Abstract
Background
Classic teaching suggests that diminished availability of oxygen leads to increased tissue oxygen extraction yet evidence to support this notion in the context of hypoxaemia, as opposed to anaemia or cardiac failure, is limited.
Methods
At 75 m above sea level, and after 7–8 days of acclimatization to 4559 m, systemic oxygen extraction [C(a−v)O2] was calculated in five participants at rest and at peak exercise. Absolute [C(a−v)O2] was calculated by subtracting central venous oxygen content (CcvO2) from arterial oxygen content in blood sampled from central venous and peripheral arterial catheters, respectively. Oxygen uptake was determined from expired gas analysis during exercise.
Results
Ascent to altitude resulted in significant hypoxaemia; median (range) 87.1 (82.5–90.7)% and 6.6 (5.7–6.8) kPa. While absolute C(a−v)O2 was reduced at maximum exercise at 4559 m [83.9 (67.5–120.9) ml litre−1 vs 99.6 (88.0–151.3) ml litre−1 at 75 m, P=0.043], there was no change in oxygen extraction ratio (OER) [C(a−v)O2/CaO2] between the two altitudes [0.52 (0.48–0.71) at 4559 m and 0.53 (0.49–0.73) at 75 m, P=0.500]. Comparison of C(a−v)O2 at peak at 4559 m and the equivalent at sea level for each participant also revealed no significant difference [83.9 (67.5–120.9) ml litre1 vs 81.2 (73.0–120.7) ml litre−1, respectively, P=0.225].
Conclusion
In acclimatized individuals at 4559 m, there was a decline in maximum absolute C(a−v)O2 during exercise but no alteration in OER calculated using central venous oxygen measurements. This suggests that oxygen extraction may have become limited after exposure to 7–8 days of hypoxaemia.
Keywords: altitude, blood gas analysis, exercise, hypoxia, oxygen, physiology
Editor's key points.
Five healthy subjects were studied at sea level and at altitude (4559 m).
Systemic oxygen extraction after peak exercise was measured.
Hypoxaemia was seen but no change in the oxygen extraction ratio.
The lack of increased tissue oxygen extraction despite hypoxaemia is not known.
Exposure to hypobaric hypoxia at high altitude presents a significant physiological challenge to oxygen transport processes within the body. This environmental stress is heightened during exercise by the additional demand of increased oxygen consumption from exercising skeletal muscles. Intuitively, one would expect that when hypoxaemic, whole body oxygen extraction would be increased to match the heightened demand for oxygen. However, during a simulated ascent of Mount Everest in a hypobaric chamber study, Operation Everest II (OE II), no such augmentation of oxygen extraction was observed either at rest or during exercise.1 Using a mathematical modelling approach based on data from OE II it was suggested that oxygen conductance within the muscle was one of the most likely factors to limit exercise at high altitude (tissue diffusion limitation).2
Whole body oxygen extraction is difficult to measure directly but can be inferred from the difference between the influent and effluent oxygen contents of an organ, or for the entire body. As proposed by Adolf Fick, comparison of blood sampled from the pulmonary vein and artery provides a measure of whole body oxygen extraction that can be used to calculate cardiac output and oxygen consumption. Estimations of these measures can be obtained in humans by obtaining simultaneous peripheral arterial and central venous blood samples.
Based on previous data, we hypothesized that absolute whole body oxygen content extraction [C(a−v)O2] derived from arterial and central venous blood samples, and relative oxygen extraction ratio (OER) [C(a−v)O2/CaO2], would be reduced at rest and during peak exercise at high altitude when compared with sea level values.
Methods
Study design and participants
Approval for this study was obtained from the University College London Committee on the Ethics of non-NHS Human Research and all participants gave written informed consent. Participants were five individuals enrolled from the Xtreme Alps 2010 expedition,3 three females and two males; median age was 37.4 years. The participants were studied at sea level in London (75 m) and then at 4559 m in the Margherita Hut high altitude research laboratory. Ascent was initially by road to Alagna (1200 m) and then, on the same day, by cable car to Punta Indren (3200 m) and on foot to the Gnifetti Hut (3647 m). After two nights spent at 3647 m, participants ascended to 4559 m. Study measurements were made on Days 5 and 6 after arriving at 4559 m (Days 7 and 8 at high altitude). Subjectively, participants were well acclimatized to high altitude with no evidence of acute high altitude illness at the time of the study and had no contraindications to undertake a maximal exercise capacity test. The median barometric pressure during the studies was 101.7 kPa at sea level and 58.1 kPa at 4559 m.
Vascular catheter insertion
All catheters were placed under full sterile conditions, local anaesthetic infiltration with 1% lidocaine. A 20 G arterial catheter (Vygon, UK) was inserted into the radial artery of the non-dominant arm. An 11 cm 18-G single lumen central venous catheter (Vygon, UK) was inserted as caudally as possible into the right internal jugular vein using ultrasound (Micromaxx, SonoSite, Inc., Bothell, WA, USA) to identify the vein. A modified Seldinger technique (without skin incision or dilatation of the vein) was used to insert the catheter. Both catheters were then connected to a pressure transducer system (Edwards Lifesciences, CA, USA) using 0.9% sodium chloride as the flushing fluid. Transducers were attached to a monitoring system (GE Marquette Dash 3000, GE Healthcare, Chalfont St Giles, UK), zeroed at the height of the right atrium and attached firmly to the participant. After completion of the exercise protocols and 30 min of recovery, vascular catheters were removed using a sterile technique.
Exercise protocol
Exercise was performed on an electronically braked cycle ergometer (Lode Corival; Lode, Groningen, Netherlands) and cardiopulmonary exercise testing system (Metamax 3b; Cortex, Leipzig, Germany) previously validated at altitude.4 At sea level a ramp increment was selected depending on the sex, age, height, weight and physical fitness of each participant, in order to obtain a test duration of ∼10 min. The same ramp increment was used at altitude. After 3 min of resting data collection, a 3 min period of unloaded pedalling preceded a ramp protocol to exhaustion. Pedalling cadence was maintained at 60 rpm. The load was removed from the ergometer when participants were no longer able to maintain a steady pedalling cadence of 60 rpm. Oxygen uptake was determined breath-by-breath from expired breath analysis. Peak was recorded as the highest average achieved during the final 20 s of exercise.
Measurements
Measurement of blood gas partial pressures, pH, and haemoglobin concentration was performed using the Rapidlab 348 analyser (Siemens Medical Solutions Diagnostics, UK) and a handheld photometric device (Hemocue® Whole Blood Hemoglobin System, HemoCue AB, Angelhoim, Sweden). We have previously validated this Siemens blood gas analyser for use at high altitude and this is described elsewhere.5 At rest and during exercise, 1 ml blood samples were obtained simultaneously from the arterial and venous catheters. Sampling was performed at the end of 3 min of unloaded cycling and then every minute during exercise until exhaustion. A final sample was obtained 1 min after cessation of exercise. Measurements comprised arterial partial pressure of oxygen , arterial partial pressure of carbon dioxide , and pH; arterial oxygen saturation was calculated by the blood gas analyser according to a standard algorithm6 as pulse oximetry is unreliable when falls to <80%.7
Calculations and statistical analysis
Arterial oxygen content was estimated as the product of haemoglobin concentration, and the volume of oxygen carried by 1 g of haemoglobin [1.39 ml g−1] plus the product of the and the solubility coefficient for oxygen (0.225). Absolute oxygen extraction C(a−v)O2 was calculated by subtracting central venous oxygen content (CcvO2) from CaO2 in blood sampled simultaneously from central venous and peripheral arterial catheters, respectively. The OER was calculated by dividing C(a−v)O2 by CaO2.
Data were treated as non-parametric and presented as median (range). Data at altitude and sea level were compared using the Wilcoxon signed-rank test, with statistical significance set at P<0.05. Analyses were performed using SPSS version 21.0 and Graphpad Prism 5.0.
Results
Data collection was successful in all five participants and no complications or adverse events occurred.
Median and at rest were higher at sea level compared with 4559 m (Table 1). Resting absolute oxygen extraction declined after ascent, however, OER remained unchanged at 4559 m (Table 1).
Table 1.
Median (range) resting oxygen extraction values at sea level and 4559 m. [Hb], haemoglobin concentration; * Significant difference between 75 and 4559 m (P<0.05)
| 75 m | 4559 m | |
|---|---|---|
| [Hb] (g dl−1) | 14.2 (13.2–15.2) | 14.7 (12.4–16.3) |
| 13.8 (11.7–14.4) | 6.6 (5.8–7.1)* | |
| 97.9 (96.7–98.2) | 87.1 (82.5–90.7)* | |
| 62.6 (53.8–64.6) | 57.7 (52.8–59.9)* | |
| CaO2 (ml litre−1) | 193.5 (183.0–210.7) | 175.2 (157.9–193.8)* |
| CcvO2 (ml litre−1) | 118.3 (111.6–137.5) | 116.6 (100.5–125.5)* |
| C(a−v)O2 (ml litre−1) | 69.2 (64.7–92.9) | 63.5 (57.4–68.3)* |
| OER | 0.36 (0.35–0.45) | 0.35 (0.33–0.38) |
| (ml min−1) | 381 (248–391) | 384 (255–455) |
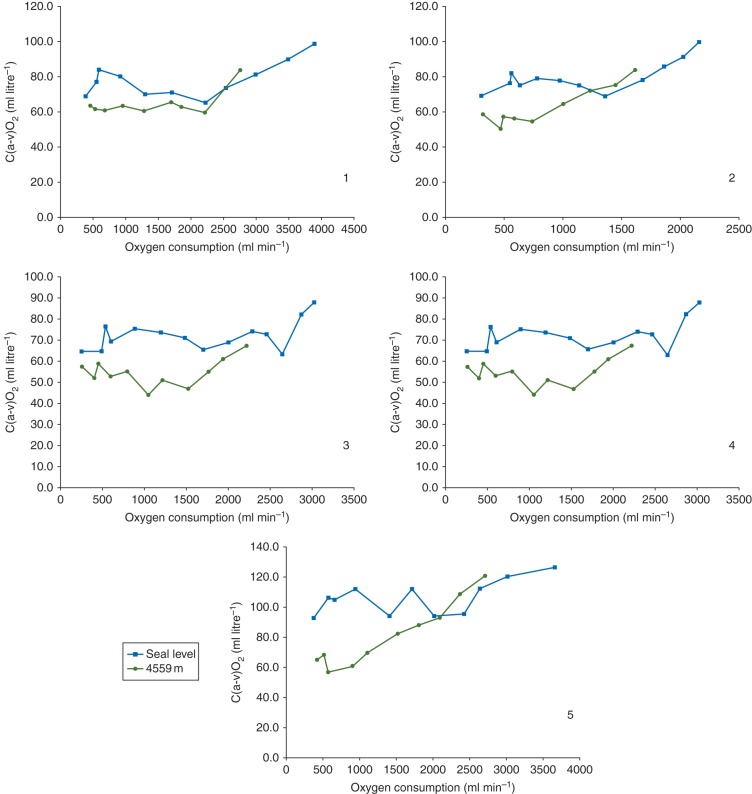
After exercise, median peak was lower at 4559 m than at 75 m (Table 2). In keeping with this, the maximum work rate achieved during exercise was reduced at 4559 m [239 (139–315) W], when compared with 75 m [286 (189–405) W; P=0.042]. During exercise at altitude and declined steadily for each individual as work rate increased during exercise while C(a−v)O2 increased to reach a maximum value at peak (Fig. 1). At peak the absolute C(a−v)O2 declined from 99.6 (88.0–151.3) ml litre−1 at sea level to 83.9 (67.5–120.9) ml litre−1 at 4559 m while OER remained unchanged, 0.53 (0.49–0.73) and 0.52 (0.48–0.71), respectively. As the maximum work rate for each participant was significantly reduced at high altitude, the C(a−v)O2 at peak for 4559 m was compared with the equivalent work rate at sea level during the ramped protocol to exhaustion (Table 3). The median difference between C(a−v)O2 calculations at these two comparable work rates was 2.6 (−5.5–15.0) ml litre−1, P=0.225.
Table 2.
Median (range) oxygen extraction values during peak exercise at sea level and 4559 m. * Significant difference between 75 and 4559 m (P<0.05)
| 75 m | 4559 m | |
|---|---|---|
| 13.0 (12.3–15.8) | 6.0 (5.7–6.8)* | |
| 97.0 (96.0–97.6) | 82.5 (75.1–85.1)* | |
| 45.4 (26.8–49.1) | 36.2 (24.6–42.0) | |
| CaO2 (ml litre−1) | 193.4 (179.0–208.7) | 165.8 (139.8–192.7)* |
| CcvO2 (ml litre−1) | 86.7 (57.4–94.5) | 73.6 (49.0–81.9)* |
| C(a−v)O2 (ml litre−1) | 99.6 (88.0–151.3) | 83.9 (67.5–120.9)* |
| OER | 0.53 (0.49–0.73) | 0.52 (0.48–0.71) |
| (ml min−1) | 3646 (2156–4537) | 2701 (1609–3355)* |
Fig 1.
Individual participant oxygen extraction [C(a−v)O2] data plotted against oxygen consumption during exercise at sea level and at 4559 m.
Table 3.
Oxygen extraction values for peak exercise at 4559 m and the equivalent work rate at sea level (SL). *The C(a−v)O2 for equivalent for each individual at SL (ml litre−1)
| Subject | C(a−v)O2 at peak VO2 (ml litre−1): 4559 m | C(a−v)O2 for equivalent work rate (ml litre−1): SL* | C(a−v)O2 (ml litre−1) difference (4559 m—SL) |
|---|---|---|---|
| 1 | 83.8 | 81.2 | 2.6 |
| 2 | 83.9 | 78.1 | 5.9 |
| 3 | 67.5 | 73.0 | −5.5 |
| 4 | 119.1 | 104.1 | 15.0 |
| 5 | 120.9 | 120.7 | 0.3 |
| Median (range) | 83.9 (67.5–120.9) | 81.2 (73.0–120.7) | 2.6 (−5.5–15.0) |
Discussion
Despite a significant reduction in , and CaO2 at rest after ascent to 4559 m [calculated inspired of 10.9 kPa], there was no increase in systemic oxygen extraction either at rest or at peak exercise. In absolute terms there was a significant decrease in systemic oxygen extraction both at rest and at peak exercise at altitude; however, in relative terms OER remained unchanged. In view of the reduced work rate at maximum exertion at high altitude a comparison was made between mean C(a−v)O2 for peak exertion at high altitude and the equivalent at sea level; no difference in oxygen extraction was detected.
The absence of an increase in whole body OER at 4559 m from the value at 75 m is consistent with data from a previous hypobaric chamber study1 and field data collected at high altitude.8,9 Others have specifically measured limb oxygen extraction during maximal exercise at high altitude, and also found no change from the sea level value.10 The consistent finding that sub-acute exposure to hypoxia is not associated with an increase in systemic oxygen extraction is surprising in view of the reduced arterial oxygen content noted in all of these studies, and goes against the traditional teaching that when oxygen delivery declines, tissues respond by extracting more oxygen from arterial blood, resulting in a lower mixed venous oxygen saturation and content.11 At rest, with only a modest reduction in CaO2, it is conceivable that there is sufficient oxygen delivery to meet tissue demands in various organs such that overall oxygen extraction is unaltered. However, the lack of an increase in oxygen extraction during exercise at altitude is less easy to explain if viewed from the perspective of balancing oxygen supply and demand. In a classic animal model study, reducing systemic oxygen delivery through isovolaemic haemodilution led to a demonstrable increase in tissue oxygen extraction in order to maintain oxygen uptake in the face of diminishing oxygen availability.12 In this experimental setting was constant throughout and it was anaemic hypoxia that impeded systemic oxygen transport. Oxygen extraction increased (in accordance with the Fick equation) until a critical level of oxygen delivery, after which oxygen uptake began to decline as oxygen extraction had reached its upper limit. It is conceivable that this process of increased oxygen extraction may not be possible (or is less effective) during a hypoxic challenge. Factors that may contribute to this lack of increase in overall whole body oxygen extraction at altitude include:
Tissue diffusion limitation of oxygen because of the reduction in partial pressure gradient from the capillary to the mitochondria that is exacerbated during exercise by a reduced capillary transit time within skeletal muscle.13,14
Localized mismatch between tissue oxygen demand and microcirculatory blood flow because of heterogenous flow patterns.
Redistribution of blood flow away from actively exercising muscle in order to ‘protect’ vital organs, a significant reduction in blood flow to other less vital organs such as the gastrointestinal tract, or both.15 Redistribution of flow may therefore result in no overall increase in systemic oxygen extraction.
Altered cellular metabolism with reduced oxygen consumption at a mitochondrial level. Oxygen consumption is determined primarily by mitochondrial oxygen metabolism rather than oxygen delivery, and at altitude alterations in mitochondrial function may limit peak oxygen consumption.16
Using the Fick equation, it is possible to calculate the cardiac output for the group, at rest and maximal exercise. Using this method the sea level values for cardiac output were 5.5 litre min−1 at rest and 36.6 litre min−1 at maximum exercise; at altitude the respective figures were 6.0 and 32.2 litre min−1. This is in keeping with previously published data when volunteers were acutely exposed to simulated high altitude.17 Thus despite a reduced cardiac output at maximum exertion at altitude in our study, there was still no demonstrable increase in whole body oxygen extraction.
Of interest we observed that the altitude-related reduction in peak work rate was proportionally smaller than that of peak, despite the work rate incrementation having been standardized for each subject. This is apparent as a reduction in the slope of the –work rate relationship, which has previously been observed in subjects performing ramp-incremental exercise with acute inhalation hypoxia .18 This may reflect an inability of oxygen transport to meet metabolic demands in the presence of reduced oxygen content,18 a change in the efficiency of oxygen utilization or altered oxygen uptake kinetics with hypoxia.
A limitation of our study was that no sample size calculation was performed before commencing the study and the number of participants was low, therefore it is possible that the lack of difference in OER between sea level and high altitude was the result of a type II error. However, the significant reduction in absolute oxygen extraction strongly suggests that there is no true increase in OER and the likelihood is that extraction is actually reduced. Another limitation of this study is the use of central venous catheters rather than pulmonary artery catheters to measure mixed venous oxygen saturation . This method measures central mixed venous oxygen saturation and is commonly used as a surrogate for clinically. While the two values are strongly correlated, tends to be lower than in healthy volunteers at rest because of the flow of well-saturated venous blood from the renal circulation that returns to the heart via the inferior vena cava; oxygen extraction is lower in the kidneys than in most other organs.19,20 We chose not to insert pulmonary artery catheters in participants as it is technically more challenging than central venous catheter insertion and is associated with significant risk of adverse events. The tip of a correctly place central venous catheter should lie in the superior vena cava just above the entrance to the right atrium. The distance from the entry site of the catheter and the superior vena cava-atrial junction is ∼16 cm in adults,21 although this depends upon the height of entry within the internal jugular vein and individual participant characteristics. The catheters used in this study were 11 cm in length but it was possible to use a ‘low’ insertion technique with ultrasound guidance in order to minimize the distance between the tip of the catheter and the right atrium. It is possible that the stresses induced by both exercise and hypobaric hypoxia may have led to disparity between measured and true . However, it is likely that this would have resulted in a relative under-estimation of oxygen extraction while resting at sea level; both exercise and hypoxia may have resulted in a relative over-estimation of oxygen extraction. That said, as an identical sampling technique was used at sea level and altitude one would expect to detect relative changes in OER if they were present, even if the absolute values of were not truly representative of .
The clinical implication of this high altitude field study is that increased tissue oxygen extraction may not be an innate physiological response to sub-acute hypoxaemia as it is in other situations of reduced oxygen delivery such as haemorrhage. Thus under conditions of hypoxaemia, strategies to maintain oxygen homeostasis at a cellular level may differ from those used in anaemia or cardiovascular failure. Simple arithmetic rebalancing of the oxygen delivery equation (the product of CaO2 and cardiac output) may well restore values back to those we associate with normal physiology but fail to address the underlying biological abnormality.
Using C(a−v)O2 derived from peripheral arterial and central venous blood samples, we have shown a decrease in absolute whole body oxygen extraction at rest and peak exercise after ascent to high altitude (4559 m) while OER remained unchanged. The cause for this lack of increase in oxygen extraction remains uncertain but may be attributable to diminished diffusion of oxygen from the microcirculation to mitochondria because of a reduced gradient.
Authors’ contributions
A.C.: study conduct, and manuscript preparation; D.S.M.: study design, study conduct, data analysis, and manuscript preparation; P.M.: study conduct and manuscript preparation; K.M.: study conduct and manuscript preparation; M.E.: study conduct and manuscript preparation; M.G.M.: study design, data analysis, and manuscript preparation; M.P.W.G.: study design, data analysis, and manuscript preparation.
Declaration of interest
None of the authors have any conflicts of interest with companies or manufacturers who will benefit from the results of the present study. M.P.W.G. leads the Xtreme-Everest oxygen research consortium which has received unrestricted grant funding from: BOC Medical (Linde Group), Ely-Lilly Critical Care, Smiths Medical, Deltex Medical, London Clinic, and Rolex. All funds were paid directly to the home institutions of researchers within the consortium. M.P.W.G. has also received honoraria for speaking (NOT related to this review) and/or travel expenses from: Baxter, Fresenius-Kabi, BOC Medical (Linde Group), Ely-Lilly Critical Care. M.G.M. is a member of the Board of Management of the BJA.
Funding
Unrestricted grant support was provided by Deltex Medical and Smiths Medical.
Acknowledgements
Xtreme Alps research group: Tom Adams, Lindsay Biseker, Adam Booth, Oliver Burdall, Alexandra Cobb, Andrew Cumpstey, Steve Dauncey, Mark Edsell, James Farrant, Martin Feelisch, Bernadette Fernandez, Oliver Firth, Edward Gilbert, Daniel Grant, Michael Grocott, Phil Hennis, Laura Jackson, Will Jenner, Jildou van der Kaaij, Maryam Khosravi, Edith Kortekaas, Denny Levett, Zeyn Mahomed, Daniel Martin, Paula Meale, Jim Milledge, Kay Mitchell, Damian Mole, Oliver Moses, Michael Mythen, Fabio Rigat, Alasdair O'Doherty, Alex Salam, Matt Sanborn, Adam Sheperdigian, Fiona Shrubb, Jo Simpson, Nick Talbot, Liesel Wandrag, Savini Wijesingha, Wilby Williamson, Tom Woolley, Heng Yow.
References
- 1.Sutton JR, Reeves JT, Wagner PD, et al. Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol. 1988;64:1309–21. doi: 10.1152/jappl.1988.64.4.1309. [DOI] [PubMed] [Google Scholar]
- 2.Wagner PD. Algebraic analysis of the determinants of VO2max. Respir Physiol. 1993;93:221–37. doi: 10.1016/0034-5687(93)90007-w. [DOI] [PubMed] [Google Scholar]
- 3.Martin DS, Gilbert-Kawai ET, Meale PM, et al. Design and conduct of ‘Xtreme Alps’: a double-blind, randomised controlled study of the effects of dietary nitrate supplementation on acclimatisation to high altitude. Contemp Clin Trials. 2013;36:450–9. doi: 10.1016/j.cct.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Levett DZ, Martin DS, Wilson MH, et al. Design and conduct of Caudwell Xtreme Everest: an observational cohort study of variation in human adaptation to progressive environmental hypoxia. BMC Med Res Methodol. 2010;10:98. doi: 10.1186/1471-2288-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–9. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmeyer S, Burnett RW, Chatburn RL, Clausen JL, Durst RA. Fractional oxyhemoglobin, oxygen content and saturation, and related quantities in blood: terminology, measurement, and reporting. National Committee for Clinical Laboratory Standards. Document C25-A. 1997;12:11. [Google Scholar]
- 7.Jubran A. Pulse oximetry. Intensive Care Med. 2004;30:2017–20. doi: 10.1007/s00134-004-2399-x. [DOI] [PubMed] [Google Scholar]
- 8.Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Why is VO2 max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003;284:R304–16. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- 9.Vogel JA, Hartley LH, Cruz JC, Hogan RP. Cardiac output during exercise in sea-level residents at sea level and high altitude. J Appl Physiol. 1974;36:169–72. doi: 10.1152/jappl.1974.36.2.169. [DOI] [PubMed] [Google Scholar]
- 10.Lundby C, Sander M, van Hall G, Saltin B, Calbet JA. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol. 2006;573:535–47. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLellan SA, Walsh TS. Oxygen delivery and haemoglobin. CEACCP. 2004;4:123–6. [Google Scholar]
- 12.Cain SM. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol. 1977;42:228–34. doi: 10.1152/jappl.1977.42.2.228. [DOI] [PubMed] [Google Scholar]
- 13.Roca J, Hogan MC, Story D, et al. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol. 1989;67:291–9. doi: 10.1152/jappl.1989.67.1.291. [DOI] [PubMed] [Google Scholar]
- 14.Wagner PD. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc. 1992;24:54–8. [PubMed] [Google Scholar]
- 15.Martin D, McCorkell S, Vercueil A, et al. Increased gastric-end tidal PCO2 gap during exercise at high altitude measured by gastric tonometry. High Alt Med Biol. 2007;8:50–5. doi: 10.1089/ham.2006.1022. [DOI] [PubMed] [Google Scholar]
- 16.Levett DZ, Radford EJ, Menassa DA, et al. Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J. 2012;26:1431–41. doi: 10.1096/fj.11-197772. [DOI] [PubMed] [Google Scholar]
- 17.Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–70. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- 18.Murphy PC, Cuervo LA, Hughson RL. A study of cardiorespiratory dynamics with step and ramp exercise tests in normoxia and hypoxia. Cardiovasc Res. 1989;23:825–32. doi: 10.1093/cvr/23.10.825. [DOI] [PubMed] [Google Scholar]
- 19.Barratt-Boyes BG, Wood EH. The oxygen saturation of blood in the venae cavae, right-heart chambers, and pulmonary vessels of healthy subjects. J Lab Clin Med. 1957;50:93–106. [PubMed] [Google Scholar]
- 20.Dahn MS, Lange MP, Jacobs LA. Central mixed and splanchnic venous oxygen saturation monitoring. Intensive Care Med. 1988;14:373–8. doi: 10.1007/BF00262891. [DOI] [PubMed] [Google Scholar]
- 21.Andrews RT, Bova DA, Venbrux AC. How much guidewire is too much? Direct measurement of the distance from subclavian and internal jugular vein access sites to the superior vena cava-atrial junction during central venous catheter placement. Crit Care Med. 2000;28:138–42. doi: 10.1097/00003246-200001000-00023. [DOI] [PubMed] [Google Scholar]