates from 2012 (n = 28). eGenomics spa type numbers are depicted (corresponding Ridom spa types are listed in Table 1). Spa types are grouped together by MLST clonal complex; subgroups CC239 and CC34 are grouped with their parent complexes (CC8 and CC30, respectively). CC, clonal complex; MLST, multilocus sequence typing; spa, staphylococcal protein A.
Spa typing data were used to infer MLST clonal complexes (CC) for the majority of isolates, with MLST performed on 42 representative isolates for confirmation (and identification of unknown spa repeat patterns). A total of 17 different clonal backgrounds were inferred, 2 of which were associated exclusively with MRSA (CC80 and 239), 8 of which were associated exclusively with MSSA (CC1, 15, 34, 88, 97, 133, 291, and 398), and 7 of which were associated with both (CC5, 6, 8, 22, 30, 121, and 1153). Overall, CC22, CC30 and CC80 accounted for 33.0%, 20.0% and 17.2% of all S. aureus isolates, respectively, with slightly lower frequencies for each in 2012 than 2008. By contrast, the proportion of MRSA within CC22 and CC30 increased in 2012 (80.8% and 40.0%, respectively) compared to 2008 (62.2% and 14.3%, respectively). This was primarily associated with the decrease of MSSA-associated spa type 113 (t005) within CC22, and the increase in MRSA-associated spa type 251 (t318) within CC30. All CC80 isolates were MRSA, and were associated with spa type 70 (t044) and SCCmec type (IVc), except for one isolate with atypical spa type 1269 (t458).
As mentioned, the overall frequency of CC22 decreased slightly from 35.7% (45/126) in 2008 to 29.2% (26/89) in 2012, but there were notable changes in the strains mapped to this clonal complex over the four-year period. Among 79 strains classified as CC22, there were a total of eight different spa types, all identified as ST22 by MLST (with the exception of a single strain that typed as ST737, a single locus variant). Within the 2008 CC22 isolates, four different spa types were observed, with spa 113 (t005) and spa 998 (t223) accounting for 31.1% and 42.2% of these, respectively. Within the 2012 CC22 isolates, four different spa types were also observed, with spa 998 (t223) accounting for 64.7% of these; by contrast, the frequency of spa 113 (t005) was much lower in 2012 (14.7%), while spa 1599 (t7063) was not detected. All of the spa 113 (t005) isolates, and three of the spa 998 (t223) isolates, were MSSA; the remainder of the CC22 isolates were MRSA.
PVL and TSST-1 detection
The genes coding for PVL (lukF/S-PV) were detected in 40.0% of all isolates, with overall prevalence increasing from 38.9% (49/126) in 2008 to 41.6% (37/89) in 2012. PVL-positive isolates were identified in six different clonal complexes (Table 1), including CC8, 22, 30, 80, 121, and 1153, with spa 251 (t318) CC30 isolates and spa 70 (t044) CC80 isolates being exclusively PVL-positive. Notably, PVL genes were not detected among any of the spa 1 (t008) CC8 isolates. The TSST-1 gene (tst) was detected in 27.4% of all S. aureus isolates, with overall prevalence decreasing from 28.6% (36/126) in 2008 to 25.8% (23/89) in 2012, and significantly higher frequency (p < 0.0001) among MRSA (39.7%) than MSSA (11.7%). Although identified in a number of isolates typed to CC30 (n = 6), CC34 (n = 1), CC80 (n = 1), and CC239 (n = 1), TSST-1 genes were associated with 70.4% (50/71) of all CC22 strains, and 91.7% (33/36) of all spa 998 (t223) strains. Notably, genes for both PVL and TSST-1 were observed in one spa 998 (t223) CC22 isolate and one spa 70 (t044) CC80 isolate, both from the 2012 collection.
A total of 17 tst-positive strains were selected in order to assay for TSST-1 toxin production, including 13 CC22 strains encompassing all observed spa, SCCmec, and PVL genotypic combinations; two representatives of each tst-positive CC30 spa type; and each of the singleton CC80 and CC239 tst-positive strains. Production of TSST-1 was determined via reverse passive latex agglutination (RPLA) as per the manufacturer’s instructions. All of the strains tested were positive for TSST-1 production, including those which also harbored genes for PVL.
SaPI characterization
In order to better characterize the tst-positive’Gaza strain’, the SaPI elements from three tst-positive spa 998 (t223) strains were sequenced, including two MRSA (from 2012) and one MSSA (from 2008). Long PCR yielded a 10.5 kb amplicon using primer set intF1 and tst-Rv, and the resulting tst-bearing SaPI elements were completely sequenced and compared against the NCBI genome database. All three SaPI elements were identical, while BLAST results indicated >99.9% similarity to the sequence of SaPI2 from S. aureus “Harrisburg” strain RN3984 [49], with only 3 nucleotide differences (SNPs) within 10,578 bp. As described in the Methods, one of the three SaPI element sequences (from strain BK 38088) was submitted to GenBank (accession number KP334121).
Discussion
The Palestinian Territories consist of two discontinuous areas: a) the West Bank, which shares a large border with Israel and Jordan; and b) the Gaza Strip, a small territory on the Mediterranean coast which shares most of its border with Israel, except for an 11-km southern border with Egypt. Since 2006, Gaza has been a semi-autonomous region, subject to border and travel restrictions enforced by Israel and Egypt. Although residents of Gaza occasionally travel to Israel for advanced medical care, Egypt is the primary portal for travel to the outside world, consisting mainly of students and foreign workers. The Egyptian border crossing in Rafah often serves as a portal for movement of people and goods, but has been increasingly subject to closure recently. As a result, the population of Gaza is somewhat more isolated than that of the West Bank, and may therefore be considered a “semi-closed” population [50].
Data regarding S. aureus and MRSA within the Palestinian Territories are generally scant [4,35]. Previous reports have mostly focused on Northern Palestine [34,51] and the West Bank [33], with relatively little detail regarding molecular epidemiology. Recently, a joint Israeli-Palestinian community-based nasal carriage study addressed the epidemiology of S. aureus in Gaza for the first time, highlighting the widespread presence of a CC22 clone dubbed the ‘Gaza strain’[36], which was subsequently described in two reports from Sicily, Italy [28,29]. In this study, we describe the molecular characteristics of clinical isolates collected from the primary tertiary-care hospital in Gaza in 2008 and 2012. We observed a similarly high prevalence of CC22, including a clone with the same spa type (998/t223) as the aforementioned Gaza strain. Moreover, we detected unusually high frequencies of CC22 isolates that harbor the gene (tst) coding for toxic shock syndrome toxin (TSST-1). To the best of our knowledge, this is the first report describing the molecular epidemiology of S. aureus clinical infection in the Gaza Strip.
The overall MRSA prevalence in this study was 56.3%, representing an increase of 3.6% from 2008 (54.8%) to 2012 (58.4%). Nosocomial MRSA prevalence in the Eastern Mediterranean was previously described as the highest in the Mediterranean region, with overall frequencies ranging as high as 52% (Egypt), 55% (Cyprus), and 56% (Jordan) [52]. Recent studies in Lebanon [53], Israel [54], Saudi Arabia [27] and Jordan [55] have noted nosocomial MRSA rates of 30%, 35%, 50%, and 62% respectively, while the only nosocomial report to date from Palestine (West Bank) described a MRSA prevalence of only 8.7% [51]. All other reports of MRSA prevalence in the Palestinian Territories have involved nasal carriage rather than infection [33,34,36], although notably, 45% of the S. aureus isolates from the aforementioned Gaza carriage study were methicillin-resistant [36]. Therefore, the MRSA frequencies described in this report may be considered among the highest described in the region thus far.
Molecular analysis revealed a surprising degree of genotypic diversity. A total of 35 different spa types corresponding to 17 MLST clonal complexes were identified, with spa type diversity increasing from 2008 to 2012. Consistent with the S. aureus literature, MRSA strains showed less genetic diversity than MSSA, and as observed in the carriage study from Gaza [36], CC22 was the predominant lineage, with spa 998 (t223) accounting for half (50.7%) of the CC22 isolates. Interestingly, much more diversity was found in the current hospital-based study than in the carriage study, possibly due to the longer sampling period in our study. Whereas spa 998 (t223) was the only CC22 spa type found in the carriage study, accounting for 64% of all MRSA isolates, in this study a total of six different CC22 spa types were identified, with spa 998 (t223) accounting for only 27.3% of all MRSA isolates. Moreover, whereas the carriage study only identified three additional MRSA clonal backgrounds (CC5, 78, and 80) and four MSSA clonal backgrounds (CC15, 22, 45, and 291), the present study identified nine MRSA-associated clonal backgrounds (CC5, 6, 8, 22, 30, 80, 121, 239, and 1153) and fifteen MSSA-associated clonal backgrounds (CC1, 5, 6, 8, 15, 22, 30, 34, 88, 97, 121, 133, 291, 398, and 1153). Nevertheless, the high prevalence of the Gaza strain in the carriage study argues in favor of a community-based origin [36], and suggests that the high levels of MRSA carriage (12–13%) observed in the community may serve as a reservoir for the nosocomial CC22 lineages described in our study [25].
Notably, the predominant clonal backgrounds and spa types observed in both Gaza studies differ markedly from those described in the West Bank and Israel. Previous reports from the West Bank have identified CC1 and CC5 [33], as well as CC239 [56], while reports from Israel have described CC5 [54,57], CC8 [54,57], and CC45 [57,58] as the dominant clonal backgrounds. Unfortunately, molecular epidemiologic data from Egypt are exceedingly rare, consisting of two studies featuring small numbers of PVL-positive CA-MRSA isolates from lineages also found in this study [59,60], and, more recently, a nasal carriage study involving outpatients in Egypt and Saudi Arabia [61]. By contrast, more detailed studies from Lebanon [2,32,53] and Jordan [24,25,32,55,62] reveal a number of spa types and clonal backgrounds also detected in this study. The contrast between the molecular epidemiology of S. aureus in Gaza and Israel is noteworthy, given that residents of Gaza frequently seek advanced medical care within Israel. Interestingly, whereas SCCmec types I, II, and V are common in Israeli hospitals [7,54], types I and V are relatively uncommon in Gaza, while type II was not detected in this study.
Biber et al [36] defined the Gaza strain (which accounted for 64% of all MRSA isolates in their study) as ST22, spa 998 (t223), SCCmec IVa, PVL-negative, and genetically distinct from the pandemic EMRSA-15 clone (as demonstrated by PFGE); however, the authors did not address whether it harbored the TSST-1 gene. In this study, although a total of 49/121 (40.5%) MRSA isolates belonged to CC22, only 31/121 (25.6%) possessed all of the aforementioned characteristics. In addition to these, one SCCmec IVa-associated spa 998 (t223) isolate was PVL-positive; one spa 998 (t223) isolate harbored SCCmec V; and three spa 998 (t223) isolates were MSSA. Notably, however, all but three of the spa 998 (t223) isolates in this study harbored TSST-1 genes, including the PVL-positive isolate. Although most CC22 strains (including EMRSA-15) do not harbor TSST-1 [63], tst-positive CC22 strains have nevertheless been described in the literature. Most recently, 8/10 (80.0%) nasal MRSA isolates obtained in 2013 from healthy preschool children in Palermo, Italy were characterized as tst-positive, spa 998 (t223), and SCCmec IVa, while 5/74 (6.8%) MSSA isolates were also spa 998 (t223) [28]. In an earlier study, the authors detected the same strain in 166/187 (88.8%) MRSA-colonized infants in a neonatal intensive care unit (NICU), describing it as “endemic” [28]. In both studies, the authors refer to the tst-positive strain as UK-EMRSA-15/“Middle Eastern Variant”, and suggest it is identical to the ‘Gaza strain’ described by Biber et al [36].
Within the Middle Eastern region, 2/16 (12.5%) EMRSA-15 isolates from Kuwaiti hospitals [26]; 4/21 (19.0%) CC22 isolates from a hospital in Abu Dhabi [23]; 6/30 (20.0%) CC22 isolates from a hospital in Riyadh (Saudi Arabia) [27]; and 5/5 (100.0%) spa 998 (t223) isolates from a community-based carriage study in Jordan [24] were all identified as tst-positive, while a more recent study in Jordan identified tst-positive spa 998 (t223) as the predominant MRSA strain among nasal isolates from healthcare workers and community-based individuals [25]. As of this writing, a carriage study in Egypt and Saudi Arabia also identified spa 998 (t223) in nasal isolates from outpatients, but presence of tst was not addressed [61]. Meanwhile, a large study in southern India uncovered 6 clinical and 7 nasal EMRSA-15 isolates which were all positive for both PVL and TSST-1 [3]. Similarly, isolates from 2005–2007 referred to the national Staphylococcal Reference Laboratory in the United Kingdom included five CC22 strains harboring both TSST-1 and PVL [30]. Lastly, the first report of an EMRSA-15 variant in the United States involved a tst-positive isolate from a 10-month old infant which was likewise described as spa 998 (t223), SCCmec IV, and PVL-negative [31].
In this study, TSST-1 genes were also detected in single isolates of CC80 and CC239, suggesting that CC22 strains may be serving as a TSST-1 reservoir for other clonal backgrounds in Gaza. The CC80 isolate also harbored PVL, and the presence of both genes in an isolate corresponding to the Gaza strain suggests the potential for greater numbers of isolates harboring genes for both toxins. These observations are supported by the detection of both PVL and TSST-1 genes in 3/5 spa 998 (t223) isolates obtained from Shohada Al-Aqsa Hospital in Gaza during the same collection period in 2012 (data not shown). Moreover, in a recent regional survey which identified TSST-1 genes in 18/31 (58.1%) CC80 strains from Jordan, 7 also harbored PVL genes [32]. As the authors of that study note, while carriage of both PVL and TSST-1 is rare in S. aureus, a previous study from Jordan also identified two CC80 isolates harboring the genes for both toxins [24]. Likewise, the aforementioned studies from India [3] and the U.K. [30] also observed simultaneous carriage within several clonal backgrounds, including CC22. Although the clinical implications of harboring both toxins are unclear at this time, a recent study suggests they may not be expressed at similar levels during infection [64].
Our study has several limitations. As mentioned earlier, very little clinical and demographic data were available, thereby limiting the possibility of epidemiological inference, including a clear distinction between healthcare-associated (HA) vs. community-associated (CA) infections. Likewise, morbidity and mortality data was not available, leaving the clinical implications of PVL- and TSST-1 bearing strains unresolved. Future studies should aim to further characterize TSST-1 harboring CC22 clones, in Gaza as well as other countries where they have been reported, in order to discern whether such strains are spreading internationally, or whether CC22 strains are acquiring TSST-1 independently. A recent phylogenomic analysis of 193 spatio-temporally diverse strains detailed the population structure of CC22, clearly distinguishing EMRSA-15 from various other lineages. However, strains from the Middle East were not included in the study, while only two MSSA strains were observed to harbor TSST-1, highlighting the unique nature of the Gaza strain among known CC22 lineages [63].
Given the pandemic spread of EMRSA-15, the potential for acquisition of TSST-1 from CC22 strains such as the ones described in this study is of significant clinical concern. Moreover, in light of recent reports from Sicily [28,29], surveillance programs should endeavor to monitor spread of the Gaza strain to other regions [25], as well as the spread of TSST-1 to other clonal backgrounds, and further occurrence of both PVL and TSST-1 within the same strain. Lastly, clinical epidemiology studies should be undertaken to determine risk factors, transmission patterns, and clinical outcomes for CC22 strains harboring TSST-1 [25], given the high rate of endemicity observed in multiple studies including this report [25,28,29]
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The first author (NAL) received support for living expenses from several institutions, including the Fulbright Scholar Program (Department of State, Bureau of Educational and Cultural Affairs, United States), the German Academic Exchange Service (DAAD, Germany), and the Erasmus Mundus External Cooperation Window (EM ECW lot III, Belgium), for support of his work during his visits to the Public Health Research Institute, Rutgers University, United States; the University Hospital of Muenster, Germany; and the Free University of Brussels, Belgium, respectively. All funding was for personal living expenses only; the agencies listed did not play any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Song Y, Du X, Li T, Zhu Y, Li M. Phenotypic and molecular characterization of Staphylococcus aureus recovered from different clinical specimens of inpatients at a teaching hospital in Shanghai between 2005 and 2010. J Med Microbiol. 2013; 62: 274–282. 10.1099/jmm.0.050971-0 [DOI] [PubMed] [Google Scholar]
- 2. Tokajian ST, Khalil PA, Jabbour D, Rizk M, Farah MJ, Hashwa FA, et al. Molecular characterization of Staphylococcus aureus in Lebanon. Epidemiol Infect. 2010; 138: 707–712. 10.1017/S0950268810000440 [DOI] [PubMed] [Google Scholar]
- 3. Nadig S, Ramachandra Raju S, Arakere G. Epidemic meticillin-resistant Staphylococcus aureus (EMRSA-15) variants detected in healthy and diseased individuals in India. J Med Microbiol. 2010; 59: 815–821. 10.1099/jmm.0.017632-0 [DOI] [PubMed] [Google Scholar]
- 4. Tokajian S. New epidemiology of Staphylococcus aureus infections in the Middle East. Clin Microbiol Infect. 2014; 20: 624–628. 10.1111/1469-0691.12691 [DOI] [PubMed] [Google Scholar]
- 5. El-Mahdy TS, El-Ahmady M, Goering RV. Molecular characterization of methicillin-resistant Staphylococcus aureus isolated over a 2-year period in a Qatari hospital from multinational patients. Clin Microbiol Infect. 2014. February; 20: 169–173. 10.1111/1469-0691.12240 [DOI] [PubMed] [Google Scholar]
- 6. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus . Lancet. 2010; 375: 1557–1568. 10.1016/S0140-6736(09)61999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alon D, Abd-Elkadir F, Chowers M, Paitan Y. MRSA SCCmec epidemiology in Israel: development and implementation of an MRSA SCCmec typing strategy. Eur J Clin Microbiol Infect Dis. 2011; 30: 1443–1452. 10.1007/s10096-011-1243-9 [DOI] [PubMed] [Google Scholar]
- 8. Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012; 15: 588–595. 10.1016/j.mib.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 9. Kock R, Schaumburg F, Mellmann A, Koksal M, Jurke A, Becker K, et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One. 2013; 8: e55040 10.1371/journal.pone.0055040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schaumburg F, Kock R, Mellmann A, Richter L, Hasenberg F, Kriegeskorte A, et al. Population dynamics among methicillin-resistant Staphylococcus aureus isolates in Germany during a 6-year period. J Clin Microbiol. 2012; 50: 3186–3192. 10.1128/JCM.01174-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verkade E, Bergmans AM, Budding AE, van Belkum A, Savelkoul P, Buiting AG, et al. Recent emergence of Staphylococcus aureus clonal complex 398 in human blood cultures. PLoS One. 2012; 7: e41855 10.1371/journal.pone.0041855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999; 37: 3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003; 41: 5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009; 53: 4961–4967. 10.1128/AAC.00579-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus . J Clin Microbiol. 2000; 38: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995; 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus . Clin Microbiol Rev. 2000; 13: 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becker K, Friedrich AW, Lubritz G, Weilert M, Peters G, Von Eiff C. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J Clin Microbiol. 2003; 41: 1434–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus . Mol Microbiol. 1998; 29: 527–543. [DOI] [PubMed] [Google Scholar]
- 20. Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus . Mol Microbiol. 2001; 41: 365–377. [DOI] [PubMed] [Google Scholar]
- 21. Novick RP, Subedi A. The SaPIs: mobile pathogenicity islands of Staphylococcus . Chem Immunol Allergy. 2007; 93: 42–57. [DOI] [PubMed] [Google Scholar]
- 22. Dauwalder O, Lina G, Durand G, Bes M, Meugnier H, Jarlier V, et al. Epidemiology of invasive methicillin-resistant Staphylococcus aureus clones collected in France in 2006 and 2007. J Clin Microbiol. 2008; 46: 3454–3458. 10.1128/JCM.01050-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber S, Ehricht R, Slickers P, Abdel-Wareth L, Donnelly G, Pitout M, et al. Genetic fingerprinting of MRSA from Abu Dhabi. ECCMID: 2010; Vienna 2010.
- 24. Al-Bakri AG, Al-Hadithi H, Kasabri V, Othman G, Kriegeskorte A, Becker K. The epidemiology and molecular characterization of methicillin-resistant staphylococci sampled from a healthy Jordanian population. Epidemiol Infect. 2013; 141: 2384–2391. 10.1017/S0950268813000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aqel AA, Alzoubi HM, Vickers A, Pichon B, Kearns AM. Molecular epidemiology of nasal isolates of methicillin-resistant Staphylococcus aureus from Jordan. J Infect Public Health. 2015; 8: 90–97. 10.1016/j.jiph.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 26. Udo EE, Al-Sweih N, Noronha B. Characterisation of non-multiresistant methicillin-resistant Staphylococcus aureus (including EMRSA-15) in Kuwait Hospitals. Clin Microbiol Infect. 2006; 12: 262–269. [DOI] [PubMed] [Google Scholar]
- 27. Monecke S, Skakni L, Hasan R, Ruppelt A, Ghazal SS, Hakawi A, et al. Characterisation of MRSA strains isolated from patients in a hospital in Riyadh, Kingdom of Saudi Arabia. BMC Microbiol. 2012; 12: 146 10.1186/1471-2180-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geraci DM, Giuffre M, Bonura C, Matranga D, Aleo A, Saporito L, et al. Methicillin-Resistant Staphylococcus aureus Colonization: A Three-Year Prospective Study in a Neonatal Intensive Care Unit in Italy. PLoS One. 2014; 9: e87760 10.1371/journal.pone.0087760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geraci DM, Bonura C, Giuffre M, Aleo A, Saporito L, Graziano G, et al. tst1-positive ST22-MRSA-IVa in healthy Italian preschool children. Infection. 2014; 42: 535–538. 10.1007/s15010-013-0583-z [DOI] [PubMed] [Google Scholar]
- 30. Kearns AM, McCormick JK, Ganner M, East C, Warner M, Hill RL, et al. Staphylococcus aureus encoding both TSST-1 and PVL in the UK. ECCMID: 2008; Barcelona 2008. [Google Scholar]
- 31. Wolter DJ, Chatterjee A, Varman M, Goering RV. Isolation and characterization of an epidemic methicillin-resistant Staphylococcus aureus 15 variant in the central United States. J Clin Microbiol. 2008; 46: 3548–3549. 10.1128/JCM.00985-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harastani HH, Tokajian ST. Community-Associated Methicillin-Resistant Staphylococcus aureus Clonal Complex 80 Type IV (CC80-MRSA-IV) Isolated from the Middle East: A Heterogeneous Expanding Clonal Lineage. PLoS One. 2014; 9: e103715 10.1371/journal.pone.0103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaibni MH, Farraj MA, Adwan K, Essawi TA. Community-acquired meticillin-resistant Staphylococcus aureus in Palestine. J Med Microbiol. 2009; 58: 644–647. 10.1099/jmm.0.007617-0 [DOI] [PubMed] [Google Scholar]
- 34. Adwan K, Jarrar N, Abu-Hijleh A, Adwan G, Awwad E, Salameh Y. Molecular analysis and susceptibility patterns of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in the northern region of Palestine. Am J Infect Control. 2013; 41: 195–198. 10.1016/j.ajic.2012.03.040 [DOI] [PubMed] [Google Scholar]
- 35. Elmanama AA, Laham NA, Tayh GA. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns. 2013; 39: 1612–1618. 10.1016/j.burns.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 36. Biber A, Abuelaish I, Rahav G, Raz M, Cohen L, Valinsky L, et al. A typical hospital-acquired methicillin-resistant Staphylococcus aureus clone is widespread in the community in the Gaza strip. PLoS One. 2012; 7: e42864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wikipedia contributors. Demographics of the Palestinian territories. Wikipedia, The Free Encyclopedia. 2014. Available: http://en.wikipedia.org/wiki/Demographics_of_the_Palestinian_territories.
- 38. Al Laham NA. Prevalence of bacterial contamination in general operating theaters in selected hospitals in the Gaza Strip, Palestine. J Infect Public Health. 2012; 5: 43–51. 10.1016/j.jiph.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 39. Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998; 36: 2548–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Becker K, Pagnier I, Schuhen B, Wenzelburger F, Friedrich AW, Kipp F, et al. Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods? J Clin Microbiol. 2006; 44: 229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mathema B, Mediavilla J, Kreiswirth BN. Sequence analysis of the variable number tandem repeat in Staphylococcus aureus protein A gene: spa typing. Methods Mol Biol. 2008; 431: 285–305. [DOI] [PubMed] [Google Scholar]
- 42. Mellmann A, Friedrich AW, Rosenkotter N, Rothganger J, Karch H, Reintjes R, et al. Automated DNA sequence-based early warning system for the detection of methicillin-resistant Staphylococcus aureus outbreaks. PLoS Med. 2006; 3: e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen L, Mediavilla JR, Oliveira DC, Willey BM, de Lencastre H, Kreiswirth BN. Multiplex real-time PCR for rapid staphylococcal cassette chromosome mec typing. J Clin Microbiol. 2009; 47: 3692–3706. 10.1128/JCM.00766-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007; 51: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004; 186: 1518–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. von Eiff C, Friedrich AW, Peters G, Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus . Diagn Microbiol Infect Dis. 2004; 49: 157–162. [DOI] [PubMed] [Google Scholar]
- 47. Said-Salim B, Mathema B, Braughton K, Davis S, Sinsimer D, Eisner W, et al. Differential distribution and expression of Panton-Valentine leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 2005; 43: 3373–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen L, Mediavilla JR, Smyth DS, Chavda KD, Ionescu R, Roberts RB, et al. Identification of a novel transposon (Tn6072) and a truncated staphylococcal cassette chromosome mec element in methicillin-resistant Staphylococcus aureus ST239. Antimicrob Agents Chemother. 2010; 54: 3347–3354. 10.1128/AAC.00001-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Subedi A, Ubeda C, Adhikari RP, Penades JR, Novick RP. Sequence analysis reveals genetic exchanges and intraspecific spread of SaPI2, a pathogenicity island involved in menstrual toxic shock. Microbiology. 2007; 153: 3235–3245. [DOI] [PubMed] [Google Scholar]
- 50. Adler A, Givon-Lavi N, Moses AE, Block C, Dagan R. Carriage of community-associated methicillin-resistant Staphylococcus aureus in a cohort of infants in southern Israel: risk factors and molecular features. J Clin Microbiol. 2010; 48: 531–538. 10.1128/JCM.02290-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adwan K, Abu-Hasan N, Adwan G, Jarrar N, Abu-Shanab B, Abu-Zant A. Nosocomial infection caused by methicillin-resistant Staphylococcus aureus in Palestine. Microb Drug Resist. 2005; 11: 75–77. [DOI] [PubMed] [Google Scholar]
- 52. Borg MA, de Kraker M, Scicluna E, van de Sande-Bruinsma N, Tiemersma E, Monen J, et al. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J Antimicrob Chemother. 2007; 60: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 53. Harastani HH, Araj GF, Tokajian ST. Molecular characteristics of Staphylococcus aureus isolated from a major hospital in Lebanon. Int J Infect Dis. 2014; 19: 33–38. 10.1016/j.ijid.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 54. Adler A, Chmelnitsky I, Shitrit P, Sprecher H, Navon-Venezia S, Embon A, et al. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Israel: dissemination of global clones and unique features. J Clin Microbiol. 2012; 50: 134–137. 10.1128/JCM.05446-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aqel AA, Ibrahim A, Shehabi A. Rare occurrence of mupirocin resistance among clinical Staphylococcus isolates in Jordan. Acta Microbiol Immunol Hung. 2012; 59: 239–247. 10.1556/AMicr.59.2012.2.8 [DOI] [PubMed] [Google Scholar]
- 56. Sabri I, Adwan K, Essawi TA, Farraj MA. Molecular characterization of methicillin-resistant Staphylococcus aureus isolates in three different Arab world countries. Eur J Microbiol Immunol (Bp). 2013; 3: 183–187. 10.1556/EuJMI.3.2013.3.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chmelnitsky I, Navon-Venezia S, Leavit A, Somekh E, Regev-Yochai G, Chowers M, et al. SCCmec and spa types of methicillin-resistant Staphylococcus aureus strains in Israel. Detection of SCCmec type V. Eur J Clin Microbiol Infect Dis. 2008; 27: 385–390. [DOI] [PubMed] [Google Scholar]
- 58. Regev-Yochay G, Carmeli Y, Raz M, Pinco E, Etienne J, Leavitt A, et al. Prevalence and genetic relatedness of community-acquired methicillin-resistant Staphylococcus aureus in Israel. Eur J Clin Microbiol Infect Dis. 2006; 25: 719–722. [DOI] [PubMed] [Google Scholar]
- 59. Enany S, Higuchi W, Okubo T, Takano T, Enany M, Yamamoto T. Brain abscess caused by Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus in Egypt, April 2007. Euro Surveill. 2007; 12: E070927.2 [DOI] [PubMed] [Google Scholar]
- 60. Enany S, Yaoita E, Yoshida Y, Enany M, Yamamoto T. Molecular characterization of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus isolates in Egypt. Microbiol Res. 2010; 165: 152–162. 10.1016/j.micres.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 61. Shady HA, Bakr AE, Hashad ME, Alzohairy MA. Staphylococcus aureus nasal carriage among outpatients attending primary health care centers: a comparative study of two cities in Saudi Arabia and Egypt. Braz J Infect Dis. 2015; 19: 68–76. 10.1016/j.bjid.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khalil W, Hashwa F, Shihabi A, Tokajian S. Methicillin-resistant Staphylococcus aureus ST80-IV clone in children from Jordan. Diagn Microbiol Infect Dis. 2012; 73: 228–230. 10.1016/j.diagmicrobio.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 63. Holden MT, Hsu LY, Kurt K, Weinert LA, Mather AE, Harris SR, et al. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013; 23: 653–664. 10.1101/gr.147710.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li Z, Stevens DL, Hamilton SM, Parimon T, Ma Y, Kearns AM, et al. Fatal S. aureus hemorrhagic pneumonia: genetic analysis of a unique clinical isolate producing both PVL and TSST-1. PLoS One. 2011; 6: e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.