Abstract
Purpose of review
Arterial and venous thrombosis are major causes of morbidity and mortality, and the incidence of thromboembolic diseases increases as a population ages. Thrombi are formed by activated platelets and fibrin. The latter is a product of the plasma coagulation system. Currently available anticoagulants such as heparins, vitamin K antagonists and inhibitors of thrombin or factor Xa target enzymes of the coagulation cascade that are critical for fibrin formation. However, fibrin is also necessary for terminating blood loss at sites of vascular injury. As a result, anticoagulants currently in clinical use increase the risk of bleeding, partially offsetting the benefits of reduced thrombosis. This review focuses on new targets for anticoagulation that are associated with minimal or no therapy-associated increased bleeding.
Recent findings
Data from experimental models using mice and clinical studies of patients with hereditary deficiencies of coagulation factors XI or XII have shown that both of these clotting factors are important for thrombosis, while having minor or no apparent roles in processes that terminate blood loss (hemostasis).
Summary
Hereditary deficiency of factor XII (Hageman factor) or factor XI, plasma proteases that initiate the intrinsic pathway of coagulation, impairs thrombus formation and provides protection from vascular occlusive events, while having a minimal impact on hemostasis. As the factor XII–factor XI pathway contributes to thrombus formation to a greater extent than to normal hemostasis, pharmacological inhibition of these coagulation factors may offer the exciting possibility of anticoagulation therapies with minimal or no bleeding risk.
Keywords: factor XI, factor XII, hemostasis, intrinsic pathway of coagulation, polyphosphate, thrombosis
Introduction
Hemostasis comprises the normal mechanisms that prevent blood loss from sites of vascular injury. Dysregulated hemostatic activity is believed to contribute to thrombotic diseases such as pulmonary embolism, myocardial infarction (MI) and stroke. Despite considerable progress on strategies to prevent or treat these diseases, thrombosis remains a major health burden in the industrialized world, and the primary cause of morbidity and mortality [1]. On a mechanistic level, hemostasis proceeds in two steps. During primary hemostasis platelets adhere to the site of trauma and become activated through binding to collagens and von Willebrand factor, and ultimately aggregate by binding to each other to form a platelet plug [2]. Platelet plug formation is enhanced and stabilized during secondary hemostasis – an intricate series of enzymatic reactions involving coagulation proteins that culminate in formation of the protease thrombin, which converts fibrinogen to fibrin to form a stable clot.
Plasma coagulation
Plasma coagulation involves a series of enzymatic steps involving a set of proteases and their cofactors. The major product of this system is the protease thrombin, which converts fibrinogen, a soluble protein, into insoluble fibrin strands. In the classical cascade/waterfall model, coagulation can be initiated by two distinct mechanisms, referred to as the extrinsic and intrinsic pathways [3,4]. The extrinsic pathway is started when coagulation factor VII (FVII) binds to the transmembrane protein tissue factor (TF) and is activated to FVIIa. Under physiologic conditions, TF is virtually absent on cells that normally have contact with plasma (blood cells and vascular endothelium), but is expressed on subendothelial cell surfaces. At vascular injury sites the TF–FVIIa complex generates small amounts of activated factor X (FXa) that initates thrombin generation and fibrin production [5]. The intrinsic pathway is initiated by activation of factor XII (FXII, Hageman factor) in a reaction involving high-molecular-weight kininogen and plasma prekallikrein. These three proteins are collectively referred to as the plasma contact system, and they initiate coagulation through a process called contact activation. When blood comes into contact with negatively charged surfaces, a conformational change in FXII results in formation of small amounts of active FXII (FXIIa). FXIIa cleaves plasma prekallikrein to generate active α-kallikrein, which reciprocally activates additional FXII in a positive feedback loop [6]. FXIIa cleaves its substrate factor XI (FXI) [7••] to form active FXIa, which in turn promotes coagulation via Ca2+-dependent activation of factor IX (FIX). The extrinsic and intrinsic pathways converge on factor X. FXa in complex with the cofactor factor Va (FVa) converts prothrombin into thrombin. Thrombin activates multiple pathways in the vascular system [8]. In addition to cleaving fibrinogen to form fibrin, thrombin can amplify its own generation by activating FXI [9,10]. The concept that thrombin formed following initiation of coagulation by the extrinsic pathway may trigger the intrinsic pathway independently of FXII has led to a revision of the cascade model.
The role of factors XI and XII in hemostasis
Contact activation-induced activation of FXII by inorganic polyanions such as glass, kaolin (a silicate) or ellagic acid is the trigger for one of the most commonly used diagnostic clotting tests, the activated partial thromboplastin time (aPTT). The aPTT is widely used in clinical practice for preoperative screening and monitoring of anticoagulation therapy. Despite its indispensible role in fibrin formation in the aPTT, FXII-initiated coagulation is not believed to have an important function in vivo, based on the clinical observation that FXII deficiency in humans and animals is not associated with abnormal bleeding despite causing a marked prolongation of the aPTT [11]. In contrast, deficiency of the extrinsic pathway trigger FVII results in severe bleeding in humans, whereas complete FVII or TF deficiency in mice is not compatible with life due to intrauterine bleeding [12,13]. Similar to FXII-deficient individuals, humans lacking the other contact proteins, plasma prekallikrein or high-molecular-weight kininogen, do not have impaired hemostasis. Individuals deficient in contact system components are usually detected during routine screening and do not report abnormal bleeding. In contrast, patients lacking FXI have a mild trauma-induced bleeding disorder (sometimes called hemophilia C) that is mostly restricted to tissues with high fibrinolytic activity. Severe FXI deficiency is a rare inherited abnormality in the general population (one in one million people), but is more common in specific populations, such as Ashkenazi Jews (one in 450) [14]. The absence of pathologic bleeding in FXII-deficient individuals, and the observation that FXI can be activated by thrombin [9,15], has led to a hypothesis that FXII-stimulated thrombin formation is not important in vivo. It is generally accepted now that coagulation in vivo is primarily if not exclusively initiated by TF/FVII [16]. FXI probably contributes to thrombin generation in low TF environments, but is likely less important when higher levels of tissue factor are present [17].
The role of factors XI and XII in thrombosis
Factor XI-deficient (FXI−/−) and FXII-deficient (FXII−/−) mice [18,19], similar to their human counterparts, have markedly prolonged aPTT clotting times, but do not exhibit abnormal spontaneous bleeding or prolonged injury-related bleeding in tail-bleeding assays or during surgical procedures [20]. FXI−/− mice were protected from carotid artery thrombus formation in a FeCl3-induced thrombosis model [21–23], suggesting the proposed thrombin–FXI feedback loop is important in thrombosis. Reconstitution of FXI-deficient mice with human FXI resolved the defect in thrombus formation, indicating that FXI functions similarly across species and that resistance to thrombus formation in FXI−/− mice is due to FXI deficiency. Unexpectedly, FXII−/− mice were also protected from thrombus formation in various arterial and venous vascular beds in response to mechanical or chemical injuries. Reconstitution of FXII−/− mice with human FXII shortened the prolonged aPTT of untreated animals and restored the capacity of animals to develop thrombosis [20]. Thrombus formation in FXII heterozygous null mice having 50% of the normal plasma FXII level was similar to wild-type controls, indicating half the normal plasma FXII concentration is sufficient to support occlusive clot formation. This is an important consideration for drug development. In contrast to many currently used anticoagulants that demonstrate increasing degrees of anticoagulation with increasing plasma concentrations, drugs targeting FXIIa may need to substantially reduce protease activity (>50%) before a therapeutic effect is observed.
Factor XII deficiency protects mice in a model of ischemic stroke [transient middle cerebral artery occlusion (tMCAO)]. FXII−/− mice had smaller cerebral infarct volumes and less fibrin deposition in microvessels without signs of intracerebral hemorrhage [24,25]. FXI−/− mice were similar to FXII−/− animals in this model, suggesting that FXIIa is operating by activating FXI, its substrate in the intrinsic pathway. The similar degree of protection in FXII−/− and FXI−/− mice, while suggesting they operate in a single pathway, does not exclude the possibility that they act independently. In a model of lethal pulmonary embolism, FXII−/−/FXI−/− double-deficient mice were protected to a similar extent to animals deficient in only one of the proteins, suggesting that FXIIa initiates fibrin production in vivo through the intrinsic pathway by activating FXI [7••]. Consistent with this, an anti-FXI monoclonal antibody that specifically targets FXIIa-mediated FXI activation efficiently interferes with intrinsic pathway-mediated fibrin formation in plasma and in thrombosis models in mice and baboons [26]. These results could be used to argue that FXI activation by thrombin, as positioned in revised coagulation models, does not contribute significantly in the thrombosis models [15]. The relative importance of FXIIa-mediated and thrombin-mediated activation of FXI in vivo is not well understood, and may vary depending on the type of injury and the vascular bed involved.
Cumulatively, the mouse models support the hypothesis that the FXII–FXI pathway is important for pathologic thrombus formation, but not hemostasis, and identify FXII and FXI as attractive drug targets for well tolerated (from a bleeding standpoint) anticoagulation therapy. This challenges the premise that pathologic thrombus formation solely represents a dysregulation of normal hemostatic mechanisms [27]. It also re-emphasizes the point that the physiologic roles of FXII are unlikely to be directly related to hemostasis. The contact system is highly conserved in mammals [28], consistent with our observations on the effects of human FXII and FXI in FXII−/− and FXI−/− mice. However, the FXII gene is absent in some vertebrate groups such as birds and fish, despite the presence of a closed circulatory system, and FXI is only found in mammals. This is consistent with the premise that these proteins are not critical elements of the hemostatic mechanism of vertebrates.
Contact system (FXII) activators
As discussed, the intrinsic pathway of coagulation is initiated by activation of FXII during contact activation on negatively charged surfaces. The identity, or even the existence, of endogenous activators of FXII in vivo has puzzled investigators for decades. Potential activators of FXII-driven fibrin production have recently been described, including collagen and extracellular RNA [29]. The absence of pathological bleeding in FXII-deficient individuals indicates either that FXII is not activated at the injured vessel walls or that FXIIa does not significantly contribute to fibrin formation at the site of injury. The reasons why RNA released from injured cells or subendothelial collagen fibers exposed at a wound site do not contribute to fibrin formation through FXIIa are not clear.
Platelet activation has been linked to FXII for more than 45 years, and activated platelets promote fibrin formation in an FXII-dependent manner in vitro [30,31]. Thrombus stability is defective in FXII−/− mice, as revealed by intravital microscopy [20], and clot firmness, as measured by thromboelastography, is reduced in mouse and human blood in which FXIIa is inhibited [7••]. We reasoned that FXII is activated specifically on procoagulant platelet surfaces in the thrombus, and not at the level of the injured vessel wall, driving fibrin production within the growing thrombus via FXI. We looked for FXII activators that are released from activated platelets and identified polyphosphate (polyP), an inorganic linear polymer of 60–100 orthophosphate units, as the endogenous FXII activator in platelets [7••]. PolyP is released from platelet dense granules and initiates thrombin generation through FXII and FXI. Targeted inhibition of polyP-mediated FXII activation efficiently and selectively protects mice from platelet-triggered thrombosis without increased bleeding. PolyP, therefore, may be a novel target for anticoagulation therapy. It has been proposed that thrombin formation on the surface of a growing clot away from the original TF trigger in the blood vessel wall is supported by TF expressed on platelets and other blood components. The results with polyP suggest an alternative TF-independent mechanism for promoting thrombus growth. Some substances, such as misfolded proteins [32] and heparin released from allergen-activated mast cells [33], appear to initiate FXIIa-mediated activation of plasma prekallikrein to α-kallikrein without activating FXI. This specific activation of the kallikrein–kinin system results in generation of the proinflammatory peptide hormone bradykinin [34,35] without enhanced thrombin generation and, therefore, appears to be a distinct process from polyP-mediated FXII activation.
Thrombosis in patients with inherited deficiency of FXI and FXII
There are few studies that systematically compare the incidence or severity of thromboembolic events (stroke, MI, pulmonary embolism) in humans with severe FXII deficiency and normal individuals. The first reported FXII-deficient individual, John Hageman, died from a pulmonary embolism [36], and several subsequent small clinical studies described an increased risk of thrombosis in FXII-deficient humans [37–39]. However, re-analyses subsequently showed that thrombosis in FXII-deficient patients were probably related to other risk factors, and not to FXII deficiency [40]. Larger case-controlled studies in the Netherlands and Switzerland did not find a correlation between FXII deficiency and a higher thrombotic risk. None of these studies addressed the possibility that FXII deficiency reduced thrombotic risk [41,42]. Recent clinical studies have demonstrated a reduced incidence of ischemic stroke in humans with severe FXI deficiency. Like FXI−/− mice [24,43], FXI-deficient humans appear to be protected from cerebral ischemia [44], supporting an important role for the intrinsic pathway in arterial thrombosis in humans. This effect may be vascular bed-specific, as FXI deficiency does not appear to reduce risk of MI [45,46•]. The protective effect is not restricted to arterial beds, as FXI deficiency reduces the risk of deep vein thrombosis [47].
Anticoagulation strategies
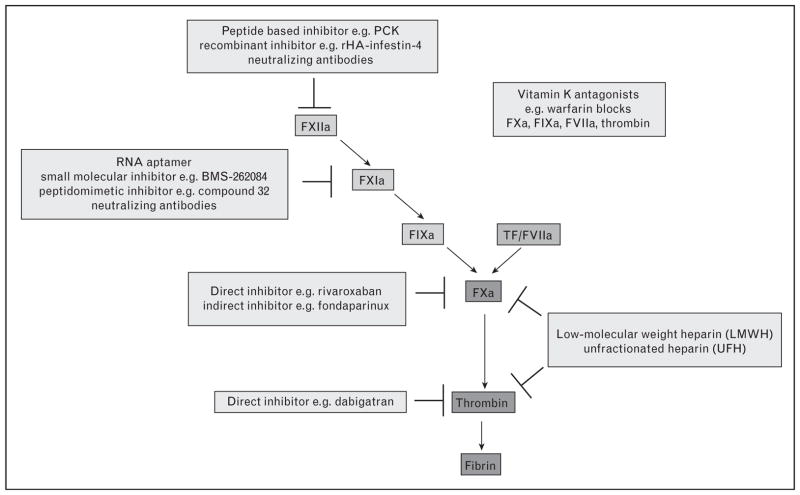
Current anticoagulation therapy is based primarily on heparins, vitamin K antagonists or indirect inhibitors of FXa like the synthetic pentasaccharide fondaparinux (Fig. 1). The need for regular monitoring and dose adjustments for some of these drugs (unfractionated heparin and warfarin), or for parenteral administration (heparins, fondaparinux) are inconvenient. Furthermore, use of these drugs is associated with an increased risk of life-threatening bleeding. New oral anticoagulations with lower, but still significant, bleeding risk include direct inhibitors of thrombin (e.g. dabigatran etexilate) or FXa (e.g. rivaroxaban). The antithrombotic effects and bleeding risks for these drugs have been assessed in several clinical trials [48]. Bleeding is an expected consequence with these compounds, because they all target proteins that are critical components of the hemostatic mechanism. In contrast, the limited roles of FXI and FXII in hemostasis suggest that agents targeting these proteases should not increase the incidence of major bleeds.
Figure 1. New and current anticoagulant targets in the coagulation pathway.
The coagulation cascade is a series of sequential reactions in which zymogens are converted to active serine proteases ultimately resulting in the production of thrombin and covalently cross-linked fibrin. Blood coagulation can be triggered via the tissue factor (TF)/FVIIa-extrinsic and the FXII-driven intrinsic pathways. Anticoagulant targets that are in current use and in development (light gray) are shown.
Wild-type mice treated with the peptidergic FXIIa inhibitor D-Pro-Phe-Arg chloromethylketone (PCK), similar to FXII−/−-deficient mice, are protected from pulmonary embolism and ischemic stroke without increased bleeding [7••,24]. Whereas PCK is not highly selective for FXIIa, recombinant FXIIa inhibitors based on the fourth domain of infestin (infestin-4, a nonclassic Kazal-type serine protease inhibitor protein from Tryatoma infestans) [49] fused to human albumin (rHA-infestin-4) have been developed. rHA-infestin-4 is a potent FXIIa inhibitor in human, mouse and rat plasmas, but does not prolong bleeding times in rodents, even after infusion of large doses. Pretreatment of mice with rHA-infestin-4 resulted in markedly reduced cerebral infarction in the tMCAO model [50]. Similarly, the recombinant contact phase inhibitor Ir-CPI, a Kunitz-type protein from the salivary glands of the tick Ixodes ricinus, blocks FXIIa, FXIa, and α-kallikrein and protects mice from venous thromboembolism [51]. A nonpeptidic 3-carboxamide-coumarin (COU254) inhibitor selectively blocked FXII activity in plasma [52], but was not effective in the tMCAO model in mice [53]. Other protein inhibitors with some degree of selectivity for FXIIa, including cabbage seed protease inhibitor [54], the pumpkin seed inhibitor CMTI-V [55,56], trypsin inhibitor from corn (CTI) [57], inhibitors from hematophagous insects [58], and ecotin (an inhibitor found in the periplasm of E. coli) [59] are effective FXIIa inhibitors in plasma, but also inhibit other coagulation proteases including thrombin, FXIa, and α-kallikrein. A monoclonal antibody against FXIIa that interferes with the FXIIa–FXI interaction has antithrombotic properties and might be useful to prevent thrombosis in patients (US Patent Application 20090304685, Pritchard D.).
Several strategies targeting FXI/FXIa, including antisense oligonucleotides (ASOs) knockdown of FXI expression, neutralizing antibodies, peptidomimetic and small molecule inhibitors, have been reviewed recently [60•]. Considerable progress has been made on understanding the antithrombotic effects of monoclonal antibodies to FXI using thrombosis models in mice, rabbits and baboons. In mice, antibody 14E11 prevented carotid artery occlusion induced by FeCl3 challenge to a similar degree as total FXI deficiency, and also had a modest beneficial effect in a TF-induced pulmonary embolism model [26]. 14E11 blocks FXI activation by FXIIa, but does not affect FIX activation by FXIa. Treatment of rabbits with antibody XI-5108 reduced thrombus formation in jugular veins [61], and on injured neointima [62]. In baboons, anti-FXI antibodies, including the monoclonal antibody O1A6, prevented occlusion of thrombogenic vascular grafts by reducing platelet and fibrin deposition [63,64]. O1A6 blocks FXIa activation of FIX. Potent ketoarginine-based peptidomimetics that irreversibly inhibit FXIa, such as compound 32, were efficacious in a rat model of venous thrombosis [65]. The irreversible small-molecule FXIa inhibitor BMS-262084 protected rats from FeCl3-induced arterial and venous thrombosis [66]. Naturally occurring inhibitors clavatadine A and B from marine sponges inhibit FXIa by covalent binding to the active center in FXI in vitro [67], and might serve as leading structure for drug development. A new and exciting method to inhibit FXI is based on antisense technology [68••]. Highly specific ASOs bind complementary sequences on the mRNA of a protein of interest by base pair hybridization. Subsequent selective cleavage and degradation of the target mRNA leads to a reduction in target protein level [69]. In a phase III trial to treat hypercholesterolemia, ASOs directed against the mRNA of apolipoprotein B were potent, selective and well tolerated [70]. The ASO ISIS 404071 reduced FXI expression in mice for up to 2 weeks, with no detectable effect on other coagulation factors. Similar results were obtained with FXI antisense oligonucleotides FXI-AS1 and FXI-AS2 in cynomolgus monkeys [71] (Table 1). Knocking down FXI expression would probably be a relatively well tolerated approach to anticoagulation therapy because of the relatively mild bleeding disorder associated with FXI deficiency. In cases in which bleeding did develop or surgical intervention is required, fresh frozen plasma or a plasma-derived FXI concentrate is available to rapidly restore the FXI level. A phase I trial of the anti-FXI ASO ISIS-FXIRx in humans is currently underway. Inhibition of FXI and possibly FXII by ASOs might serve as future strategies for the treatment and prevention of thromboembolic disease.
Table 1.
Potential thromboprotection of new factor XII and factor XI inhibitors in vivo
| Target | Inhibitor | Arterial thrombosis | Venous thrombosis | Ischemic stroke |
|---|---|---|---|---|
| FXIIa | Infestin-4 from Triatoma infestans fused to recombinant human albumin (rHA-infestin-4) [50] | × | × | × |
| Nonpeptidic inhibitor (3-carboxamide-coumarin, COU254) [53] | × | |||
| FXIIa/α-kallikrein | Peptide-based inhibitor (D-Pro-Phe-Arg chloromethylketone, PCK) [7••,24] | × | × | |
| FXIIa/FXIa/α-kallikrein | Recombinant Ixodes ricinus inhibitor (Ir-CPI) [51] | × | × | |
| FXI | Antisense oligonucleotides (ASOs) [68••] | × | × | |
| Neutralizing antibodies [4,26,61,63] | × | × | ||
| FXIa | Small molecule inhibitor, e.g. BMS-262084 [66] | × | × | |
| Peptidomimetic inhibitor, e.g. compound 32 [65] | × |
Conclusion
Novel strategies are required to treat and prevent thrombotic disorders. Currently available antithrombotic agents are associated with some risk of severe bleeding complications, as they target components of the coagulation mechanism that are required for hemostasis. The intrinsic pathway proteases FXI and FXII appear to play a critical role in development of pathological thrombus formation, while having limited (or no effect) on physiologic hemostasis. Pharmacological targeting of these proteins with a new generation of drugs may make antithrombotic therapy safer, and thus broaden the range of clinical indications and scenarios in which therapy can be applied.
Key points.
Factors XI and XII are essential for arterial thrombosis but have minor/no role for hemostasis.
Factors XII–factor XI pathway is activated by polyphosphates in thrombosis.
Hereditary deficiency in factors XI and XII abolishes thrombosis in animal models.
Experimental factor XI and XII inhibitors interfere with pathological thrombosis and open the perspective for a well tolerated anticoagulation.
Pharmacological inhibitors of factors XI and XII have minimal/no increased bleeding.
Acknowledgments
The work was supported in parts by grants from Vetenskapsrådet (K2010-64x-21462-01-3), Hjärt Lungfonden (200906.42), Stockholms läns landsting (ALF, 20090540), Cancerfonden (100615), the Federal Ministry of Education and Research (BMBF)-funded ERARE and 01EO1003 programs, Aroseniusfonden and Stiftung für Pathobiochemie und Molekulare Diagnostik (DGKL).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 377–378).
- 1.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 5.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108:1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colman RW. Contact activation (kallikrein-kinin) pathway: multiple physiologic and pathophysiologic activities. In: Colman RW, Mader VJ, Clowes AW, et al., editors. Hemostasis and thrombosis: basic principles and clinical practice. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 107–130. [Google Scholar]
- 7••.Muller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. The study identifies polyphosphate (an inorganic polymer) as the activator of the FXII-driven intrinsic pathway of coagulation on procoagulant platelets. Polyphosphates initiate thrombosis in animal models and patients and provide procoagulant activity to propagate arterial thrombi in a FXII-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 9.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 10.Gailani D, Sun MF, Cheng Q, et al. Evidence against a protein in plasma that is a product of a factor XI mRNA splice variant missing exons 6 and 7. Blood. 2010;116:1185–1186. doi: 10.1182/blood-2010-01-265702. author reply 1186–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen ED, Chan JC, Idusogie E, et al. Mice lacking factor VII develop normally but suffer fatal perinatal bleeding. Nature. 1997;390:290–294. doi: 10.1038/36862. [DOI] [PubMed] [Google Scholar]
- 13.Bugge TH, Xiao Q, Kombrinck KW, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders RE, Shiltagh N, Gomez K, et al. Structural analysis of eight novel and 112 previously reported missense mutations in the interactive FXI mutation database reveals new insight on FXI deficiency. Thromb Haemost. 2009;102:287–301. doi: 10.1160/TH09-01-0044. [DOI] [PubMed] [Google Scholar]
- 15.Kravtsov DV, Matafonov A, Tucker EI, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissey JH. Tissue factor: in at the start…and the finish? J Thromb Haemost. 2003;1:878–880. doi: 10.1046/j.1538-7836.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 17.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86:3035–3042. [PubMed] [Google Scholar]
- 18.Gailani D, Lasky NM, Broze GJ., Jr A murine model of factor XI deficiency. Blood Coagul Fibrinolysis. 1997;8:134–144. doi: 10.1097/00001721-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Pauer HU, Renne T, Hemmerlein B, et al. Targeted deletion of murine coagulation factor XII gene: a model for contact phase activation in vivo. Thromb Haemost. 2004;92:503–508. doi: 10.1160/TH04-04-0250. [DOI] [PubMed] [Google Scholar]
- 20.Renne T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 22.Wang X, Cheng Q, Xu L, et al. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Smith PL, Hsu MY, et al. Effects of factor XI deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4:1982–1988. doi: 10.1111/j.1538-7836.2006.02093.x. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschnitz C, Stoll G, Bendszus M, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham M, Kleinschnitz C, Helluy X, et al. Enhanced cortical reperfusion protects coagulation factor XII-deficient mice from ischemic stroke as revealed by high-field MRI. Neuroimage. 2010;49:2907–2914. doi: 10.1016/j.neuroimage.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renne T, Gailani D. Role of factor XII in hemostasis and thrombosis: clinical implications. Expert Rev Cardiovasc Ther. 2007;5:733–741. doi: 10.1586/14779072.5.4.733. [DOI] [PubMed] [Google Scholar]
- 28.Doolittle RF. Coagulation in vertebrates with a focus on evolution and inflammation. J Innate Immun. 2011;3:9–16. doi: 10.1159/000321005. [DOI] [PubMed] [Google Scholar]
- 29.Maas C, Oschatz C, Renne T. Activators of the contact system 2.0. Semin Thromb Hemost. doi: 10.1055/s-0031-1276586. in press. [DOI] [PubMed] [Google Scholar]
- 30.Walsh PN. The role of platelets in the contact phase of blood coagulation. Br J Haematol. 1972;22:237–254. doi: 10.1111/j.1365-2141.1972.tb08803.x. [DOI] [PubMed] [Google Scholar]
- 31.Muller F, Renne T. Platelet polyphosphates: the nexus of primary and secondary hemostasis. Scand J Clin Lab Invest. 2011;71:82–86. doi: 10.3109/00365513.2010.550312. [DOI] [PubMed] [Google Scholar]
- 32.Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34:258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Renne T, Schuh K, Muller-Esterl W. Local bradykinin formation is controlled by glycosaminoglycans. J Immunol. 2005;175:3377–3385. doi: 10.4049/jimmunol.175.5.3377. [DOI] [PubMed] [Google Scholar]
- 35.Renne T, Dedio J, David G, Muller-Esterl W. High molecular weight kininogen utilizes heparan sulfate proteoglycans for accumulation on endothelial cells. J Biol Chem. 2000;275:33688–33696. doi: 10.1074/jbc.M000313200. [DOI] [PubMed] [Google Scholar]
- 36.Ratnoff OD. The demise of John Hageman. N Engl J Med. 1968;279:760–761. [Google Scholar]
- 37.Girolami A, Randi ML, Gavasso S, et al. The occasional venous thromboses seen in patients with severe (homozygous) FXII deficiency are probably due to associated risk factors: a study of prevalence in 21 patients and review of the literature. J Thromb Thrombolysis. 2004;17:139–143. doi: 10.1023/B:THRO.0000037670.42776.cd. [DOI] [PubMed] [Google Scholar]
- 38.Bach J, Endler G, Winkelmann BR, et al. Coagulation factor XII (FXII) activity, activated FXII, distribution of FXII C46T gene polymorphism and coronary risk. J Thromb Haemost. 2008;6:291–296. doi: 10.1111/j.1538-7836.2008.02839.x. [DOI] [PubMed] [Google Scholar]
- 39.Halbmayer WM, Mannhalter C, Feichtinger C, et al. The prevalence of factor XII deficiency in 103 orally anticoagulated outpatients suffering from recurrent venous and/or arterial thromboembolism. Thromb Haemost. 1992;68:285–290. [PubMed] [Google Scholar]
- 40.Girolami A, Morello M, Girolami B, et al. Myocardial infarction and arterial thrombosis in severe (homozygous) FXII deficiency: no apparent causative relation. Clin Appl Thromb Hemost. 2005;11:49–53. doi: 10.1177/107602960501100105. [DOI] [PubMed] [Google Scholar]
- 41.Koster T, Rosendaal FR, Briet E, Vandenbroucke JP. John Hageman’s factor and deep-vein thrombosis: Leiden thrombophilia Study. Br J Haematol. 1994;87:422–424. doi: 10.1111/j.1365-2141.1994.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 42.Zeerleder S, Schloesser M, Redondo M, et al. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency: a study on 73 subjects from 14 Swiss families. Thromb Haemost. 1999;82:1240–1246. [PubMed] [Google Scholar]
- 43.Renne T, Oschatz C, Seifert S, et al. Factor XI deficiency in animal models. J Thromb Haemost. 2009;7 (Suppl 1):79–83. doi: 10.1111/j.1538-7836.2009.03393.x. [DOI] [PubMed] [Google Scholar]
- 44.Salomon O, Steinberg DM, Koren-Morag N, et al. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 45.Salomon O, Steinberg DM, Dardik R, et al. Inherited factor XI deficiency confers no protection against acute myocardial infarction. J Thromb Haemost. 2003;1:658–661. doi: 10.1046/j.1538-7836.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 46•.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7 (Suppl 1):84–87. doi: 10.1111/j.1538-7836.2009.03395.x. Recent and comprehensive review about clinics and therapy of hereditary FXI defiency in patients. [DOI] [PubMed] [Google Scholar]
- 47.Salomon O, Steinberg DM, Zucker M, et al. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 48.Weitz JI. Factor Xa and thrombin as targets for new oral anticoagulants. Thromb Res. 2011;127 (Suppl 2):S5–S12. doi: 10.1016/S0049-3848(10)70147-X. [DOI] [PubMed] [Google Scholar]
- 49.Campos IT, Amino R, Sampaio CA, et al. Infestin, a thrombin inhibitor present in Triatoma infestans midgut, a Chagas’ disease vector: gene cloning, expression and characterization of the inhibitor. Insect Biochem Mol Biol. 2002;32:991–997. doi: 10.1016/s0965-1748(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 50.Hagedorn I, Schmidbauer S, Pleines I, et al. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121:1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- 51.Decrem Y, Rath G, Blasioli V, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robert S, Bertolla C, Masereel B, et al. Novel 3-carboxamide-coumarins as potent and selective FXIIa inhibitors. J Med Chem. 2008;51:3077–3080. doi: 10.1021/jm8002697. [DOI] [PubMed] [Google Scholar]
- 53.Kraft P, Schwarz T, Pochet L, et al. COU254, a specific 3-carboxamide-coumarin inhibitor of coagulation factor XII, does not protect mice from acute ischemic stroke. Exp Transl Stroke Med. 2010;2:5. doi: 10.1186/2040-7378-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter TH, Everson BA, Ratnoff OD. Cabbage seed protease inhibitor: a slow, tight-binding inhibitor of trypsin with activity toward thrombin, activated Stuart factor (factor Xa), activated Hageman factor (factor XIIa), and plasmin. Blood. 1990;75:108–115. [PubMed] [Google Scholar]
- 55.Hojima Y, Pierce JV, Pisano JJ. Pumpkin seed inhibitor of human factor XIIa (activated Hageman factor) and bovine trypsin. Biochemistry. 1982;21:3741–3746. doi: 10.1021/bi00259a003. [DOI] [PubMed] [Google Scholar]
- 56.Krishnamoorthi R, Gong YX, Richardson M. A new protein inhibitor of trypsin and activated Hageman factor from pumpkin (Cucurbita maxima) seeds. FEBS Lett. 1990;273:163–167. doi: 10.1016/0014-5793(90)81075-y. [DOI] [PubMed] [Google Scholar]
- 57.Mahoney WC, Hermodson MA, Jones B, et al. Amino acid sequence and secondary structural analysis of the corn inhibitor of trypsin and activated Hageman Factor. J Biol Chem. 1984;259:8412–8416. [PubMed] [Google Scholar]
- 58.Campos IT, Tanaka-Azevedo AM, Tanaka AS. Identification and characterization of a novel factor XIIa inhibitor in the hematophagous insect, Triatoma infestans (Hemiptera: Reduviidae) FEBS Lett. 2004;577:512–516. doi: 10.1016/j.febslet.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 59.Ulmer JS, Lindquist RN, Dennis MS, Lazarus RA. Ecotin is a potent inhibitor of the contact system proteases factor XIIa and plasma kallikrein. FEBS Lett. 1995;365:159–163. doi: 10.1016/0014-5793(95)00466-m. [DOI] [PubMed] [Google Scholar]
- 60•.Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388–392. doi: 10.1161/ATVBAHA.109.197178. The comprehensive review gives an overview about coagulation factor XI and its inhibitors and summarizes current strategies to target this protease as an anticoagulant therapy. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Yamashita A, Moriguchi-Goto S, et al. Inhibition of factor XI reduces thrombus formation in rabbit jugular vein under endothelial denudation and/or blood stasis. Thromb Res. 2010;125:464–470. doi: 10.1016/j.thromres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita A, Nishihira K, Kitazawa T, et al. Factor XI contributes to thrombus propagation on injured neointima of the rabbit iliac artery. J Thromb Haemost. 2006;4:1496–1501. doi: 10.1111/j.1538-7836.2006.01973.x. [DOI] [PubMed] [Google Scholar]
- 63.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 64.Tucker EI, Marzec UM, White TC, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin J, Deng H, Jin L, et al. Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem. 2006;49:7781–7791. doi: 10.1021/jm060978s. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher WA, Seiler SE, Steinbacher TE, et al. Antithrombotic and hemostatic effects of a small molecule factor XIa inhibitor in rats. Eur J Pharmacol. 2007;570:167–174. doi: 10.1016/j.ejphar.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 67.Buchanan MS, Carroll AR, Wessling D, et al. Clavatadine A, a natural product with selective recognition and irreversible inhibition of factor XIa. J Med Chem. 2008;51:3583–3587. doi: 10.1021/jm800314b. [DOI] [PubMed] [Google Scholar]
- 68••.Zhang H, Lowenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–4692. doi: 10.1182/blood-2010-04-277798. Zhang et al. described a new approach targeting FXI by RNA interference for prevention of arterial and venous thrombosis without increasing the risk of bleeding. FXI antisense oligonucleotide may be a potential strategy to interfere with thrombosis in humans. [DOI] [PubMed] [Google Scholar]
- 69.Crooke ST. Antisense strategies. Curr Mol Med. 2004;4:465–487. doi: 10.2174/1566524043360375. [DOI] [PubMed] [Google Scholar]
- 70.Kastelein JJ, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 71.Mac Leod R, Crosby J, Zhao C. Pharmacological characterization and structure activity relationship of FXI antisense oligonucleotides in cynomolgus monkeys. Blood (ASH Annual Meeting Abstracts) 2009:114. [Google Scholar]